Summary:
Glycemic control represents a modifiable preoperative risk factor in surgery. Traditionally, hemoglobin A1c (HbA1c) and plasma glucose are utilized as measures of glycemic control. However, studies show mixed results regarding the ability of these conventional measures to predict adverse surgical outcomes. This may be explained by the time window captured by HbA1c and serum glucose: long-term and immediate glycemic control, respectively. Fructosamine, glycosylated albumin, and 1,5-anhydroglucitol constitute alternative metrics of glycemic control that are of growing interest but are underutilized in the field of surgery. These nontraditional measures reflect the temporal variations in glycemia over the preceding days to weeks. Therefore, they may more accurately reflect glycemic control within the time window that most significantly affects surgical outcomes. Additionally, these alternative measures are predictive of negative outcomes, even in the nondiabetic population and in patients with chronic renal disease and anemia, for whom HbA1c performs poorly. Adopting these newer metrics of glycemia may enhance the value of preoperative evaluation, such that the effectiveness of any preoperative glycemic control interventions can be assessed, and adverse outcomes associated with hyperglycemia better predicted. The goal of this review is to provide an update on the preoperative management of glycemia and to describe alternative metrics that may improve our ability to predict and control for the negative outcomes associated with poor glycemic control.
INTRODUCTION
Diabetes mellitus and hyperglycemia are known risk factors for adverse surgical outcomes1–4 and improved preoperative glycemic control within days to weeks of surgery can reduce the incidence of several postoperative complications.5,6 Therefore, accurate estimation of glycemic control within days to weeks of surgery may offer prescient knowledge of unfavorable outcomes. The cost of surgery is heavily influenced by the presence of complications which can increase expenditure up to 5 times more than the initial charge.7 Additionally, the introduction of a value-based pay system that penalizes poor outcomes, such as readmissions and surgical site infection,8 heightens the issue. Therefore, lowering negative events in the postoperative course can result in notable savings. Thus, glycemic control, a modifiable factor, is an attractive option for intervention.
In current clinical practice, hemoglobin A1c (HbA1c) and plasma glucose are used to assess preoperative glycemic control. HbA1c provides an estimate of average blood glucose levels over the preceding 3 months. Hence, it is unlikely to adequately reflect the fluctuations in glycemia in the days to weeks preceding surgery. This may explain the mixed results obtained in studies that have attempted to correlate HbA1c with adverse surgical outcomes.9–12 Conversely, plasma glucose is simply a snapshot of control that is highly variable. Thus, the search continues for ideal measures of glycemic control. In this regard, fructosamine, glycosylated albumin (GA), and 1,5-anhydroglucitol (1,5-AG) have shown promise. However, their use has been largely limited to optimizing medical management of patients with diabetes, frequently as an alternative glycemic marker in situations when HbA1c cannot be used.
In this article, we review the current options for assessing glycemic control in a preoperative setting. Additionally, we describe these emerging measures of glycemic control and explore the potential benefit of their inclusion in surgical practice.
PERIOPERATIVE HYPERGLYCEMIA
Perioperative hyperglycemia is an independent predictor of adverse surgical outcomes, irrespective of diabetes status. It has been associated with postoperative infections following cardiac surgery,5 increased mortality in emergent surgery,13 and longer hospital stay following joint replacement.14 Within plastic surgery, it has been linked to surgical site infection and wound dehiscence in body contouring, craniofacial procedures, and surgery in the extremities.2 These effects culminate in increased morbidity and overall healthcare cost.15
Surgical site infections and wound dehiscence are common consequences of hyperglycemia with the former constituting the greatest proportion of annual costs of all hospital-acquired infections, a payment metric for CMS reimbursement,15 and the most common reason for unplanned readmissions.16 Furthermore, the microvascular and macrovascular complications associated with chronic hyperglycemia make managing the surgical patient with diabetes, or hyperglycemia, a formidable challenge. Given the burden placed on health care by surgical complications, there is clear value in determining patients with modifiable risk factors before surgery.
