Abstract
Background:
Laparoscopic left hemihepatectomy (LLH) has been widely accepted as a minimally invasive alternative to open liver surgery. We assessed the benefits and drawbacks of LLH compared with open left hemihepatectomy (OLH) using meta-analysis.
Methods:
Relevant literature was retrieved using PubMed, Embase, Cochrane, and Ovid Medline databases. Multiple parameters of efficacy and safety were compared between the treatment groups. Results are expressed as odds ratio (OD) or mean difference (MD) with 95% confidence interval (95% CI) for fixed- and random-effects models.
Results:
The meta-analysis included 13 trials involving 1163 patients. Compared with OLH, LLH significantly reduced intraoperative blood loss (MD, −91.01; 95% CI, −139.12 to −42.89; P = .0002), transfusion requirement (OR, 0.24; 95% CI, 0.11–0.54; P = .0004), time to oral intake (MD, −0.80; 95% CI, −1.27 to −0.33; P = .0008), and hospital stay (MD, −3.94; 95% CI, −4.85 to −3.03; P < .0001). However, operative time; complications rate; and postoperative alanine transferase, albumin, and total bilirubin levels did not differ significantly between the 2 surgical groups (P > .05). For hepatolithiasis treatment, there were no significant differences in operative time, residual stones, stone recurrence, and complications rate between the groups (P > .05), but LLH resulted in lower incisional infection rate (OR, 0.44; 95% CI, 0.22–0.89; P = .02) than OLH. The LLH group demonstrated higher bile leakage rate (OR, 1.79; 95% CI, 1.14–2.81; P = .01) and incurred greater hospital costs (MD, 618.56; 95% CI, 154.47–1082.64; P = .009).
Conclusions:
LLH has multiple advantages over OLH and should thus be considered as the first choice for left hemihepatectomy.
Keywords: hepatolithiasis, laparoscopic, left hemihepatectomy
1. Introduction
The ideal liver surgery technique should be simple, minimally invasive, and reliable. Additionally, it should allow fast functional recovery, cause minimal pain, and be affordable for patients. Laparoscopic hepatectomy (LH) fulfills these requirements.[1,2] Indeed, LH has matured and become a widely applicable treatment option for benign and malignant liver lesions.[3–9] Left hepatectomy is the most common procedure in many laparoscopic series worldwide and is particularly suitable for minimally invasive surgery because the left hemiliver is more likely to be exposed during surgery owing to its smaller volume in the abdominal cavity, relatively independent and acute angle tract, and clear vasculature gradation.[10]
Left-sided hepatectomy, including left lateral sectionectomy and left hemihepatectomy, is the main procedure performed for most patients with lesions in the left side of the liver. Our previous study confirmed these advantages, and we proposed that laparoscopic left lateral hepatic lobectomy should be considered as the gold standard for treatment of left hepatic lobe lesions.[8] However, there has been no comprehensive meta-analysis directly comparing the benefits and drawbacks of laparoscopic left hemihepatectomy (LLH) to open left hemihepatectomy (OLH); therefore, no broad consensus on which approach is superior has been reached. Furthermore, there are no standard guidelines on indications for the laparoscopic approach. The purpose of this study was to compare the efficacy and safety between LLH and OLH by a meta-analysis of published trials.
2. Material and methods
2.1. Literature search
A systematic search was conducted using PubMed, Embase, Cochrane Database of Systematic Reviews, and Ovid Medline with the following keywords: hepatectomy, left hemihepatectomy, left-side hepatectomy, laparoscopic hepatectomy, and laparoscopic versus open. The search included articles published between the date of electronic database creation and May 30, 2018. Additionally, we searched the reference lists of selected papers and systematic reviews for potentially relevant studies missed by the original search. Two reviewers independently selected the eligible studies. Disagreements on article inclusion were resolved by discussion with a third reviewer. The study protocol followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and Cochrane Handbook for Diagnostic Test Accuracy Reviews. Institutional review board approval and patient consent were waived because of the retrospective, anonymized nature of the study. The quality of each study was assessed using the Newcastle–Ottawa scale (NOS).[11] A star system of NOS (range, 0–9 stars) was developed for evaluation (Table 1). The study was approved by the ethics committee.
Table 1.
Operative outcomes of included trials.

2.2. Inclusion and exclusion criteria
All case–control studies comparing LLH to OLH were selected for further review. Studies were included if they involved left hemihepatectomy with no requirement for additional procedures. The selected studies also reported malignancy, time to oral intake (days), blood transfusion, mortality, complications, operative time (minutes), length of hospital stay (days), hospital expense (dollars), blood loss (mL), number of residual stones, stone recurrence, and/or postoperative alanine transferase (ALT), albumin, and total bilirubin (TB) levels. Specific complications included bile leakage, incisional infection, bleeding, ascites, pneumonia, intra-abdominal fluid collection, incisional hernia, abscess, urinary tract infection, ulcer, and pulmonary embolus.
Articles not reporting any of these outcomes as well as editorials, review articles, and animal studies were excluded. Neither authorship nor publisher information influenced article selection.
2.3. Statistical analysis
Analyses were performed using Review Manager version 5.1 (RevMan, Cochrane Collaboration, Oxford, UK). Group differences in dichotomous data are expressed as odds ratios (ORs) and group differences in continuous data as mean differences (MDs), both with 95% confidence intervals (CIs). Continuous variables were pooled using the inverse variance method and dichotomous variables using the Mantel–Haenszel method. Statistical heterogeneity was evaluated by the χ2 test. P < .05 (2-tailed) was considered statistically significant for all tests. If heterogeneity was significant, we used the random-effects model. Otherwise, we used the fixed-effects model. If data were reported as median and range rather than mean and standard deviation (SD), the mean and SD were estimated as described previously.[12]
3. Results
3.1. Study selection and characteristics
Figure 1 illustrates the search process and final selection of relevant studies. We analyzed 13 trials[13–25] involving 1163 patients that met all the criteria (Table 1). These included 10 trials comparing LLH to OLH for hepatolithiasis, 1 trial for hepatocellular carcinoma, and 1 trial for benign and malignant lesions, while 1 trial did not report the lesions.
