Abstract
Background:
It is uncertain if dexmedetomidine has more favorable pharmacokinetic profile than the traditional sedative drug propofol in patients who undergo endovascular therapy for acute stroke. We conducted a prospective randomized control trial to compare the safety and efficacy of dexmedetomidine with propofol for patients undergoing endovascular therapy for acute stroke.
Methods:
A total of 80 patients who met study inclusion criteria were received either propofol (n = 45) or dexmedetomidine (n = 35) between January 2016 and August 2018. We recorded the favorable neurologic outcome (modified Rankin score <3) both at discharge and 3 months after stroke, National Institute of Health Stroke scale (NIHSS) at 48 hours post intervention, modified thrombolysis in myocardial infarction score on digital subtraction angiography, intraprocedural hemodynamics, recovery time, relevant time intervals, satisfaction score of the surgeon, mortality, and complications.
Results:
There were no significant differences between the 2 groups (P > .05) with respect to heart rate, respiratory rate, and SPO2 during the procedure. The mean arterial pressure (MAP) was significantly low in the propofol group until 15 minutes after anesthesia was induced. No difference was recorded between the groups at the incidence of fall in MAP >20%, MAP >40% and time spent with MAP fall >20% from baseline MAP. In the propofol group, the time spent with MAP fall >40% from baseline MAP was significantly long (P < .05). Midazolam and fentanyl were similar between the 2 groups (P > .05) that used vasoactive drugs. The time interval from stroke onset to CT room, from stroke onset to groin puncture, and from stroke onset to recanalization/end of the procedure, was not significantly different between the 2 groups (P > .05). The recovery time was longer in the dexmedetomidine group (P < .05). There was no difference between the groups with respect to complications, favorable neurological outcome, and mortality both at hospital discharge and 3 months later, successful recanalization and NIHSS score after 48 hours (P > .05). However, the satisfaction score of the surgeon was higher in the dexmedetomidine group (P < .05).
Conclusions:
Dexmedetomidine was undesirable than propofol as a sedative agent during endovascular therapy in patients with acute stroke for a long-term functional outcome, though the satisfaction score of the surgeon was higher in the dexmedetomidine group.
Keywords: acute stroke, dexmedetomidine, endovascular therapy, monitored anesthesia care, propofol
Key Points
Both dexmedetomidine and propofol have been used in patients for acute stroke.
It is uncertain which one is better for patients with acute stroke.
Dexmedetomidine was undesirable than propofol in patients with acute stroke.
1. Introduction
In 2013, approximately 7.0 million people had an ischemic stroke, and the mortality rates reached up to 40% in the USA.[1] Acute stroke is caused by blockage of brain blood vessels. Patients who suffer from coronary heart disease with atrial fibrillation are more vulnerable to mural thrombosis.[2]
Previous studies confer that endovascular therapy combined with intravenous thrombolysis could be preferable than intravenous thrombolysis alone for acute stroke, in addition to the evolution of devices and pharmaceuticals. It was illustrated that endovascular arterial revascularization for acute stroke improves clinical outcome at 90-days post thrombectomy of patients with large vessel occlusion.[3–5] Because of a higher degree of accuracy and better visualization of cerebrovascular anatomy, cerebral angiography (CA) is recognized as the gold standard for diagnosis and treatment of patients with acute stroke.[6] However, many patients are often agitated and unable to cooperate with this invasive procedure, regarding mild to moderate pain or discomfort, thereby increasing the procedure-related risk.[7,8]
Many factors can influence the outcomes of stroke such as age, initial stroke severity, time from stroke onset to vessel recanalization, blood pressure, and temperature.[9] Whether the anesthetic technique has an impact or not on the neurological outcome of patients undergoing endovascular therapy for acute stroke is debatable.[10] The ideal anesthetic scheme is to improve patients’ comfort and hemodynamic stability, reduce movement and minimize the risk of complications. Local anesthesia, monitored anesthesia care (MAC) and general anesthesia are commonly used methods at present.[11] A previous study confers general anesthesia may be associated with poor neurologic outcome and the brain can still be exposed to hypoxia due to a supply-demand imbalance of oxygen under sedation.[12] Patients with acute stroke widely use midazolam, propofol, opioids, or a combination of these drugs during CA; however, varying degrees of adverse effects are met.[13–15] Propofol is the most widely used drug though it could result in hemodynamic fluctuation. Dexmedetomidine (a highly selective centrally agonist of α 2 adrenergic receptor) has been used in many diagnostic and therapeutic procedures safely and effectively for dose-dependent sedation, anxiolysis and lack of respiratory depression, although clinically significant side-effects include bradycardia and hypotension.[16] A previous study determined dexmedetomidine post-treatment could also provide neuroprotection against subarachnoid hemorrhage.[17]
The aim of our study is to compare the safety and efficacy of dexmedetomidine with propofol for patients undergoing endovascular therapy for acute stroke with a strict periprocedural blood pressure control and normoventilation.
