Abstract
Background:
Capsular contracture remains a common complication after implant-based breast reconstruction. Previous work has suggested that the use of acellular dermal matrix (ADM) reduces the rate of capsular contracture, though little is understood about the underlying mechanism. As myofibroblasts are believed to be the key cells implicated in contracture formation, we hypothesized that ADM would result in a reduction in periprosthetic myofibroblast concentration.
Methods:
Five patients who underwent immediate prepectoral tissue expander placement with anterior ADM coverage and an inferior cuff were included. At the second stage, tissue samples were obtained of both ADM and capsule from each reconstructed breast. Samples were then prepared for hematoxylin and eosin staining and immunohistochemistry for myofibroblast identification (alpha smooth muscle actin and vimentin positive and desmin negative) and analysis. Experimental values are presented as mean ± SD unless otherwise stated. Statistical significance was determined using unpaired t test.
Results:
Successful incorporation of ADM was noted in all cases. A significant reduction in myofibroblast concentration was noted in the ADM versus the capsule (P = 0.0018). This was paralleled by significantly thicker periprosthetic capsule formation overlying the formerly raw pectoralis major muscle, that is, not covered by ADM (P < 0.0001).
Conclusions:
In the presence of ADM, there are significantly fewer myofibroblasts in breast capsules and thinner capsules on histology. Given the central role of myofibroblasts in the development of clinically significant capsular contracture, this study unmasks a possible mechanism for the protective effect of ADM with respect to capsular contracture development.
INTRODUCTION
Implant-based breast reconstruction is the most common reconstructive modality after mastectomy in the United States. However, the heterogeneity of outcomes within this group is quite substantial and is related to the timing of reconstruction (staged reconstruction versus direct-to-implant reconstruction), plane of implant placement, and use of acellular dermal matrices (ADM).1–6 In recent years, prepectoral breast reconstruction has become increasingly popular due to the ease of technique, decreased postoperative pain, minimal morbidity, and prevention of animation deformity.7 However, critics comment on the fact that “prepectoral” reconstruction (ie, subcutaneous implant placement) was performed in the 1970s and eventually abandoned due to high complication rates.8–10 A main reason for the transition from prepectoral to submuscular device placement was the high incidence of capsular contracture associated with subcutaneous device placement.8–10 Substantial advances have been made since, with one of the most important being the introduction of acellular dermal matrices in breast reconstruction.11
A particularly challenging problem in implant-based reconstruction is the development of capsular contracture.2,3,12,13 Its clinical manifestation ranges from a palpable to a visible and painful deformity, with a reported incidence of >10%.2,3,12,13 In fact, it remains one of the most commonly reported complications in implant-based breast surgery.2,12 All surgical implants undergo some degree of encapsulation due to a natural foreign body reaction by the surrounding native tissues. Clinically significant breast capsular contracture, however, results in discomfort, cosmetic deformity, and need for revision surgery.2,3,12,13
The exact mechanism for this troubling complication is not yet completely understood. It has been suggested that the hypertrophic, circumferential scar is driven by the stimulation of myofibroblasts that are known to be present within the periprosthetic capsule.14,15 Various strategies have been used to attempt to reduce the risk of capsular contracture. There is an increasing body of evidence that supports a multimodal therapy of site change, implant change, and the use of ADM to reduce the reoccurrence of capsular contracture.3,5,13,14,16–18
ADM was first introduced by Breuing and Warren11 as an adjunct to improve outcomes after implant-based breast reconstruction. Subsequent clinical studies demonstrated that ADM was associated with a decrease in the rate of postoperative capsular contracture.5,19 The integration of ADM into host tissue has been demonstrated histologically and, thus, has further supported its preventative effect on capsular contracture development.3,13,16,20,21
Here, we investigate the periprosthetic microenvironment to better understand the effect of ADM on the microenvironment cellular activity, specifically on the key cell implicated in capsular contracture—the myofibroblast. The interplay of dermal presence and secondary contracture is well known. In the 1970s, Rudolph22 demonstrated that wounds covered with full-thickness skin grafts resulted in reduced myofibroblast activity in comparison to wounds covered with split-thickness skin grafts or those that healed by secondary intention. Applying these observations to breast reconstruction, we thus hypothesized that the presence of acellular dermis reduces the myofibroblast concentration in the periprosthetic microenvironment, thus, contributing to a decreased capsular contracture rate after ADM use. We also questioned whether the presence of acellular dermal matrix increased angiogenesis relative to the periprosthetic capsule alone. Our primary aim was to determine if the presence of ADM resulted in a reduced periprosthetic myofibroblast concentration in patients undergoing breast reconstruction.