Although studies have demonstrated benefits from postoperative glycemic control protocols,17–19 preoperative control may be more consequential20,21 with lower risk for hypoglycemia. Therefore, the focus continues to shift toward screening and optimizing glycemic control in surgery candidates. The ability to assess glycemic control in the days to weeks preceding surgery has great relevance in perioperative risk assessment, particularly in elective procedures where there is an opportunity for optimization. A recent study found that early treatment of diabetes starting up to 13 days before surgery resulted in improved intraoperative and postoperative glycemic control and a shorter length of hospital admission.6 Such short to medium term change in glycemic control is unlikely to be reflected in HbA1c. Therefore, metrics that assess glycemic control over the short to medium term may possess greater utility than HbA1c.
TRADITIONAL MEASURES OF GLYCEMIC CONTROL
Hemoglobin A1c
HbA1c is the gold standard measurement tool for monitoring glycemic control. It forms when hemoglobin in circulating erythrocytes binds with glucose and offers a consistent assessment of glycemia over the preceding 3 months that is little influenced by brief peaks and troughs in plasma glucose, or brief periods of stress or illness. HbA1c is highly prognostic for long-term diabetes-related morbidity in the general diabetic population22,23 and provides a metric for the medical management decisions for patient with diabetes. Unfortunately, these benefits of HbA1c may not be as applicable in perioperative risk assessment and optimization of surgical candidates, given that the goals differ from those of long-term medical management of diabetes. The lack of consensus in the literature on the association between HbA1c and postoperative complications9–11,21 further attests to this limitation. Although diabetes status is an independent risk factor for postoperative infections, multiple studies have shown that HbA1c does not correlate with infection, wound healing, or operative complication rate.10,12,24 Thus, HbA1c may not adequately reveal the adverse effects of hyperglycemia, potentially masking opportunities for beneficial interventions in candidates for elective surgical procedures.
Furthermore, the accuracy of HbA1c is affected by abnormal erythrocyte turnover (Table 1)25–27 and recent data suggest that the lifespan of an erythrocyte can vary sufficiently among individuals to alter the accuracy of HbA1c measurement.28 HbA1c underestimates hyperglycemia in patients of African American race, chronic kidney disease, anemia, hemoglobinopathies, and patients on dialysis. This limitation is significant given that some of these demographics are overrepresented among surgical candidates. Therefore, it is likely that patients belonging to these groups would benefit from alternative assays that are independent of erythrocyte lifespan.
Table 1.
Inaccuracies with HbA1c Measurements

Plasma Glucose
Plasma glucose, measured as fasting or capillary plasma concentrations, is commonly used to assess perioperative control and offer information on immediate glycemic control. Plasma glucose offers a direct measure through a readily available assay with premeals levels of glucose ≥140 mg/dL and random values ≥180 mg/dL used as the thresholds for hyperglycemia in the inpatient setting.19
The literature is replete with studies that show adverse surgical outcomes in patients with deranged preoperative, intraoperative, or postoperative plasma glucose. Nevertheless, the efficacy of random or fasting plasma glucose measurement in predicting the incidence of postoperative infections and wound complications10,24 remains inconclusive. This may be because plasma glucose testing offers only a snapshot of glycemia and is not sensitive to rapid fluctuations in glycemia or sustained variations that are temporally removed from the test. Therefore, there is a high incidence of false negatives and low test sensitivity. Additionally, plasma glucose can be elevated with increased stress and metabolic demand, conditions associated with surgery. This effect is present in patients with and without diabetes alike and, hence, can lead to a high rate of false positive results, decreasing test specificity.
