Abstract
Background:
Geographical differences in breast implant selection approaches exist, and clinical data to guide the process are limited. Developing knowledge of implant-related risk factors further complicates the process. This analysis aimed to establish expert consensus on considerations for breast implant selection in Australia and New Zealand based on practice patterns in those countries.
Methods:
A modified Delphi method was used to gain consensus from experts in breast augmentation surgery in Australia and New Zealand. Panelists anonymously completed an initial questionnaire on current considerations in implant selection, discussed a summary of their responses in a live meeting, and completed a final consensus survey based on their live recommendations.
Results:
Seven panelists completed the final consensus survey. Consensus recommendations included ensuring consideration of proper surgical technique (pocket formation, positioning of implant) and patient tissue and anatomical characteristics, weighing relative expected results of various surface textures, sizes, and degrees of cohesivity, and careful contemplation of the migration risk.
Conclusions:
This modified Delphi exercise provided consensus recommendations on the key factors involved in implant selection from the perspective of plastic surgeons with practices in Australia and New Zealand. A primary recommendation was that the choice of implant for each patient should be individualized to patient tissue and anatomical characteristics.
INTRODUCTION
Implant selection for breast augmentation surgery is a multifactorial process that involves choosing from an array of implant shapes, sizes, surface textures, and filler attributes; considering anatomy, tissue characteristics, and aesthetic objectives of patients; and accounting for a surgeon’s preferences and experience.1–6 Despite abundant clinical experience with the multitude of breast implant types, there is limited high-quality, up-to-date evidence comparing these devices and helping surgeons to choose between them.7,8 Selecting the appropriate implant, however, is important to optimize surgical outcomes, and a general principle has gained acceptance in the surgical community to guide this process—matching the implant to the needs and characteristics of the patient.5,7,9–12 Although breast augmentation preoperative methodologies and surgical techniques have been described in detail,2,9,10,13 there is opportunity for additional refinement and direction with respect to matching the implant to the patient.9 For example, considerable geographical differences in surgical approach may influence implant selection decisions.14,15 Surgeons in Australia and New Zealand (ANZ), in particular, have distinct practice patterns, including a tendency to use larger implants than surgeons in Europe or Asia and less pocket irrigation with triple-antibiotic solution than other regions of the world.15 Additionally, developing knowledge of factors associated with capsular contracture and breast implant−associated anaplastic large cell lymphoma (BIA-ALCL) in ANZ16 has led to a more critical review of implant selection in these countries. Refinement of surgical techniques and implant considerations is ongoing.
Breast augmentations are the foremost surgical cosmetic procedure in Australia, exceeding 17,000 surgeries in 2016,17 and a sharp increase in overall cosmetic surgeries in New Zealand has also been reported.18 Subsequently, guidance on implant selection approaches for surgeons in ANZ is a high priority. As advancements in intraoperative techniques, such as the use of bacterial mitigation strategies, increasingly reduce rates of complications,19–21 there exists a greater opportunity to focus on defining principles to guide implant selection.
The present analysis aimed to establish expert consensus on considerations for breast implant selection in ANZ based on practice patterns in those countries, identifying the specific implant features and surgical techniques that account for patient characteristics and desired surgical outcomes in current practice.
METHODS
The consensus panel was composed of Mark R. Magnusson, FRACS (Plast), Queensland, Australia, who conceived the concept for this initiative, obtained sponsorship agreement from Allergan plc (Dublin, Ireland), and served as the panel chairperson, and 7 experienced, senior surgeons from ANZ. Surgeons were selected based on criteria specified by the chairperson, including high volume of breast augmentation surgeries performed; expansive knowledge of and experience with different types of breast implants, surgical technique, and other factors affecting surgical outcomes; and, to mitigate bias of opinion, diversity in practices (ie, academic and community based) and preferences regarding implant selection (eg, brand, texture, and shape). Recommendations presented in this article represent the panel’s expert opinion based on their collective clinical experience.
Modified Delphi Method
The consensus-gathering process followed a modified Delphi method (Fig. 1). The Delphi method is a well-established technique for reaching a consensus of opinion among participants with expertise on a particular issue. This method utilizes multiple iterative rounds of questioning, including an initial inquiry to gather data, formulation of answers into items for further consideration, and reformulation to obtain majority opinion.22,23
Fig. 1.

