Abstract
Background:
Neuropathic pain is one of the more severe types of chronic pain and presents a great challenge as response to medical therapy remains often unpredictable. With the opioid epidemic and the search for ways to avoid narcotics, physicians are seeking other modalities to treat neuropathic pain. In recent years, surgeons have explored various surgical avenues to improve outcomes. The aim of this review was to evaluate the current clinical evidence regarding the efficacy of fat grafting for the treatment of neuropathic pain.
Methods:
A critical review was conducted to examine the current clinical evidence of fat grafting as a therapy for neuropathic pain caused by neuromas, peripheral neuralgia, migraine and headaches, neuropathic scar pain, and postmastectomy pain syndrome.
Results:
The precise mechanism role of fat grafting in modulating neuropathic pain remains unclear, but it appears to reduce pain levels through the anti-inflammatory effects of adipose-derived stem cells and mechanical cushioning by fat.
Conclusions:
Fat grafting is an emerging therapy for chronic neuropathic pain of various etiologies. Although promising results have been reported, sample size and level of evidence of current studies are low. The encouraging results, however, are worthy of further clinical and scientific study. The minimally invasive nature of fat grafting and favorable risk profile make this an attractive therapy for neuropathic pain.
INTRODUCTION
Neuropathic pain is a pathological condition arising as a direct consequence of a lesion or disease affecting the somatosensory system.1 Neuropathic pain has various etiologies including carpal tunnel syndrome, complex regional pain syndrome, herpes zoster, diabetic neuropathy, phantom limb, traumatic nerve injury (including postsurgical and postmastectomy pain), trigeminal neuralgia, neuroma, entrapment syndromes, and painful scars.2
The prevalence of neuropathic pain in the world was estimated between 0.9% and 17.9%,3 whereas the incidence rate was reported as 0.82% per year.4 Neuropathic pain is characterized by paresthesias, spontaneous ongoing shooting pain, electric shock-like sensations, burning sensation, hyperalgesia, mechanical and thermal allodynia, and temporal summation.2
Neuropathic pain is regarded as one of the more severe types of chronic pain5 and presents a great challenge, as response to medical therapy remains often unpredictable.6 Available therapy revolves around many types of drugs, including antidepressants, antiepileptics, opioids, botulinum toxin type A, nitrate derivates, nicotinic agonists, lidocaine plasters, capsaicin patches, and others.6 Unfortunately, drug treatment is usually chronic and multimodal because of the difficulty in eliminating the pain, but the results remain unsatisfactory because they do not address the underlying etiology.7,8
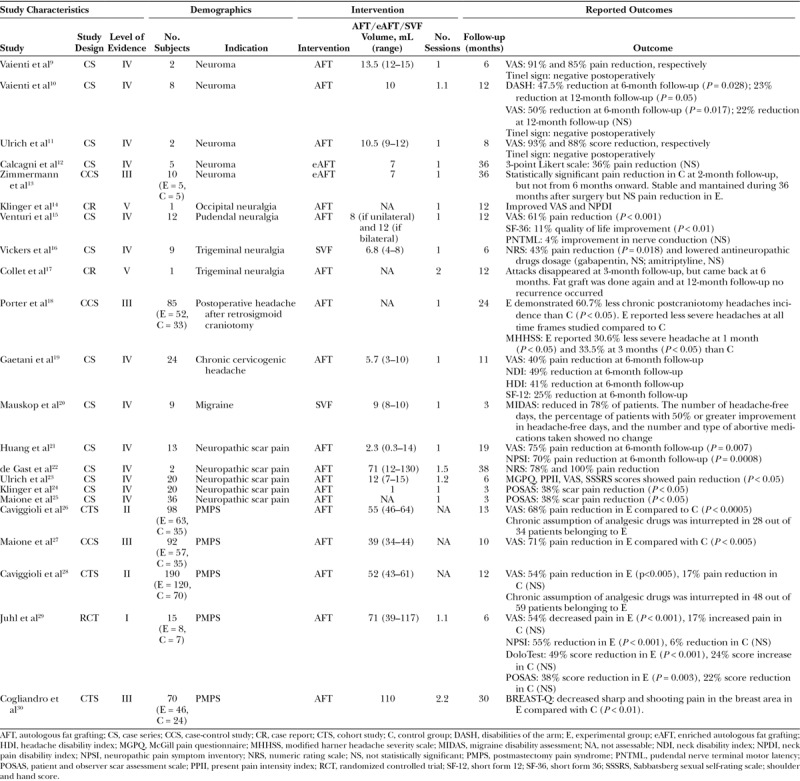
In recent years, surgeons have explored various surgical avenues to improve outcomes in neuropathic pain patients and have demonstrated that in selected cases fat grafting was found to be beneficial (Table 1). For this reason, this review aims to summarize the evidence about the use of fat grafting in the treatment of neuropathic pain.