EMERGING MEASURES OF GLYCEMIC CONTROL
Fructosamine
Fructosamine is the collective term for plasma protein ketoamines, serum proteins formed by spontaneous nonenzymatic glycation,29 whose levels become elevated in the presence of hyperglycemia. The test constitutes of a simple assay taken from a blood sample or fingerprick test.30 Due to its relatively short half-life compared with HbA1c, fructosamine is a better measure of glycemia control in the preceding 2- to 3-week period.29,31 Recent studies in patients undergoing total joint arthroplasty demonstrated that elevated preoperative fructosamine is an independent risk factor for postsurgical infection.32,33 In this patient population, raised fructosamine levels were more predictive of postoperative infections than elevated HbA1c. Furthermore, the authors demonstrated a correlation between elevated preoperative fructosamine and the risks of readmission and reoperation.33 HbA1c showed no such correlation, indicating that fructosamine may be superior to HbA1c for preoperative risk assessment of surgical candidates. Unlike HbA1c, fructosamine remains reliable in patients undergoing hemodialysis,34 whereby elevated fructosamine levels have been shown to be predictive of infections and hospitalization among patients on hemodialysis.35
Commonly, a threshold of plasma fructosamine ≥270 µmol/L is indicative of hyperglycemia. This threshold is estimated to correspond with HbA1c ≥6.5%.36,37 Plasma levels of fructosamine are affected by conditions such as myeloma and cirrhosis that alter the amount or distribution of serum proteins.34 Although methods have been developed by utilizing plasma albumin for correcting the measured fructosamine in this subset of patients, no formal correction method is currently recommended.31,35 Despite this, fructosamine still offers a more accurate glycemic index in patients with renal impairment and dialysis than HbA1c.34,36
Glycosylated Albumin
Glycosylated, or glycated, albumin (GA) is formed through specific nonenzymatic glycation of albumin.38 Because albumin is the most abundant serum protein, GA constitutes a large proportion of fructosamine but is a measure in its own right with its own direct assay,39 indicating that GA may contribute clinically independent information. Similar to fructosamine, GA is elevated in the setting of hyperglycemia and reflects intermediate-term glycemic control over the preceding 2–3 weeks.25,40 However, GA has been shown to have a stronger correlation with HbA1c and fasting glucose than fructosamine, and a formula which provides accurate HbA1c equivalents for measured values of GA is currently in use40.
Many studies have focused on the accuracy of GA in scenarios where HbA1c measurements are suboptimal, particularly in those with renal impairment. GA is a more accurate glycemic indicator than HbA1c in patients with diabetes with chronic kidney disease.41 A study of the predictive value of various measures of glycemic control for cardiovascular events showed a strong correlation between levels of GA and the risk of hospitalization for cardiovascular events and an 11% increase in duration of hospitalization for every 5% increase in GA levels.42 Furthermore, GA was superior to HbA1c and plasma glucose as a predictor of hospitalization in this patient population. GA is also thought to be reliable in patients with anemia, unlike HbA1c.27 In addition to its capabilities as a glycemic marker, GA may act as a surrogate marker for infection susceptibility and diminished healing capability. It has shown associations with reduced immune function and increased oxidative stress.34
GA levels are measured through serum assay, and a fingerprick blood test has also shown validity for testing.43 However, there is limited instrument availability in the United States and there is no standardized assay currently available, with great inter- and intravariation in reference ranges. GA has a different limitation profile to that of both HbA1c and fructosamine. Values are affected by conditions that alter serum albumin metabolism, such as smoking, hepatic cirrhosis, thyroid disease, hypertriglyceridemia, and hyperuricemia.44 A compensatory mechanism results in an extended half-life of albumin in conditions of low concentration; therefore, GA is overestimated. However, this effect is somewhat mitigated by its measurement values which are reported as a ratio or percentage of total albumin (GA/albumin).34,44
1,5-Anhydroglucitol
1,5-Anhydroglucitol (1,5-AG) is a 6-carbon monosaccharide that is not metabolized. Its serum level is determined by its relative oral ingestion and renal excretion.45,46 While present in most foods,45,47 it is excreted, unchanged, through the renal system. In incidences of elevated plasma glucose, glucose competes with 1,5-AG for reabsorption in the renal tubules, resulting in increased 1,5-AG urinary excretion. Thus, circulating levels of 1,5-AG are inverse to the plasma glucose levels. This process is independent of glycation and, therefore, is not subject to the heterogeneity of glycation rates. 1,5-AG reflects postprandial glucose control over the last week48,49 and, hence, offers a short-term measure of glycemic control.