2. Materials and methods
2.1. Patients
We obtained the Institutional Review Board of Liaocheng People's Hospital approval for this prospective randomized control trial. This study was also registered at chictr.org (ChiCTR-IPR-16008494). A computer-generated randomization table was used to allocate the patients into 2 equal groups by an independent anesthetist.
We restricted our analysis to single-center data to reduce the selection bias. Patients undergoing endovascular therapy for acute stroke with MAC were enrolled in this study between January 2016 and August 2018 after obtaining the patients’ and their families’ consent if they met the following inclusion criteria: time of stroke onset <6.5 hours, age 40 years or older, National Institute of Health Stroke scale (NIHSS) < 20, occlusion in the anterior circulation identified with computed tomographic angiography or digital subtraction angiography (DSA). Exclusion criteria were occlusion in the posterior circulation, neurological recovery or recanalization before endovascular therapy, modified Rankin Scale (mRS) score ≥4, and contraindications for intravenous thrombolysis. Electronic chart and DoCare Clinic electronic anesthesia recording system data were used.
2.2. MAC management
All patients were transported to CT room where the neurological examination (NIHSS) and CT examination were performed simultaneously. Patients needed for endovascular therapy were then transported to the stroke unit to carry out intravenous thrombolysis. Oxygen supplementation at 4 L·min−1 was achieved through a facemask after arriving at the cath lab. We adopted the Ramsay sedation scale (RSS) and visual analog scale (VAS) to assess the level of sedation and pain during surgery, respectively. Patients in the dexmedetomidine group received dexmedetomidine at the rate of 0.2 to 0.7 μg·kg−1·h−1. Patients in the propofol group received propofol at the rate of 2 to 4 mg·kg−1·h−1. The procedure was started when RSS reached 4. All patients received local anesthetic infiltration with 5 mL lidocaine 1% through the femoral arterial puncture. Anticoagulation was maintained with 2500 U·h−1 of heparin during the procedure. Thrombectomy devices were used at the discretion of a Neurointerventionalist according to device availability and cerebrovascular anatomy of the patients. Hence, the Neurointerventionalist documented the rate of successful recanalization with the modified thrombolysis in myocardial infarction (mTICI > 2a; 0–2a, not successful recanalization; 2b-3, successful recanalization) scores.[18]
The infusion rate increased by 0.1 μg·kg−1·h−1 of dexmedetomidine or 0.5 mg·kg−1·h−1 of propofol, respectively. As the previous study implies, a recurrent bolus of midazolam 0.02 mg·kg−1 was given repeatedly every 5 minutes to a maximum dose of 2.5 mg if RSS <3 or if movement occurred during the procedure, while fentanyl 1 μg·kg−1 was given repeatedly every 5 minutes to a maximum dose of 0.2 mg if VAS >4. The patients were put under general anesthesia if they did not reach the ideal status after the maximum dose of midazolam and fentanyl.[19] All patients were transferred to the stroke unit after surgery. The goal of this treatment was to maintain systolic blood pressure between 140 and 180 mm Hg and mean arterial pressure (MAP) >80 mm Hg before recanalization in all the patients.[20] During the procedure, bradycardia and tachycardia were defined as heart rate (HR) <60 bpm or >120 bpm increase from baseline and treated by atropine 0.2 mg or esmolol 0.4 mg·kg−1 iv, respectively. Hypertension and hypotension were treated by urapidil (10–15 mg) or ephedrine (6–10 mg). Respiratory events such as oxygen desaturation and airway obstruction were also recorded. Oxygen desaturation (SpO2 <90%) and airway obstruction were defined as noisy breathing with paradoxical chest expansion. Neck repositioning, jaw thrust, airway insertion or endotracheal intubation was carried out if the SpO2 decreased to <90%.[21]
2.3. Data collection
The good neurologic outcome (mRS <3) was recorded both at discharge and 3 months after stroke. NIHSS at 48 hours postintervention, mTICI score on DSA, intraprocedural hemodynamics, relevant time intervals (from stroke onset to CT, from stroke onset to groin puncture, from stroke onset to recanalization/end of procedure), the amount of rescue midazolam, fentanyl or vasoactive drugs, recovery time, surgeon satisfaction score (on a 10-point scale: 0 = poor, 10 = excellent), mortality and complications were also recorded.