METHODS
Institutional Review Board approval was obtained before conducting the study. Samples were obtained from patients who had undergone prepectoral tissue expander placement using a technique of anterior ADM coverage with an inferior cuff. Thus, at the time of tissue expander removal and secondary reconstruction, the surgical field provided for a unique opportunity to investigate the effect of ADM on the underlying wound bed as ADM covered pectoralis major muscle was located side-by-side with prepectoral capsule (distinct from ADM) formation (Fig. 1). Between August and October 2017, we obtained tissue samples from both (1) the periprosthetic capsule disparate from the ADM (denoted as “capsule” hereafter) and (2) the incorporated ADM itself (denoted as “ADM” hereafter) from each breast in 5 consecutive patients undergoing tissue expander removal and secondary breast reconstruction (Table 1). After excision of the respective specimen, samples were kept on ice and transported to the laboratory.
Fig. 1.
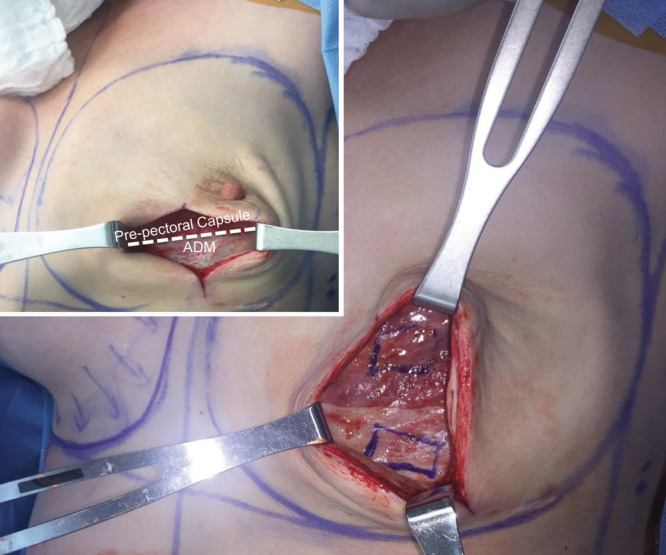
Intraoperative tissue sampling technique. Note that the inferior-cuff technique of ADM insertion allows for an intraindividual side-by-side analysis of ADM-covered pectoralis major muscle and formerly raw surface that has formed a prepectoral capsule.
Table 1.
Patient Demographics
Hematoxylin and Eosin Histology
Portions of capsule and ADM were designated for hematoxylin and eosin (H&E) staining. Specimens were fixed in 10% neutral buffered formalin for 16 hours at 4°C. They were then washed with phosphate-buffered saline (PBS), embedded in paraffin, and sectioned. Sections were stained with H&E and imaged using a Leica DM5000 B Light microscope (Leica Microsystems; Buffalo Grove, IL) at the 10× objective.
Immunofluorescent Histology
A portion of each sample was designated for immunofluorescent staining. The specimens were fixed in 10% neutral buffered formalin for 16 hours at 4°C. They were then washed 5 times in PBS over 5 hours before being placed into 30% sucrose in PBS. Specimens were soaked in the 30% sucrose for 7 days. They were then embedded in cryoembedding medium (OCT, Sakura Finetek, Alphen aan der Rijn, Netherlands). The 6-µm-thick sections were cut and placed on glass slides. The slides were soaked in PBS for 5 minutes to remove OCT. Hydrophobic squares were drawn around the sections (PAP pen; Abcam, Cambridge, UK), and the sections were permeabilized in 0.2% Triton X-100 (Sigma Aldrich, St Louis, MO) and then washed 3 times with 0.05% Tween-20 (Sigma Aldrich) in PBS (PBST). Blocking solution (Power Block; BioGenex, Fremont, CA) was placed over the sections for 1 hour. Sections were then incubated with primary antibodies against alpha smooth muscle actin (α-SMA) (goat anti-α-SMA antibody, cat Ab21027, 1:20; Abcam), desmin (mouse antidesmin antibody, cat Ab8470, 1:20; Abcam), and vimentin (rabbit antivimentin antibody; cat Ab137321, 1:20; Abcam) dissolved in blocking solution overnight at 4°C. The next morning, the sections were washed 3 times with PBST. The sections were then incubated with secondary antibodies (donkey anti-goat IgG Alexa Fluor 647, cat 150131, 1:400; Abcam; donkey anti-rabbit IgG Alexa Fluor 488, cat 150073, 1:400; Abcam; goat anti-chicken IgY H&L Alexa Fluor 555, cat Ab150170, 1:400; Abcam), washed 3 times with PBST, and mounted with 4´,6-diamidino-2-phenylindole (DAPI)-containing aqueous mounting medium (Fluoroshield with DAPI; Abcam).