Studies posit that 1,5-AG offers a better record of glucose control than HbA1c50 and can better differentiate patients with excessive glycemic variability in the presence of near-normal HbA1c values.45 This offers a particularly attractive tool in elective surgical patients, enhanced by the ability of 1,5-AG to note small hyperglycemic changes over as short a timeframe as 1 week, where fructosamine and HbA1c fail to detect the same changes.51 This accuracy can also be applied to the rest of the population as 1,5-AG has a higher sensitivity than HbA1c for detection of clinically significant hyperglycemia in subjects without diagnosed diabetes.52
1,5-AG is excreted in the presence of glycosuria. Therefore, a reduction in 1,5-AG levels marks hyperglycemia but remain unaffected by hypoglycemia. 1,5-AG most strongly correlates with standard glycemic measures at the highest plasma glucose concentrations53 and is a poor correlator when used in the nondiabetic population. As a result, its clinical use may be limited to those with overt hyperglycemia. Additional limitations suggested are diet, nutritional status, and renal impairment,54,55 but to what extent remains unknown,48,56 and further research is likely required to clarify these influences. A simple automated assay for 1,5-AG exists in the United States as GlycoMark46 and noninvasive assays, such as saliva and urine, have been shown to correlate highly with serum levels.57,58 1,5-AG has been measured and used clinically in Japan for over a decade to monitor short-term glycemic control,53 and it is FDA approved for use in short-term glucose monitoring in the United States, with appropriate widely recognized clinical target values for 1,5-AG pending.
DISCUSSION
The prevalence of diabetes mellitus increases annually59,60 and poses a substantial healthcare burden in terms of prolonged length of hospital stay and postoperative complications; often used metrics of surgical success and safety. Although an ideal measure of glycemic control – fast, accurate, and without limitation – may be currently beyond our capabilities, emerging assays offer an inexpensive alternative to more traditional glycemia measures61 (Table 2). The case for their inclusion in surgical practice is 3-fold: their timeframes of measurement are more pertinent to surgery than current measures; they possess prognostic ability for morbidity and adverse outcomes; and they have sustained accuracy in settings where HbA1c use is problematic.
Table 2.
Comparison of Properties of Novel Emerging Glycemic Markers

Traditional measures offer information on long-term and immediate glycemic control, in the form of HbA1c and serum glucose, respectively, which lends minimal value in a preoperative setting. Whereas their nontraditional counterparts reflect control over the preceding days to weeks and, thus, better mimic the labile nature of glycemia.29,40,48 The relationship is somewhat analogous to that of albumin and prealbumin, with the latter reflecting a nutritional state in a more acute timeframe, making it a more timely and sensitive indicator.62 This may also be true of these newer glycemic measures which, due to their shorter period of action, may predict poor outcomes. Studies show that interventions up to 2 weeks before surgery improve perioperative glycemia.6 Therefore, this may present a critical preoperative time period for glycemic control. Thus, the use of medium or short-term glycemic control metrics may hold value in preoperative evaluation (Fig. 1).
Fig. 1.
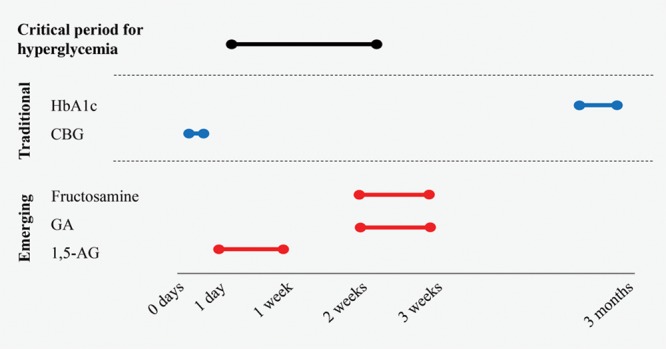
Time periods of glycemic control measurements for traditional and emerging glycemic indices. CBG, capillary blood glucose.
