Abstract
Background:
Mastectomy flap and nipple–areola complex (NAC) ischemia can be devastating complications after nipple-sparing mastectomy (NSM). Predictors of reconstructive failure with major skin envelope ischemia and implications for decision-making remain to be fully elucidated.
Methods:
All cases of implant-based reconstruction after NSM from 2006 to June 2018 with mastectomy flap necrosis or NAC necrosis requiring debridement were reviewed. Data on patient demographics, operative characteristics, additional complications, and the nature and management of ischemic complications were collected and analyzed.
Results:
Out of 1045 NSMs, 70 cases (6.7%) had major ischemic complications. Fifty-two cases (74.3% of major ischemic complications) had isolated major mastectomy flap necrosis, 7 (10%) had full NAC necrosis and 11 (15.7%) had both. Five cases (7.1%) underwent implant exchange at the time of debridement and 15 cases (21.4%) required explantation. Explanted cases had significantly lower body mass index (22.3 versus 24.7, P = 0.013) and larger debridement size (49.5 cm2 versus 17.6 cm2, P = 0.0168). Additionally, explanted cases had a higher rate of acellular dermal matrix/mesh (100% versus 45.5%, P < 0.0001), prior radiation (20.0% versus 0%, P = 0.0083), immediate implants (46.7% versus 20.0%, P = 0.0491), major infection (30.0% versus 1.8%, P = 0.028), and both major mastectomy flap/NAC necrosis (33.3% versus 10.9%, P = 0.0494).
Conclusions:
NSM cases with major ischemia requiring explantation had a lower body mass index and significantly higher rate of preoperative radiation, immediate implant placement, use of acellular dermal matrix/mesh, and concomitant major infection. These variables should be taken into account when discussing risks with patients preoperatively and assessing the quality of mastectomy flaps and subsequent reconstructive choices intraoperatively.
INTRODUCTION
Nipple-sparing mastectomy (NSM) provides the opportunity to optimize esthetic outcomes after breast reconstruction with high patient satisfaction and quality of life.1–3 NSM, however, carries an inherently greater risk for mastectomy flap ischemia compared with traditional mastectomy techniques. This tendency is secondary to preservation of the majority or entirety of the skin envelope which creates a larger surface area to be perfused, may increase traction on flaps in larger breasts, and can contribute to the inability to excise a significant amount of skin if compromised in certain situations.
Reported rates of nipple–areola complex (NAC) and mastectomy flap necrosis in NSM vary, ranging from 2.1% to 7% and 1.2% to 8.1%, respectively.4–17 When combined, overall rates of major ischemic complications, typically defined as full-thickness necrosis requiring debridement, can be significant. In a procedure aimed at preserving the entirety of the natural breast skin envelope and NAC, this loss can be a source of significant distress for the patient and surgeon. In addition, full-thickness loss of tissue in implant-based reconstruction ultimately threatens prosthesis exposure, implant loss, and reconstructive failure.
Risk factors for postoperative ischemic complications have been studied at length. Intrinsic or patient-specific factors include body mass index (BMI) and breast morphology (breast size, mastectomy weight, and ptosis).6,11,15,18 Extrinsic influences such as radiation, incision pattern, mastectomy flap thickness, smoking, and the type of reconstruction must also be considered.6,9,19–22 Although the contribution of these elements to the development of NAC or mastectomy flap necrosis has been quantified, the outcomes of these complications and their implications for the overall reconstruction are less well described.
There is a broad spectrum of consequences to major ischemic events, from simple in-office debridement and closure, to the need for explantation and delayed reconstruction. Major skin envelope necrosis has been reported as the etiology for explantation in up to 79.1% of reconstructive failures in large NSM series.11 Identification of modifiable operative choices and adaptable postoperative decisions that influence the course of these complications can help encourage a more favorable outcome in amenable cases.
This study examines the characteristics of major ischemic complications after NSM and the factors that contribute to reconstructive failure in implant-based reconstruction to determine the optimal management of these difficult cases and minimize their associated morbidity.
METHODS
Data Collection and Analysis
A retrospective review was performed of all patients who underwent immediate alloplastic breast reconstruction after NSM at a single institution from 2006 to June 2018. All patients with postoperative major NAC or mastectomy flap necrosis, defined as necrosis requiring either in-office or operating room debridement, were included.
Data on patient demographics, adjuvant, and neoadjuvant therapies, and mastectomy and reconstructive operative characteristics, was collected and analyzed. Additionally, details of major ischemic complications including time to debridement, debridement size and setting, additional complications, and need for implant exchange or explantation were analyzed. Cases requiring explantation were compared with cases with major ischemic complications that were salvaged. Cases salvaged after implant exchange were additionally compared with those requiring explantation and delayed reconstruction.
Statistical Analysis
Descriptive statistics and measure of central tendency were used to describe absolute and mean results, respectively. Unpaired Student’s t tests were used to analyze continuous data sets, whereas Fisher’s exact test was used to compare proportional responses. All statistical analyses were performed using GraphPad Software, Inc. (La Jolla, CA). A P-value of less than 0.05 was considered significant.