Flow diagram of the modified Delphi method used in this analysis.
Initial Inquiry Phase
Dr Magnusson developed an open-ended questionnaire on current considerations in breast implant selection and disseminated it to the panel for review and comment. The questionnaire comprised 10 preliminary topics for consideration: implant surface, gel, shape, projection, planes of implant insertion, patient tissue characteristics (eg, qualities of skin envelope), deformity (eg, constriction, tuberous breast, and massive weight loss), activity levels, body mass index, and breast reconstruction. For each topic, panelists were queried on patient or planning considerations, relative indications and contraindications, and rationale for responses.
An independent medical writer summarized questionnaire responses in a blinded fashion. This summary was presented to panelists in a live meeting for further discussion to obtain agreement on topics for a final consensus survey. At least 2 responders were required to mention a specific point of possible consensus within a topic area for the topic to be considered for the final survey.
Consensus Survey Development and Administration
Based on the responses from the live meeting, an independent medical writer developed a consensus survey pertaining to current considerations in implant selection. The panelists completed this survey via an interactive document using a 5-point Likert-type scale to indicate level of agreement with each survey item. The scale of responses included the following choices: strongly disagree, disagree, no opinion, agree, or strongly agree or unimportant, of little importance, moderately important, important, or very important.
Survey Tabulation and Formulation of Consensus
Survey responses were collected and tabulated in an anonymous manner. Consensus was defined using a threshold of 70% for the rate of responses in which panelists reported agree/important and strongly agree/very important.
RESULTS
The responses to the initial questionnaire resulted in 42 possible points of consensus for consideration in breast implant selection. Additional points of consensus were added based on discussion of the initial survey results at the live meeting, resulting in 5 topics and 58 items for the consensus survey: (1) implant surface area nomenclature and relationship to tissue integration (3 items); (2) implant migration risk factors (9 items); (3) implant characteristics (20 items); (4) patient characteristics (17 items); and (5) operative factors (9 items). Members of the consensus panel and Dr Magnusson completed the final consensus survey (n = 7).
Implant Surface Area Nomenclature and Relationship to Tissue Integration
Respondents did not achieve consensus on the usefulness of quantitative nomenclature (ie, surface area of texture expressed in square millimeter) and class nomenclature (surface area classified as smooth, micro, macro, and macro-plus) to categorize implant surface textures. Only 57.1% of panelists agreed or strongly agreed that quantitative nomenclature was useful for defining the surface area of breast implants. No respondents strongly agreed that class nomenclature was useful. Five responses indicated no opinion on the usefulness of quantitative and class nomenclature. Classification of implant surface/roughness according to the categories high, intermediate, low, and minimal, based on direct measurement of implant surface area and roughness, was identified as the preferred nomenclature of the panel during their postsurvey discussions.24 There was 100% consensus that increased implant surface area leads to a higher amount of tissue integration.
Implant Migration Risk Factors
Panelists achieved consensus that the top risk factors for implant migration were surgical formation of the pocket and positioning of the implant, implant size, and patient soft tissue characteristics and ptosis of any degree (Fig. 2). All panelists reported that implant surface texture was moderately important or important as a risk factor for implant migration, but none identified it as very important. At least half of the respondents reported that implant cohesivity has little importance in the risk of displacement of an implant.
Fig. 2.
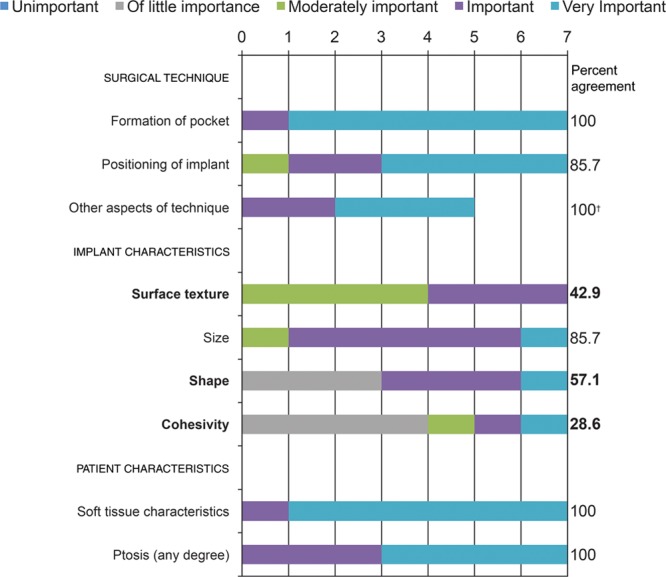
Risk factors involved in implant migration: panelists’ responses and percent agreement (N = 7). Items not reaching the consensus threshold* are in bold font. *Consensus defined using a threshold of 70% for the rate of responses in which panelists reported very important and important. †Of only 5 respondents, 2 panelists did not complete this item.