This theory is supported by the literature which demonstrates the strong prognostic capabilities of these novel measures,42,63–65 regardless of diabetes status.65 They have demonstrated a high predictive value for morbidities, such as cardiovascular events, infection, hospitalization rate, and mortality.64–66 These alternative markers also show correlation with microvascular complications,22,66 a pertinent concern as small vessel disease negatively affects wound healing. Hence, fructosamine, GA, and 1,5-AG levels seem prescient to poor outcomes and pose a valuable preoperative evaluation tool for surgery. Converse to HbA1c, the emerging metrics of glycemic control show great fluctuations, reflecting the rapid changes in blood glucose, and are thus better markers of poor glycemic control38,50 with the capability to detect deteriorations in glycemic control earlier. Moreover, they have utility in those without diabetes.53 This is of particular interest in patients with prediabetes where there is potential to offer a forewarning to the surgeon.
Additionally, conventional glycemic indices have limited efficacy in certain patient demographics overrepresented in the surgical population, such as those suffering from anemia or renal impairment. However, these novel metrics act through a mechanism independent of erythrocyte lifespan and they, therefore, may have added utility in cases where HbA1c measurements prove inaccurate.35,42,50 Furthermore, these novel assays are rapid, simple, and inexpensive, further enhancing potential health savings.
However, all three measures are subject to the same flaw. Although fructosamine, GA, and 1,5-AG are associated with risk factors for diabetes mellitus in a similar manner as traditional glycemic measures,36 they show a reverse relationship with body mass index (BMI).36,67 Some clinicians have suggested that this discordance is secondary to altered serum protein turnover in patients with obesity, which results in a distorted measurement of fructosamine and GA, whereas the inaccuracy in 1,5-AG may reflect the increased food intake in obese population. However, this may have further implications for its use in monitoring glycemic control in the obese populations.
Given the pay-for-performance model implemented by Centers for Medicare & Medicaid Services to incentivize better outcomes, reducing the rate of complications is a key topic on the health agenda and these novel measures present a fresh avenue for research. Their assays are readily available, inexpensive, and simple. Currently, there is no definitive evidence base to guide surgeons in identifying high-risk patients or informing patients of their risks. Delineation of a critical value, above which hyperglycemia is detrimental, may enable high-risk patients to be identified and then optimized before surgery. Future directions may also be aimed at investigating associations between surgical complications and these new measures.
CONCLUSIONS
Nontraditional measures of glycemic control, fructosamine, GA, and 1,5-AG are an exciting new area of research with much potential for perioperative surgical application. Whereas HbA1c and plasma glucose offer mixed efficacy for postoperative outcomes, emerging glycemic measures yield a closer reflection of the temporal variability of glycemia due to their shorter half-lives. Therefore, fluctuations in their concentrations occur before changes in HbA1c, so deteriorations in glycemic control are detected earlier. They possess prognostic function alongside their use as glycemic indices and have the potential to forecast adverse outcomes and identify high-risk patients. Additionally, they offer accurate alternatives in circumstances where HbA1c has reduced validity, such as renal disease and anemia. The properties of these emerging measures of glycemic control can be implemented into surgical practice to improve preoperative evaluation.
Footnotes
Published online 16 May 2019.