Patient Selection and Surgical Technique
NSM is discussed as an option with all women presenting for breast reconstruction after prophylactic mastectomy and in patients undergoing therapeutic mastectomy with tumor-to-nipple distances greater than 1 cm.23 Candidacy for nipple-sparing techniques is determined in conjunction with the breast surgeon and the patient. Relative contraindications included neoadjuvant chemotherapy, preoperative radiation, morbid obesity, severe macromastia, grade III ptosis, or significant chest wall/NAC asymmetry. Although none of these factors in isolation contraindicated NSM, the presence of multiple risk factors led to recommendation of non-NSM techniques or staged reduction in appropriate patients. Patients desiring implant-based reconstruction were offered 1- or 2-stage reconstructions based on breast morphologic characteristics and intraoperative evaluation of mastectomy flaps.12
Operative techniques for dual-plane and total submuscular reconstruction are as previously detailed.24 Intraoperative evaluation of mastectomy flaps was based on clinical examination of skin and NAC perfusion including skin-edge bleeding, flap thickness, and amount of visible dermis. Indocyanine green angiography is not routinely utilized given the use of a low-volume, dilute epinephrine-containing local anesthetic before mastectomy.
Postoperative management of skin envelope ischemia was based on individual surgeon preferences. Typically, any indication of postoperative skin or NAC ischemia was treated with local wound care with moist petroleum-based gauze or antibiotic ointment and nonadherent gauze dressing changes. If tissue expanders (TEs) were placed at the initial operation, they were deflated to relieve any excess skin tension. Adjunctive therapies such as topical nitroglycerin paste or hyperbaric oxygen treatments were rarely utilized. The decision to proceed with debridement was based on surgeon assessment of estimated extent and thickness of skin necrosis, and the presence of underlying vascularized tissue versus prosthesis/non-vascularized matrix.
RESULTS
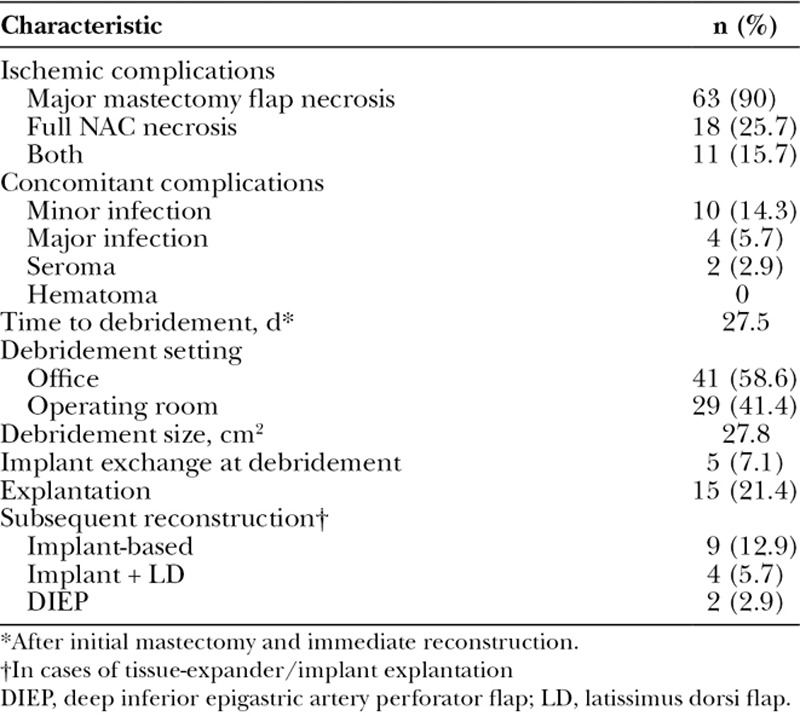
During the study period, 1045 cases of immediate prosthetic-based reconstruction were performed after NSM. Of these, 70 cases (6.7%) had major ischemic complications (Table 1). Sixty-three cases (90%) had major mastectomy flap necrosis, 18 (25.7%) had full NAC necrosis and 11 (15.7%) had both. Four cases (5.7%) were in active smokers and 21 cases (30%) in formers smokers. Average mastectomy weight was 645.7 g. Most cases were 2-stage TE reconstructions (74.3%) and used either biologic or synthetic mesh/matrix support (57.1%). All TEs were textured, integrated-port devices, and all implants were smooth, round implants. Average follow-up length was 39.1 months.
Table 1.
Patient Demographics and Intraoperative Characteristics of NSM Cases With Major Ischemic Complications

The most common complication associated with major necrosis was minor infection treated with oral antibiotics in 10 cases (14.3%), followed by major infection treated with intravenous antibiotics in 4 cases (5.7%) (Table 2). The average time to debridement from the initial mastectomy and immediate reconstruction was 27.5 days, and the average debridement size was 27.8 cm2. One patient underwent hyperbaric oxygen treatment and eventually required debridement of a 9 cm2 area of mastectomy flap necrosis in the office. No patients had topical nitroglycerin applied postoperatively.
Table 2.
Reconstructive Complications and Management of NSM Cases With Major Ischemic Complications
Five cases (7.1%) underwent implant exchange at the time of debridement and 15 cases (21.4%) required explantation upon debridement. Of the cases requiring explantation, all underwent successful delayed secondary reconstruction with implant-based or autologous techniques (Fig. 1).
Fig. 1.