Implant Characteristics
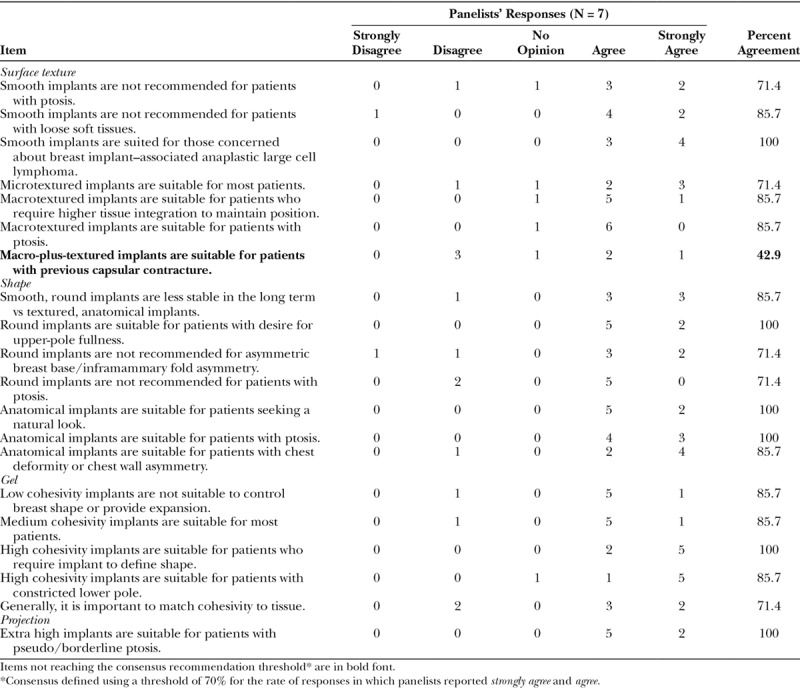
Consensus regarding the specific applications for particular implant surface textures, shapes, filler materials, and projection was achieved for most items (Table 1). There was general agreement that it is important to match the cohesivity of the gel to the tissue of the patient. Most panelists agreed that smooth, round implants are more likely to migrate than textured implants. Consensus was lacking for one item; Macro-plus-textured implants are suitable for patients with previous capsular contracture, with 42.9% of the panelists agreeing on this application.
Table 1.
Implant Characteristics: Panelist Responses and Percent Agreement
Patient Characteristics
Consensus was reached on the importance of patient characteristics in implant selection, such as lax soft tissues and ptosis/pseudoptosis, with consensus absent for the importance of activity restrictions for patients with lax soft tissues or the role of mastopexy in minimizing the importance of tissue characteristics in implant selection (Fig. 3). Consensus survey findings on recommendations for special cases related to patient anatomy and tissue characteristics are summarized in Figure 4. Responses showed that 71.4% strongly agreed that an anatomical implant may be considered an option for patients with tuberous breasts. There was also consensus that patients with a tight envelope require a high cohesivity gel—again, that the firmness of the implant gel should correspond to the tissue of the patient. There was 100% agreement that the longer term concerns for patients with grade 2 ptosis include further ptosis. Consensus was not reached on the implant type best suited to patients with massive weight loss. However, panelists agreed that the dual-plane insertion technique should be considered for patients with a body mass index >25 kg/m2.
Fig. 3.