Figure 1.
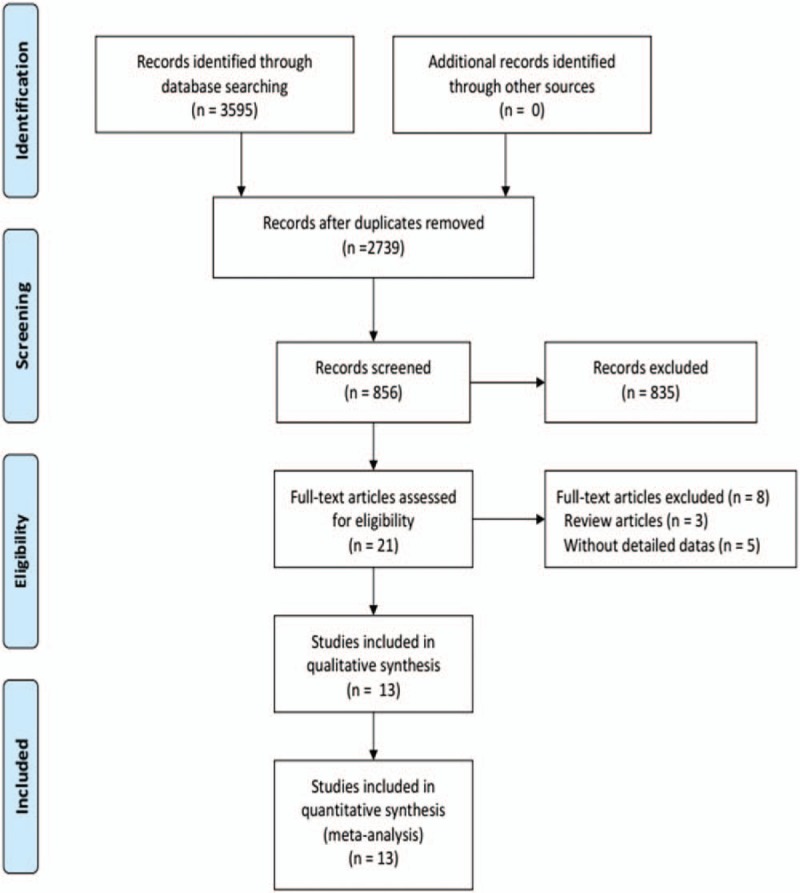
Flow diagram of study selection.
3.2. Overall comparison of LLH versus OLH
We first compared intraoperative parameters, postoperative parameters, hospital costs, and indices of liver function between the LLH and OLH cohorts. Information on operative time (minutes) was provided in all 13 trials and did not differ significantly between the LLH and OLH groups (MD, 11.67; 95% CI, −9.56 to 32.91; P = .28; Fig. 2A). Twelve trials reported information on blood loss and 6 trials on transfusion information. Compared with the OLH group, the LLH group demonstrated significantly reduced blood loss (MD, −91.01; 95% CI, −139.12 to −42.89; P = .0002; Fig. 2B) and need for blood transfusion (OR, 0.24; 95% CI, 0.11–0.54; P = .0004; Fig. 2C).
Figure 2.
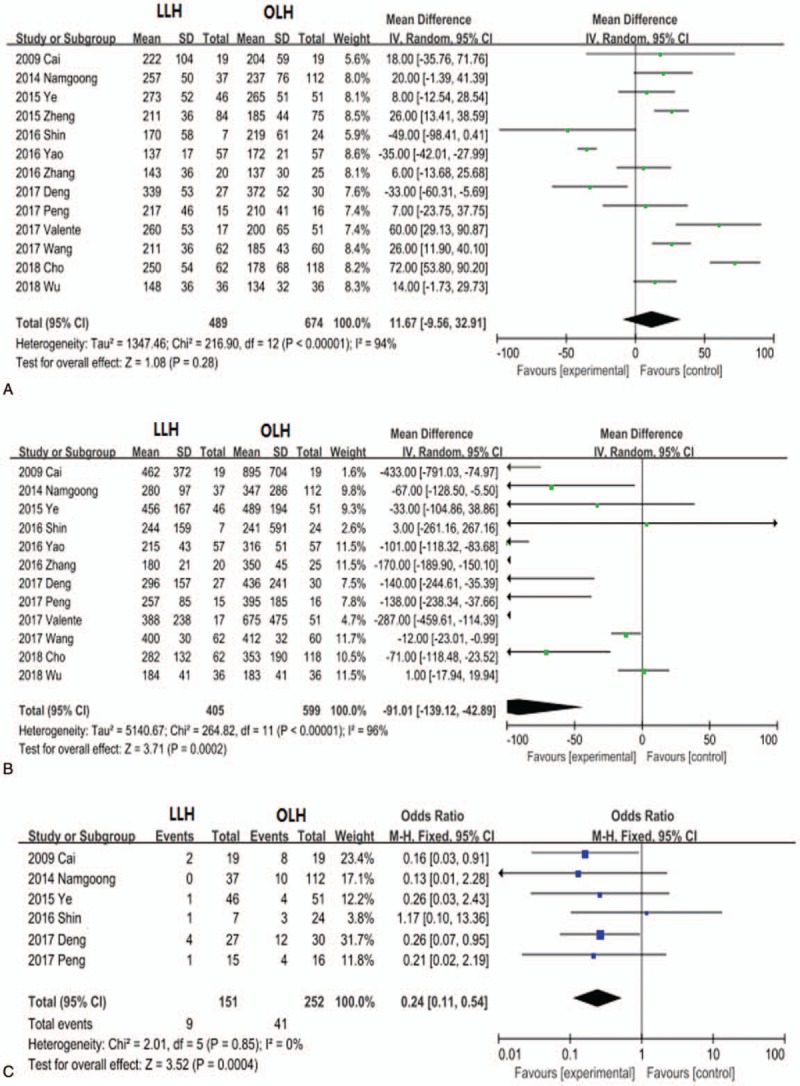
Forest plots comparing the intraoperative parameters operative time (A), blood loss (B), and blood transfusion (C) between the entire LLH and OLH groups. LLH = laparoscopic left hemihepatectomy, OLH = open left hemihepatectomy.