Table 1.
Summary of All Studies
NEUROMA
Aside from drug therapies, people have generally treated neuromas with surgical excision. However, results of surgical treatment alone are poor, being ineffective in up to 67% of cases.31
Vaienti et al9,10 described favorable outcomes using neuroma excision in combination with perineural autologous fat grafting (Coleman’s technique) to treat painful neuromas of the upper limb, either refractory to medical therapy or featured by multiple recurrence after previous unsuccessful excision. They showed that 7 out of 8 patients had a statistically significant reduction of neuropathic pain at 6 months illustrated by an improved disabilities of the arm shoulder and hand score (preoperative disabilities of the arm score = 46.6; 6 months postoperative disabilities of the arm score = 24.8, P = 0.028) and visual analogic scale [preoperative visual analogue scale (VAS) = 6.8; 6 months postoperative VAS = 3.4, P = 0.017).10 However, no statistical significant difference was found at 12 months follow-up in VAS score. The authors hypothesized that the beneficial results of fat grafting are due to biological and mechanical properties, such as improved vascularization and creation of a cushion around the nerve stump, reducing nerve compression and stimulation.9,10 Ulrich et al11 published in 2012 a short case series describing the optimal neuropathic pain relief achieved using autologous fat grafting (Coleman’s technique) in 2 women affected by postepisiotomy painful neuromas, which recurred after previous surgical excision. The 2 patients experienced a marked drop in the VAS score (75% and 68% preoperatively versus 5% and 8% postoperatively, respectively) at 7 and 9 months, respectively. Furthermore, Calcagni et al12 described in a recent case series, the use of autologous stromal vascular fraction (SVF)-enriched fat grafting after excision of painful neuromas of superficial radial nerve to treat neuropathic pain. Five patients with a mean duration of preoperative pain of 67.5 months were clinically evaluated before surgery and at 2-, 6-, 12-, and 36-month follow-up visits using a 3-point Likert scale. An overall pain reduction score was observed from 2.2 to 1.4 three years after the surgery but did not reach statistical significance. Pain reached a steady level 2 months after the surgery with no relapse up to 36 months. The same group published later a controlled-cohort study, comparing the aforementioned new technique to intramuscular transposition technique in painful neuromas of the superficial branch of the radial nerve.13 Despite an initial significant pain reduction in the transposition group, no statistically significant difference was found after 6 months. However, the SVF-enriched fat grafting group showed a steady and maintained pain reduction during the whole follow-up (36 months) suggesting a potential benefit. The authors speculated that SVF accelerates neuronal regeneration and prevents disorganized axonal outgrowth thanks to enhanced vascularization and reduced inflammatory processes, which leads to decreased fibrosis and hypertrophy of the adjacent tissues. Furthermore, the fat graft is thought to protect the nerve stump preventing contraction of the surrounding tissues and nerve entrapment.12 Although these case series provide some evidence of the benefits of fat grafting in the management of neuromas, the small sample size and lack of a control group prevents any conclusions. Further studies are therefore indicated.
