Supplemental Digital Content is available in the text
Keywords: breastfeeding, cost-utility, HIV, infant feeding, transmission
Abstract
Objective:
The aim of the study was to determine whether exclusive breastfeeding or exclusive formula feeding is more cost-effective when a Canadian mother with HIV is adherent to antiretroviral therapy and has full virologic suppression.
Design:
Current Canadian guidelines recommend that mothers with HIV practice exclusive formula feeding. This contradicts the updated World Health Organization (WHO) guidelines which recommend that mothers with HIV should breastfeed for ≥12 months while receiving support for antiretroviral therapy adherence. Due to the economic and health risks and benefits associated with each modality, there remains expert disagreement on whether the WHO recommendations should be adopted in high-income countries.
Methods:
A microsimulation model was developed to estimate lifetime costs and effectiveness (i.e., infant's quality-adjusted life years) of a hypothetical group of 1,000,000 initially healthy, HIV-negative infants, if the mother with HIV was on antiretroviral therapy with full virologic suppression and either exclusive breastfeeding or exclusive formula feeding. The model was developed from the economic perspective of the Ontario Ministry of Health, taking into account direct costs associated with infant feeding modality as well as related indirect costs born out of the child's lifetime health outcomes. Uncertainties related to model parameters were evaluated using one-way and probabilistic sensitivity analyses.
Results:
In comparison to exclusive formula feeding, exclusive breastfeeding was the dominant feeding modality (i.e., less costly and more effective) yielding cost-savings of $13,812 per additional quality-adjusted life year gained. Neither one-way nor probabilistic sensitivity analyses altered the conclusions.
Conclusions:
Despite the risk of HIV transmission, exclusive breastfeeding was more cost-effective than exclusive formula feeding. These findings merit review of current infant feeding guidelines for mothers with HIV living in high-income countries.
1. Introduction
Today, half of the 35.3 million people living with HIV globally are women of childbearing age (defined as 15–44 years old).[1,2] In Canada, a quarter of the estimated 75,500 individuals living with HIV at the end of 2014 were women.[3] Owing largely to the increased use and success of combination antiretroviral therapy (cART), the quality of life and life expectancy of individuals with HIV has drastically improved, and is almost comparable to HIV-negative individuals.[1,4] Furthermore, a growing number of women living with HIV in Ontario, Canada, have expressed the desire to have children.[4,5] According to annual national surveillance data, there were 232 HIV-positive mother with infant pairs in Canada in 2014; 81 of them being from Ontario. In the 10 preceding years, the number of children born to women living with HIV in Ontario has risen by over 20% from 67 (2004) to 81 (2014), reaffirming the overall trend in pregnancies in this population.[6]
Following the birth of their children, mothers living with HIV (MLWH) in Canada are currently advised to avoid breastfeeding, regardless of plasma HIV viral load and use of cART.[7] However, in August 2016, WHO released updated guidelines on infant feeding and HIV, indicating that “mothers living with HIV who are on [c]ART and adherent to therapy should breastfeed exclusively for the first 6 months, and then add complementary feeding until 12 months of age.”[8] In the new WHO guidelines, a systematic review was conducted showing that during the breastfeeding period, when cART was used, the risk of postnatal HIV transmission was <0·5%.[9] These findings have been confirmed in the PROMISE study, and a recent meta-analyses by Bispo et al, where use of cART and infant prophylaxis were both shown to be safe and effective at preventing HIV transmission during breastfeeding.[10,11] Given this new information, the updated guideline removed restrictions on the avoidance of breastfeeding, even when nutritionally adequate and safe replacement feeding options are available.[8]
Importantly, the new WHO recommendations recognize that although exclusive formula feeding (EFF) completely eliminates the risk of HIV transmission through breast milk, there are known benefits of breastfeeding for both infant and maternal health.[9] Breast milk is necessary for maintaining infant health, as it plays an important role on the developing infant gut, provides ideal nutrition, and protects the infant from adverse health outcomes in the short and long term.[12–14] In addition, previous analyses in the general population have found that improved rates of exclusive breastfeeding (EBF) translate to significant savings in the health care system.[15,16] This data, therefore, indicates not only a health trade-off between EBF and EFF in this population, but also an economic one. Although the WHO has taken a supportive stance on breastfeeding by MLWH, most regional guidelines and recommendations in high-income countries still recommend EFF.[7,16–19]
The relationship between perinatal HIV transmission risks and economic consequences relating to infant feeding modality has not previously been explored in depth. Prior research has primarily focused on the clinical safety and cost-effectiveness of breastfeeding in cART-adherent mothers, specifically in low- and middle-income countries.[20–24] Currently, there is limited research addressing health economic evaluations on EBF and EFF for MLWH in high-income countries, such as Canada, where there is universal access to cART and formula milk. When mothers are adherent to cART and have their viral loads regularly monitored during and postpregnancy, particularly while breastfeeding, it is possible that short- and long-term risks and costs associated with HIV transmission do not outweigh the risks and costs related to formula feeding. This study aims to explore this relationship, identify the safest and most cost-effective infant feeding modality, and to help inform economically sound guidelines on infant feeding.