Disclosure: Elias K. Spanakis has received continuous glucose monitoring (CGM) supplies from DEXCOM for the conduction of inpatient clinical trials. Elias K. Spanakis is also a recipient of the VA MERIT award (#1I01CX001825-01) from the US Department of Veterans Affairs Clinical Sciences Research and Development Service. The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Ibrahim AM, Shuster M, Koolen PG, et al. Analysis of the National Surgical Quality Improvement Program database in 19,100 patients undergoing implant-based breast reconstruction: complication rates with acellular dermal matrix. Plast Reconstr Surg. 2013;132:1057–1066. [DOI] [PubMed] [Google Scholar]
- 2.Goltsman D, Morrison KA, Ascherman JA. Defining the association between diabetes and plastic surgery outcomes: an analysis of nearly 40,000 patients. Plast Reconstr Surg Glob Open. 2017;5:e1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamba R, Gupta V, Shack RB, et al. Evaluation of diabetes mellitus as a risk factor for major complications in patients undergoing aesthetic surgery. Aesthet Surg J. 2016;36:598–608. [DOI] [PubMed] [Google Scholar]
- 4.Dortch JD, Eck DL, Ladlie B, et al. Perioperative glycemic control in plastic surgery: review and discussion of an institutional protocol. Aesthet Surg J. 2016;36:821–830. [DOI] [PubMed] [Google Scholar]
- 5.Golden SH, Peart-Vigilance C, Kao WH, et al. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22:1408–1414. [DOI] [PubMed] [Google Scholar]
- 6.Garg R, Schuman B, Bader A, et al. Effect of preoperative diabetes management on glycemic control and clinical outcomes after elective surgery. Ann Surg. 2018;267:858–862. [DOI] [PubMed] [Google Scholar]
- 7.Vonlanthen R, Slankamenac K, Breitenstein S, et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg. 2011;254:907–913. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services. Hospital-Acquired Condition (HAC) reduction program. Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HAC/Hospital-Acquired-Conditions.html. Accessed December 31, 2018 [DOI] [PubMed]
- 9.Adams AL, Paxton EW, Wang JQ, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001 to 2009. J Bone Joint Surg Am. 2013;95:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King JT, Jr, Goulet JL, Perkal MF, et al. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg. 2011;253:158–165. [DOI] [PubMed] [Google Scholar]
- 11.Shohat N, Muhsen K, Gilat R, et al. Inadequate glycemic control is associated with increased surgical site infection in total joint arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2018;33:2312. [DOI] [PubMed] [Google Scholar]
- 12.Blankush JM, Leitman IM, Soleiman A, et al. Association between elevated pre-operative glycosylated hemoglobin and post-operative infections after non-emergent surgery. Ann Med Surg (Lond). 2016;10:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. [DOI] [PubMed] [Google Scholar]
- 14.Marchant MH, Jr, Viens NA, Cook C, et al. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91:1621–1629. [DOI] [PubMed] [Google Scholar]
- 15.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173:2039–2046. [DOI] [PubMed] [Google Scholar]
- 16.Merkow RP, Ju MH, Chung JW, et al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313:483–495. [DOI] [PubMed] [Google Scholar]
- 17.Qaseem A, Humphrey LL, Chou R, et al. ; Clinical Guidelines Committee of the American College of Physicians. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;154:260–267. [DOI] [PubMed] [Google Scholar]
- 18.Buchleitner AM, Martínez-Alonso M, Hernández M, et al. Perioperative glycaemic control for diabetic patients undergoing surgery. Cochrane Database Syst Rev. 2012;12:CD007315. [DOI] [PubMed] [Google Scholar]
- 19.Umpierrez GE, Hellman R, Korytkowski MT, et al. ; Endocrine Society. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:16–38. [DOI] [PubMed] [Google Scholar]
- 20.Rayfield EJ, Ault MJ, Keusch GT, et al. Infection and diabetes: the case for glucose control. Am J Med. 1982;72:439–450. [DOI] [PubMed] [Google Scholar]