The time to oral intake and length of hospital stay were evaluated in 6 and 12 studies, respectively. Patients in the LLH group exhibited a shorter time to oral intake (MD, −0.80; 95% CI, −1.27 to −0.33; P = .0008; Fig. 3A) and shorter hospital stay (MD, −3.94; 95% CI, −4.85 to −3.03; P < .0001; Fig. 3B). Information on hospitalization costs was reported in 4 trials. Patients in the OLH group incurred less hospitalization costs than those in the LLH group (average, $618.56; 95% CI, 154.47–1082.64; P = .009; Fig. 3C). Twelve trials provided information on complications (Table 2). Neither overall complication rate (OR, 0.74; 95% CI, 0.55–1.00; P = .05; Fig. 3D) nor severe grade III + IV complication rate (OR, 0.69; 95% CI, 0.30–1.60; P = .39) differed significantly between the 2 groups. There were no statistically significant differences in postoperative ALT (MD, −9.14; 95% CI, −39.75 to 21.47; P = .56), albumin (MD, 2.32; 95% CI, −2.56 to 7.21; P = .35), and TB levels (MD, −0.09, 95% CI −0.30 to 0.12; P = .39) between the LLH and OLH groups.
Figure 3.
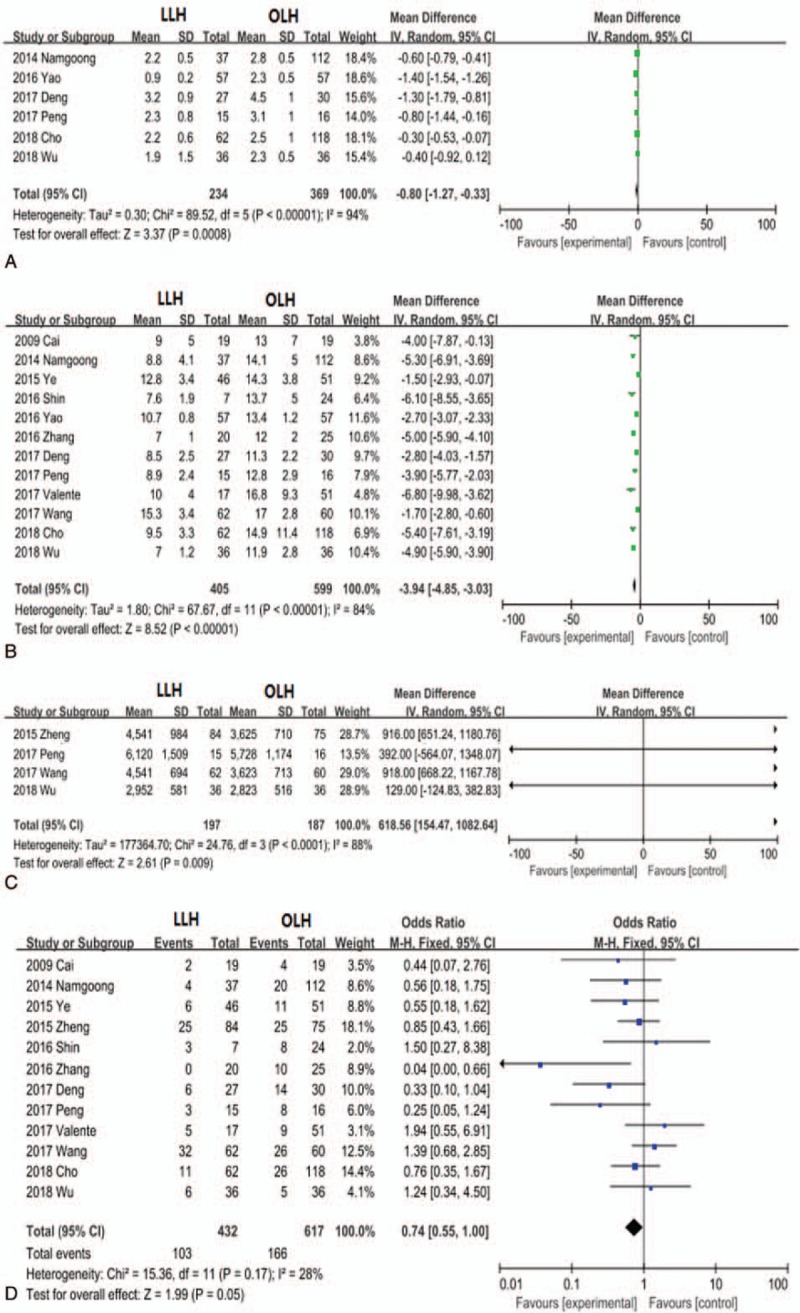
Forest plots comparing postoperative parameters time to oral intake (A), length of hospital stay (B), hospitalization charges (C), and complications (D) between the entire LLH and OLH treatment groups. LLH = laparoscopic left hemihepatectomy, OLH = open left hemihepatectomy.