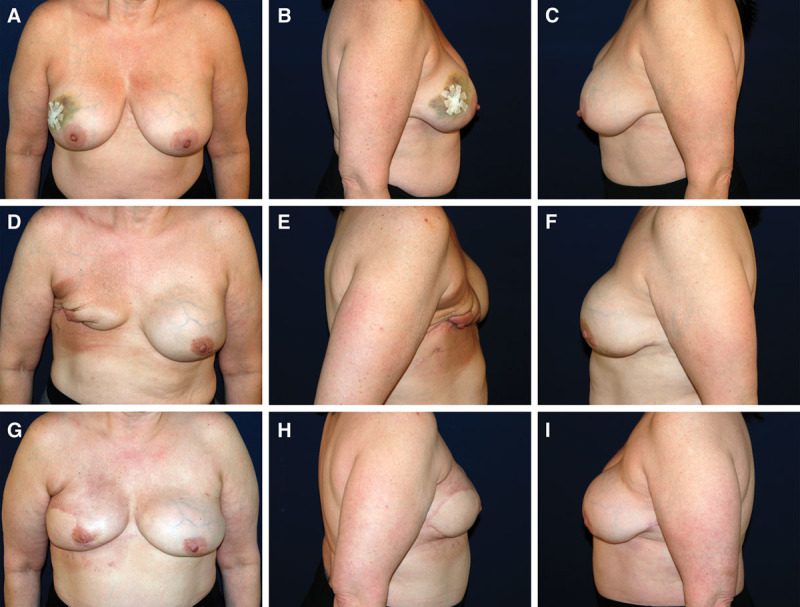
53-year-old female with a history of right breast cancer who underwent bilateral nipple-sparing mastectomy and dual-plane immediate implant reconstruction with 475 cc smooth, round implants. Postoperative course complicated by significant right mastectomy flap necrosis and implant exposure requiring explantation. A–C, Preoperative photographs. D–F, Postoperative photographs after right implant explantation and right chest radiation therapy. G–I, Postoperative photographs 1 year after bilateral secondary reconstruction with DIEP flaps. DIEP, deep inferior epigastric artery perforator.
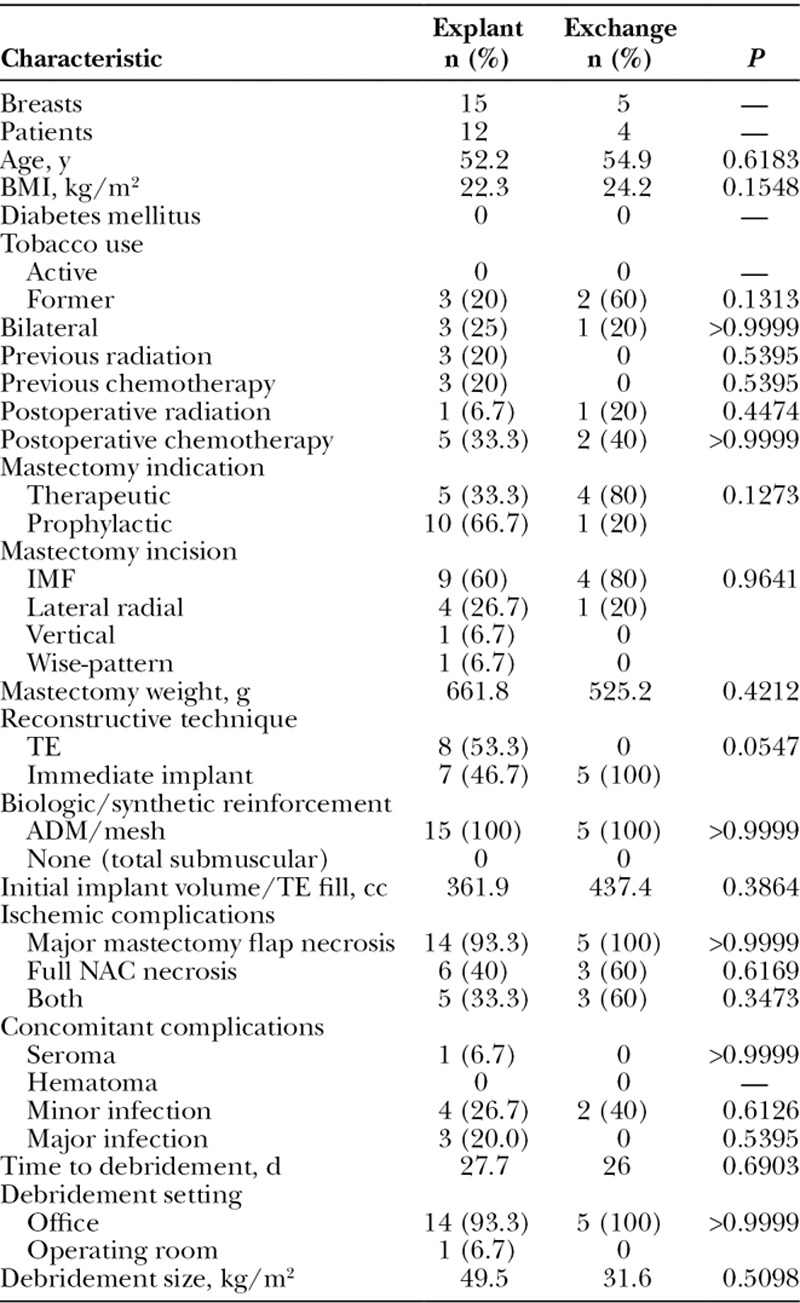
Cases of major mastectomy flap or NAC necrosis requiring explantation had a significantly lower BMI (22.3 versus 24.7, respectively; P = 0.013) and a higher rate of preoperative radiation (20% versus 0%, respectively; P = 0.0083) compared with those who did not require explantation (55 cases) (Table 3). Operative characteristics also differed between the 2 cohorts. Cases requiring explantation had a significantly higher rate of immediate implant placement (45.6% versus 20%, P = 0.0491) and utilization of acellular dermal matrix (ADM)/mesh (100% versus 45.5%, P < 0.0001) (Fig. 2). Both major mastectomy flap necrosis and NAC necrosis together (33.3 versus 10.9%, P = 0494) and the occurrence of major infection (20 % versus 1.8%, P = 0.0288) were more likely to occur in explanted cases. Finally, explanted cases had a larger average surface area of necrosis debrided (49.5 cm2 versus 17.6 cm2, P = 0.0168) and were more likely to have debridement performed in the operating room as opposed to the office compared with cases without explantation (93.3% versus 82.7%, respectively; P < 0.0001). Comparison of explanted cases to those who underwent implant exchange at the time of debridement showed no differences between the 2 groups (Table 4).