PERIPHERAL NEURALGIA
Fat grafting demonstrated a promising result in the treatment of neuropathic pain in patients affected by peripheral neuralgia, specifically in occipital,14 pudendal,15 and trigeminal neuralgia.16,17 In 2009, Klinger et al14 used fat grafting (Coleman’s technique) in a patient affected by postsurgical, chronic, drug-resistant occipital neuralgia, within the clinically painful affected area. According to the VAS score and to the Neck Pain Disability Index, a steady improvement was observed at 1-year follow-up. Because anatomical scar entrapment was identified as the etiology of persistent neuropathic pain in this instance, the authors hypothesized that autologous fat grafting was able to remodel the architecture of scar tissue (scar entrapment release) and to secrete factors that prolonged analgesia. Moreover, a prospective clinical trial was published in 2015 by Venturi et al15 concerning pudendal neuralgia treated with fat grafting (Coleman’s technique) injected in Alcock’s canal of the affected side. They reported that 10 out of 12 patients with pudendal neuralgia demonstrated at 12 months a statistically significant improvement in VAS scores (8.1 versus 3.2, P < 0.001), and Short Form 36 Health Survey scores (85.0 versus 75.5, P < 0.01). However, this innovative treatment did not lead to any benefit in 2 patients. Interestingly, a progressive pain reduction until 6 months postoperatively was observed, suggesting a potential active biological role played by the implanted cells, besides the mechanical cushion effect. Similar to prior studies, the authors speculated that growth factor and cytokine secretion by adipose-derived stem cells (ASCs) promote angiogenesis and hinder inflammation, and also stimulate the repair of damaged nerves. Small sample and lack of a control group represent the main weaknesses of this trial.
Fat grafting was also found to be beneficial in the treatment of trigeminal neuralgia.16,17 Collet et al17 published a case report in 2013 on a patient suffering from essential trigeminal neuralgia of right V3 (30–35 pain episodes a day) previously treated without success with carbamazepine, gabapentin, and amitriptyline at the time of consult. The labial and cheek region were treated with fat grafting (Coleman’s technique). The patient described the evanescence of pain occurring after an even and gradual reduction of attacks over 2 months, nevertheless attacks recurred after 6 months. After a second round of fat grafting, her pain disappeared within 3 weeks, without recurrence at 24 months follow-up, despite stopping her antineuropathic analgesics. In 2014, Vickers et al16 conducted a study on 9 subjects with neuropathic trigeminal pain and treated them with SVF grafting. Most patients enrolled had tried multiple medications without relief. At 6 months follow-up visits, 5 out of 9 patients had reduced both pain intensity score (7.5 versus 4.3, P = 0.018, using a 10-point Likert scale) and had either stopped or lowered the dose of antineuropathic medication (P = 0.053). Again, no control group was available, and placebo effect is possible, especially because of the use of a surgical procedure. However, according to the authors, a 6-month follow-up featured by a steady and progressive reduction in pain should lessen the placebo effect, particularly in subjects with a history of unsuccessful multiple drug treatments.
Although several medications and procedures are available as a treatment for peripheral neuralgia, they demonstrated varying success rate and side effects. Although further evidences are needed in this direction before any conclusion can be reached, fat grafting has been shown to be a promising safe and effective treatment, especially in drug-resistant peripheral neuralgia.
MIGRAINE AND HEADACHE
Fat grafting was also tested as a way to reduce severity and frequency of migraine and headache. Porter et al18 conducted a controlled clinical study in 2009 enrolling 85 patients undergoing retrosigmoid craniotomy for removal of cerebellar-pontine tumors. The control group included patients with standard wound closure, whereas in the study group, abdominal fat graft was placed over the bone pate at the time of wound closure. The study group experienced less severe postoperative headache after retrosigmoid craniotomy compared with the control group, with statistical significance (P < 0.05) at 1- and 3-month follow-up. No statistical difference was found at 6-month, 1- and 2-year follow-up. Additionally, almost all of the nongrafted patients who experienced postcraniotomy headache (90.9%) transitioned to the chronic state, compared with only 42.9% of patients of the fat graft group (P< 0.05). Gaetani et al19 conducted an observational study in 24 subjects with chronic cervicogenic headaches or occipital neuralgia threated with autologous fat grafting in the zone adjacent to the nerve emergence corresponding to the clinical tender point. At 6-month follow-up, the VAS score (P = 0.0008), Neck Pain Disability Index (P = 0.0006), Headache Disability Index (P = 0.001), and SF-12 (P = 0.003) showed a statistically significant decrease compared with preoperative scores. Furthermore, a reduction was found in duration of headache (P = 0.0006), days of medical treatment (P = 0.0011), and work days loss (P = 0.0015). Of 24 subjects, 22 reported to be strongly satisfied with their treatment. Because chronic cervicogenic headaches and occipital neuralgia are often characterized by persistent pain due to the involvement of the great occipital nerve, with concurrent myofascial spasm and the consequent nerve entrapment within the trapezoid tunnel, subsequently the authors hypothesized that different molecules secreted by transplanted ADSC express an antifibrotic and anti-inflammatory effect improving tissue differentiation and nerve entrapment, leading to successful clinical result. Another recent case series by Mauskop et al20 injected 8–10 mL of SVF into the temporalis, occipitalis, neck, and trapezius muscles of 9 subjects suffering from chronic migraine refractory to medical treatment. Pain was improved in 7 out of 9 patients at the 3-month follow-up. Nevertheless, the number of headache-free days, the percentage of patients with 50% or greater improvement in headache-free days, and the number and type of abortive medications taken showed no change. The authors of both studies associated these improvements to the anti-inflammatory and immunoregulatory properties of SVF. Furthermore, they hypothesized that endothelial progenitors derived from adipose tissue may replace or positively increase the activity and the number of endothelial cells, which have been addressed as a possible dysfunctional element in the pathogenesis of migraine. However, due to the small number of patients, the allowance of concomitant therapies, changes in those therapies during the study, and lack of a control group and use of placebo, definitive conclusions cannot be drawn.