2. Methods
2.1. Study design
This study followed recommendations from the ISPOR Task Force best practice guidelines for cost-effectiveness analyses (CEAs) where possible, as well as the CHEERS guidelines for reporting CEA results. A microsimulation model was developed to determine the cost-effectiveness profile of EBF versus EFF in a Canadian context. The modality of infant feeding was exclusive (i.e., no other solids or liquids) for the first 6 months of life, followed by the introduction of complementary food, and cessation of EFF/EBF at 12 months of age.[8,9] Although it is acknowledged that cART reduces the risks of HIV transmission even during mixed feeding, and that exclusive feeding is extremely difficult for some mothers to adhere to in reality, this study aimed to assess only exclusive forms of feeding modality. Mixed feeding methods were excluded due to the difficulties in quantifying “mixed” feeding practices, and because some studies show that mixed feeding carries a higher chance of HIV transmission than EBF or EFF.[24,25] The model was restricted to include only MLWH with high adherence to cART and fully suppressed viral loads.[7,8] Although access to these resources varies widely across Canadian communities, assumptions made here serve as useful starting points for assessing the need to consider infant feeding alternatives for MLWH.
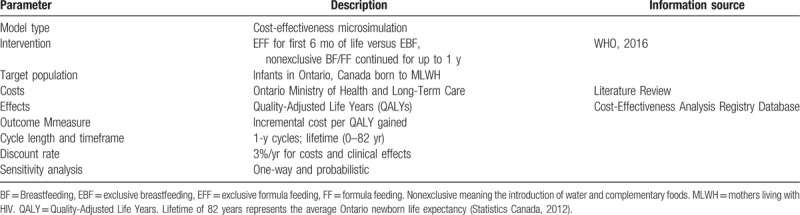
The clinical and economic trajectories of a hypothetical group of 1,000,000 initially healthy, HIV-negative neonates were simulated and summed for all individuals over the course of the model (infant's lifetime). This number of simulations was large enough to achieve stabilized estimates without exceeding computational capacity. The lifetime horizon was divided into cycle lengths of 1 year. This annual duration provided a time interval that was short enough to capture differences in clinical effects and costs between the cycles. The assumption and recommendation that infant breast/formula feeding be carried out for 1 year also helped make this time interval a clinically meaningful one. Model parameter descriptions are outlined in Table 1. This model was created using TreeAge Pro 2015 software and results obtained are expressed in terms of incremental costs per quality-adjusted life years (QALYs) gained.[26]
Table 1.
Study design.
Currently, there are no randomized clinical trials comparing the health outcomes of EBF and EFF in infants potentially exposed to HIV in high-income countries. Due to the known health benefits of breastfeeding and lack of contraindications, it is no longer deemed ethically feasible to randomize healthy infants to receive breast or formula milk. Thus, this study relied on currently published literature (observational studies in particular) to inform the parameters of the microsimulation. Also for this reason, neither an ethics committee nor an institutional review board was necessitated for this analysis to be conducted.
2.2. Health states and transition probabilities
To identify the health states and transitions appropriate for inclusion into the microsimulation model (Fig. 1), a systematic review was originally designed and carried out to obtain previously published systematic and meta-analyses on the health risks of breastfeeding versus formula feeding (see Fig. 1, Supplemental Content, for flow diagram summarizing the inclusion and exclusion of studies used to obtain mutually exclusive health states and their associated transition probabilities). The databases MEDLINE (Ovid), PubMed, and Scopus were searched systematically for all publications between January 2000 and May 2015. The following concepts were included in the search: “breastfeeding,” “infant feeding,” “formula feeding,” “breast milk,” “formula milk,” “mortality,” “morbidity,” “health,” “hospitalization,” “systematic review,” and “meta-analysis.” Grey literature, seminar papers, and abstracts were not considered. Reference lists from key review articles were also searched. In this stage, the search results yielded 539 systematic reviews and/or meta-analyses. After removal of duplicates, the first selection of articles was made based on title and abstracts. Studies were excluded if they addressed only a particular issue or health condition, such as those solely focusing on the association between infant feeding and obesity, or if they focused exclusively on less developed countries. The remaining 64 articles were retrieved for more in-depth review of the intervention and health outcomes assessed. Following this inclusion/exclusion, 4 articles stood out as the most influential and comprehensive based on the number of health conditions considered and unambiguous assessment of evidence quality. Of these analyses, the report from the U.S. Agency for Healthcare Research and Quality (AHRQ) was selected due to its high-quality comprehensiveness, time and place of publication, explicit reference list, analysis of studies included, and applicability to developed countries.[27] This 200-page technical report screened over 9000 abstracts. A total of 43 primary studies on infant health outcomes, 43 primary studies on maternal health outcomes, and 29 systematic reviews or meta-analyses that covered approximately 400 individual studies were included (see Table 1, Supplemental Content, which cites the studies from the AHRQ). However, the AHRQ study did not evaluate first-hand the quality of the primary studies included in those analyses. Therefore, all primary studies included within the AHRQ were retrieved and reviewed (see Table 2, Supplemental Content, which cites all the primary studies extracted from the systematic reviews and meta-analysis cited in the AHRQ article). The methodological quality of these studies was assessed based on Quality of Reporting of Meta-analyses statement, Meta-analysis Of Observational Studies in Epidemiology guidelines, and supplemented criteria created by the AHRQ authors. Studies graded as “poor” were excluded, whereas those with sufficient information to acquire transition probabilities necessary for the microsimulation were included. Once probabilities were derived from the primary studies, STATA was used to pool these estimates using a random-effects model in consideration of heterogeneity between studies (see Table 3, Supplemental Content, which shows the pooled probabilities, their confidence intervals, and the references used to generate the estimates; see Table 2 for pooled probability values).[28,29]
Figure 1.