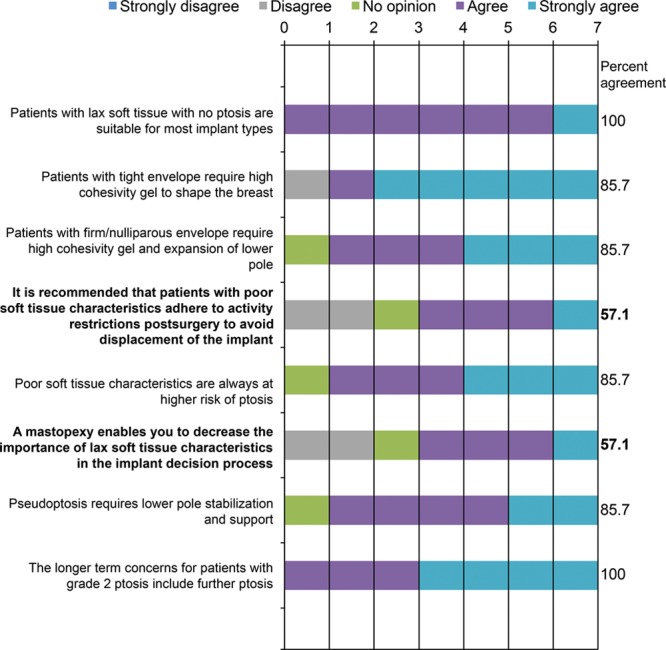
Patient tissue characteristics: panelists’ responses and percent agreement (N = 7). Items not reaching the consensus threshold* are in bold font. *Consensus defined using a threshold of 70% for the rate of responses in which panelists reported strongly agree and agree.
Fig. 4.
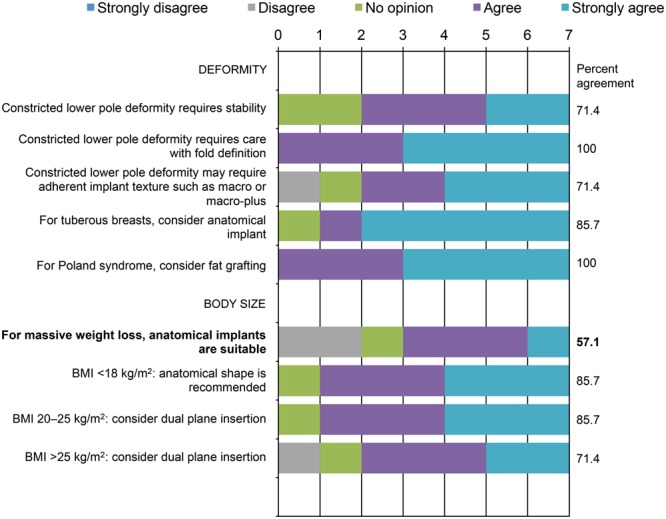
Special patient populations: panelists’ responses and percent agreement (N = 7). Items not reaching the consensus recommendation threshold* are in bold font. *Consensus defined using a threshold of 70% for the rate of responses in which panelists reported strongly agree and agree. BMI, body mass index.
Operative Factors
Consensus was reached on the optimal implant plane based on patient characteristics. All panelists agreed or strongly agreed that dual-plane implant placement resulted in fewer complications (Fig. 5).
Fig. 5.

Operative factors: panelists’ responses and percent agreement (N = 7).
DISCUSSION
Consensus recommendations based on expert opinion are important to the surgical community when few or no data are available. Regional practice differences make it especially difficult to apply general consensus recommendations based on data or on global expert opinion. This report presents the first consensus recommendations on implant selection based on the clinical experience of plastic surgeons from ANZ.
