Abstract
Background:
Keloids are benign proliferative scars that often occur among individuals of color, and are thought to be the result of excessive collagen deposition that occurs after injury to the skin. The treatment of these scars is difficult with often poor outcomes. This study aimed to evaluate the effectiveness of surgical excision followed by in-office superficial radiation therapy (SRT) as a method to improve keloid remission.
Methods:
Participants for this study were recruited from June 2016 through February 2017 with 48 subjects enrolled and completed this study. All keloids were surgically resected and participants received 3 consecutive days of a customized dose of SRT, with a maximum cumulative dosage of 18 Gy. Patients were followed over the course of 12 months to monitor outcomes.
Results:
In this cohort, we found 39 (81%) to have achieved successful remission with 9 (19%) being classified as refractory. There were no adverse effects or medical complications reported as a part of this study.
Conclusion:
Study outcomes support the clinical benefits of surgical excision followed by SRT as a practical and efficient treatment for keloids.
INTRODUCTION
Keloid scars can be characterized as benign, rubbery or fibrous nodules that are the result of aberrant wound healing caused by any insult to the reticular dermis, such as ear piercing, acne, and burns.1 These lesions are known to occur in anyone; however, they are typically seen among those with darker pigmented skin such as Asians and Africans. The exact prevalence of these scars is unknown and they can occur at any time; however, there is a high incidence during the second and third decade of life.2 Along with physical discomfort, specifically, itching and pain, there is often concurrent psychological distress due to disfigurement and body image.3
The pathogenesis of these lesions remains poorly understood; however, clinicians have long held that the formation of a keloid begins during the transition from the early inflammatory phase to the late inflammatory phase of wound repair.4 During this stage of the healing process, neutrophils are replaced with macrophages that in turn release abnormal amounts of cytokines that prolong tissue inflammation. This protracted inflammatory response is believed to be a key factor that contributes to the formation of these scars.5 Histopathological examination of keloids reveals the presence of thickened and hyalinized collagen, an excessive production of fibroblasts and collagen deposition or a fibrosis of the dermal layer.6
The successful treatment of these scars have challenged surgeons for decades with surgical excision, in particular, for larger keloids yielding recurrence rates from 45% to 100%.7 To address these clinical failures, various adjuvant therapies have evolved including the use of steroid injection, silicone and pressure sheeting, gels, and cryotherapy with varying results.8
An important modality, radiation therapy, has been used to treat keloids for decades beginning initially with superficial x-rays in the 1920s, followed by postoperative recommendations for the prevention of recurrence in the 1940s and 1950s.9,10 The use of radiotherapy has been significantly evolved since its initial inception and is generally accepted as a method of treatment; however, there is a continued lack of consensus among clinicians regarding types of radiation and dosing protocols that are deemed to be safe and effective as a method to enhance postsurgical outcomes in the treatment of keloids.11 Systematic reviews of studies examining the use of different forms and dosages of radiotherapy including single-fraction, electron beam, and high dose rate versus low dose rate brachytherapy or interstitial radiation, for keloids have yielded some promising results.12 Of particular significance is that in several of the studies, there was a wide variation in the units of gray (Gy) used to treat the keloids and complication rates among those treated.13–15 In addition, advances in computer-driven radiation therapy have afforded patients with access to new therapies that allow clinicians to safely target keloids with x-rays that are more robust, permeable, and utilize a precision medicine approach. An important driving factor in applying a precision medicine approach is that recent innovations in superficial radiation therapy (SRT) also include a better understanding of fractionation, the science of dividing radiation doses for maximum safety and efficacy. The SRT-100 device (K063456*) received Food and Drug Administration (FDA) approval in 2013 for the specific treatment of keloid scars. This evolution in science allows clinicians to personalize treatments enhancing the accurate delivery of dose of radiation based on clinical parameters and anatomical information.
