Summary:
Indocyanine green (ICG) lymphography is a useful imaging modality for evaluation of lymphedema and detection of lymphatic vessels. It also allows us to ensure patency of the anastomosed vessels intraoperatively. However, strong light from the operating microscope usually disturbs ICG fluorescence imaging. Only some built-in ICG camera systems with specific operating microscopes make real-time ICG lymphography possible in lymphaticovenular anastomosis (LVA). We applied a new high-resolution ICG videolymphography system, which is separated from the operating microscope. Because the system can divide near-infrared fluorescence light of ICG from visible light of the operating microscope, real-time ICG videolymphography-navigated LVA under operating microscope illumination is possible regardless types of operating microscopes. The study involved 10 patients with upper extremity lymphedema characterized by International Society of Lymphology stage 2 and treated by 3 lymphaticovenular anastomoses at the forearm (30 lymphaticovenular anastomoses incorporating 30 lymphatic vessels) under real-time ICG videolymphography. The rate of intraoperative detection of lymphatic vessels using real-time ICG videolymphography was 86.7% (0.25–0.85 mm in diameter), and that of lymph flow through the lymphaticovenular anastomoses was 76.7%. None of lymphatic vessels and no flow were detected under the microscope light by means of another non-built-in ICG lymphography camera. Real-time ICG videolymphography in LVA is beneficial, because the surgeon could find lymphatic vessels easily by checking dual images of original view and ICG fluorescent view and ensure accuracy of the LVA in a suture by a suture without any pauses of the surgical procedures.
Lymphedema is a chronic and progressive disease mainly caused by cancer and its treatments.1–7 Lymphaticovenular anastomosis (LVA) is a generally effective, minimally invasive surgical treatment for lymphedema.8–18 However, LVA is still challenging procedure for microsurgeons especially for upper extremity lymphedema (UEL), because very small (mostly less than 0.50 mm in diameter in the upper extremity) and thin-walled lymphatic vessels are difficult to detect and anastomose even under an operating microscope.9–14,19,20
Indocyanine green (ICG) lymphography is a minimally invasive imaging modality that is useful for the preoperative detection of lymphatic vessel by mapping linear patterns on the patient’s limb.4,7,11–21 However, ICG lymphography still has difficulty in assisting intraoperative detection of lymphatic vessels in performing LVA, because strong light from the operating microscope usually disturbs ICG fluorescence imaging at the incision sites.21–24 Only some built-in ICG camera systems with specific operating microscopes make real-time ICG lymphography possible in LVA.
We applied a new operating microscope-separated ICG imaging system device, which can divide near-infrared fluorescence light of ICG from visible light of the operating microscope, and performed real-time ICG videolymphography-navigated LVA under operating microscope illumination.
In this study, the feasibility for using a new ICG videolymphography system to visualize flow of lymph in both real time and standard time under the operating microscope light is demonstrated. The study was conducted under the approval from the Ethics Committee of St. Marianna University School of Medicine (approval number: 4071).
PATIENTS AND METHODS
The study included 10 patients with International Society of Lymphology (ISL) stage 2 breast cancer treatment-related UEL in whom, between March 2018 and July 2018, 3 LVAs had been created at the forearm.25 Three 1.5-cm-long incisions for LVA were decided using preoperative dynamic ultrasonography and preoperative lymph mapping using ICG lymphography device currently in use (PDE-Neo; Hamamatsu Photonics, Hamamatsu, Japan) to analyze effective muscle pumping areas at the arm; we previously reported this LVA method as the dynamic-LVA method.26 All 30 LVAs in the study patients were created by the same surgeon (YS). A new ICG videolymphography devise and current ICG lymphography device were used in evaluating LVA procedures intraoperatively.
Real-time ICG Videolymphography
Two hours before the surgery, 0.1 mL of ICG (Diagnogreen 0.25%; Daiichi Pharmaceutical, Tokyo, Japan) was injected intradermally at the second web space of the hand, at the anterior border of the styloid process of radius, and at the anterior border of the styloid process of ulna. A new device of ICG videolymphography (LIGHTVISION; Shimazu Corporation, Kyoto, Japan) was set to visualize each incision site under magnification with specific configuration of the system (intensity of ICG fluorescent ranged from 8 to 22 and intensity of visible light ranged from 0 to 8) (Fig. 1). All LVAs were created using a Carl Zeiss surgical microscope OPMI Pentero under 5–20% power of the microscope illumination of the xenon light.