Table 2.
Description of included trials and the specific complications.

3.3. LLH versus OLH for hepatolithiasis
Ten trials compared LLH to OLH for hepatolithiasis patients. According to overall results, the operative time was not statistically different between the LLH and OLH groups (MD, 1.38; 95% CI, −19.98 to 22.73; P = .90; Fig. 4A), but the LLH group demonstrated significantly reduced blood loss (MD −0.91; 95% CI, −1.39 to −0.44; P = .0002; Fig. 4B), need for blood transfusion (OR, 0.24; 95% CI, 0.11–0.54; P = .0004), time to oral intake (MD, −0.91; 95% CI, −1.39 to −0.44; P = .0002; Fig. 4A), and hospital stay (MD, −3.46; 95% CI, −4.40 to −2.53; P < .001). Also consistent with overall results, hospital expenses were high in the LLH group (MD, 618.56; 95% CI, 154.47–1082.64; P = .009) than in the OLH group.
Figure 4.
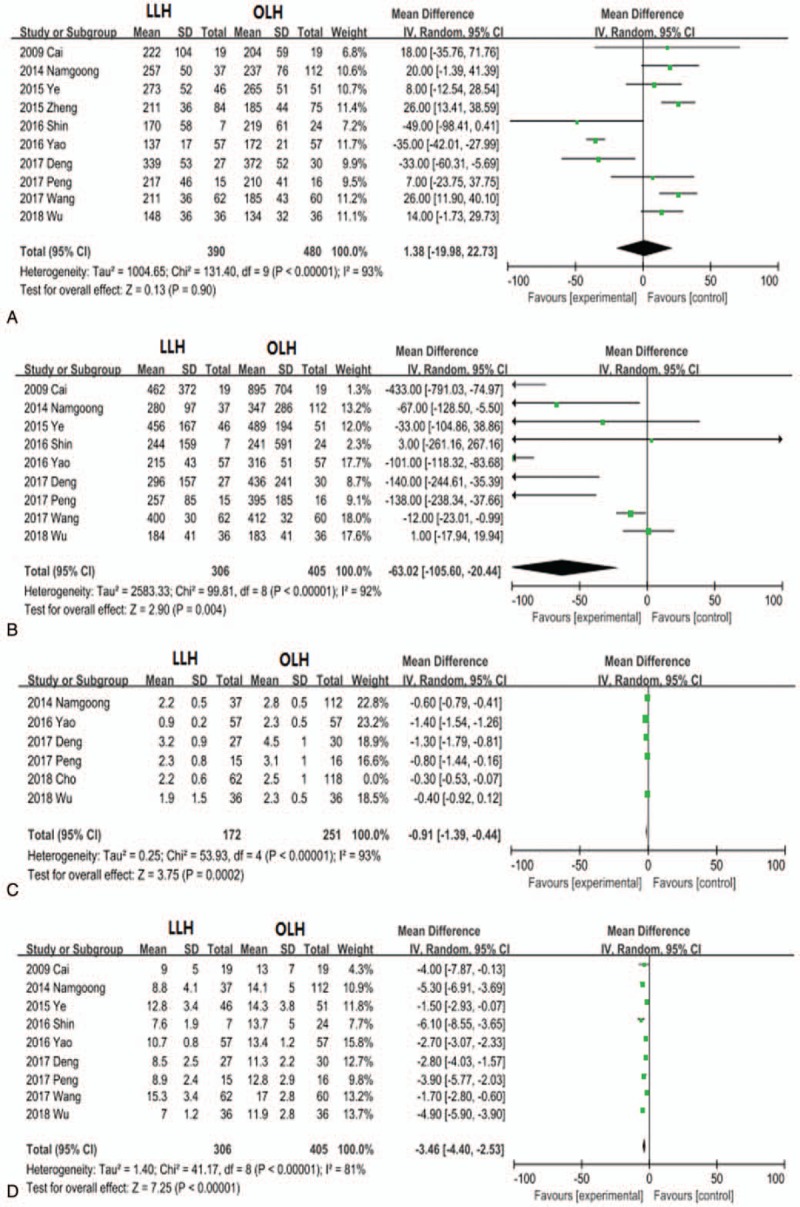
Forest plots comparing the hepatolithiasis outcomes operative time (A), blood loss (B), blood transfusion (C), and length of hospital stay (D).
Nine trials for hepatolithiasis patients provided information on complications. The overall complication rate did not differ significantly between the LLH and OLH groups (OR, 0.81; 95% CI, 0.58–1.15; P = .24; Fig. 5A). Residual stone incidence was evaluated in 10 studies, and stones recurrence was evaluated in 5 studies. There were no significant group differences in the residual stone rate (OR, 0.86; 95% CI, 0.53–1.42; P = .56; Fig. 5B) or stone recurrence rate (OR, 0.69; 95% CI, 0.26–1.82; P = .45; Fig. 5C).
Figure 5.