Implant Surface Area
The complexity of breast implant surface texture is generally related to implant surface area; surface texture plays an important role in determining breast tissue response to the implant.25,26 The consensus panel agreed that greater implant surface area leads to greater tissue integration in the clinical setting; however, based on the panelists’ clinical experience, the presence of implant texture may not always correlate with integration into the patient’s tissue. Survey responses did not demonstrate a strong need to communicate varying degrees of implant surface area using current quantitative nomenclature, which may have reflected their shortcomings. However, the panel considers it important to implement a standardized language to communicate surface area based on texture complexity and to compare data across studies, as the literature suggests.27 Standardized nomenclature may be particularly relevant given recent reviews demonstrating a potential association of surface texture and BIA-ALCL, including an analysis of BIA-ALCL cases in ANZ.16,28 Publicity surrounding ALCL in ANZ has implications for practice patterns regarding specific breast implant types and for discussions with patients about implant surface texture options. Such recent developments have led to the proposed classification of implant texture by measuring implant surface area and roughness (high, intermediate, low, and minimal) as previously described.24
Implant Migration Risk Factors
Implant selection may be driven by the perceived risk that a particular type of implant will migrate from its original position after surgery.29 Selection of an implant with demonstrated tissue adhesion properties and placement in a precisely proportioned pocket, with adequate tissue coverage, have been shown to reduce the risk of postsurgical implant malposition.2,3,29 In the panel’s experience, capsular contracture is seen less frequently as surgical techniques are refined. The literature confirms this observation.19,20 A prospective, multinational study that examined the collective experience of 8 surgeons with approximately 42,000 macrotextured devices implanted using a 14-point, anti-infective surgical technique, with a mean follow-up period of 11.7 years, found an overall capsular contracture rate of 2.2%.20 In the view of the panel, surgical measures to reduce the bacterial load around implants have helped to minimize risk of capsular contracture,21 and implant migration has instead moved to the forefront as a primary concern in breast augmentation. Although consensus was not established on the importance of implant texture, shape, and gel cohesivity in the risk of migration, there was a consensus (85.7%) on implant size, and all panelists reported texture to be at least moderately important or important as a risk factor in mitigating implant migration. Larger implants have been associated with increased complications compared with smaller implants,30,31 and the added weight of a larger implant could result in downward displacement over time.32 The responses to the role of texture in implant migration may reflect the greater use of textured implants in ANZ.33 Surgical technique (eg, formation of pocket and positioning of implant) was considered a key factor in minimizing risk of migration and other complications. Implant migration is a risk when there are low levels of tissue integration, as with smooth surface implants.34 However, risk of migration may be reduced by limiting dissection and providing soft tissue reinforcement. High-risk patients may do well with implants with greater tissue integration, but based on the clinical experience of the panel, gel cohesivity may also contribute to implant stability and its role should be investigated further. Overall, any implant can result in a suboptimal outcome if either the surgical technique or the quality of the patient’s soft tissue is poor.
Implant Characteristics
A fundamental part of the implant selection process is considering the physical characteristics of the implant itself. Features that are important in differentiating between implant choices include shape, size, filler material, proportional fill, and surface texture.3,6,7 Surface texture, developed by implant manufacturers to encourage better integration of the implant into surrounding tissue,25 has been an area of increased focus. There is evidence that more complex surface textures may lower the risk of implant rotation and capsular contracture compared with smooth implant shell surfaces.8,35 Implant surface textures have been described in 4 categories, according to increasing surface roughness27 and increasing depth and complexity of surface characteristics and increasing surface area.26 Although there was consensus that textured, anatomical implants may provide more stability than smooth, round implants in the long term, individual implant characteristics such as cohesivity and surface texture have not been adequately studied in clinical settings to assess their impact on implant stability, and thus, no absolute determinations about the performance of one texture versus another can be made. The panel considered round, textured implants to have the same outlook with regard to comparative stability and published studies. In addition, there are some patient cases in which smooth, round implants are the most appropriate choice—for example, when there is poor soft tissue coverage.3 Furthermore, the level of stability desired may vary from patient to patient; for example, an implant that moves with the breast may be preferable to an implant with strong stability if the patient has lax or aging breast tissue that may result in a waterfall deformity (ie, natural breast tissue drooping over the stable breast implant). Finally, in the panelists’ opinion, their inability to achieve consensus that macro-plus-textured implants are suitable for patients with previous capsular contracture may reflect some cautionary views about highly textured implants and risk of ALCL, particularly in ANZ where this is a leading topic of research and discussion. The overall opinion of the panel is that, when considering the many device options, the goal is to match the implant to the patient.
Patient Characteristics
A critical component of selecting an implant is through analysis of a patient’s existing anatomical features, including weight and height, chest wall size and shape, breast volume and shape, and skin and soft tissue characteristics.7,36 Implant selection must be individualized to the patient, accounting for various patient types and tissue characteristics. For example, patients with a tight skin envelope may have more choices when it comes to selecting an implant. The panel achieved consensus on most aspects of patient characteristics and their impact on implant selection. However, there were some differences of opinion surrounding the appropriate implant style for patients who have had massive weight loss because the skin and tissue of individual patients respond to massive weight loss differently.