A recent resurgence in the use of radiation therapy originated with the use of in-office SRT in the treatment of nonmelanoma cancers such as basal and squamous cell by-passing the need for patients to seek treatment in a hospital setting, thereby creating a new opportunity to expand upon these treatments for keloids. An important consideration in the use of SRT is that in comparison to high-energy machines used in radiation oncology, SRT is low energy, targets the skin sparing deeper structures, a factor ideal in the treatment of conditions that affect cutaneous maladies.16 One important consideration is that the cost of SRT treatments varies for each individual and is dependent on factors such as size of keloid and the extent of treatment necessary. In addition, it should be noted that few studies have investigated the long-term consequences of SRT, and therefore, all treatment options should be explored and risk versus benefit of selected modality fully disclosed.17
METHODS
The aim of this prospective pilot study was to evaluate the benefit of postoperative SRT as an adjuvant treatment for keloids. The study was approved by an independent external institutional review board. All subjects signed written informed consent before being enrolled and were included in the study after they received medical clearance to participate in protocol. We enrolled male and female participants with keloids that originated from postsurgical or traumatic etiology (eg, acne vulgaris). Pregnant and/or breast feeding women and patients under 18 years of age were excluded from this study. In addition, large keloids, for example, those that required multiple surgeries and extensive grafting and who required neoadjuvant SRT were also excluded. Enrollment in this feasibility study was capped at 50 and to reduce bias, only the consenting study investigator was aware of participant enrollment at the time of procedures. Participants for this study were enrolled from June 2016 through February 2017 and prospectively followed for 1 year postsurgical and SRT treatment. Before surgery, a brief survey was conducted with targeted questions about patient demographics, including family history of keloids. Data pertaining to characteristics of keloid included how they occurred, length of time since they first developed, and if there were any prior treatments. All subjects were evaluated with the Kyoto scar scale (KSS), a validated measure that is extensively used clinically and in research during before intervention and at follow-up to assess the redness, hardness and elevation (objective signs 0–2 points each), and subjective symptoms such as itching and pain of the wound site (0–1 point each).18 The scale is scored as follows; excellent (0 points), good (1–2 points), fair (3 points), and poor (4–8 points). Scars were surgically excised, and to improve aesthetic outcomes, a running subcuticular closure was used with absorbable sutures. Postoperatively, participants were instructed to return to the office for 3 consecutive days following surgery for SRT treatment. Custom lead shields were applied to protectsurrounding tissue and minimize any radiation exposure to the surrounding tissue. A cumulative dose of 18 Gy was precisely delivered using in-office an FDA approved SRT-100 to each of the surgically removed keloids beginning 24 hours postoperative and as a part of the protocol subjects were required to return for 2 additional consecutive days for treatment. The dose was selected based upon our experience and is within the dosage range of similar irradiation protocols that have achieved positive outcomes.19,20 Participants were then followed at 3, 6, and 12 months to assess for any adverse effects, complications and for signs of recurrence by a trained member of our clinical team. We also asked participants to apply Keloid Care, a proprietary cream specifically developed by our team to the wound daily. Data collection was completed in March of 2018 and data were then analyzed using SPSS software (Version 24, Chicago, Ill.). Data were analyzed using frequency tests, cross tabulation, and descriptive statistics.