NEUROPATHIC SCAR PAIN
In 2015, Huang et al21 described the successful use of fat grafting (Coleman’s technique) to treat neuropathic scar pain in 13 patients. They used the VAS, DN4, and Neuropathic Pain Symptom Inventory scores to survey patient’s pain. The 3 scores showed statistically significant decreased pain levels at 24 weeks. However, the 2 oldest patients (over 50 years of age) did not demonstrate improvement in pain levels, leading to the hypothesis that age might impact the activity of the ASCs. However, these findings were not confirmed in other clinical studies treating neuropathic pain. In fact, fat grafting used to treat trigeminal neuralgia was found to be most beneficial in patients over 80 years old.16 Moreover, in vitro study showed that ASCs exhibit similar characteristics regardless of the donor age, except for a modest decline in growth rate with age.32 In their study on neuropathic scar pain, Huang et al21 showed that most significant improvements were evoked in pain and paresthesia and dysesthesia (P < 0.001). The authors agreed on the possible mechanical role played by fat grafting in building a protective lining around the damaged nerve, which inhibits nerve stimulation and abnormal sensations. Simultaneously, autologous fat grafting may reduce scar inflammation and relieve neuropathic pain because AD-MSC-secreting cytokines such as IL-10 inhibit the production of inflammatory cytokines. A limitation of this study is the small extension of the scar areas addressed. de Gast et al22 reported the beneficial effect of lipofilling to treat neuropathic scar pain in 2 patients caused by cosmetic surgery (face lift and breast augmentation) and resistant to multiple medical therapies. The first patient demonstrated a 7-point reduction (using a 10-point Likert scale) at 48-month follow-up; the second patient underwent lipofilling twice at distance of 5 months because of partial efficacy, but ultimately had a complete resolution of her neuropathic scar pain demonstrated by a 9-point reduction at 28-month follow-up. Both patients would strongly recommend this treatment. Authors attributed alleviation of pain not only to the padding effect of fat, but also to the tissue regeneration and immunomodulation promoted by ASCs. Ulrich et al23 investigated the usefulness of fat grafting in treating chronic scar pain after episiotomy in 20 patients suffering from dyspareunia and other scar pain related problems. A significant improvement of pain and sexual function was confirmed at 1-, 3-, and 6-month follow-up (P < 0.05). Klinger et al24 conducted a retrospective study of 20 patients affected by chronic scar pain due to retracted and painful scars from burn injuries, road trauma, domestic accidents, or surgery procedures treated with fat grafting (Coleman’s technique). A mean scar pain reduction of 38% (P < 0.05) was registered using the patient and observer scar assessment scale questionnaire at the 3-month follow-up. Similar results were observed by Maione et al25 who showed a 38% scar pain reduction (P < 0.05) following fat grafting (Coleman’s technique) in a prospective clinical trial of 36 patients who complained of post short stature surgical correction painful scars. According to the authors, a possible explanation is that at the basis of the tissue remodeling process, there is the local action of cytokines, growth factors, angiogenic factors, enzymes, and cellular component contained in lipoaspirate, which leads to the formation of new blood vessels with scar tissue remodeling. The aforementioned outcomes indicate how fat grafting might be an encouraging tool against drug-resistant neuropathic scar pain. However, any conclusion remains limited because of the low level of evidence and few studies available so far. Randomized controlled trials (RCTs) are necessary to better confirm the results described above.