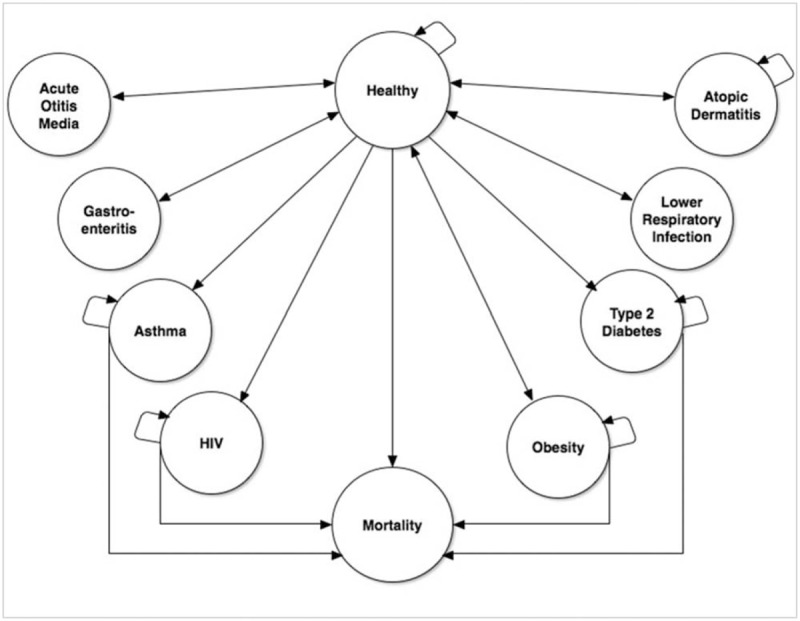
Microsimulation diagram. This figure presents the possible pathways which a simulated patient may take. Arrows connecting 2 different states indicate allowed transitions. Arrows leading from a state to itself indicate that the patient may remain in that state in consecutive cycles.
Table 2.
Model parameter values.
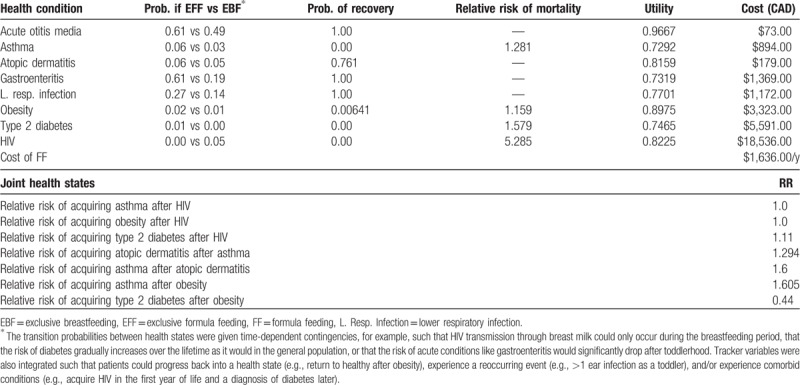
After application of the inclusion and exclusion criteria to all primary studies, the health state transitions for this analysis were the following: acute otitis media (AOM), nonspecific gastroenteritis, severe lower respiratory tract infections, atopic dermatitis, asthma, obesity, and type 2 diabetes. Death was an “absorbing” health state that individuals in the model could transition to in each cycle. The probability of entering the death state either reflected Ontario Age-Specific Mortality Rates or an increased risk of mortality, dependent on the nature of their health condition (see Tables 4–7, Supplemental Content, which show the relative risk of death contingent on health condition).
The transition probabilities between health states were also given time-dependent contingencies, for example, such that HIV transmission through breast milk could only occur during the breastfeeding period. The risks of acquiring any of the acute conditions (AOM, atopic dermatitis, nonspecific gastroenteritis, lower respiratory tract infection) was more likely in toddlers and easily recoverable in the developed country context. Accordingly, the risk of acquiring these conditions was eliminated after the second cycle in both feeding groups. For chronic conditions, risks in the general population were adjusted according to infant feeding modality to reflect disease patterns over time. Lifetime risk of diagnosis of asthma and type 2 diabetes in the general population were extrapolated from 2 separate studies that quantified these baseline risks, and multiplied by the relative risk of acquiring these conditions based on the pooled probabilities extracted from the AHRQ report, its systematic reviews, and their primary studies (Table 2).[30,31] Thus, the general population risks over the ages were multiplied by intervention-related relative risks to derive a probability that varied as the patient aged. Unfortunately, no appropriate studies were found that approximated the probability of obesity over the ages. As a result, the risk of obesity was a constant average over the life course (i.e., the risk was not time-dependent) for both the EBF and EFF groups. However, patients were allowed to return to a lean, healthy state based on annual probabilities for weight loss.[32] Tracker variables were also integrated throughout the model to allow for comorbid conditions (e.g., acquire an infection in the first year of life and a diagnosis of diabetes later). Clinical histories were also recorded such that the presence of one condition could impact another health state in terms of transition probabilities, costs, and effects (see Table 8, Supplemental Content, for relative risk of comorbidities alongside references; or summary of these risks in Table 2).