RESULTS
A total of 48 subjects were enrolled in this feasibility study and were prospectively followed postoperatively for 1 year to assess recurrence of the excised keloid(s). In this study, 2 participants with missing data and that were lost to follow-up were excluded from the study. Participant characteristics are described in Table 1. There were 19 (40%) males and 29 (60%) females. The mean age of the participants was 39 years (SD ±14.27) with the range from 18 to 67. Fifty percent of participants reported a family history of keloids, of which 71% (34) were self-identified as being African American. Half of the participants reported that scars were greater than 3 years old with the primary reason for the origin of the keloid being a surgical procedure; however, skin trauma such as shaving and the presence of acne vulgaris also significantly contributed to the development of these scars (Fig. 1). The location of the removed keloid was recorded and we found that most of scars our team removed were located on the sternum 19 (40%), an area of high tension that is known to be associated with the development of keloids.21 Figure 2 outlines the specific location of the keloids that were treated. Scars ranged in size from 0.5 to 9.5 cm. Additionally, because nicotine is a vasoconstrictor that reduces the natural blood flow of nutrients to the skin and is known to impair healing, we were interested in participants’ history of smoking. Only 3 subjects reported that they were active smokers with one of the participants having a recurrence of the keloid. We assessed the keloid scar as being successfully treated based upon 3 separate factors. All participants’ scars were evaluated by the KSS, visual inspection by one of our trained surgical staff and self-reported patient satisfaction. Forty-eight scars were excised and followed with our radiation protocol. A total of 39 (81%) keloids were found to have achieved successful remission with 9 (19%) being classified as refractory (Table 1). Our KSSs (Fig. 3) were in agreement with these results with participants scoring an 8 or a poor score before having the keloid removed and 39 (81%) scoring as either 0–2 range with one subject rated as fair or 3. Figure 4 illustrates before and after protocol outcomes.
Table 1.
Participant Characteristics

Fig. 1.
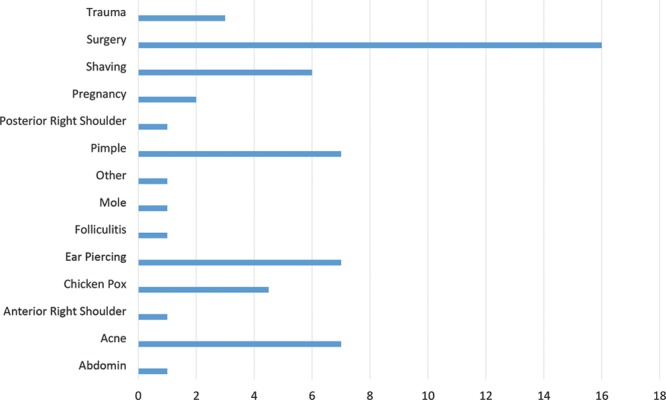
Primary cause of skin injury.
Fig. 2.

The site of keloid location.
Fig. 3.
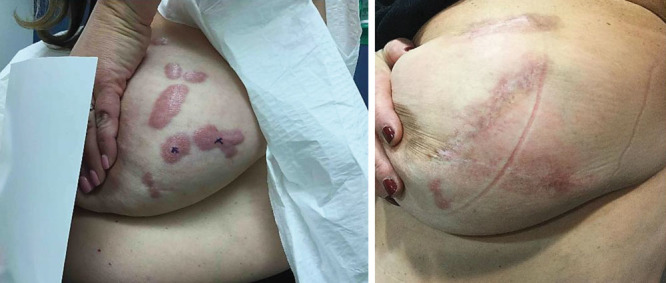
Kyoto Scar Scale Scores.
Fig. 4.
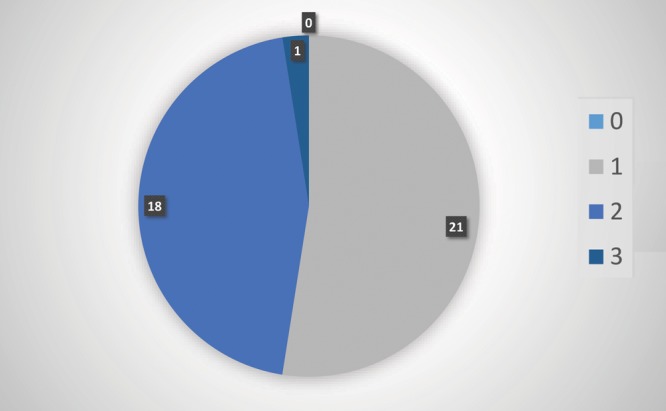
Preoperative and postoperative photograph of keloid.