POSTMASTECTOMY PAIN SYNDROME
Strong data exist of the benefits of fat grafting in the treatment of postmastectomy pain syndrome (PMPS). The International Association for the Study of Pain defined PMPS as a chronic pain condition with characteristics resembling neuropathic pain. Its incidence is between 20% and 60% of patients undergoing mastectomy or lumpectomy for breast cancer33–35 and therefore is of great concern for both patients and surgeons. Chronic pain is defined as PMPS if it responds to 3 criteria: pain properties, location, and timing. Pain should have neuropathic characteristics with unpleasant and peculiar sensations described as numbness, pins and needles sensations, burning, or stabbing. Its location should be recorded at the same side of surgery, in the axilla, arm, shoulder, or chest wall area. It should last more than 3 months after surgery.26 After mastectomy, reconstructive surgery should aim to restore not only the patient’s breast but also the patient’s quality of life to preoperative conditions: an effective and safe technique that aims to cope with PMPS was investigated.
To the best of our knowledge, Caviggioli et al26 was the first group to report in 2011 the positive outcome achieved in treating PMPS with fat grafting (Coleman’s technique). A total of 98 patients (those lost to follow-up are excluded) affected by PMPS were enrolled in a prospective controlled clinical trial. All of the patients underwent mastectomy with axillary dissection and adjuvant radiotherapy; however, 63 subjects were treated with fat grafting and 35 patients did not receive further surgical intervention (control group). The authors demonstrated a statistically significant decrease in pain levels with VAS score reduction of 3.23 in the experimental group and 1.04 in the control group. Furthermore, analgesic medications were stopped in 82% of patients belonging to the experimental group whose antalgic drug intake was recorded. No information about the antalgic therapy was reported for the control group. Nerve entrapment liberation is suggested as a possible pathophysiologic explanation for pain control improvement in PMPS. The secretion of anti-inflammatory molecules that improve tissue differentiation and scar softness might be responsible for the analgesic effect. Another possible explanation is the mechanical effect of adding soft tissue to scars. In 2014, the same group extended the therapeutic use of autologous fat grafting (Coleman’s technique) to patients who underwent breast conservation surgery (lumpectomy) and adjuvant radiotherapy.27 A total of 92 subjects (experimental group = 57, control group = 35) completed the controlled clinical trial demonstrating a statistically significant greater reduction in pain levels in the fat grafting group compared with the control group (3.1 versus 0.9, P < 0.005). They hypothesized that fat grafting induces stimulating changes in the microenvironment, architectural remodeling of scar tissue, enhancing regeneration of nervous system, and neovessels formation. Because no randomization was performed in these 2 studies, selection bias might be present. Nevertheless, Caviggioli et al28 published a review of their experience in 2016, uniting the results obtained in 201126 and 201427 and to exclude selection bias they statistically compared VAS values in case patients and in control patients before treatment, showing absence of statistical difference
More evidence about the use of autologous fat grafting in persistent pain after breast cancer treatment comes from a single-site, not blinded, randomized controlled study conducted by Juhl et al.29 A total of 15 patients who had undergone unilateral mastectomy to treat breast cancer were included in the study: 8 patients who underwent fat grafting and 7 patients who did not undergo further intervention (control group). All the subjects were examined at the baseline and at 3 and 6 months by using the DoloTest, VAS score, neuropathic pain symptom inventory score, and patient and observer scar assessment scale. All the scores found statistically significant improvement at 6-month follow-up compared with baseline scores in the study group, on the contrary no statistically significant differences resulted in the control group at 6 months. Interestingly, according to the VAS score, this study detected an average pain reduction of 54% at 6 months from the day of fat grafting, and this result is similar to previous findings reported by Caviggioli et al26 and Maione et al.27 Recently, in 2017, Cogliandro et al30 enrolled 70 patients who underwent mastectomy with implant-based reconstruction in a not-randomized controlled clinical trial. The experimental group (46 patients) who received fat grafting (Coleman’s technique) demonstrated a statistically significant improvement in BREAST-Q scores related to pain in the muscles of the chest, sharp and shooting pains in the breast area. Although main weaknesses of the study are lack of randomization or use of placebo, the favorable effect of fat grafting against neuropathic pain was reported.