Table 3.
Comparison of Patient Demographics, Intraoperative Techniques, and Characteristics of Ischemic Complications in Patients Requiring Explantation
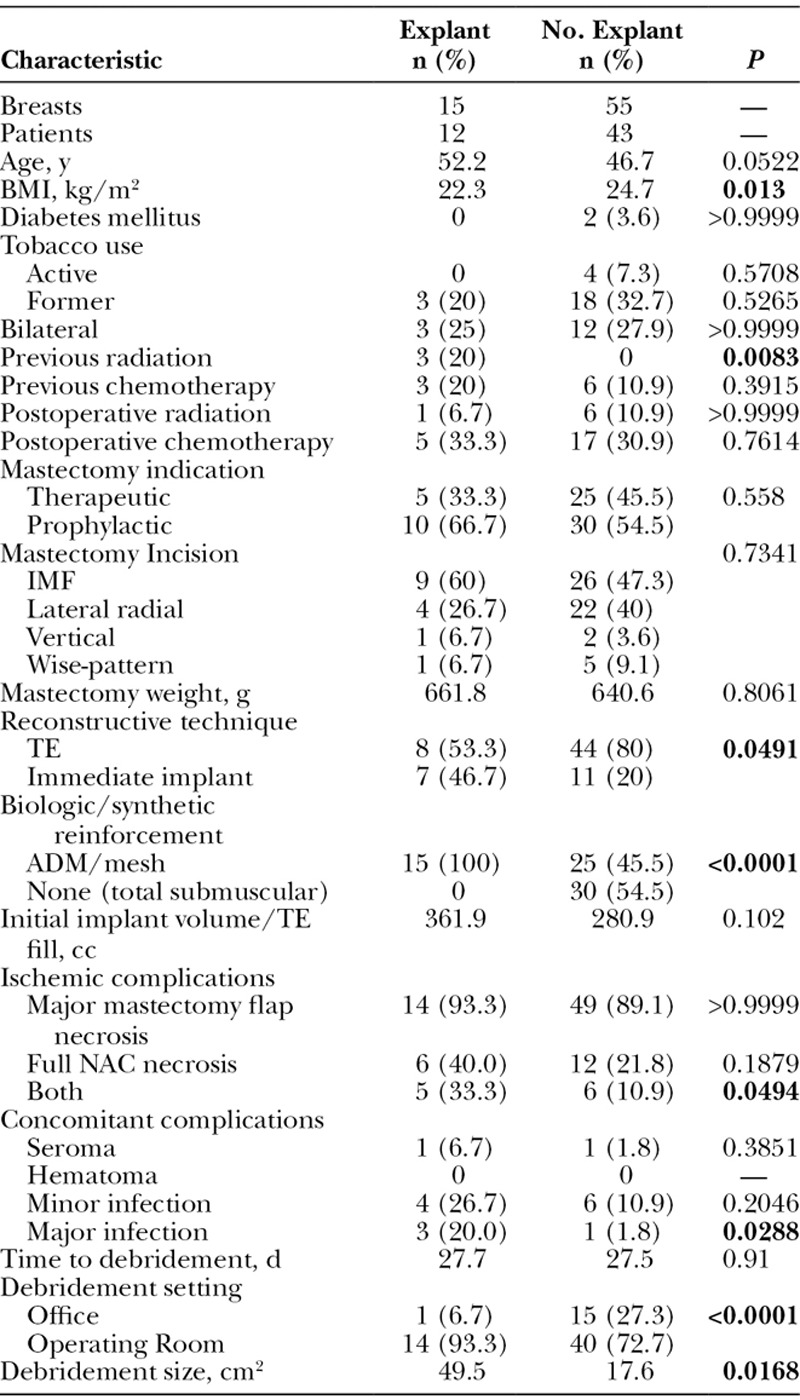
Fig. 2.