DISCUSSION
The removal of keloid scars has long burdened both patient and clinician with few successful treatment options. Surgeons continue to debate the “gold standard” of treatment of these lesions offering multimodal therapies combining surgical resection with several methods including steroid injections, pressure therapy, topical creams, and laser removal with variable outcomes.22 There have been several additional studies examining the utility of irradiation in the treatment of keloids with varying conclusions. Specific differences include the type and dose of external beam radiotherapy, for example, high dose rate brachytherapy versus low dose rate brachytherapy which can have adverse effects such as radionecrosis to healthy tissue and possible carcinogenesis.23 We sought to explore the utility of superficial radiation as it specifically administers a “low” dose that can be controlled more directly and efficiently to the target area providing less irradiation to the surrounding tissue thereby sparing patients unnecessary complications. The treatment method described in this paper resulted in an 81% of our subjects achieving remission a rate which is comparable to the success reported in several studies that explored the use of similar techniques specifically for keloids.11–25
This study implemented an FDA approved in-office SRT following surgical excision as an adjuvant therapy. We followed all participants for a total of 12 months to determine if the methods of surgical excision followed by in-office SRT effectively prevented the recurrence of the keloids. Our study provides further evidence that surgery plus adjuvant in-office low-dose radiation therapy within 24 hours and delivered over 3 consecutive days of the procedure resulted in significant improvement in outcomes of these often treatment resistant scars. The protocol we developed utilized 18 Gy of radiation therapy and study outcomes support this to be within the range of previously studied effective cumulative dose.19 The authors, however, conclude that given our limited sample size, a larger prospective randomized control trial would be the next step in confirming results.
It should be noted that although our results offer positive support for the use of surgical excision plus adjuvant SRT in the remission of keloids, more work needs to be done to flush out the best and most reliable and more importantly repeatable method for the treatment of these cutaneous malformations. We previously reported results of 2 retrospective studies to explore the benefits of SRT.20–26 In an initial review, we looked at 44 surgically excised keloids; however, the reported clinical protocol also included the application of autologous platelet-rich-plasma (PRP) directly to the surgical wound. The use of PRP is well established as safe and its application has shown positive wound healing properties.27 In the first review, we achieved a 95.5% remission at 3 months, follow-up was between 3 and 12 months. In a second review, we retrospectively analyzed the outcomes of 49 patients that had sought out treatment for the management of keloid scars localized to the auricle. The treatment protocol included surgical excision followed by SRT and also included the application of PRP directly to the surgical wound. Results were promising with a 94% nonrecurrence rate over a 2-year period. This is an important contribution to this prospective feasibility study; however, to improve generalizability and to reduce any bias, we are currently conducting a larger-scale randomized control trial to test differences between surgical excision and SRT as compared to surgical excision, PRP followed by SRT.