Operative Factors
All members of the panel agreed that the dual-plane technique is the most commonly used and may result in fewer complications. The panelists also achieved consensus on the applications for subglandular, subfascial, and premuscular approaches to implant placement. However, it is important for surgeons to be aware of subtleties of subglandular and subfascial techniques in actual surgical practice. Regional practice differences may result in survey bias in relation to choosing smooth, round implants over textured, round, or anatomical implants, and surgeons in ANZ typically prefer and have more experience with textured, anatomical implants compared with surgeons in other regions of the world (eg, Asia).15 Local biases exist, as well. The anecdotal experience of the panel suggests that surgeons in Victoria, Australia, use smooth implants more often than those in Queensland and New South Wales. In each state, surgical practice, refinement, and education in techniques for achieving consistent results with a chosen implant reinforce that implant choice over time.
Implant Selection: Summary
Surgical technique, adequate planning, precise execution, careful control of pocket dissection, and incorporating appropriate steps to reduce bacterial burden provide a foundation to achieve favorable outcomes in breast augmentation surgery. Implant selection is a balance of experience, geography, patient goals, and tissue characteristics. There are, however, clinical indicators that make the consideration of certain implant characteristics more relevant. In most instances, multiple implant options may lead to successful outcomes if the procedure is well planned and executed; good implant selection will not compensate for poor technique. Table 2 summarizes the panel’s recommendations on implant and patient characteristics that may be important in optimizing implant selection. Finally, the role of surgical experience over time cannot be discounted when working toward the goal of optimizing implant selection.
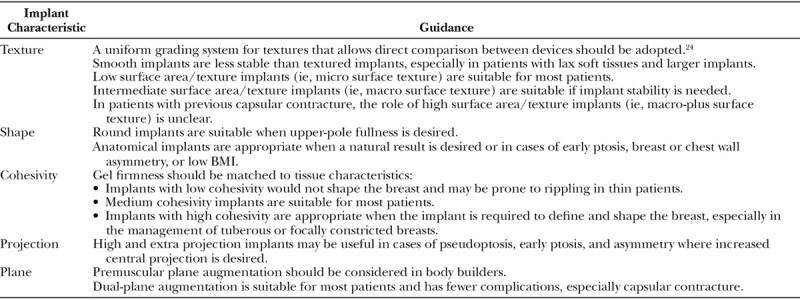
Table 2.
Panel Recommendations to Guide Implant Selection
Study Limitations
As with all Delphi studies, there are no accepted consensus thresholds and no accepted criteria for the selection of participants.23,37 Inability to achieve consensus does not mean that the item lacks support or that the item should be completely ruled out as a consideration in choosing an implant. Regardless of these limitations, the consensus recommendations reached in this study may provide a foundation for re-evaluation and modification of a plastic surgeon’s current perioperative and operative procedures. Consensus exercises focusing on prescriptive approaches to decision making, such as preoperative planning measurements and surgical technique, may provide additional guidance to surgeons.
CONCLUSIONS
Using a modified Delphi method, consensus recommendations were reached on key factors in choosing an implant, including patient characteristics, appropriate surgical technique, and appraisal of implant migration risk. The most important consideration is matching the implant to the patient. This requires careful assessment of a patient’s anatomical characteristics in the context of the physical characteristics of the desired implant and local practice preferences. Although consensus was reached by a panel of experts practicing in ANZ, many of the recommendations can be applied globally. As anticipated, much of what we consider important in implant selection derives from expert opinion rather than higher levels of evidence. We support ongoing high-level study of implant-based breast surgery to provide definitive answers to the questions that were addressed.
Footnotes
Published online 3 May 2019.
Supported by Allergan plc, Dublin, Ireland. Writing and editorial support was provided to the authors by Linda Romagnano, PhD, of Peloton Advantage, LLC, an OPEN Health Company. Parsippany, New Jersey, and was funded by Allergan plc. Neither honoraria nor other forms of payment were made for authorship.