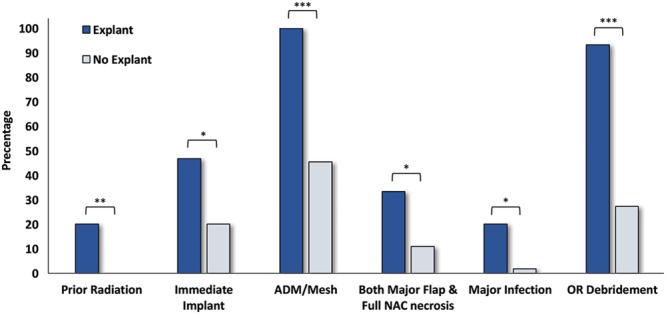
Categorical variables with significant differences between explanted and nonexplanted cases in nipple-sparing mastectomy and immediate reconstruction with major ischemic complications. OR, operating room. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 4.
Comparison of Patient Demographics, Intraoperative Techniques, and Characteristics of Ischemic Complications in Patients Requiring Explantation Versus Exchange
DISCUSSION
Major ischemic complications of the skin envelope after NSM and implant-based reconstruction at the very least compromise esthetic outcomes and often can threaten the entire reconstruction and delay adjuvant treatments. Fortunately, severe cases of major necrosis are rare; however, some degree of NAC or mastectomy flap necrosis requiring debridement is not an uncommon phenomenon. Review of our series of implant-based reconstruction after NSM revealed an overall major necrosis rate of 6.7%, which compares favorably with outcomes of prior series.6,9–11
Multiple factors have been associated with an increased risk of ischemic complications after NSM.6,9,11,15,18–20 Interestingly, the incidence of certain risk factors was low in this isolated cohort of cases with major necrosis. The percentage of active tobacco users was only 5.7%, likely due to an overall low rate of active smokers at our institution (4.1%). However, we have previously found an association of between an extended period of prior smoking history and major necrosis after NSM,25 which correlates with the high percentage of former smokers in this group (30%). Similarly, the lower incidence of other potential risk factors may reflect the general characteristics and surgical preferences at our institution. Patients selected for NSM typically demonstrate a more favorable risk profile based on relative contraindications for nipple-sparing techniques. Patients were relatively young and average BMI was low. Periareolar incisions are associated with a significantly higher rate of NAC necrosis9,22,26,27 but are generally avoided at our institution. Although these factors certainly influence the potential for postoperative ischemia, the more critical issues appear to be the intrinsic morphology of the breast, its implications for perfusion and how this perfusion is altered during mastectomy and reconstruction.
Breast size, as quantified by various metrics including mastectomy weight, is a significant predictor of ischemic complications of the NAC and skin envelope.15,18 Increasing breast size, and to a certain extent the degree of ptosis,11 results in a greater surface area to be perfused, an increased distance perforators must travel, increased traction on mastectomy flaps during mastectomy and greater manipulation of the NAC during reconstruction. The average mastectomy weight in this series was 645.7 g, categorized as an intermediate mastectomy weight which has been previously associated with a significantly increased risk of both major mastectomy flap necrosis and full-thickness NAC necrosis.18
Likely the most critical variable in the development of ischemic complications is the quality of mastectomy flaps. Assessment of mastectomy flap quality can be performed by examining clinical signs of perfusion, evaluating mastectomy flap thickness, and quantifying perfusion with fluorescence angiography-based imaging. Relative mastectomy flap thickness plays a particularly influential role in determining the preservation of the superficial perfusion from the internal mammary artery perforators running in the subcutaneous fat by performing the mastectomy in the appropriate plane, just superficial to the breast capsule.21 Unfortunately, mastectomy flap thickness was not able to be analyzed retrospectively as the majority of cases in this cohort did not have postoperative magnetic resonance imaging (MRIs) available for measurement of flap thickness. The few cases with available postoperative MRIs did demonstrate thin flaps less than 8 mm in thickness21 (Fig. 3).
Fig. 3.

Postoperative MRI of implant reconstruction complicated by major mastectomy flap necrosis with minimal subcutaneous tissue present in the majority of thin mastectomy flaps.
Approximately 21% of cases with major ischemic complications required explantation and secondary reconstruction, significantly higher than typical overall explantation rates in larger NSM series.10,11,24 Cases requiring explantation were compared with those who avoided implant removal to determine whether certain variables may influence the ability to salvage a reconstruction complicated by major necrosis. Several variables were found to be significantly different between the explant and no explant cohorts that related to preoperative patient characteristics, intraoperative decision-making, and postoperative complications.
The only intrinsic patient-related variable found to be significantly different between the 2 cohorts was BMI, which was paradoxically lower in the explantation cohort. Higher BMI has consistently been associated with an increased risk of postoperative complications after implant-based breast reconstruction.6,28–30 However, in the context of tissue loss and debridement, excess tissue in patients with higher BMIs may prove favorable. A paucity of skin or soft tissue after debridement and TE deflation can favor the removal of a prosthesis to allow for adequate healing without prosthesis exposure. Patients with higher BMIs and greater redundancy in available soft tissue, on the other hand, may allow for the primary closure of debridement defects that would otherwise compromise a reconstruction. Notably, average BMI for both cohorts remained within the normal range.