2.3. Costs and effects
A third-party payer perspective, through the Ontario Ministry of Health and Long-Term Care (MOHLTC), was adopted for this study. The MOHLTC offers free formula milk for 1 year to new MLWH as infant formula is currently considered a health good among MLWH given the perinatal transmission risks associated with breastfeeding.[33] The third-party payer perspective is consistent with Canadian economic evaluation guidelines, and more suitable than a societal perspective because the outcomes of this study apply only to a specific subgroup of the population.[34] Direct costs of each infant feeding method were captured based on the cost of formula milk, as well as the frequency of physician visits and diagnostic services needed for mother and infant during the breast/formula feeding period. As there are extremely few known and closely monitored cases of MLWH exclusively breastfeeding in the Ontario context, local and global experts in the field, namely clinicians, were consulted for this information.[35] These inputs were based on guidelines and practices currently followed by other high-income countries (e.g., the United Kingdom where EBF is allowed for MLWH in rare circumstances). The cost of physician visits and diagnostic testing for mother and child were based on costing data made available from Maple Leaf Medical Clinic in Toronto, a typical HIV clinic in Ontario.
Health state-related costs (as opposed to the cost of feeding modality itself) were also captured. These included expenses relating to hospital care, medication, rehabilitative devices, diagnostic tests, and physician/specialist visits, covered by the MOHLTC. These estimates were captured through a restrictive literature review, searching specifically for articles that reported direct average annual costs, per person, to the healthcare system over the child's lifetime following diagnosis. Costs included both symptomatic and asymptomatic versions of the health condition in question. Specific data extracted from the literature included the actual dollar value of treating the illness, geographical location of costing, and year and currency that costs were reported. All past costs were adjusted to 2015 Canadian dollar values using historical Consumer Price Index values, similarly any costs reported in a foreign currency were converted to Canadian dollars using the December 2015 exchange rate (see Table 9, Supplemental Content, for cost data inputs and references).
Each branch in the decision model has an incremental effect reflecting the value of being in that state for one cycle. Utility values used to acquire QALYs were calculated as mean values based on the Cost-Effectiveness Analysis Registry (CEAR).[36] Utility values that integrated comorbid conditions were excluded (e.g., if a CEAR study reported combined utility values for stroke and diabetes, this study would not have been used because solitary utility values were needed). However, a range of severity was accounted for when estimating the mean utility for the health conditions.
Future costs and effects were discounted at a yearly rate of 3% in the base case analyses. In addition, a half-cycle correction was used throughout the model to account for the fact that transitions between health states are a continuous process throughout the model's cycle length. Baseline model parameter values (e.g., probabilities, costs, effects, and relative risks) are reported in Table 2.
2.4. Sensitivity analysis
Both deterministic and probabilistic sensitivity analyses (PSAs) were conducted to test the uncertainty of our model parameters. A one-way sensitivity analysis was conducted on the cost and effect discount rates, the cost of formula, and the probability of HIV transmission. Each one-way sensitivity analysis was conducted using 1000 iterations. Discount rates were evaluated at 0% and 7%; cost of formula was evaluated at a half and double the base case value; and probability of HIV transmission was assessed through a net benefit approach to determine the threshold at which the cost-effectiveness decision reverses. Parameter uncertainty was also examined simultaneously in a probabilistic sensitivity analysis by using 1,000 samples and 10,000 trials for the Monte Carlo simulations. Each of the cost, effect, and intervention-related transition probabilities was assigned distributions for the PSA according to the characteristics of the parameters. The parameters that dictated these distributions were based on the precision of the estimates in the literature, using the reported/calculated means and standard deviations. Cost parameters were modeled using a gamma distribution, which is constrained on the interval of 0 to positive infinity.[35,36] To do this, distribution parameters alpha (α) and lambda (λ) were calculated by TreeAge Pro from the mean and standard deviation of cost estimates using the following formulas:
α = (mean)2/(standard deviation)2
λ = (mean)/(standard deviation)2.
State utilities and intervention-related transition probabilities were modeled using a beta distribution, which is constrained on the interval of 0 and 1.[37,38] Distribution parameters alpha (α) and beta (β) were calculated again by TreeAge Pro from the mean and standard deviation of the estimates using the following formulas:
α = (mean)2 × (1−mean)/(standard deviation)2
β = mean × (1−mean)/(standard deviation)2 − α.
To ensure that the distribution functions reflected the range of uncertainty that was unique to each feeding modality, the base case estimates were set as the mean values in the PSA. This condition permits the expected value of each scenario, over all iterations of the Monte Carlo simulation, to converge to base case input values. Wide confidence intervals were used for cost estimates by halving and doubling the high and low values to determine the range of the confidence interval and subsequently, the standard deviation. Once distributions were attributed to model parameters, and PSA run, these bootstrapped simulations were plotted along the cost-effectiveness plane.
3. Results
After running the baseline simulation model, it was found that for infants in the EBF arm, individuals accumulated mean costs of $55,111 and 57.89 QALYs. EFF infants, however, accumulated mean costs of $74,182 and 56.51 QALYs per individual. Consequently, results of this baseline analysis demonstrate that EBF was both less expensive and possibly more effective, yielding estimated cost-savings of $13,812 for each additional QALY.