Disclosure: Dr Magnusson is an advisor to Allergan and has an educational relationship with Mentor (Johnson & Johnson). Dr Connell is a clinical consultant and research coordinator for Allergan and Airxpanders. Dr Layt is a paid consultant to Allergan. Dr Ashton received personal fees from Allergan for both work related and unrelated to this study. Dr Deva is a consultant and research coordinator for Allergan, Mentor (Johnson & Johnson), Sientra, Motiva, and Acelity and has a patent pending for an antibiofilm irrigation solution. Dr Miroshnik received personal fees from Allergan during the conduct of the study and from Mentor (Johnson & Johnson) outside the submitted work. Drs Farrow and Januszkiewicz have no conflicts of interest to disclose.
REFERENCES
- 1.Adams WP, Jr, Small KH. The process of breast augmentation with special focus on patient education, patient selection and implant selection. Clin Plast Surg. 2015;42:413–426. [DOI] [PubMed] [Google Scholar]
- 2.Hedén P, Brown MH, Luan J, et al. Delphi study consensus recommendations: patient selection and preoperative planning measurements for Natrelle 410. Plast Reconstr Surg Glob Open. 2015;3:e556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedén P, Montemurro P, Adams WP, Jr, et al. Anatomical and round breast implants: how to select and indications for use. Plast Reconstr Surg. 2015;136:263–272. [DOI] [PubMed] [Google Scholar]
- 4.Mallucci P, Branford OA. Design for natural breast augmentation: the ICE principle. Plast Reconstr Surg. 2016;137:1728–1737. [DOI] [PubMed] [Google Scholar]
- 5.Tebbetts JB. A system for breast implant selection based on patient tissue characteristics and implant-soft tissue dynamics. Plast Reconstr Surg. 2002;109:1396–1409; discussion 1410. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell GP, Scheflan M, Spear S, et al. Benefits and limitations of macrotextured breast implants and consensus recommendations for optimizing their effectiveness. Aesthet Surg J. 2014;34:876–881. [DOI] [PubMed] [Google Scholar]
- 7.Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg. 2014;133:567e–583e. [DOI] [PubMed] [Google Scholar]
- 8.Namnoum JD, Largent J, Kaplan HM, et al. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. 2013;66:1165–1172. [DOI] [PubMed] [Google Scholar]
- 9.Adams WP, Jr, Mckee D. Matching the implant to the breast: a systematic review of implant size selection systems for breast augmentation. Plast Reconstr Surg. 2016;138:987–994. [DOI] [PubMed] [Google Scholar]
- 10.Berry MG, Cucchiara V, Davies DM. Breast augmentation: part III–preoperative considerations and planning. J Plast Reconstr Aesthet Surg. 2011;64:1401–1409. [DOI] [PubMed] [Google Scholar]
- 11.Montemurro P, Cheema M, Hedén P, et al. Do not fear an implant’s shape: a single surgeon’s experience of over 1200 round and shaped textured implants in primary breast augmentation. Aesthet Surg J. 2018;38:254–261. [DOI] [PubMed] [Google Scholar]
- 12.Tebbetts JB, Adams WP. Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process. Plast Reconstr Surg. 2005;116:2005–2016. [PubMed] [Google Scholar]
- 13.Maxwell GP, Brown MH, Hedén P, et al. Delphi consensus recommendations: intraoperative technique and postoperative management of patients with Natrelle 410 implants. Plast Reconstr Surg Glob Open. 2015;3:e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes WJ, Timmons MJ, Kauser S. Techniques used by United Kingdom consultant plastic surgeons to select implant size for primary breast augmentation. J Plast Reconstr Aesthet Surg. 2015;68:1364–1369. [DOI] [PubMed] [Google Scholar]
- 15.Heidekrueger PI, Sinno S, Hidalgo DA, et al. Current trends in breast augmentation: an international analysis. Aesthet Surg J. 2018;38:133–148. [DOI] [PubMed] [Google Scholar]
- 16.Loch-Wilkinson A, Beath KJ, Knight RJW, et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: high-surface-area textured implants are associated with increased risk. Plast Reconstr Surg. 2017;140:645–654. [DOI] [PubMed] [Google Scholar]
- 17.International Society of Aesthetic Plastic Surgery. The international study on aesthetic/cosmetic procedures performed in 2016. Available at: http://www.isaps.org/Media/Default/Current%20News/GlobalStatistics2016.pdf. Accessed September 25, 2017.