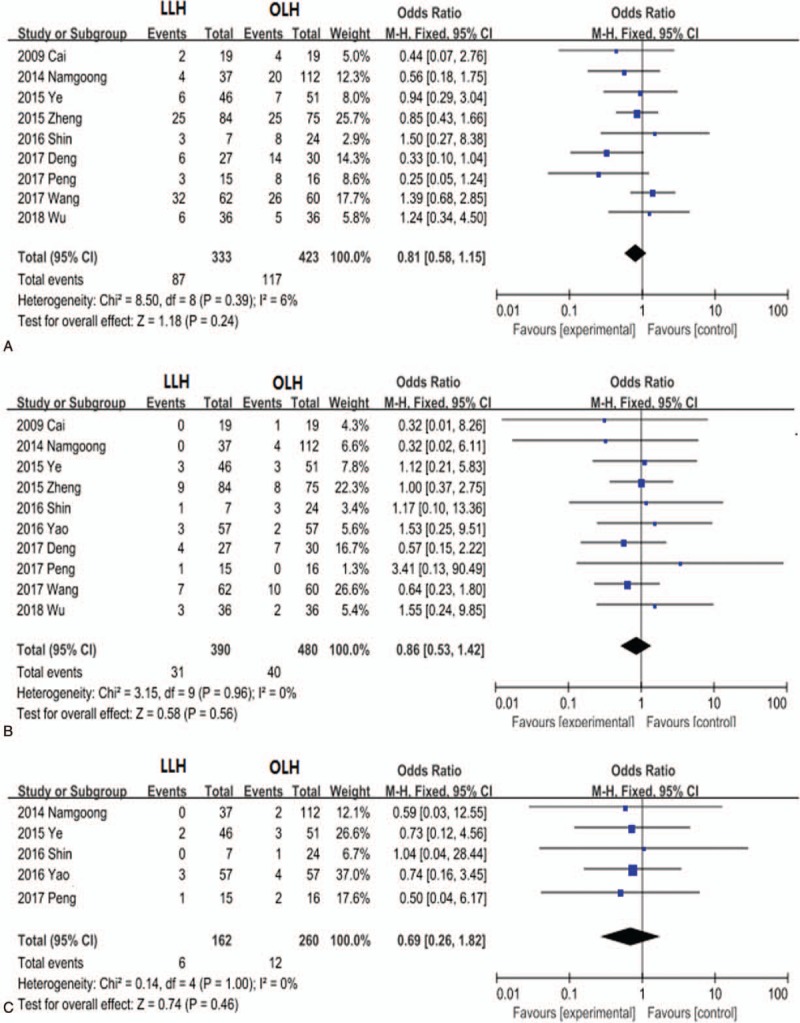
Forest plots comparing the hepatolithiasis outcomes complications rate (A), residual stones (B), and stone recurrence (C).
Further subgroup analyses revealed that the LLH group exhibited significantly lower incisional infection rate (OR, 0.44; 95% CI, 0.22–0.89; P = .02; Fig. 6 B) than the OLH group, while incidences of intra-abdominal fluid collection (OR, 1.26; 95% CI, 0.52–3.03; P = .61; Fig. 6 C), abdominal infection (OR, 1.09; 95% CI, 0.60–1.98; P = .78; Fig. 6 D), and pneumonia (OR, 0.49; 95% CI, 0.13–1.83; P = .29; Fig. 6 E) did not differ significantly between the groups. In contrast, bile leakage rate was higher in the LLH group (OR, 1.79; 95% CI, 1.14–2.81; P = 0.01; Fig. 6 A) than in the OLH group
Figure 6.
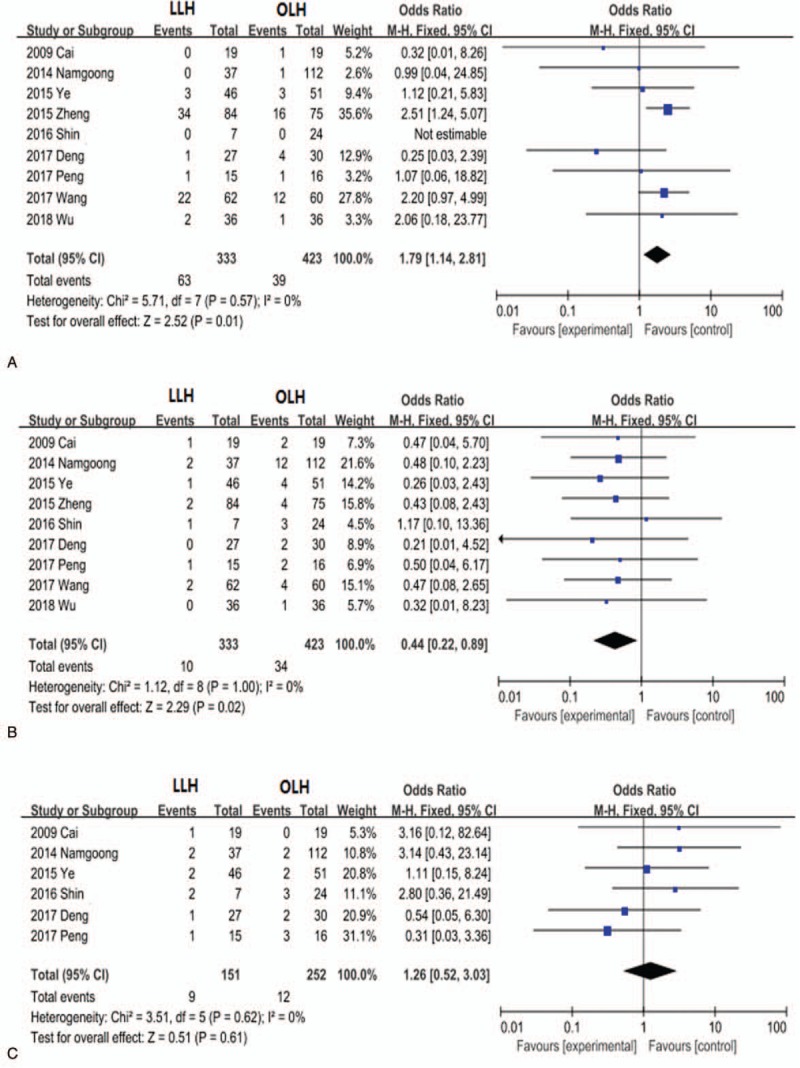
Forest plots comparing hepatolithiasis complications bile leakage (A), incisional infection (B), intra-abdominal fluid collection (C), abdominal infection (D), and pneumonia (E).