A subset of slides from each sample was stained with the primary antibody anti-human CD31 (or PECAM, cat Ab28364, 1:30; Abcam), which labels endothelial cells, and the secondary antibody donkey anti-rabbit (IgG Alexa Fluor 488, cat 150073, 1:400). Immunofluorescent images were obtained using the Zeiss LSM700 confocal microscope. Three random images were taken per section at 20× magnification, with 3 slides per sample and patient.
Morphometric and Densitometric Analysis
The samples were then analyzed using ImageJ (NIH, Bethesda, MD) for the presence of myofibroblasts. Here, we used the DAPI stain to represent all cells. Myofibroblasts were detected by noting the presence of staining for vimentin and α-SMA and the absence of desmin. Two authors (MRB and DI), blinded to the sample type, counted by hand the number of cells (identified by the presence of a nucleus) positive for both vimentin and α-SMA and negative for desmin. Ten representative samples of each specimen at 20× magnification were included for analysis. Samples were also analyzed for the degree of vascularization, indicated by positive staining for CD31. The area in pixels of fluorescence-positive area per total scanned area at 20× magnification (750 µm × 750 µm) was quantified using ImageJ and compared between ADM and periprosthetic capsule specimens. This analysis was conducted in 10 representative samples of each specimen at 20× magnification.
Regarding H&E staining and quantification, the capsule edge of each specimen was marked and actual capsular thickness was measured in pixels for each image and converted to millimeters. Three serial microscopic images at 10× magnification were captured for each patient, and average score per patient was then determined.
Statistical Analyses
Continuous parametric experimental data were presented using the mean ± SD unless otherwise stated. For each patient, the (1) periprosthetic capsule disparate from the ADM and (2) ADM itself were analyzed; thus, each patient acted as their own internal control. Paired t tests were used to compare means between groups. A P value of <0.05 was considered statistically significant, and all statistical analyses were conducted on the GraphPad Prism software (San Diego, CA).
RESULTS
Patient Characteristics
Specimen of 5 patients with a mean age of 52.6 years (range 40–70 years) and a mean BMI of 31.1 kg/m2 (range 24.8–35.3 kg/m2) was collected. Four patients had primary breast cancer. One patient presented with recurrent disease after breast-conserving therapy and, thus, was the only patient with a history of radiation therapy (XRT). Three patients underwent bilateral mastectomy with reconstruction, whereas 2 patients underwent unilateral reconstruction. The mean interval between the reconstructive procedures was 5.2 months (range 4–6 months) (Table 1). After tissue expander removal and ADM/capsule collection, 1 patient underwent silicone implant placement, 2 patients underwent delayed immediate-free abdominal flap transfer, and 2 patients underwent hybrid breast reconstruction with free abdominal flap transfer and simultaneous implant insertion. Of note, incorporation of the ADM was noted in all cases.
Capsule Thickness
Prepectoral capsules and ADM specimens were harvested in the study patients during the second reconstructive stage. Here, we see that ADM capsules were significantly thinner than their periprosthetic counterparts using the H&E analysis (P < 0.0001, Fig. 2).
Fig. 2.