Other important preoperative factors included prior radiation, which was significantly higher in the explant cohort. Radiation therapy has known acute and chronic detrimental effects on wound healing,31 which have been shown to increase complication rates in implant-based reconstruction6,32,33 and similarly compromise the ability to salvage reconstructions in wounds requiring debridement.
Intraoperative decisions also had an important impact on reconstructive failure. The use of ADM or mesh was significantly associated with explantation in cases with major necrosis and was present in 100% of cases with implant loss. Until it becomes incorporated, ADM should be considered as a foreign body, no different that the underlying prosthesis. Full-thickness necrosis overlying ADM in dual-plane reconstructions can be considered nearly equivalent to implant exposure. More prompt debridement is therefore required rather than a “watch and wait” approach which may allow for secondary healing, subsequently decreasing the area requiring debridement. These findings align with previous studies examining conservative management of NAC ischemia. Dent et al.34 examined the success of expectant management of NAC ischemia in NSM and found ADM use to be significantly associated with failure of conservative treatment. Of note, many of these cases were single-stage reconstructions that typically require the use of ADM or mesh for coverage.
The association of ADM with explantation in NSMs with major ischemia also has important implications for implant-based prepectoral reconstruction. Most reported cases of prepectoral reconstruction involve covering at least the anterior prosthesis surface with ADM,14,16,35–39 or placing the prosthesis directly under mastectomy flaps.13 As with full-thickness necrosis overlying ADM in dual-plane reconstruction, major necrosis in prepectoral reconstruction can have more dire consequences without the availability of interpositional vascularized muscle. All cases of mastectomy flap necrosis or late dehiscence in a series of prepectoral reconstructions reported by Bernini et al.35 required explantation. Similarly, though overall rates of complications were low, Nahabedian et al.16 reported 100% of explantations were secondary to mastectomy flap necrosis compared to 67% in dual-plane reconstructions. In a series of prepectoral implant-based reconstruction by Highton et al.,39 all 5 cases of major skin necrosis required explantation, though the additional risk of immediate implant reconstruction must be considered. Prepectoral breast reconstruction has still demonstrated a good safety profile14,16,40; however, these outcomes must be interpreted in the context of low rates of major ischemic complications.
Other intraoperative factors associated with explantation included the reconstructive modality, with immediate implant placement being significantly more associated with reconstructive failure. The inability to remove fluid as with a TE reconstruction to decrease the size of the prosthesis and “increase” the amount of available skin envelope will further increase the likelihood of implant removal to facilitate successful primary closure of a wound. Along the same lines, cases requiring explantation had more extensive necrosis, signified by more frequent involvement of both the NAC and the mastectomy skin and a much larger debridement size that would prohibit wound closure without removal of the prosthesis. Certain risk factors such as BMI, preoperative radiation, and major infection, trended toward but did not have statistical significance in comparison of explant and exchange cohorts. These trends suggest similar possible mechanisms that contributed to failure of implant exchange though the small sample size in this cohort limited statistical analysis.
When faced with a concern for severe NAC or mastectomy skin flap ischemia, consideration of risk factors for explantation can help facilitate decision-making to potentially minimize the possibility of more severe complications. Although a specific variable in isolation may not be of much consequence, a global assessment that reveals multiple concerning issues in the presence of a poorly vascularized skin envelope warrants a more “defensive” approach to reconstruction. In this regard, the primary goal is to minimize potential morbidity by taking measures to optimize viability of the NAC and mastectomy flaps and preserve structures vital to reconstructive and cosmetic outcomes.
The presence of intraoperative ischemia of the breast envelope in combination with risk factors such as a paucity of excess skin or a history of prior radiation should prompt caution in preventing further compromise of tissue perfusion. Single-stage implant reconstruction can be converted to TEs, and TEs should be inflated with low volumes, if at all, to minimize any tension on skin flaps. The use of ADM or mesh must also be evaluated, particularly if areas of potential ischemia will be overlying these materials. In such cases, we will often consider conversion to a total submuscular approach utilizing serratus fascia to protect the underlying prosthesis. In cases with significant concern for skin envelope ischemia intraoperatively, delaying the reconstruction can be considered in both prepectoral and subpectoral reconstructions to allow for adequate perfusion of the skin envelope before prosthesis placement.41 Adjunctive postoperative therapies, such as the use of nitroglycerin ointment, may also be utilized if there is concern for ischemia intraoperatively.42 Other interventions, such as nipple delay, can be useful in patients that are determined to be high risk preoperatively.43,44
Importantly, these possibilities must be discussed at length with the patient, to ensure a process of informed and shared decision-making. Although certain risk factors such as prior radiation may warrant preoperative discussion on increased risk for implant loss, other factors such as a paucity of soft tissue in a low-BMI patient may be better suited for discussion in the presence of major necrosis postoperatively. Though the potential for a prolonged reconstructive process or an initial result farther from the ideal outcome is never desired, when framed in the context of optimizing the final reconstructive and cosmetic outcome, it is almost always understood and accepted.