Figure 6 (Continued).
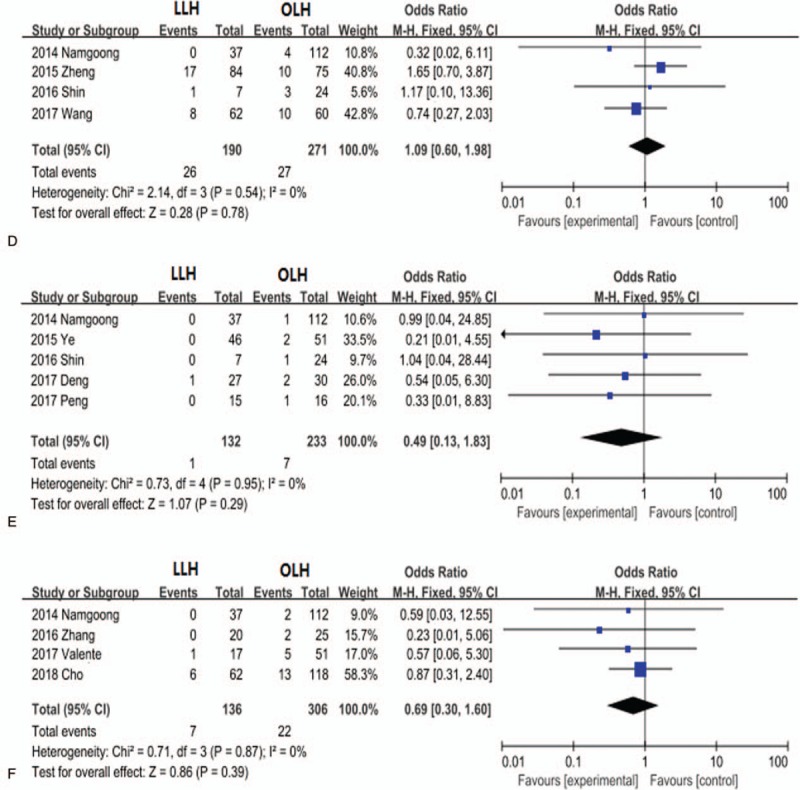
Forest plots comparing hepatolithiasis complications bile leakage (A), incisional infection (B), intra-abdominal fluid collection (C), abdominal infection (D), and pneumonia (E).
3.4. Publication bias
Funnel plots for blood transfusion, blood loss, time to oral intake, hospital stay, bile leakage, and incisional infection showed basic symmetry, indicating no substantial publication bias.
4. Discussion
In the past, the drawbacks of laparoscopic hepatectomy included relatively greater technical complexity, longer operative time, and higher incidence of postoperative complications, such as bleeding, than those in open hepatectomy.[26] The liver is rich in blood vessels and blood supply, causing frequent bleeding during resection, and it is difficult to precisely control bleeding by laparoscopy. Furthermore, the lack of tactile feedback and requirement for greater hand-eye coordination increase surgical difficulty and prolong the operative time. The availability of the equipment needed for laparoscopic hepatectomy is also limited compared to the basic equipment for open hepatectomy. With recent advances in laparoscopic technology and equipment, all these problems have been mitigated, and the feasibility and safety of laparoscopic hepatectomy have been confirmed by several large-scale studies. However, none of these studies focused specifically on LLH.[5–9] The left hemiliver exhibits a relatively simple intrahepatic tract and clear borders with the surrounding organs, anatomical features favorable for laparoscopic surgery. Nonetheless, there was still a lack of clinical evidence supporting these proposed advantages from large-sample multi-center studies. In this work, we found that LLH indeed has many advantages over OLH, including reduced blood loss, less frequent transfusion requirement, shorter hospital stay, lesser time to oral intake, and lower frequencies of certain specific complications.
Contrary to expectations based on previous studies, the control of bleeding during LLH was actually superior to open hepatectomy, possibly due to improved intraoperative magnification for surgical manipulations, better pressure control of the pneumoperitoneum, and new coagulating devices.[27] Indeed, the LLH group was less likely to require blood transfusion and experienced less blood loss compared to the OLH group, with no substantial difference in the operative time. We believe that occasional bleeding and hepatic vein injury are the most common hepatectomy risks regardless of the approach. Hence, detailed preoperative evaluations, including computed tomography, magnetic resonance imaging, 3D visualization technology, and especially intraoperative ultrasound, to accurately reveal the size and location of the lesion, as well as individual variations in blood vessels and the biliary tract, are essential to reduce bleeding risk regardless of the surgical approach. The rapid development of laparoscopic equipment, such as the Endo-GIA stapler, not only greatly reduce the operative time but also effectively prevent bleeding.[28] Moreover, ultrasonic shears, argon beams, vessel sealing devices (eg, LigaSure), microwave coagulators, laparoscopic ultrasound systems, and suturing techniques are improving constantly, leading to a rapid global increase in the popularity of laparoscopic hepatectomy. In turn, increasing use of the procedure has resulted in enhanced surgical expertise and standardization of surgical steps.