- 21.Underwood P, Askari R, Hurwitz S, et al. Preoperative A1C and clinical outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care. 2014;37:611–616. [DOI] [PubMed] [Google Scholar]
- 22.Selvin E, Francis LM, Ballantyne CM, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care. 2011;34:960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrinello CM, Sharrett AR, Maruthur NM, et al. Racial differences in and prognostic value of biomarkers of hyperglycemia. Diabetes Care. 2016;39:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endara M, Masden D, Goldstein J, et al. The role of chronic and perioperative glucose management in high-risk surgical closures: a case for tighter glycemic control. Plast Reconstr Surg. 2013;132:996–1004. [DOI] [PubMed] [Google Scholar]
- 25.Koga M. Glycated albumin; clinical usefulness. Clin Chim Acta. 2014;433:96–104. [DOI] [PubMed] [Google Scholar]
- 26.Rubinow KB, Hirsch IB. Reexamining metrics for glucose control. JAMA. 2011;305:1132–1133. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Min WK, Chun S, et al. Glycated albumin may be a possible alternative to hemoglobin A1c in diabetic patients with anemia. Clin Chem Lab Med. 2011;49:1743–1747. [DOI] [PubMed] [Google Scholar]
- 28.Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112:4284–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153–2163. [PubMed] [Google Scholar]
- 30.Cefalu WT, Wang ZQ, Redmon E, et al. Clinical validity of a self-test fructosamine in outpatient diabetic management. Diabetes Technol Ther. 1999;1:435–441. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27:1761–1773. [DOI] [PubMed] [Google Scholar]
- 32.Burekovic A, Dizdarevic-Bostandzic A, Godinjak A. Poorly regulated blood glucose in diabetic patients-predictor of acute infections. Med Arch. 2014;68:163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shohat N, Tarabichi M, Tischler EH, et al. Serum fructosamine: a simple and inexpensive test for assessing preoperative glycemic control. J Bone Joint Surg Am. 2017;99:1900–1907. [DOI] [PubMed] [Google Scholar]
- 34.Zheng CM, Ma WY, Wu CC, et al. Glycated albumin in diabetic patients with chronic kidney disease. Clin Chim Acta. 2012;413:1555–1561. [DOI] [PubMed] [Google Scholar]
- 35.Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int Suppl. 2010;117:S41–S45. [DOI] [PubMed] [Google Scholar]
- 36.Poon AK, Juraschek SP, Ballantyne CM, et al. Comparative associations of diabetes risk factors with five measures of hyperglycemia. BMJ Open Diabetes Res Care. 2014;2:e000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvin E, Warren B, He X, et al. Establishment of community-based reference intervals for fructosamine, glycated albumin, and 1,5-anhydroglucitol. Clin Chem. 2018;64:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rondeau P, Bourdon E. The glycation of albumin: structural and functional impacts. Biochimie. 2011;93:645–658. [DOI] [PubMed] [Google Scholar]
- 39.Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin Chem. 2012;58:1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koga M, Murai J, Saito H, et al. Prediction of near-future glycated hemoglobin levels using glycated albumin levels before and after treatment for diabetes. J Diabetes Investig. 2011;2:304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inaba M, Okuno S, Kumeda Y, et al. ; Osaka CKD Expert Research Group. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896–903. [DOI] [PubMed] [Google Scholar]
- 42.Murea M, Moran T, Russell GB, et al. Glycated albumin, not hemoglobin A1c, predicts cardiovascular hospitalization and length of stay in diabetic patients on dialysis. Am J Nephrol. 2012;36:488–496. [DOI] [PubMed] [Google Scholar]
- 43.Rendell M, Brannan C, Nierenberg J, et al. Fingerstick glycosylated hemoglobin, plasma protein, and albumin. Diabetes Care. 1987;10:629–632. [DOI] [PubMed] [Google Scholar]
- 44.Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751–762. [DOI] [PubMed] [Google Scholar]
- 45.Yamanouchi T, Tachibana Y, Akanuma H, et al. Origin and disposal of 1,5-anhydroglucitol, a major polyol in the human body. Am J Physiol. 1992;263:E268–E273. [DOI] [PubMed] [Google Scholar]