When full-thickness necrosis has developed postoperatively, early excisional intervention for smaller wounds can salvage reconstructions. Implant exchange may be warranted in uncomplicated cases with larger areas of necrosis if the skin envelope is still sufficient. In this study, there were no significant differences between explant and exchange cohorts, though the low sample size in the exchange group likely precluded certain differences from being observed. Early intervention is particularly important in the setting of necrosis overlying ADM or mesh, to prevent contamination and infection of the prosthesis. Major infection was significantly associated with explantation and minor infection trended toward reconstructive failure in this series. Infection should be treated aggressively when associated with major necrosis. Salvage rates remained low in this scenario (25%), though comparable with prior studies.45
Limitations of this study include its retrospective analysis of data which prevented certain important variables, such as mastectomy flap quality and thickness, from being quantifiably analyzed. In addition, though the overall study population was relatively large, certain subgroups had a low sample size which may have limited the observed differences between cohorts. Finally, patient satisfaction and objective analysis of cosmetic outcomes are important metrics that require further evaluation.
CONCLUSIONS
Although all surgeons strive for the ideal, it is equally important to understand how to manage the nonideal situation. Major ischemic complications following NSM and implant-based reconstruction are altogether undesirable events but can result in outcomes ranging from minor in-office procedures to loss of a reconstruction. Predisposition to poor wound healing, the use nonvascularized support materials, excess strain on the skin envelope, concomitant major infection, and larger affected areas further increase the risk of reconstructive failure in the setting of major ischemic complications. Early recognition of potential ischemia and subsequent avoidance or mitigation of these factors may therefore help efficiently and appropriately to treat these difficult cases in an effort to minimize morbidity while preserving the reconstruction and optimizing the final outcome.
Footnotes
Published online 23 May 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Djohan R, Gage E, Gatherwright J, et al. Patient satisfaction following nipple-sparing mastectomy and immediate breast reconstruction: an 8-year outcome study. Plast Reconstr Surg. 2010;125:818–829. [DOI] [PubMed] [Google Scholar]
- 2.Bailey CR, Ogbuagu O, Baltodano PA, et al. Quality-of-life outcomes improve with nipple-sparing mastectomy and breast reconstruction. Plast Reconstr Surg. 2017;140:219–226. [DOI] [PubMed] [Google Scholar]
- 3.Didier F, Radice D, Gandini S, et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat. 2009;118:623–633. [DOI] [PubMed] [Google Scholar]
- 4.Reish RG, Lin A, Phillips NA, et al. Breast reconstruction outcomes after nipple-sparing mastectomy and radiation therapy. Plast Reconstr Surg. 2015;135:959–966. [DOI] [PubMed] [Google Scholar]
- 5.Sorkin M, Qi J, Kim HM, et al. Acellular dermal matrix in immediate expander/implant breast reconstruction: a multicenter assessment of risks and benefits. Plast Reconstr Surg. 2017;140:1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg. 2014;133:496–506. [DOI] [PubMed] [Google Scholar]
- 7.Mastroianni M, Lin AM, Smith BL, et al. Nipple loss following nipple-sparing mastectomy. Plast Reconstr Surg. 2016;138:24e–30e. [DOI] [PubMed] [Google Scholar]
- 8.Spear SL, Shuck J, Hannan L, et al. Evaluating long-term outcomes following nipple-sparing mastectomy and reconstruction in the irradiated breast. Plast Reconstr Surg. 2014;133:605e–614e. [DOI] [PubMed] [Google Scholar]
- 9.Endara M, Chen D, Verma K, et al. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg. 2013;132:1043–1054. [DOI] [PubMed] [Google Scholar]
- 10.Orzalesi L, Casella D, Santi C, et al. Nipple sparing mastectomy: surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast. 2016;25:75–81. [DOI] [PubMed] [Google Scholar]
- 11.De Vita R, Zoccali G, Buccheri EM, et al. Outcome evaluation after 2023 nipple-sparing mastectomies: our experience. Plast Reconstr Surg. 2017;139:335e–347e. [DOI] [PubMed] [Google Scholar]
- 12.Choi M, Frey JD, Alperovich M, et al. “Breast in a Day”: examining single-stage immediate, permanent implant reconstruction in nipple-sparing mastectomy. Plast Reconstr Surg. 2016;138:184e–191e. [DOI] [PubMed] [Google Scholar]
- 13.Salibian AH, Harness JK, Mowlds DS. Staged suprapectoral expander/implant reconstruction without acellular dermal matrix following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;139:30–39. [DOI] [PubMed] [Google Scholar]
- 14.Sbitany H, Piper M, Lentz R. Prepectoral breast reconstruction: a safe alternative to submuscular prosthetic reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;140:432–443. [DOI] [PubMed] [Google Scholar]
- 15.Chirappapha P, Petit JY, Rietjens M, et al. Nipple sparing mastectomy: does breast morphological factor related to necrotic complications? Plast Reconstr Surg Glob Open. 2014;2:e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahabedian MY, Cocilovo C. Two-stage prosthetic breast reconstruction: a comparison between prepectoral and partial subpectoral techniques. Plast Reconstr Surg. 2017;140(6S Prepectoral Breast Reconstruction):22S–30S. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:e77–e86. [DOI] [PubMed] [Google Scholar]