The overall complication rate did not differ significantly between the LLH and OLH groups. However, subgroup analysis revealed a significantly lower incisional infection rate in the LLH group than in the OLH group. Residual stone and stone recurrence rates did not differ between the groups. Furthermore, the 2 groups did not differ significantly in postoperative ALT, albumin, and TB levels, suggesting no difference in the extent of perioperative liver injury or functional outcome. The only 2 unfavorable outcomes of LLH were higher bile leakage and greater total hospital expenses. Greater hospital expenses are understandable as laparoscopy requires numerous advanced instruments, such as trocars, which are not required for conventional open surgery. However, in some cases, greater surgical costs may be compensated by shorter hospital stay and less frequent need for blood transfusion.[25,29] Alternatively, bile leakage may actually be underestimated because color distortion of the laparoscope camera and display could make smaller bile leakage volumes difficult to detect.[30] At the same time, inflammation and edema due to cholangitis increase bile wall thickness relative to blood vessels. When the hepatic parenchyma was separated with ultrasonic shears, only the blood vessels were coagulated, so the small bile duct may reopen after surgery. Therefore, the bile duct should be handled with care during ultrasonic scalpel use. The targeted section of the liver should also be carefully examined after dissection.[31] For patients with high risk of bile leakage, such as those with severe cholangitis or perihepatitis, the use of T tube drainage is recommended for prevention and treatment.[32]
Usually, we distinguish the left and right hemiliver by the hepatic ischemic line, but the middle hepatic vein is occasionally damaged due to deviation. Hence, it is very important to determine the direction of the middle hepatic vein before dissection. We first find the branch of the middle hepatic vein and then look for the trunk along the branch. Laparoscopic ultrasound can ensure complete avoidance of the middle hepatic vein while providing images of intrahepatic intubation.[33] The LLH protocol used at our center and critical recommendations are as follows:
-
(1)
detailed preoperative evaluation by videography;
-
(2)
individual isolation and ligation of the left hepatic artery and left portal vein before hepatic parenchymal dissection (which is aided by their anatomic superiority and ease of left hepatic artery and portal branch division);
-
(3)
identification of the middle hepatic vein branch and then location of the trunk;
-
(4)
careful resection of the hepatic parenchyma using ultrasonic shears without pulling on the tissue (placement of T tubes is recommended for patients with severe cholangitis to prevent small bile duct injury before complete coagulation);
-
(5)
maintenance of central venous pressure between 4 and 6 cm H2O, the optimal intraoperative range for reducing bleeding and hepatic vein reflux.[34]
Limitations of this study are typical of most meta-analyses, including inter-trial heterogeneity, selection bias, and publication bias. Sources of heterogeneity include variations in patient inclusion, patient condition, parameter definition, and surgical expertise. Second, few trials included were randomized and controlled. Third, although we tried to identify all relevant data, some potentially relevant studies were excluded due to lack of reported data. Finally, this study was based only on reports published in English and Chinese.
This direct comparison indicates that LLH can improve multiple efficacy and safety metrics for left hemihepatectomy compared to OLH, such as wound infection rate, blood loss, time to oral intake, and hospital duration, without increased operative time or complications. Only hospital cost and bile leakage were greater for LLH. Therefore, our findings suggest overall enhanced recovery after surgery. We thus recommend LLH as the first choice for the treatment of left hemiliver lesions.
Author contributions
Conceptualization: Xiangbao Yin, Dilai Luo, Yong Huang.
Data curation: Xiangbao Yin, Dilai Luo, Yong Huang.
Formal analysis: Xiangbao Yin, Dilai Luo, Yong Huang, Mingwen Huang.
Funding acquisition: Xiangbao Yin, Yong Huang.
Investigation: Xiangbao Yin, Yong Huang.
Methodology: Xiangbao Yin, Yong Huang.
Project administration: Xiangbao Yin, Dilai Luo, Yong Huang, Mingwen Huang.
Resources: Xiangbao Yin, Yong Huang, Mingwen Huang.
Software: Xiangbao Yin, Dilai Luo, Yong Huang, Mingwen Huang.
Supervision: Xiangbao Yin, Dilai Luo, Yong Huang, Mingwen Huang.
Validation: Xiangbao Yin, Yong Huang, Mingwen Huang.
Visualization: Xiangbao Yin, Yong Huang, Mingwen Huang.
Writing – original draft: Xiangbao Yin, Yong Huang.
Writing – review and editing: Xiangbao Yin, Dilai Luo, Yong Huang, Mingwen Huang.
Footnotes
Abbreviations: CIs = confidence intervals, LH = laparoscopic hepatectomy, LLH = laparoscopic left hemihepatectomy, MDs = mean differences, NOS = Newcastle–Ottawa scale, OLH = open left hemihepatectomy, ORs = odds ratios, SD = standard deviation.
This work was supported by the National Natural Science Foundation of China (No. 81760514).
The authors declare that they have no competing interests.