Although the placebo analgesic effect could not be excluded and RCTs are mandatory before deducing any conclusion, the aforementioned studies boast high level of evidence, thus the promising results obtained warrant further investigation.
BENEFITS, LIMITATIONS, AND FUTURE CONSIDERATIONS
Current surgical interventions for treating localized neuropathic pain include nerve decompression, neurolysis, neurectomy, or nerve reconstruction. Nerve decompression is commonly applied to many peripheral nerves, including the median nerve at the carpal tunnel, and the ulnar nerve at the cubital tunnel and generally finds indication in cases of identifiable anatomic narrowing or compression from adjacent tissues. Neurolysis for entrapment syndromes is commonly performed for the peroneal nerve at the fibular neck, or the tibial nerve at the tarsal tunnel. It is also performed commonly for neuromas-in-continuity, such as those following injury to a mixed motor and sensory nerve. To achieve a high success rate, nerve decompression and neurolysis can be performed once a precise point of entrapment has been highlighted.36 Neurectomy can be considered in patients who have pain in the distribution of purely sensory nerves. It has to be considered as a permanent step and carries the risk of neuroma formation. In addition results decrease with long-term follow-up and patients need to be carefully instructed about care of the new areas of numbness. Nerve repair or reconstruction can be considered in patients with intractable pain from a nerve injury. Dysesthetic neuropathic pain is common in patients who undergo neurolysis or neurectomy in the immediate postoperative period, which typically but not always resolves over several days to weeks.36 The aforementioned techniques are pure surgical procedures. Alternatives include neurostimulation techniques, acupuncture, and nerve block injections.37
Fat grafting has proven to be a minimally invasive, easy to perform, safe, tolerable, and effective procedure in reducing or completely eliminating persistent neuropathic pain. This technique demonstrated significant improvement of symptoms in addition to lowering or entirely stopping antineuropathic therapy to enable an improved quality of life with fewer drug side effects.
Although the role of fat grafting in modulating neuropathic pain is still unclear, many authors hypothesized the anti-inflammatory effect of ASCs in addressing the underlying pathogenesis of neuropathic pain in selected cases, thanks to the capability of ASCs to secrete anti-inflammatory cytokines.38–40 Several in vitro and animal studies demonstrated different ways through which ADSC could play a role in alleviating neuropathic pain. There is evidence that ADSC might differentiate in Schwann cell phenotype and be able to promote neurite overgrowth, thus be beneficial for peripheral nerve injuries.41 ADSCs are also able to produce anti-inflammatory cytokines at the nerve injury site and revert nociceptive hypersensitivity.39,42,43 Experimental models of neuropathy including burn-induced neuropathic pain and sciatic nerve injuries demonstrated marked improvement after ADSC application.44–46
Another possible explanation of the benefits of fat grafting drawn from the aforementioned studies considers both the ASCs biological and mechanical properties in inducing tissue softening and maturation, which release nerve entrapment and stimulation. However, the mechanism remains uncertain due to the low level of evidence of the studies available in the literature so far. Most of the studies include small sample size and lack randomization. Only one RCT was conducted.29
Future studies should be randomized, examine a larger cohort, and include control groups to provide a stronger level of evidence and to try to answer important questions such as the time of efficacy after treatment, volume of fat grafting, patient selection, and site of injection.
CONCLUSIONS
Fat grafting is an emerging therapy for chronic neuropathic pain of various etiologies. This is an exciting area because of the benefits of fat grafting and the potential of reduction of long-term medical therapy. Promising results have been reported, but they are limited by the small sample size and low level of evidence of current studies. The encouraging results, however, are worthy of further clinical and scientific study.