In comparison to EFF, EBF remained cost-saving across almost all sensitivity analyses. Discount rates for costs and effects were evaluated individually at 0% and 7%. One-way sensitivity analyses were also conducted on the HIV transmission risk probability (Fig. 2). With a willingness to pay value of $10,000/QALY, EBF was no longer the more cost-effective strategy if the risk of HIV transmission in the first year of life was >23·4%. Increases in the cost of formula were also explored in the sensitivity analyses, with the result that the cost-effectiveness of EBF increased in line with the cost of formula.
Figure 2.
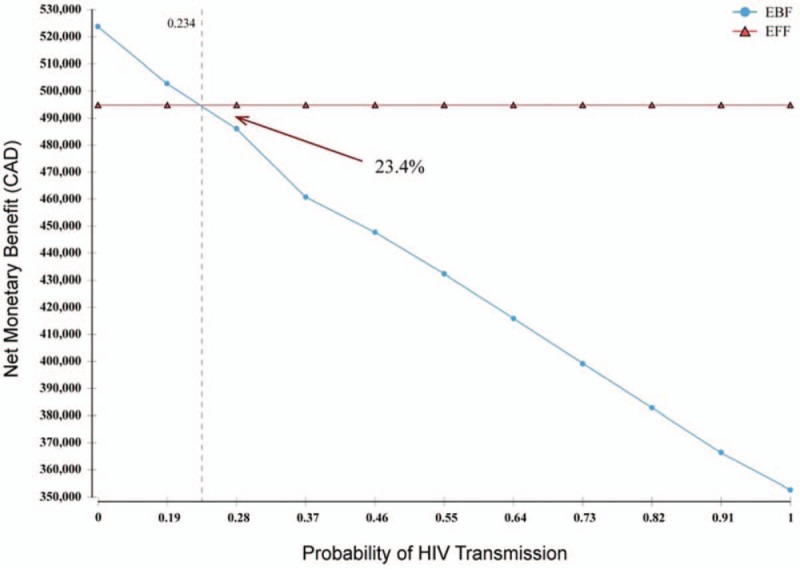
One-way sensitivity analysis on HIV transmission risk through exclusive breastfeeding, with willingness to pay value of $10,000/Quality Adjusted Life Year. This figure presents the net benefit of each infant feeding modality in relation to one another at different HIV transmission risks. Thus, when the risk is >23.4%, EFF presents a larger net benefit than EBF; therefore, EBF is no longer cost-effective if the transmission risk was that high.
Probabilities, utilities, and cost parameters were tested over their range of plausible values using a tornado diagram, at a conservative willingness to pay value of $10,000 (see Fig. 2, Supplemental Content, Tornado Analysis [Net Benefits], with Willingness to Pay Values of $10,000). Results of this analyses showed that the cost of HIV, cost of type 2 diabetes, utility value of type 2 diabetes, utility value of atopic dermatitis, and cost of AOM exerted the greatest influence over the relative costs and effectiveness of the model. When costs, utilities, and intervention-related transition probabilities were simultaneously varied in accordance with their respective probability distributions in the PSA, EFF is dominated by EBF as the more effective and cost-saving feeding modality. The probabilistic simulation in Fig. 3 illustrates the 95% confidence ellipse for the Incremental Cost-Effectiveness Ratio (ICER).
Figure 3.
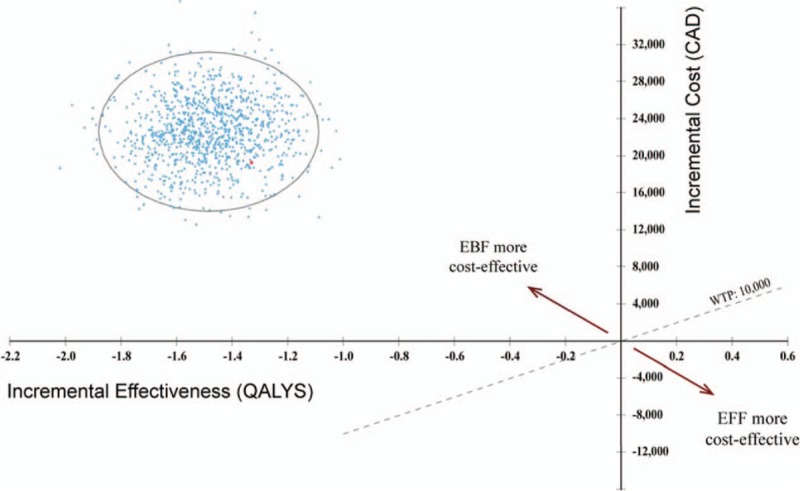
Incremental cost-effectiveness 95% confidence interval ellipse, exclusive formula feeding (EFF) versus exclusive breastfeeding (EBF). This figure presents the uncertainty of the incremental cost-effectiveness ratio (ICER) generated by this model using the probabilistic sensitivity analysis, with the red dot depicting the base case ICER estimate. The diagonal WTP line denotes the maximum acceptable ICER at an assumed WTP of $10,000. Estimates above this line indicate that EFF is costlier and less effective than EBF, thus EBF is the more cost-effective strategy (as is the case here). Conversely, estimates below this line would indicate that EFF is less costly and more effective, which would deem EFF as the more cost-effective strategy in comparison to EBF.