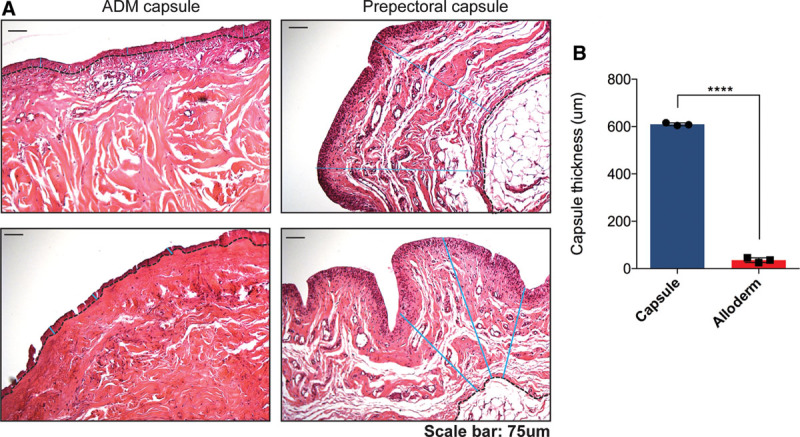
Capsule quantification. (A) H&E staining of ADM (top left) and capsule (top right) specimens following paraffin embedding. The capsule edge was marked and actual capsular thickness was measured in pixels for each image and converted to mm. Graph (right) demonstrates a significantly thinner capsule in ADM capsule (p<0.0001).
Myofibroblast Presence
Myofibroblasts have been shown to be the predominant cell in breast capsule formation. To examine the myofibroblast presence in the capsules, we analyzed the presence of myofibroblasts using immunofluorescent staining for α-SMA, vimentin, and desmin. Here, we see that there are significantly lower numbers of myofibroblasts present in the ADM versus the capsule (P = 0.0018) (Fig. 3). As scarring is a complex multisystemic process, we then examined each patient’s ADM versus capsule separately, so that there was an inherent internal control.
Fig. 3.
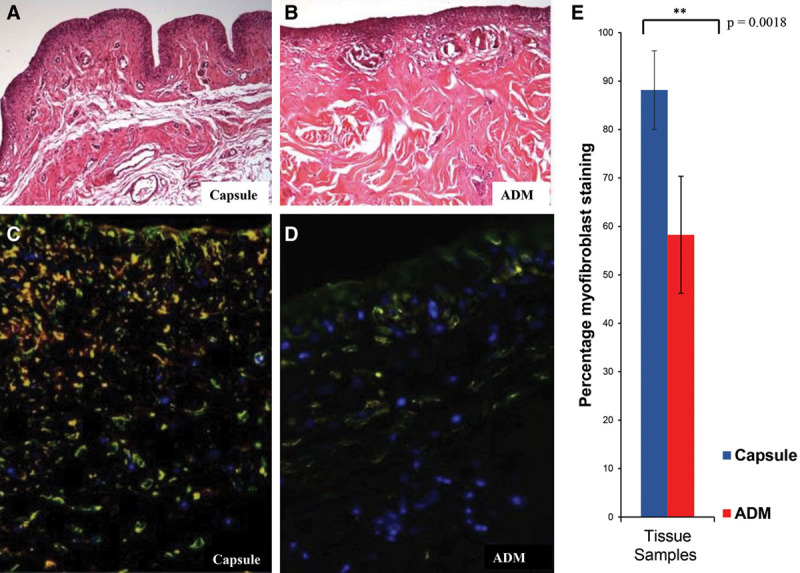
H&E staining of capsule (A) and ADM (B) specimens after paraffin embedding. Immunohistochemical composite staining of α-SMA (red) and vimentin (green) positive cells in capsule specimen (C) and ADM (D). The overlap of the green and red channels in this composite image appears as yellow (myofibroblasts). Desmin (white) positive cells were not counted. E, Percentage of myofibroblast staining in the cellular microenvironment of capsule (blue) and ADM (red). Here, we see that there was a significant myofibroblast predominance in the capsule vs the ADM, P = 0.0018.
Here, we found that patients numbered 1–4 had a significantly decreased presence of myofibroblasts in the ADM versus the capsule (P < 0.001) (Fig. 4), supporting our hypothesis. Patient 5 also had a trend of increased myofibroblast presence in the capsule versus the ADM; however, this was not a significant finding (P > 0.05).
Fig. 4.