Figure 1.
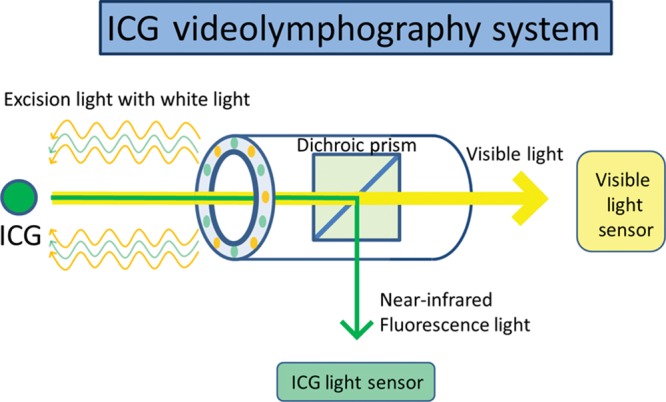
The system of a new ICG videolymphography. A new ICG videolymphography system has a special dichroic prism, which can divide near-infrared fluorescence light of ICG from visible light, and each light is assessed by 2 different sensors.
RESULTS
LVA was performed under local anesthesia in all 10 patients. Patients’ age ranged from 31 to 73 (mean 55.8 ± 13.1) and body mass index (BMI) ranged from 23.6 to 30.5 (mean 25.9 ± 2.2). Five patients revealed ISL stage 2a UEL and other 5 patients revealed ISL stage 2b UEL. Each 3 LVAs were successfully created in 10 study patients (30 lymphaticovenular anastomoses incorporating 30 lymphatic vessels at the forearm). In all 30 anastomoses, intraoperative real-time ICG fluorescent detection of lymphatic vessels and flow through the anastomoses were accessed per ICG fluorescent features (linear, stardust, and diffuse) (Table 1). Under the new ICG videolymphography, the rate of real-time detection of lymphatic vessels was 86.7% (0.25–0.85 mm in diameter) (Fig. 2) and that of lymph flow through the lymphaticovenular anastomoses was 76.7% (Fig. 3). None of lymphatic vessels and no flow were detected under the microscope light by means of another ICG lymphography modality currently in use.
Table 1.
Intraoperative Findings of Lymphatic Vessels in Real-time ICG Videolymphography

Figure 2.
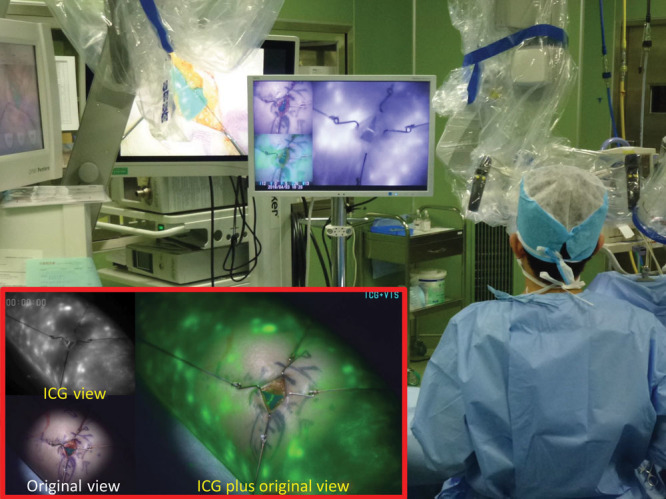
Detection of the lymphatic vessel in stardust patterns using real-time ICG videolymphography imaging. ICG fluorescence images are clearly visualized by an ICG videolymphography under the microscope illumination of the xenon light. The system monitors the operation field real-timely by 3 different simultaneous images: original view with visible light, which include no ICG fluorescence images, ICG view with near-infrared fluorescence light images, and ICG plus original view, which combines original view and ICG view with enhancing ICG fluorescence as green color in the image.
Figure 3.