4. Discussion
In this cost-effectiveness simulation, we found that EBF, despite the potential risk of HIV transmission, provides immunological protection resulting in it being more effective and more cost-saving as an infant feeding modality than EFF in a setting in Ontario when MLWH are on ART and have virologic suppression. In comparison to EFF, EBF yielded cost-savings of $13,812 per additional infant QALY. Unlike previously published studies, the present model design and results accounted for HIV and non-HIV survival, morbidity, and economic outcomes over the lifetime of the infant, in the high-income country context. Similar studies are summarized in Table 10, Supplemental Content (Other Cost-Effectiveness Studies on Breast-/Formula Feeding and HIV), along with their results to allow comparison to this analysis. Apart from the Maredza et al study, these assessments are most pertinent to resource-limited settings where there are apparent disadvantages to formula feeding.[20] These economic evaluations also present limited incorporation of morbidities, which are prominent in the non-HIV, infant feeding literature, and yet are critical when addressing overall costs and benefits between formula feeding and breastfeeding.
Our results support the recommendations outlined in the 2016 WHO guidelines on HIV and infant feeding, which recommends that MLWH should breastfeed for ≥12 months and may continue breastfeeding for up to 24 months or longer while being fully supported for ART adherence. This contrasts with Canadian guidelines, which recommend EFF for all MLWH.[7] As Canada contemplates changes in its HIV and infant feeding guidelines and practices, this paper provides cost-effectiveness evidence that can inform relevant policy decisions. Other policy implications of our findings may include a shift in the provision of replacement feeding, coherent, and consistent messaging to patients, counselors, and clinicians, as well as urgent action in providing cART prophylaxis to women in Canada still lacking appropriate access to care. Practice implications of our findings include fostering open discussion of mother's feeding preferences, discussing with the mother the currently known and unknown risks of EBF in the context of HIV, and the adoption of harm reduction strategies regardless of the modality of infant feeding.[35] Although the results of this study suggest that EBF is cost-effective for MLWH, there are other considerations related to the individual circumstances of the mother that can influence the choice to breastfeed. Consequently, it is important to ensure that mothers who cannot, or choose not to, breastfeed are provided with sufficient information and support on formula feeding to meet their individual needs. This choice model for MLWH advocates for a shared decision-making approach to care by engaging in open discussion of the mother's preferences and knowledge, providing education and support for her decision.
As with most decision analytic models in cost-effectiveness analyses, this study is limited in its ability to model the clinical complexities of the present problem and estimate all probabilities, costs, and utility values, especially over the lifetime, with accuracy and precision and warrants caution. With respect to EBF, several assumptions were made ‘ relying on the best available current evidence. As much of the data were based on observational studies, there are potential deficiencies in study designs (e.g., misclassification of exposure, confounding factors) that may compromise the internal validity and generalizability of the findings. With regard to misclassification of exposure, studies with unclear or unstated feeding exposure were excluded, whereas studies that looked at shorter durations (<6 months) of exclusive feeding or lower levels of exclusivity (<100% exclusivity) were still included. A further limitation of this analysis is that it does not incorporate any external benefits derived from dynamic spread of infection at the population level or account for further differences in subpopulations. There are various factors, such as adult-to-adult HIV transmission (when the infant has grown up) or at-risk populations, which may introduce variability in both the cost and effects of interventions. Given that Markov microsimulation models are equipped to handle patient characteristic variances such as smoking status, income level, cART adherence level, and extent of feeding exclusivity, the incorporation of these variables is high priority for future analyses to help project costs and effects more accurately. Another limitation of this analysis is that it does not address the potential costs to the healthcare system if an individual experiences antiretroviral toxicity or resistance. Although the cost and utility averages do incorporate varying degrees of condition severity and may capture these values, it is possible that those who acquire HIV perinatally experience worse utility values than those who acquire HIV during adulthood and by other methods, such as injection drug use. Ideally, both costs and effects of EBF and EFF can be elicited directly from patients in observational studies or clinical trials. This study may additionally be underestimating the actual cost-effectiveness of EBF through its omission of the potential economic consequences of EBF as they relate to potential maternal health outcomes (e.g., reduction of breast and ovarian cancer).[16,27] Future studies could also consider the cost-effectiveness of EBF versus EFF in the context of HIV by also incorporating the mothers’ perspective in terms of benefit (cost and health) and preference of infant feeding modality, which could impact adherence and the cost-effective consequences.
Results from the sensitivity analyses provide some insight into the generalizability of the findings. Settings with higher infant mortality, less access to quality care for the infant, and higher risk of perinatal transmission may benefit more from formula feeding where there is reliable access to formula and clean water. This may play a substantial role in rural areas of Canada and in instances where the mother may be more likely not to adhere to an exclusive form of infant feeding or to have higher viral counts. As consequence of this and the other limitations of this study, it is strongly recommended that MLWH be provided with appropriate and adequate counseling and treatment to support their infant feeding strategy of choice.
Despite these limitations, this study has several noteworthy strengths. It answers an important question that has not been addressed in the Canadian context before, whether EBF or EFF is more cost-effective when there is risk of perinatal HIV transmission. Furthermore, unlike previous studies on infant feeding modality in the context of HIV, this study examines potential health outcomes over the lifetime horizon with consideration of not only mortality but also morbidity. The framework and techniques used in this complex model, consequently, lay the foundation for other health economists to conduct similar cost-utility analyses relevant to their specific context. This model may also be increasingly useful as the treatment and prognosis of HIV diagnosis improves in coming years.