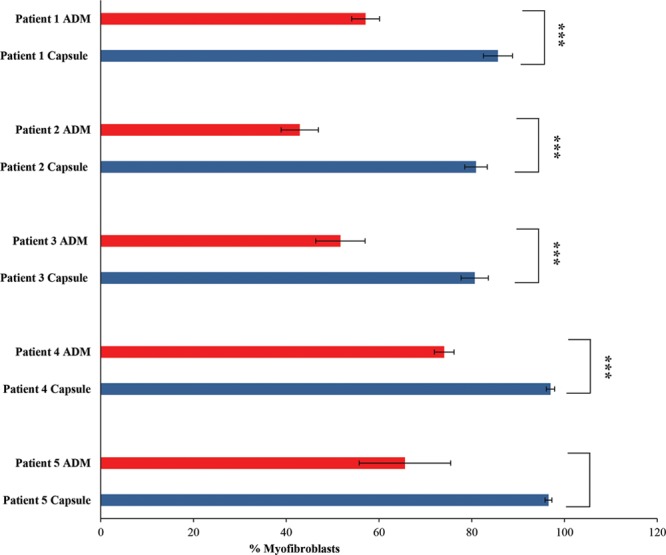
Intraindividual results of percentage of myofibroblast staining (after immunofluorescence detection as shown in Fig. 3) in the cellular microenvironment of capsule (blue) and ADM (red) showing patients 1–5. A significant reduction in myofibroblasts in the ADM vs capsule was seen in patients 1–4.
Vascularization
The vascularization of the ADM and periprosthetic capsule specimens was assessed by CD31 staining. Our results indicated that there was no significant difference in vascularization between the ADM and periprosthetic capsule specimens (0.37 ± 0.29 versuus 0.48 ± 0.62 fluorescence-positive area, P = 0.82) (Fig. 5).
Fig. 5.
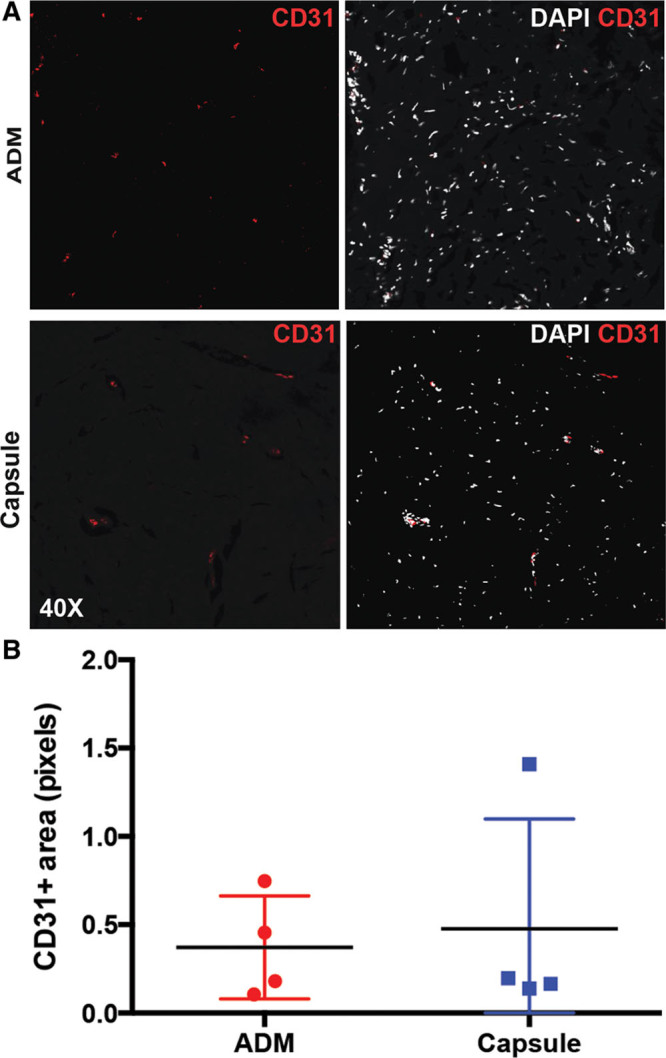
CD31 immunofluorescence staining indicating the degree of vascularization in a representative ADM (A, top) and peri-prosthetic capsule specimen from a single patient (A, bottom). There was no significant difference in CD31 staining between ADM and capsule specimens (0.37 ± 0.29 vs. 0.48 ± 0.62 fluorescence-positive area, p = 0.82) (B).
DISCUSSION
Capsular contracture is a common complication after implant-based breast reconstruction.2,12 It can result in cosmetic deformity, pain, patient morbidity, and additional surgical procedures. Previous studies have suggested that the use of ADM is associated with a reduced rate of capsular contracture.5,6,19 The mechanism, however, is poorly understood. Here, we demonstrate that well-known cellular mechanisms may be at play. The protective effect of the surgical treatment of open wounds with skin grafts with respect to subsequent scar contracture is well known. Skin grafts have been shown to accelerate the myofibroblast life cycle, leading to accelerated disappearance of the myofibroblasts from the wound bed.22 In addition, Brown, Garner, and Young demonstrated that the capacity of a skin graft to inhibit wound contraction is directly proportional to the amount of structurally intact dermal collagen present in the graft.23 We believe that the same mechanism may be at play in implant-based breast surgery.