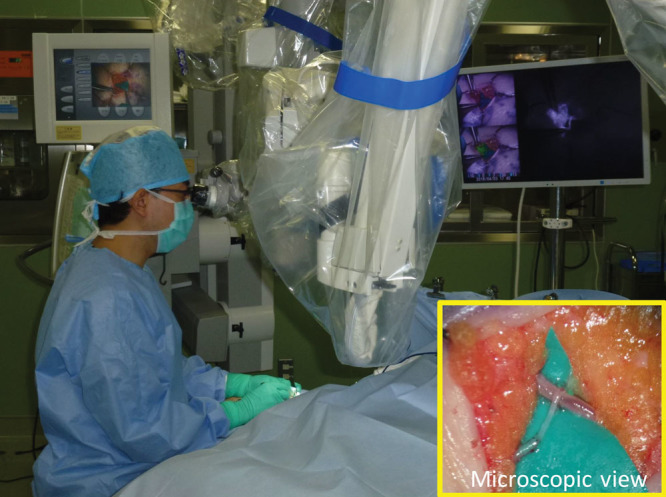
Real-time ICG videolymphography imaging in lymphaticovenular anastomosis. The lymphatic vessel with a diameter of 0.50 mm under fat tissue is easily detected and anastomosed to a subcutaneous vein with a diameter of 0.40 mm. Lymph-to-venous flow of lymph is observed real-timely in performing the anastomosis.
DISCUSSION
ICG lymphography is useful as a minimally invasive imaging modality for evaluating the severity of lymphedema and for detecting the lymphatic vessels preoperatively.4,7,11–24
In conventional ICG-navigated LVA, regions in which preoperative ICG lymphography reveals linear patterns are recommended for LVA because lymphatic vessels in these areas are typically nonsclerotic, especially in patients with early-stage UEL.20,27–29 However, preoperative detection of lymphatic vessels using ICG lymphography was difficult in most of late-stage UEL patients, because linear patterns were usually concealed by stardust patterns or diffuse patterns in progressive lymphedema.22–24,26,29–32 Furthermore, ICG lymphography has limitations in detecting lymphatic vessels real-timely, because strong light of an operating microscope and stardust patterns around the incision sites usually influences delicate ICG fluorescent imaging. In this study, real-time ICG videolymphography under operating microscope light could detect 20 lymphatic vessels for LVAs from 24 stardust or diffuse pattern incision sites intraoperatively (83.3% detection rate).
Lymphatic vessels at the arm in UEL patients are relatively small in diameter than that of lower extremity lymphedema patients.9–14,20,26,32 When lymphatic vessels are under 0.30 mm in diameter, it is not easy to detect lymphatic vessels from connective tissue fibers or nerves in lymphedematous fibrotic subcutaneous tissues even for experts. With our real-time ICG videolymphography, lymphatic vessels were easily detected from these structures.
ICG lymphography has limitation in detecting lymphatic vessels intraoperatively.26,31,32 Yang et al33 reported that 36.1% of lymphatic vessels were not enhanced intraoperatively by ICG fluorescence. Detection rate of lymphatic vessels in real-time ICG videolymphography in this study was 86.7%. This result suggests that our videolymphography could detect most of lymphatic vessels, which can be enhanced by ICG fluorescence. Further study is needed to ascertain whether technical improvements in ICG lymphography could increase the intraoperative detection rate of lymphatic vessels.
In real-time ICG videolymphography, the surgical procedure of LVA could be evaluated in a suture by a suture, because flow of lymph enhanced by ICG fluorescence was visible in the anastomosis of lymphatic vessel and vein real-timely (see video, Supplemental Digital Content 1, which shows the real-time intraoperative ICG videolymphography in lymphaticovenular anastomosis. By real-time ICG fluorescence imaging, lymphatic vessels are easily detected and flow of lymph in the anastomosis is visible in a suture by a suture. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or at http://links.lww.com/PRSGO/B68). Real-time assessment of lymphatic flow at the anastomosis could improve the accuracy of surgical procedures.
Video Graphic 1.