- 18.New Zealand Association of Plastic Surgeons. FAQs: What are the most popular cosmetic surgery procedures undertaken in New Zealand? Available at: http://plasticsurgery.org.nz/consumer-information/faqs/. Accessed February 20, 2018.
- 19.Jewell ML, Adams WP., Jr Betadine and breast implants. Aesthet Surg J. 2018;38:623–626. [DOI] [PubMed] [Google Scholar]
- 20.Adams WP, Jr, Culbertson EJ, Deva AK, et al. Macrotextured breast implants with defined steps to minimize bacterial contamination around the device: experience in 42,000 implants. Plast Reconstr Surg. 2017;140:427–431. [DOI] [PubMed] [Google Scholar]
- 21.Deva AK. Reply: chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: implications for breast implant-associated lymphoma. Plast Reconstr Surg. 2015;135:1059e–1060e. [DOI] [PubMed] [Google Scholar]
- 22.Chung KC. Discussion: managing late periprosthetic fluid collections (seroma) in patients with breast implants: a consensus panel recommendation and review of the literature. Plast Reconstr Surg. 2011;128:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Practical Assess Res Eval. 2007;12:1–8. [Google Scholar]
- 24.Jones P, Mempin M, Hu H, et al. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast Reconstr Surg. 2018;142:837–849. [DOI] [PubMed] [Google Scholar]
- 25.Harvey AG, Hill EW, Bayat A. Designing implant surface topography for improved biocompatibility. Expert Rev Med Devices. 2013;10:257–267. [DOI] [PubMed] [Google Scholar]
- 26.Atlan M, Nuti G, Wang H, et al. Breast implant surface texture impacts host tissue response. J Mech Behav Biomed Mater. 2018;88:377–385. [DOI] [PubMed] [Google Scholar]
- 27.Barr S, Hill EW, Bayat A. Functional biocompatibility testing of silicone breast implants and a novel classification system based on surface roughness. J Mech Behav Biomed Mater. 2017;75:75–81. [DOI] [PubMed] [Google Scholar]
- 28.Leberfinger AN, Behar BJ, Williams NC, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. JAMA Surg. 2017;152:1161–1168. [DOI] [PubMed] [Google Scholar]
- 29.Montemurro P, Papas A, Hedén P. Is rotation a concern with anatomical breast implants? A statistical analysis of factors predisposing to rotation. Plast Reconstr Surg. 2017;139:1367–1378. [DOI] [PubMed] [Google Scholar]
- 30.Tsai TL, Castillo AC, Moliver CL. Breast striae after cosmetic augmentation. Aesthet Surg J. 2014;34:1050–1058. [DOI] [PubMed] [Google Scholar]
- 31.Govrin-Yehudain J, Dvir H, Preise D, et al. Lightweight breast implants: a novel solution for breast augmentation and reconstruction mammaplasty. Aesthet Surg J. 2015;35:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tebbetts JB, Teitelbaum S. High- and extra-high-projection breast implants: potential consequences for patients. Plast Reconstr Surg. 2010;126:2150–2159. [DOI] [PubMed] [Google Scholar]
- 33.Hopper I, Parker E, Pellegrini B, et al. The Australian Breast Device Registry 2016 Report. 2018Melbourne, Australia: Department of Epidemiology and Preventive Medicine, Monash University. [Google Scholar]
- 34.Valencia-Lazcano AA, Alonso-Rasgado T, Bayat A. Characterisation of breast implant surfaces and correlation with fibroblast adhesion. J Mech Behav Biomed Mater. 2013;21:133–148. [DOI] [PubMed] [Google Scholar]
- 35.Calobrace MB, Schwartz MR, Zeidler KR, et al. Long-term safety of textured and smooth breast implants. Aesthet Surg J. 2017;38:38–48. [DOI] [PubMed] [Google Scholar]
- 36.Nava MB, Rocco N, Tunesi G, et al. Decisional pathways in breast augmentation: how to improve outcomes through accurate pre-operative planning. Gland Surg. 2017;6:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67:401–409. [DOI] [PubMed] [Google Scholar]