5. Conclusion
The results of this study demonstrate that even in high-resource settings where there can be high adherence to ART, optimal adherence to infant feeding modality, proper access to healthcare services, and safe and consistent provision of formula milk, EBF could represent a potentially economically sound health strategy for MLWH. With acknowledgment that findings of this analysis were determined from modeling, and confirmation is required from clinical studies, it is recommended that a review be undertaken of current HIV infant feeding guidelines in high-incomes countries. At minimum, MLWH should be provided nonjudgmental environments for open discussion of their breastfeeding intentions, and be supported in their infant feeding strategy of choice.
Acknowledgments
The authors also wish to thank the following individuals for their contribution to the editing and conception of this paper: Adam B. Johnson and Dr. David Naimark. We also acknowledge the University of Toronto and Women's College Research Institute for providing funding for this study.
Author contributions
RK contributed to all parts of this article, including conception and design, literature reviews, writing, model development, statistical analysis, and editing. PCC helped with the health economic and quantitative methods of this paper, particularly study design, interpretation of data, alongside critical revisions of the manuscript. AL and PMS both helped with drafting, revision, and final approval of the manuscript. MRL advised with clinical expert opinion, conception and study design, interpretation of data, and critical revision of the manuscript particularly the background and discussion.
Conceptualization: Reyhaneh Keshmiri, Peter C. Coyte, Mona R. Loutfy.
Data curation: Reyhaneh Keshmiri, Peter C. Coyte, Mona R. Loutfy.
Formal analysis: Reyhaneh Keshmiri, Peter C. Coyte, Mona R. Loutfy.
Funding acquisition: Reyhaneh Keshmiri, Mona R. Loutfy.
Investigation: Reyhaneh Keshmiri, Peter C. Coyte, Mona R. Loutfy.
Methodology: Reyhaneh Keshmiri, Peter C. Coyte, Mona R. Loutfy.
Project administration: Reyhaneh Keshmiri.
Supervision: Audrey Laporte, Prameet M. Sheth, Mona R. Loutfy.
Writing – original draft: Reyhaneh Keshmiri, Audrey Laporte, Prameet M. Sheth, Mona R. Loutfy.
Writing – review and editing: Reyhaneh Keshmiri, Peter C. Coyte, Audrey Laporte, Prameet M. Sheth, Mona R. Loutfy.
Supplementary Material
Footnotes
Abbreviations: AHRQ = Agency for Healthcare Research and Quality, AOM = acute otitis media, cART = combination antiretroviral therapy, CEA = cost-effectiveness analysis, CEAR = Cost-Effectiveness Analysis Registry, EBF = exclusive breastfeeding, EFF = exclusive formula feeding, ICER = incremental cost-effectiveness ratio, MLWH = mothers living with HIV, MOHLTC = Ontario Ministry of Health and Long-Term Care, PSA = probabilistic sensitivity analyses, QALY = quality-adjusted life year, WHO = World Health Organization.
COI/Disclosures: All authors are required to provide a conflict of interest statement and should complete a standard form, which is available at http://download.thelancet.com/flatcontentassets/authors/icmje-coi-form.pdf. This form can be uploaded with the manuscript of faxed in.
Funding: University of Toronto and Women's College Research Institute
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].UNAIDS. A focus on women: a key strategy to preventing HIV among children. Issue Brief. 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/JC2538_preventingHIVamongchildren_en_0.pdf. Accessed May 2, 2019. [Google Scholar]
- [2].World Health Organization. Women's health: Fact sheet No. 334. 2013. Available at: https://apps.who.int/mediacentre/factsheets/fs334/en/. Accessed May 2, 2019. [Google Scholar]
- [3].Public Health Agency of Canada. HIV and AIDS in Canada: Surveillance Report to December 31, 2014. Ottawa: Minister of Public Works and Government Services Canada; 2015. [Google Scholar]
- [4].Zhang Y, Margolese S, Yudin MH, et al. Desires, need, perceptions, and knowledge of assisted reproductive technologies of HIV-positive women of reproductive Age in Ontario, Canada. ISRN Obstet Gynecol 2012;2012:853503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Loutfy MR, Hart TA, Mohammed SS, et al. Fertility desires and intentions of HIV-positive women of reproductive age in Ontario, Canada: a cross-sectional study. PLoS One 2009;4:e7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Singer J, Bitnun A, Lee T, et al. Canadian Perinatal HIV Surveillance Program (CPHSP): analysis of trends in perinatal HIV transmission, treatment in pregnancy and demographics in Canada, 2013. Poster presented at Canadian Association for HIV Research (CAHR); May 1, 2014; Toronto, Canada. [Google Scholar]
- [7].Money D, Tulloch K, Boucoiran I, et al. Guidelines for the care of pregnant women living with HIV and interventions to reduce perinatal transmission: executive summary. J Obstet Gynaecol Can 2014;36:721–34. [DOI] [PubMed] [Google Scholar]
- [8].World Health Organization, United Nations Children's Fund. Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva: World Health Organization; 2016. Available at: https://apps.who.int/iris/bitstream/handle/10665/246260/9789241549707-eng.pdf. Accessed May 2, 2019. [Google Scholar]