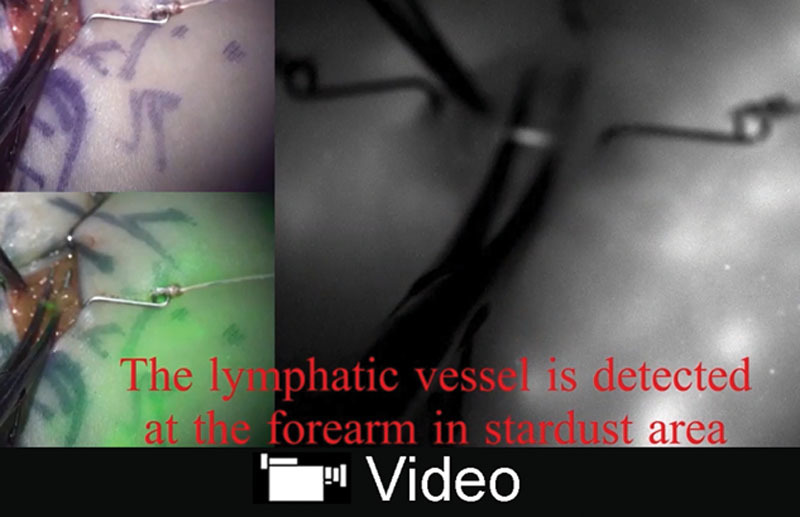
See video, Supplemental Digital Content 1, which shows the real-time intraoperative ICG videolymphography in lymphaticovenular anastomosis. By real-time ICG fluorescence imaging, lymphatic vessels are easily detected and flow of lymph in the anastomosis is visible in a suture by a suture. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or at http://links.lww.com/PRSGO/B68.
CONCLUSION
Real-time ICG videolymphography is beneficial, because the surgeon could find lymphatic vessels easily by checking dual-imaging of original and ICG fluorescent views and ensure accuracy of the LVA intraoperatively.
ACKNOWLEDGMENTS
We thank Miyuki, Hanano, Mayoko, and all members of our department for their kind support during preparation of this article.
Footnotes
Published online 24 May 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245:452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakorafas GH, Peros G, Cataliotti L, et al. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol. 2006;15:153–165. [DOI] [PubMed] [Google Scholar]
- 3.DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. [DOI] [PubMed] [Google Scholar]
- 4.Akita S, Mitsukawa N, Rikihisa N, et al. Early diagnosis and risk factors for lymphedema following lymph node dissection for gynecologic cancer. Plast Reconstr Surg. 2013;131:283–290. [DOI] [PubMed] [Google Scholar]
- 5.Ferrandina G, Mantegna G, Petrillo M, et al. Quality of life and emotional distress in early stage and locally advanced cervical cancer patients: a prospective, longitudinal study. Gynecol Oncol. 2012;124:389–394. [DOI] [PubMed] [Google Scholar]
- 6.Biglia N, Librino A, Ottino MC, et al. Lower limb lymphedema and neurological complications after lymphadenectomy for gynecological cancer. Int J Gynecol Cancer. 2015;25:521–525. [DOI] [PubMed] [Google Scholar]
- 7.Akita S, Nakamura R, Yamamoto N, et al. Early detection of lymphatic disorder and treatment for lymphedema following breast cancer. Plast Reconstr Surg. 2016;138:192e–202e. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y. Studies on lymphatic venous anastomosis in lymphedema. Nagoya J Med Sci. 1969;32:1–21. [Google Scholar]
- 9.O’Brien BM, Sykes P, Threlfall GN, et al. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg. 1977;60:197–211. [DOI] [PubMed] [Google Scholar]
- 10.Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg. 2000;16:437–442. [DOI] [PubMed] [Google Scholar]
- 11.Granzow JW, Soderberg JM, Kaji AH, et al. Review of current surgical treatments for lymphedema. Ann Surg Oncol. 2014;21:1195–1201. [DOI] [PubMed] [Google Scholar]
- 12.Garza R, 3rd, Skoracki R, Hock K, et al. A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies. BMC Cancer. 2017;17:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garza RM, Chang DW. Lymphovenous bypass for the treatment of lymphedema. J Surg Oncol. 2018;118:743–749. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Narushima M, Yoshimatsu H, et al. Minimally invasive lymphatic supermicrosurgery (MILS): indocyanine green lymphography-guided simultaneous multisite lymphaticovenular anastomoses via millimeter skin incisions. Ann Plast Surg. 2014;72:67–70. [DOI] [PubMed] [Google Scholar]
- 15.Seki Y, Yamamoto T, Yoshimatsu H, et al. The superior-edge-of-the-knee incision method in lymphaticovenular anastomosis for lower extremity lymphedema. Plast Reconstr Surg. 2015;136:665e–675e. [DOI] [PubMed] [Google Scholar]