Footnotes
Published online 3 May 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCE
- 1.Andrews JP, Marttala J, Macarak E, et al. Keloids: the paradigm of skin fibrosis—pathomechanisms and treatment. Matrix Biol. 2016;51:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43 (Suppl 1):S3–S18. [DOI] [PubMed] [Google Scholar]
- 3.Brown BC, McKenna SP, Siddhi K, et al. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg. 2008;61:1049–1058. [DOI] [PubMed] [Google Scholar]
- 4.Sahl WJ, Jr, Clever H. Cutaneous scars: part I. Int J Dermatol. 1994;33:681–691. [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Akaishi S, Hyakusoku H, et al. Are keloid and hypertrophic scar different forms of the same disorder? A fibroproliferative skin disorder hypothesis based on keloid findings. Int Wound J. 2014;11:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Murphy GF, Akaishi S, et al. Keloids and hypertrophic scars: update and future directions. Plast Reconstr Surg Glob Open. 2013;1:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman B, Bieley HC. Adjunct therapies to surgical management of keloids. Dermatol Surg. 1996;22:126–130. [DOI] [PubMed] [Google Scholar]
- 8.Paul A, Kumar N, Moirangthem T, et al. Comparative study of intralesional triamcinolone alone and in combination with 5-flurourouracil for the treatment of keloid and hypertrophic scars. Hellenic J Surg. 2017;89:13–17. [Google Scholar]
- 9.Levitt W, Gillies H. Radiotherapy in the prophylaxis and treatment of keloid. Lancet. 1942;239. [Google Scholar]
- 10.Kruger A. Keloids and their treatment with spcecial reference to the radiotherapy. Strahlentherapie. 1954;93:426–433. [PubMed] [Google Scholar]
- 11.Cheraghi N, Cognetta A, Jr, Goldberg D. Radiation therapy for the adjunctive treatment of surgically excised keloids: a review. J Clin Aesthet Dermatol. 2017;10:12–15. [PMC free article] [PubMed] [Google Scholar]
- 12.Goutos I, Ogawa R. Brachytherapy in the adjuvant management of keloid scars: literature review. Scars Burn Heal. 2017;3:2059513117735483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K, Son D, Kim J. Radiation therapy following total keloidectomy: a retrospective study over 11 Years. Arch Plast Surg. 2015;42:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeling BH, Whitsitt J, Liu A, et al. Keloid removal by shave excision with adjuvant external beam radiation therapy. Dermatol Surg. 2015;41:989–992. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa R, Mitsuhashi K, Hyakusoku H, et al. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg. 2003;111:547–553; discussion 554. [DOI] [PubMed] [Google Scholar]
- 16.McGregor S, Minni J, Herold D. Superficial radiation therapy for the treatment of nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2015;8:12–14. [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe CM, Cognetta AB., Jr Radiation therapy (RT) for nonmelanoma skin cancer (NMSC), a cost comparison: clarifying misconceptions. J Am Acad Dermatol. 2016;75:654–655. [DOI] [PubMed] [Google Scholar]
- 18.Yamawaki S, Naitoh M, Yoshikawa K, Ishiko T, Suzuki S. Kyoto Scar Scale for assessment of keloids following surgery and irradiation. Sosyo. 2011;2:112–117. [Google Scholar]
- 19.Ogawa R, Miyashita T, Hyakusoku H, et al. Postoperative radiation protocol for keloids and hypertrophic scars: statistical analysis of 370 sites followed for over 18 months. Ann Plast Surg. 2007;59:688–691. [DOI] [PubMed] [Google Scholar]
- 20.Jones ME, Hardy C, Ridgway J. Keloid management: a retrospective case review on a new approach using surgical excision, platelet-rich plasma, and in-office superficial photon x-ray radiation therapy. Adv Skin Wound Care. 2016;29:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akaishi S, Akimoto M, Ogawa R, et al. The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg. 2008;60:445–451. [DOI] [PubMed] [Google Scholar]
- 22.Durani P, Bayat A. Levels of evidence for the treatment of keloid disease. J Plast Reconstr Aesthet Surg. 2008;61:4–17. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa R, Yoshitatsu S, Yoshida K, et al. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124:1196–1201. [DOI] [PubMed] [Google Scholar]
- 24.Yamawaki S, Naitoh M, Ishiko T, et al. Keloids can be forced into remission with surgical excision and radiation, followed by adjuvant therapy. Ann Plast Surg. 2011;67:402–406. [DOI] [PubMed] [Google Scholar]
- 25.Ragoowansi R, Cornes PG, Moss AL, et al. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. Plast Reconstr Surg. 2003;111:1853–1859. [DOI] [PubMed] [Google Scholar]
- 26.Jones ME, McLane J, Adenegan R, et al. Advancing Keloid Treatment: A Novel Multimodal Approach to Ear Keloids. Dermatol Surg. 2017;43:1164–1169. [DOI] [PubMed] [Google Scholar]
- 27.Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010;83:1–9. [PMC free article] [PubMed] [Google Scholar]