Footnotes
Published online 21 May 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. [DOI] [PubMed] [Google Scholar]
- 2.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. [DOI] [PubMed] [Google Scholar]
- 3.van Hecke O, Austin SK, Khan RA, et al. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–662. [DOI] [PubMed] [Google Scholar]
- 4.Dieleman JP, Kerklaan J, Huygen FJ, et al. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain. 2008;137:681–688. [DOI] [PubMed] [Google Scholar]
- 5.Smith BH, Torrance N, Bennett MI, et al. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin J Pain. 2007;23:143–149. [DOI] [PubMed] [Google Scholar]
- 6.Attal N, Cruccu G, Baron R, et al. ; European Federation of Neurological Societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–1e88. [DOI] [PubMed] [Google Scholar]
- 7.Fortino VR, Pelaez D, Cheung HS. Concise review: stem cell therapies for neuropathic pain. Stem Cells Transl Med. 2013;2:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. [DOI] [PubMed] [Google Scholar]
- 9.Vaienti L, Merle M, Villani F, et al. Fat grafting according to Coleman for the treatment of radial nerve neuromas. Plast Reconstr Surg. 2010;126:676–678. [DOI] [PubMed] [Google Scholar]
- 10.Vaienti L, Merle M, Battiston B, et al. Perineural fat grafting in the treatment of painful end-neuromas of the upper limb: a pilot study. J Hand Surg Eur Vol. 2013;38:36–42. [DOI] [PubMed] [Google Scholar]
- 11.Ulrich D, van Doorn L, Hovius S. Fat injection for treatment of painful neuroma after episiotomy. Int J Gynaecol Obstet. 2011;115:290–291. [DOI] [PubMed] [Google Scholar]
- 12.Calcagni M, Zimmermann S, Scaglioni MF, et al. The novel treatment of SVF-enriched fat grafting for painful end-neuromas of superficial radial nerve. Microsurgery. 2018;38:264–269. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann S, Fakin RM, Giovanoli P, et al. Outcome of stromal vascular fraction-enriched fat grafting compared to intramuscular transposition in painful end-neuromas of superficial radial nerve: preliminary results. Front Surg. 2018;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinger M, Villani F, Klinger F, et al. Anatomical variations of the occipital nerves: implications for the treatment of chronic headaches. Plast Reconstr Surg. 2009;124:1727–1728; author reply 1728. [DOI] [PubMed] [Google Scholar]
- 15.Venturi M, Boccasanta P, Lombardi B, et al. Pudendal neuralgia: a new option for treatment? Preliminary results on feasibility and efficacy. Pain Med Malden Mass. 2015;16:1475–1481. [DOI] [PubMed] [Google Scholar]
- 16.Vickers ER, Karsten E, Flood J, et al. A preliminary report on stem cell therapy for neuropathic pain in humans. J Pain Res. 2014;7:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collet C, Haen P, Laversanne S, et al. Trigeminal neuralgia: a new therapy? Med Hypotheses. 2013;81:1088–1089. [DOI] [PubMed] [Google Scholar]
- 18.Porter RG, Leonetti JP, Ksiazek J, et al. Association between adipose graft usage and postoperative headache after retrosigmoid craniotomy. Otol Neurotol. 2009;30:635–639. [DOI] [PubMed] [Google Scholar]
- 19.Gaetani P, Klinger M, Levi D, et al. Treatment of chronic headache of cervical origin with lipostructure: an observational study. Headache. 2013;53:507–513. [DOI] [PubMed] [Google Scholar]
- 20.Mauskop A, Rothaus KO. Stem cells in the treatment of refractory chronic migraines. Case Rep Neurol. 2017;9:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang SH, Wu SH, Chang KP, et al. Alleviation of neuropathic scar pain using autologous fat grafting. Ann Plast Surg. 2015;74(suppl 2):S99–S104. [DOI] [PubMed] [Google Scholar]
- 22.de Gast H, Torrensma B, Fitzgerald E, et al. The treatment of chronic neuropathic pain: bio (regenerative) pain treatment through lipofilling. A short communication case series. Pain Physician. 2016;19:E495–E498. [PubMed] [Google Scholar]
- 23.Ulrich D, Ulrich F, van Doorn L, et al. Lipofilling of perineal and vaginal scars: a new method for improvement of pain after episiotomy and perineal laceration. Plast Reconstr Surg. 2012;129:593e–594e. [DOI] [PubMed] [Google Scholar]
- 24.Klinger M, Caviggioli F, Klinger FM, et al. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24:1610–1615. [DOI] [PubMed] [Google Scholar]