- 46.Nowatzke W, Sarno MJ, Birch NC, et al. Evaluation of an assay for serum 1,5-anhydroglucitol (GlycoMark) and determination of reference intervals on the Hitachi 917 analyzer. Clin Chim Acta. 2004;350:201–209. [DOI] [PubMed] [Google Scholar]
- 47.Koga M, Murai J, Saito H, et al. Habitual intake of dairy products influences serum 1,5-anhydroglucitol levels independently of plasma glucose. Diabetes Res Clin Pract. 2010;90:122–125. [DOI] [PubMed] [Google Scholar]
- 48.Dungan KM. 1,5-Anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 2008;8:9–19. [DOI] [PubMed] [Google Scholar]
- 49.Stettler C, Stahl M, Allemann S, et al. Association of 1,5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care. 2008;31:1534–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peixoto EM, Bozkurt NC, Messinger S, et al. The use of 1.5-anhydroglucitol for monitoring glycemic control in islet transplant recipients. Cell Transplant. 2014;23:1213–1219. [DOI] [PubMed] [Google Scholar]
- 51.Yamanouchi T, Ogata N, Tagaya T, et al. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet. 1996;347:1514–1518. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Yuan Y, Zhang Y, et al. Serum 1,5-anhydroglucitol level as a screening tool for diabetes mellitus in a community-based population at high risk of diabetes. Acta Diabetol. 2017;54:425–431. [DOI] [PubMed] [Google Scholar]
- 53.Juraschek SP, Steffes MW, Miller ER, 3rd, et al. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 2012;35:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juraschek SP, Miller ER, 3rd, Appel LJ, et al. Effects of dietary carbohydrate on 1,5-anhydroglucitol in a population without diabetes: results from the OmniCarb trial. Diabet Med. 2017;34:1407–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Bai Y, Yang R, et al. Serum 1,5-anhydroglucitol concentrations remain valid as a glycemic control marker in diabetes with earlier chronic kidney disease stages. Exp Clin Endocrinol Diabetes. 2017. doi: 10.1055/s-0043-122142. [DOI] [PubMed] [Google Scholar]
- 56.Buse JB, Freeman JL, Edelman SV, et al. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003;5:355–363. [DOI] [PubMed] [Google Scholar]
- 57.Mook-Kanamori DO, Selim MM, Takiddin AH, et al. 1,5-Anhydroglucitol in saliva is a noninvasive marker of short-term glycemic control. J Clin Endocrinol Metab. 2014;99:E479–E483. [DOI] [PubMed] [Google Scholar]
- 58.Niwa T, Yamamoto N, Maeda K, et al. Gas chromatographic–mass spectrometric analysis of polyols in urine and serum of uremic patients. Identification of new deoxyalditols and inositol isomers. J Chromatogr. 1983;277:25–39. [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention. Long-term trends in diabetes. 2017. https://www.cdc.gov/diabetes/statistics/slides/long_term_trends.pdf. Accessed November 21, 2018.
- 60.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017: estimates of diabetes and its burden in the United States. Available at https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed November 21, 2018.
- 61.Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep. 2014;14:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unal D, Orhan O, Eroglu C, et al. Prealbumin is a more sensitive marker than albumin to assess the nutritional status in patients undergoing radiotherapy for head and neck cancer. Contemp Oncol (Pozn). 2013;17:276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selvin E, Rawlings A, Lutsey P, et al. Association of 1,5-anhydroglucitol with cardiovascular disease and mortality. Diabetes. 2016;65:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shafi T, Sozio SM, Plantinga LC, et al. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care. 2013;36:1522–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Selvin E, Rawlings AM, Lutsey PL, et al. Fructosamine and glycated albumin and the risk of cardiovascular outcomes and death. Circulation. 2015;132:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyashita Y, Nishimura R, Morimoto A, et al. Glycated albumin is low in obese, type 2 diabetic patients. Diabetes Res Clin Pract. 2007;78:51–55. [DOI] [PubMed] [Google Scholar]