- 16.Seki Y, Kajikawa A, Yamamoto T, et al. Single lymphaticovenular anastomosis for early-stage lower extremity lymphedema treated by the superior-edge-of-the-knee incision method. Plast Reconstr Surg Glob Open. 2018;6:e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang JC, Wu SC, Chiang MH, et al. Targeting reflux-free veins with a vein visualizer to identify the ideal recipient vein preoperatively for optimal lymphaticovenous anastomosis in treating lymphedema. Plast Reconstr Surg. 2018;141:793–797. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi A, Hayashi N, Yoshimatsu H, et al. Effective and efficient lymphaticovenular anastomosis using preoperative ultrasound detection technique of lymphatic vessels in lower extremity lymphedema. J Surg Oncol. 2018;117:290–298. [DOI] [PubMed] [Google Scholar]
- 19.Chang DW, Masia J, Garza R, 3rd, et al. Lymphedema: surgical and medical therapy. Plast Reconstr Surg. 2016;138(suppl 3):209S–218S. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Yamamoto N, Yoshimatsu H, et al. Factors associated with lymphosclerosis: an analysis on 962 lymphatic vessels. Plast Reconstr Surg. 2017;140:734–741. [DOI] [PubMed] [Google Scholar]
- 21.Ogata F, Narushima M, Mihara M, et al. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg. 2007;59:180–184. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Narushima M, Doi K, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011;127:1979–1986. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Matsuda N, Doi K, et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow stage and concept of subclinical lymphedema. Plast Reconstr Surg. 2011;128:314e–321e. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Yamamoto N, Doi K, et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011;128:941–947. [DOI] [PubMed] [Google Scholar]
- 25.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. 2009 consensus document of the International Society of Lymphology. Lymphology. 2009;42:51–60. [PubMed] [Google Scholar]
- 26.Seki Y, Kajikawa A, Yamamoto T, et al. The dynamic-lymphaticovenular anastomosis method for breast cancer treatment-related lymphedema: creation of functional lymphaticovenular anastomoses with use of preoperative dynamic ultrasonography. J Plast Reconstr Aesthet Surg. 2019;72:62–70. [DOI] [PubMed] [Google Scholar]
- 27.Koshima I, Kawada S, Moriguchi T, et al. Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg. 1996;97:397–405; discussion 406. [DOI] [PubMed] [Google Scholar]
- 28.Mihara M, Hara H, Hayashi Y, et al. Pathological steps of cancer-related lymphedema: histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One. 2012;7:e41126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara H, Mihara M, Seki Y, et al. Comparison of indocyanine green lymphographic findings with the conditions of collecting lymphatic vessels of limbs in patients with lymphedema. Plast Reconstr Surg. 2013;132:1612–1618. [DOI] [PubMed] [Google Scholar]
- 30.Seki Y, Yamamoto T, Kajikawa A. Lymphaticovenular anastomosis for breast cancer treatment-related lymphedema: three-line strategy for an optimal outcome. J Plast Reconstr Aesthet Surg. 2018;71:e13–e14. [DOI] [PubMed] [Google Scholar]
- 31.Mihara M, Seki Y, Hara H, et al. Predictive lymphatic mapping: a method for mapping lymphatic channels in patients with advanced unilateral lymphedema using indocyanine green lymphography. Ann Plast Surg. 2014;72:706–710. [DOI] [PubMed] [Google Scholar]
- 32.Gentileschi S, Servillo M, Albanese R, et al. Lymphatic mapping of the upper limb with lymphedema before lymphatic supermicrosurgery by mirroring of the healthy limb. Microsurgery. 2017;37:881–889. [DOI] [PubMed] [Google Scholar]
- 33.Yang JC, Wu SC, Chiang MH, et al. Intraoperative identification and definition of “functional” lymphatic collecting vessels for supermicrosurgical lymphatico-venous anastomosis in treating lymphedema patients. J Surg Oncol. 2018;117:994–1000. [DOI] [PubMed] [Google Scholar]