References
- [1].Vanounou T, Steel JL, Nguyen KT, et al. Comparing the clinical andeconomic impact of laparoscopic versus open liver resection. Ann Surg Oncol 2010;17:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Polignano FM, Quyn AJ, de Figueiredo RS, et al. Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc 2008;22:2564–70. [DOI] [PubMed] [Google Scholar]
- [3].Chen J, Li H, Liu F, et al. Surgical outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma for various resection extent. Medicine (Baltimore) 2017;96:e6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tomassini F, Scuderi V, Colman R, et al. The single surgeon learning curve of laparoscopic liver resection: a continuous evolving process through stepwise difficulties. Medicine (Baltimore) 2016;95:e5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kasai M, Cipriani F, Gayet B, et al. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery 2018;16:985–95. [DOI] [PubMed] [Google Scholar]
- [6].Liu X, Min X, Ma Z, et al. Laparoscopic hepatectomy produces better outcomes for hepatolithiasis than open hepatectomy: an updated systematic review and meta-analysis. Int J Surg 2018;51:151–63. [DOI] [PubMed] [Google Scholar]
- [7].Xu J, Hu C, Cao HL, et al. Meta-analysis of laparoscopic versus open hepatectomy for live liver donors. PLoS One 2016;11:e0165319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu Z, Ding H, Xiong X, et al. Laparoscopic left lateral hepatic sectionectomy was expected to be the standard for the treatment of left hepatic lobe lesions: a meta-analysis. Medicine (Baltimore) 2018;97:e9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yu YD, Kim KH, Jung DH, et al. Robotic versus laparoscopic liver resection: a comparative study from a single center. Langenbecks Arch Surg 2014;399:1039–45. [DOI] [PubMed] [Google Scholar]
- [10].Lee SE, Jang JY, Lee JM, et al. Selection of appropriate liver resection in left hepatolithiasis based on anatomic and clinical study. World J Surg 2008;32:413–8. [DOI] [PubMed] [Google Scholar]
- [11].Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 2013;7:e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;20:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cai XJ, Wang YF, Liang YL, et al. Laparoscopic left hemihepatectomy: a safety and feasibility study of 19 cases. Surg Endosc 2009;23:2556–62. [DOI] [PubMed] [Google Scholar]
- [14].Namgoong JM, Kim KH, Park GC, et al. Comparison of laparoscopic versus open left hemihepatectomy for left-sided hepatolithiasis. Int J Med Sci 2014;11:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ye X, Ni K, Zhou X, et al. Laparoscopic versus open left hemihepatectomy for hepatolithiasis. J Surg Res 2015;199:402–6. [DOI] [PubMed] [Google Scholar]
- [16].Zheng SM, Li H, Zhou XH, et al. Comparison of laparoscopic and open hemihepatectomy for hepatolithiasis. Chin J Hepatobiliary Surg 2015;21:709–11. [Google Scholar]
- [17].Shin YC, Jang JY, Kang MJ, et al. Comparison of laparoscopic versus open left-sided hepatectomy for intrahepatic duct stones. Surg Endosc 2016;30:259–65. [DOI] [PubMed] [Google Scholar]
- [18].Yao J, Zhou NJ. Efficacy and prognosis of laparoscopic and open left hepatectomy in the treatment of patients with hepatic duct stones. Proc Clin Med 2016;25:654–7. [Google Scholar]
- [19].Zhang Y, Huang J, Chen XM, et al. A comparison of laparoscopic versus open left hemihepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech 2016;26:146–9. [DOI] [PubMed] [Google Scholar]
- [20].Deng GM, Zhang YM, Zhou ZT, et al. A comparison between total laparoscopic and open left hemihepatectomy for left intrahepatic bile duct stones. Chin J Min Inv Surg 2017;17:331–4. [Google Scholar]
- [21].Peng L, Xiao J, Liu Z, et al. Laparoscopic left-sided hepatectomy for the treatment of hepatolithiasis: a comparative study with open approach. Int J Surg 2017;40:117–23. [DOI] [PubMed] [Google Scholar]
- [22].Valente R, Sutcliffe R, Levesque E, et al. Fully laparoscopic left hepatectomy-a technical reference proposed for standard practice compared to the open approach: a retrospective propensity score model. HPB (Oxford) 2018;20:347–55. [DOI] [PubMed] [Google Scholar]
- [23].Wang JC, Shang BH. Laparoscopic and open left hemihepatectomy for the treatment of intrahepatic bile duct stones. Chin J Curr Adv Gen Surg 2017;20:213–5. [Google Scholar]
- [24].Cho HD, Kim KH, Hwang S, et al. Comparison of pure laparoscopic versus open left hemihepatectomy by multivariate analysis: a retrospective cohort study. Surg Endosc 2018;32:643–50. [DOI] [PubMed] [Google Scholar]
- [25].Wu YH, Li XG, Zhang YM. The clinical efficacy comparison of laparoscopic and open left hemihepatectomy for the treatment of intrahepatic bile duct stones. J Taishan Med Coll 2018;39:55–6. [Google Scholar]
- [26].Biertho L, Waage A, Gagner M. Laparoscopic hepatectomy. Ann Chir 2002;127:164–70. [DOI] [PubMed] [Google Scholar]
- [27].Morise Z, Kawabe N, Kawase J, et al. Pure laparoscopic hepatectomy for hepatocellular carcinoma with chronic liver disease. World J Hepatol 2013;5:487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schemmer P, Friess H, Dervenis C, et al. The use of endo-GIA vascular staplers in liver surgery and their potential benefit: a review. Dig Surg 2007;24:300–5. [DOI] [PubMed] [Google Scholar]
- [29].Medbery RL, Chadid TS, Sweeney JF, et al. Laparoscopic vs open right hepatectomy: a value-based analysis. J Am Coll Surg 2014;218:929–39. [DOI] [PubMed] [Google Scholar]
- [30].Huang SW, Lin CH, Lee MS, et al. Residual common bile duet stones on direct peroral cholangioscopy using ultraslim endoscope. World J Gastroentero 2013;19:4966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Frattaroli FM, Lai Q, Coiro S, et al. Mirizzi syndrome in a patient with an accessory hepatic duct. Clin Ter 2013;164:139–41. [DOI] [PubMed] [Google Scholar]
- [32].Wills VL, Gibson K, Karihaloot C, et al. Complications of biliary T-tubes after choledochotomy. ANZ J Surg 2002;72:177–80. [DOI] [PubMed] [Google Scholar]
- [33].Araki K, Conrad C, Ogiso S, et al. Intraoperative ultrasonography of laparoscopic hepatectomy: key technique for safe liver transection. J Am Coll Surg 2014;218:e37–41. [DOI] [PubMed] [Google Scholar]
- [34].Montorsi M, Santambrogio R, Bianchi P, et al. Perspectives and draw backs of minimally invasive surgery for hepatocellular carcinoma. Hepatogastroenterology 2002;49:56–61. [PubMed] [Google Scholar]