- [9].Chikhungu LC, Bispo S, Rollins N, et al. HIV-free survival at 12-24 months in breastfed infants of HIV-infected women on antiretroviral treatment. Trop Med Int Health 2016;21:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taha TE, James MM, Hoover DR, et al. Association of recent HIV infection and in utero HIV-1 transmission: findings from the PEPI-Malawi trial. AIDS 2011;25:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bispo S, Chikhungu L, Rollins N, et al. Postnatal HIV transmission in breastfed infants of HIV-infected women on ART: a systematic review and meta-analysis. J Int AIDS Soc 2017;20:21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fernández L, Langa S, Martín V, et al. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res 2013;69:1–0. [DOI] [PubMed] [Google Scholar]
- [13].Bernardo H, Cesar V, World Health Organization. Long-term effects of breastfeeding: a systematic review. 2013. Available at https://apps.who.int/iris/bitstream/handle/10665/79198/9789241505307_eng.pdf. Accessed May 20, 2019. [Google Scholar]
- [14].Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obst Gynecol 2009;2:222–31. [PMC free article] [PubMed] [Google Scholar]
- [15].Ball TM, Wright AL. Health care costs of formula-feeding in the first year of life. Pediatrics 1999;103Suppl. 1:870–6. [PubMed] [Google Scholar]
- [16].Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–41. [DOI] [PubMed] [Google Scholar]
- [17].Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed May 2, 2019. [Google Scholar]
- [18].Loutfy M, Kennedy VL, Poliquin V, et al. No. 354-Canadian HIV pregnancy planning guidelines. J Obstet Gynaecol Can 2018;40:94–114. [DOI] [PubMed] [Google Scholar]
- [19].Taylor GP, Anderson J, Clayden P, et al. British HIV Association and Children's HIV Association position statement on infant feeding in the UK 2011. HIV Med 2011;12:389–93. [DOI] [PubMed] [Google Scholar]
- [20].Maredza M, Bertram MY, Saloojee H, et al. Cost-effectiveness analysis of infant feeding strategies to prevent mother-to-child transmission of HIV in South Africa. Afr J AIDS Res 2013;12:151–60. [DOI] [PubMed] [Google Scholar]
- [21].Maclean CC, Stringer JSA. Potential cost-effectiveness of maternal and infant antiretroviral interventions to prevent mother-to-child transmission during breast-feeding. J Acquir Immune Defic Syndr 2005;38:570–7. [DOI] [PubMed] [Google Scholar]
- [22].Rauner MS, Brailsford SC, Flessa S. Use of discrete-event simulation to evaluate strategies for the prevention of mother-to-child transmission of HIV in developing countries. J Oper Res Soc 2005. 222–33. [Google Scholar]
- [23].Yu W, Li C, Fu X, et al. The cost-effectiveness of different feeding patterns combined with prompt treatments for preventing mother-to-child HIV transmission in South Africa: estimates from simulation modeling. PLoS One 2014;9:e102872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 2007;369:1107–16. [DOI] [PubMed] [Google Scholar]
- [25].Mofenson LM, Flynn PM, Aldrovandi GM, et al. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics 2013;131:391–6. [DOI] [PubMed] [Google Scholar]
- [26].TreeAge Pro [computer program]. Version R2.0. Williamstown, MA: TreeAge Software; 2015. [Google Scholar]
- [27].Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007. 1–86. [PMC free article] [PubMed] [Google Scholar]
- [28].Stata Statistical Software [computer program]. Version 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- [29].Gidwani R. Deriving Transition Probabilities for Decision Models. Health Economics Resource Center; 2014. Available at: https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=2401. Accessed May 2, 2019. [Google Scholar]
- [30].Narayan KV, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–90. [DOI] [PubMed] [Google Scholar]
- [31].To T, Wang C, Guan J, et al. What is the lifetime risk of physician-diagnosed asthma in Ontario, Canada? Am J Respir Crit Care Med 2010;181:337–43. [DOI] [PubMed] [Google Scholar]
- [32].Fildes A, Charlton J, Rudisill C, et al. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Public Health 2015;105:e54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Women & HIV/AIDS Initiative. Positive Women & Breastfeeding. Available at: http://www.whai.ca/resources/whai-breastfeeding-fact-sheet.pdf. Accessed May 2, 2019. [Google Scholar]
- [34].Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada. 4th ed. Ottawa: CADTH; 2017. Available at: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. Accessed May 2, 2019. [Google Scholar]
- [35].Levison J, Weber S, Cohan D. Breastfeeding and HIV-infected women in the United States: harm reduction counseling strategies. Clin Infect Dis 2014;59:304–9. [DOI] [PubMed] [Google Scholar]
- [36].Cost-Effectiveness Analysis Registry. Boston: Tufts Medical Center; 2015. Available at: https://cevr.tuftsmedicalcenter.org/databases/cea-registry. Accessed May 2, 2019. [Google Scholar]
- [37].Gray AM, Clarke PM, Wolstenholme JL, et al. Applied Methods of Cost-Effectiveness Analysis in Healthcare. Oxford: OUP; 2010. [Google Scholar]
- [38].Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group–6. Med Decis Making 2012;32:722–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