After mastectomy and tissue expander/implant insertion, the tissue in contact with the device is essentially a raw wound bed that heals by secondary intention, that is, scar/capsule formation. Capsular contracture is believed to be the clinical corollary of the intrinsic propensity of the resulting capsule to contract. Incorporation of ADM in the reconstruction, however, allows the internal breast wound to be “grafted,” thus potentially halting the unfavorable sequelae of contracture formation, analogous to skin grafting an open wound.22
We hypothesized that the presence of acellular dermis reduces myofibroblast cellular predominance, as was shown decades ago by Rudolph22 in a study of wound healing. Indeed, by examining both the periprosthetic capsule and the ADM cellular microenvironment histologically, we found that the presence of acellular dermis resulted in a significant reduction in myofibroblast concentration in the periprosthetic tissues and thinner capsules. Importantly, we were able to demonstrate the favorable effects of ADM on myofibroblast concentration using intraindividual controls. Interestingly, we examine the posterior capsule and perhaps, there are different forces acting on the anterior versus posterior capsule that may indeed alter the niche microenvironment and the resident cells. A further study could focus on the different cellular profiles present at the different areas of capsule in an effort to better understand capsular contracture formation. In addition, it would be interesting to explore the microenvironmental changes in capsules at different phases of development, as the capsules examined in our study developed 4–6 months postplacement.
Our findings provide an explanation for the clinical observations of decreased capsular contracture formation after ADM use in implant-based breast surgery.4,5,16,24 Despite these favorable observations, we wish to disclose limitations of the present study. These include a small sample size and the fact that we only examined myofibroblast concentration. Future studies will focus on myofibroblast activity and proliferation in a larger number of patients. Another limitation is the age of the capsule. Specimens were obtained after a mean of 5.2 months. Hence, we cannot draw any conclusions regarding the long-term developments that occur in the periprosthetic tissues.
Despite the abundance of myofibroblasts in the periprosthetic capsule specimens, we did not observe any significant difference in vascularization between ADM and periprosthetic capsule specimens. Activated fibroblasts, including myofibroblasts, are a source of potent antiangiogenic factors such as thrombospondins 1 and 2.25 Although myofibroblasts are upregulated in the setting of fibrosis, fibrosis has been associated with both an increase and a decrease in angiogenesis.26 This is reflected in our results where vascularization appeared more mixed in the capsule versus ADM specimens. Furthermore, the small number of patients and variable time since implant placement and ADM difference between patients may mask any time-dependent effects of fibrosis and vascularization in the setting of breast implants and ADM. The complex relationship between myofibroblasts, angiogenesis, and fibrosis remains to be explored in future studies in a larger cohort.
CONCLUSIONS
The presence of ADM significantly reduces the predominance of myofibroblasts in breast capsules on histology. Myofibroblasts are the major cell implicated in clinically significant capsular contracture. This study supports the clinical finding that ADM reduces capsular contracture development and explains a mechanism for this clinical finding.
Footnotes
Published online 17 May 2019.
Disclosure: Dr. Momeni is a consultant for Allergan, AxoGen, and Stryker. The other authors have no financial interest to declare in relation to the content of this article. No payment was received for the study.