- 25.Maione L, Memeo A, Pedretti L, et al. Autologous fat graft as treatment of post short stature surgical correction scars. Injury. 2014;45(suppl 6):S126–S132. [DOI] [PubMed] [Google Scholar]
- 26.Caviggioli F, Maione L, Forcellini D, et al. Autologous fat graft in postmastectomy pain syndrome. Plast Reconstr Surg. 2011;128:349–352. [DOI] [PubMed] [Google Scholar]
- 27.Maione L, Vinci V, Caviggioli F, et al. Autologous fat graft in postmastectomy pain syndrome following breast conservative surgery and radiotherapy. Aesthetic Plast Surg. 2014;38:528–532. [DOI] [PubMed] [Google Scholar]
- 28.Caviggioli F, Maione L, Klinger F, et al. Autologous fat grafting reduces pain in irradiated breast: a review of our experience. Stem Cells Int. 2016;2016:2527349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juhl AA, Karlsson P, Damsgaard TE. Fat grafting for alleviating persistent pain after breast cancer treatment: a randomized controlled trial. J Plast Reconstr Aesthet Surg. 2016;69:1192–1202. [DOI] [PubMed] [Google Scholar]
- 30.Cogliandro A, Barone M, Tenna S, et al. The role of lipofilling after breast reconstruction: evaluation of outcomes and patient satisfaction with BREAST-Q. Aesthetic Plast Surg. 2017;41:1325–1331. [DOI] [PubMed] [Google Scholar]
- 31.Stokvis A, Coert JH, van Neck JW. Insufficient pain relief after surgical neuroma treatment: prognostic factors and central sensitisation. J Plast Reconstr Aesthet Surg. 2010;63:1538–1543. [DOI] [PubMed] [Google Scholar]
- 32.Sowa Y, Imura T, Numajiri T, et al. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev. 2012;21:1852–1862. [DOI] [PubMed] [Google Scholar]
- 33.Alves Nogueira Fabro E, Bergmann A, do Amaral E Silva B, et al. Post-mastectomy pain syndrome: incidence and risks. Breast. 2012;21:321–325. [DOI] [PubMed] [Google Scholar]
- 34.Stevens PE, Dibble SL, Miaskowski C. Prevalence, characteristics, and impact of postmastectomy pain syndrome: an investigation of women’s experiences. Pain. 1995;61:61–68. [DOI] [PubMed] [Google Scholar]
- 35.Smith WC, Bourne D, Squair J, et al. A retrospective cohort study of post mastectomy pain syndrome. Pain. 1999;83:91–95. [DOI] [PubMed] [Google Scholar]
- 36.Lipinski LJ, Spinner RJ. Neurolysis, neurectomy, and nerve repair/reconstruction for chronic pain. Neurosurg Clin N Am. 2014;25:777–787. [DOI] [PubMed] [Google Scholar]
- 37.Hatch MN, Cushing TR, Carlson GD, et al. Neuropathic pain and SCI: identification and treatment strategies in the 21st century. J Neurol Sci. 2018;384:75–83. [DOI] [PubMed] [Google Scholar]
- 38.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005;19:547–566. [DOI] [PubMed] [Google Scholar]
- 39.Sacerdote P, Niada S, Franchi S, et al. Systemic administration of human adipose-derived stem cells reverts nociceptive hypersensitivity in an experimental model of neuropathy. Stem Cells Dev. 2013;22:1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waldner M, Zhang W, James IB, et al. Characteristics and immunomodulating functions of adipose-derived and bone marrow-derived nesenchymal stem cells across defined guman leukocyte antigen Bbarriers. Front Immunol. 2018;9:1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingham PJ, Kalbermatten DF, Mahay D, et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–274. [DOI] [PubMed] [Google Scholar]
- 42.Franchi S, Castelli M, Amodeo G, et al. Adult stem cell as new advanced therapy for experimental neuropathic pain treatment. Biomed Res Int. 2014;2014:470983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siniscalco D, Giordano C, Galderisi U, et al. Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Front Integr Neurosci. 2011;5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang SH, Wu SH, Chang KP, et al. Autologous fat grafting alleviates burn-induced neuropathic pain in rats. Plast Reconstr Surg. 2014;133:1396–1405. [DOI] [PubMed] [Google Scholar]
- 45.Lee HY, Lee HL, Yun Y, et al. Human adipose stem cells improve mechanical allodynia and enhance functional recovery in a rat model of neuropathic pain. Tissue Eng Part A. 2015;21:2044–2052. [DOI] [PubMed] [Google Scholar]
- 46.Haselbach D, Raffoul W, Larcher L, et al. Regeneration patterns influence hindlimb automutilation after sciatic nerve repair using stem cells in rats. Neurosci Lett. 2016;634:153–159. [DOI] [PubMed] [Google Scholar]