- 18.Frey JD, Salibian AA, Karp NS, et al. The impact of mastectomy weight on reconstructive trends and outcomes in nipple-sparing mastectomy: progressively greater complications with larger breast size. Plast Reconstr Surg. 2018;141:795e–804e. [DOI] [PubMed] [Google Scholar]
- 19.Algaithy ZK, Petit JY, Lohsiriwat V, et al. Nipple sparing mastectomy: can we predict the factors predisposing to necrosis? Eur J Surg Oncol. 2012;38:125–129. [DOI] [PubMed] [Google Scholar]
- 20.Carlson GW, Chu CK, Moyer HR, et al. Predictors of nipple ischemia after nipple sparing mastectomy. Breast J. 2014;20:69–73. [DOI] [PubMed] [Google Scholar]
- 21.Frey JD, Salibian AA, Choi M, et al. Mastectomy flap thickness and complications in nipple-sparing mastectomy: objective evaluation using magnetic resonance imaging. Plast Reconstr Surg Glob Open. 2017;5:e1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey JD, Salibian AA, Levine JP, et al. Incision choices in nipple-sparing mastectomy: a comparative analysis of outcomes and evolution of a clinical algorithm. Plast Reconstr Surg. 2018;142:826e–835e. [DOI] [PubMed] [Google Scholar]
- 23.Frey JD, Salibian AA, Lee J, et al. Oncologic trends, outcomes, and risk factors for locoregional recurrence: an analysis of tumor-to-nipple distance and critical factors in therapeutic nipple-sparing mastectomy. Plast Reconstr Surg. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Frey JD, Choi M, Salibian AA, et al. Comparison of outcomes with tissue expander, immediate implant, and autologous breast reconstruction in greater than 1000 nipple-sparing mastectomies. Plast Reconstr Surg. 2017;139:1300–1310. [DOI] [PubMed] [Google Scholar]
- 25.Frey JD, Alperovich M, Levine JP, et al. Does smoking history confer a higher risk for reconstructive complications in nipple-sparing mastectomy? Breast J. 2017;23:415–420. [DOI] [PubMed] [Google Scholar]
- 26.Rawlani V, Fiuk J, Johnson SA, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can J Plast Surg. 2011;19:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyer HR, Ghazi B, Daniel JR, et al. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg. 2012;68:446–450. [DOI] [PubMed] [Google Scholar]
- 28.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121:1886–1892. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen KT, Hanwright PJ, Smetona JT, et al. Body mass index as a continuous predictor of outcomes after expander-implant breast reconstruction. Ann Plast Surg. 2014;73:19–24. [DOI] [PubMed] [Google Scholar]
- 31.Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. [DOI] [PubMed] [Google Scholar]
- 32.Spear SL, Seruya M, Rao SS, et al. Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plast Reconstr Surg. 2012;130:1–9. [DOI] [PubMed] [Google Scholar]
- 33.Cordeiro PG, Albornoz CR, McCormick B, et al. What is the optimum timing of postmastectomy radiotherapy in two-stage prosthetic reconstruction: radiation to the tissue expander or permanent implant? Plast Reconstr Surg. 2015;135:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dent BL, Small K, Swistel A, et al. Nipple-areolar complex ischemia after nipple-sparing mastectomy with immediate implant-based reconstruction: risk factors and the success of conservative treatment. Aesthet Surg J. 2014;34:560–570. [DOI] [PubMed] [Google Scholar]
- 35.Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous direct-to-implant breast reconstruction: surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open. 2015;3:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones G, Yoo A, King V, et al. Prepectoral immediate direct-to-implant breast reconstruction with anterior AlloDerm coverage. Plast Reconstr Surg. 2017;140(6S Prepectoral Breast Reconstruction):31S–38S. [DOI] [PubMed] [Google Scholar]
- 37.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:162–167. [DOI] [PubMed] [Google Scholar]
- 38.Vidya R. Prepectoral breast reconstruction or muscle-sparing technique with the Braxon porcine acellular dermal matrix. Plast Reconstr Surg Glob Open. 2017;5:e1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Highton L, Johnson R, Kirwan C, et al. Prepectoral implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2017;5:e1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterjee A, Nahabedian MY, Gabriel A, et al. Early assessment of post-surgical outcomes with pre-pectoral breast reconstruction: a literature review and meta-analysis. J Surg Oncol. 2018;117:1119–1130. [DOI] [PubMed] [Google Scholar]
- 41.Sbitany H. Important considerations for performing prepectoral breast reconstruction. Plast Reconstr Surg. 2017;140(6S Prepectoral Breast Reconstruction):7S–13S. [DOI] [PubMed] [Google Scholar]
- 42.Turin SY, Li DD, Vaca EE, et al. Nitroglycerin ointment for reducing the rate of mastectomy flap necrosis in immediate implant-based breast reconstruction. Plast Reconstr Surg. 2018;142:264e–270e. [DOI] [PubMed] [Google Scholar]
- 43.Jensen JA, Lin JH, Kapoor N, et al. Surgical delay of the nipple-areolar complex: a powerful technique to maximize nipple viability following nipple-sparing mastectomy. Ann Surg Oncol. 2012;19:3171–3176. [DOI] [PubMed] [Google Scholar]
- 44.Martinovic ME, Pellicane JV, Blanchet NP. Surgical delay of the nipple-areolar complex in high-risk nipple-sparing mastectomy reconstruction. Plast Reconstr Surg Glob Open. 2016;4:e760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reish RG, Damjanovic B, Austen WG, Jr, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. 2013;131:1223–1230. [DOI] [PubMed] [Google Scholar]