REFERENCES
- 1.Cabalag MS, Rostek M, Miller GS, et al. Alloplastic adjuncts in breast reconstruction. Gland Surg. 2016;5:158–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craft RO, Colakoglu S, Curtis MS, et al. Patient satisfaction in unilateral and bilateral breast reconstruction [outcomes article]. Plast Reconstr Surg. 2011;127:1417–1424. [DOI] [PubMed] [Google Scholar]
- 3.Lardi AM, Ho-Asjoe M, Junge K, et al. Capsular contracture in implant based breast reconstruction—the effect of porcine acellular dermal matrix. Gland Surg. 2017;6:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthet Plast Surg. 2008;32:418–425. [DOI] [PubMed] [Google Scholar]
- 5.Spear S. L, Seruya M, Clemens M. W, Teitelbaum S, Nahabedian M.Y. Acellular dermal matrix for the treatment and prevention of implant-associated breast deformities. Plast Reconstr Surg. 2011;127:1047–1058. [DOI] [PubMed] [Google Scholar]
- 6.Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128:403e–410e. [DOI] [PubMed] [Google Scholar]
- 7.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:162–167. [DOI] [PubMed] [Google Scholar]
- 8.Baker BG, Irri R, MacCallum V, et al. A prospective comparison of short-term outcomes of subpectoral and prepectoral strattice-based immediate breast reconstruction. Plast Reconstr Surg. 2018;141:1077–1084. [DOI] [PubMed] [Google Scholar]
- 9.Bonomi S, Sala L, Cortinovis U. Prepectoral breast reconstruction. Plast Reconstr Surg. 2018;142:232e–233e. [DOI] [PubMed] [Google Scholar]
- 10.Walia GS, Aston J, Bello R, et al. Prepectoral versus subpectoral tissue expander placement: a clinical and quality of life outcomes study. Plast Reconstr Surg Glob Open. 2018;6:e1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55:232–239. [DOI] [PubMed] [Google Scholar]
- 12.Headon H, Kasem A, Mokbel K. Capsular contracture after breast augmentation: an update for clinical practice. Arch Plast Surg. 2015;42:532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuehlmann B, Burkhardt R, Kosaric N, et al. Capsular fibrosis in aesthetic and reconstructive-cancer patients: a retrospective analysis of 319 cases. Clin Hemorheol Microcirc. 2018;70:191–200. [DOI] [PubMed] [Google Scholar]
- 14.Kim IK, Park SO, Chang H, et al. Inhibition mechanism of acellular dermal matrix on capsule formation in expander-implant breast reconstruction after postmastectomy radiotherapy. Ann Surg Oncol. 2018;25:2279–2287. [DOI] [PubMed] [Google Scholar]
- 15.Colak O, Ozer K, Dikmen A, et al. Evaluation of safe systemic immunosuppression created with dexamethasone in prevention of capsular contracture: a glance to distinct perspectives with Toll-like receptors. Aesthet Plast Surg. 2018;42:1133–1143. [DOI] [PubMed] [Google Scholar]
- 16.Namnoum JD, Moyer HR. The role of acellular dermal matrix in the treatment of capsular contracture. Clin Plast Surg. 2012;39:127–136. [DOI] [PubMed] [Google Scholar]
- 17.Paydar KZ, Wirth GA, Mowlds DS. Prepectoral breast reconstruction with fenestrated acellular dermal matrix: a novel design. Plast Reconstr Surg Glob Open. 2018;6:e1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan D, Rohrich RJ. Revisiting the management of capsular contracture in breast augmentation: a systematic review. Plast Reconstr Surg. 2016;137:826–841. [DOI] [PubMed] [Google Scholar]
- 19.Cheng A, Lakhiani C, Saint-Cyr M. Treatment of capsular contracture using complete implant coverage by acellular dermal matrix: a novel technique. Plast Reconstr Surg. 2013;132:519–529. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel A, Maxwell GP. AlloDerm RTU integration and clinical outcomes when used for reconstructive breast surgery. Plast Reconstr Surg Glob Open. 2018;6:e1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones G, Yoo A, King V, et al. Prepectoral immediate direct-to-implant breast reconstruction with anterior AlloDerm coverage. Plast Reconstr Surg. 2017;140(6S Prepectoral Breast Reconstruction):31S–38S. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph R. Inhibition of myofibroblasts by skin grafts. Plast Reconstr Surg. 1979;63:473–480. [DOI] [PubMed] [Google Scholar]
- 23.Klein C, Kovács A, Stuckensen T. Free arterialised venous forearm flaps for intraoral reconstruction. Br J Plast Surg. 1997;50:166–171. [DOI] [PubMed] [Google Scholar]
- 24.Chopra K, Buckingham B, Matthews J, et al. Acellular dermal matrix reduces capsule formation in two-stage breast reconstruction. Int Wound J. 2017;14:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auguste P, Vincent F, Gabbiani G, et al. Seed MP, Walsh DA. The fibroblast and myofibroblast in inflammatory angiogenesis: mechanisms and clinical correlates. In: Angiogenesis in Inflammation. 2008:Basel, Switzerland: Birkauser; 59–82. [Google Scholar]
- 26.Hanumegowda C, Farkas L, Kolb M. Angiogenesis in pulmonary fibrosis: too much or not enough? Chest. 2012;142:200–207. [DOI] [PubMed] [Google Scholar]