The intraoperative hemodynamic data (HR, MAP, SpO2, and respiratory rate [RR]) were measured every 5 minutes from baseline until the RSS returned to the baseline value. We recorded intraoperative hemodynamic data at the following time points: arrival at the operating room (T1), 5 minutes (T2), 10 minutes (T3), 15 minutes (T4), 20 minutes (T5), 25 minutes (T6), 30 minutes (T7), 35 minutes (T8), 40 minutes (T9) during the procedure. A MAP was recorded in this study for better consistency between noninvasive and invasive measurements than systolic or diastolic pressure. The occurrence of >20% and >40% fall in the MAP was recorded from baseline to total time spent under these limits.
2.4. Statistical analysis
Sample size was calculated on the basis of an expected 10% longer in the time spent with MAP fall >40% in the propofol group. For a study power of 80% (α = 0.05, β = 0.2), the required sample size per group was calculated to be 30 (PASS 11.0, NCSS Statistical Software, Kaysville, Utah). Assuming a dropout rate of 15%, the final sample size was determined to be 35 patients for each group.
The Kolmogorov–Smirnov test was used to assess the distribution of variables. Homogeneity of variance was determined using Levene tests. Quantitative data were expressed as mean and standard deviation or median and inter-quartile range. Inter-group comparisons were performed using repeated-measure analysis of variance. The Bonferroni correction was used for post-hoc multiple comparisons. The nonparametric Kolmogorov–Smirnov test was used for variables that were not normally distributed. Categorical data were expressed as frequency and percentage and analyzed using chi-squared tests or Fisher exact tests when appropriate. Probability (P) values < .05 were considered statistically significant. Statistical analysis was performed with SPSS for Windows Version 21.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Baseline characteristics
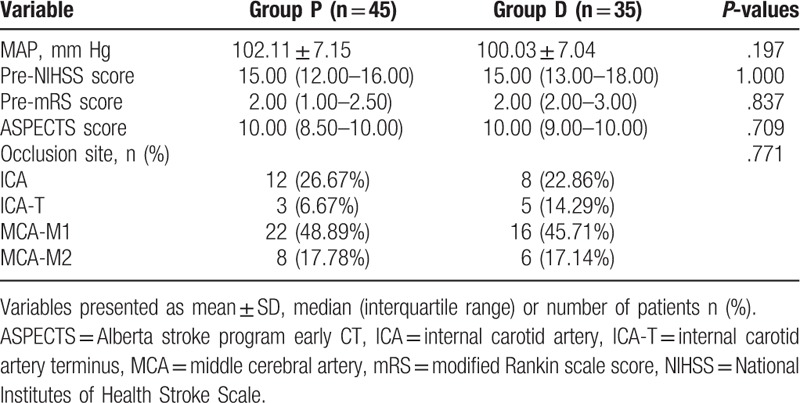
A total of 267 patients initially underwent endovascular therapy for acute stroke with MAC and were screened between January 2016 and August 2018. 183 patients were excluded due to the factors: time of stroke onset was longer than 6.5 hours in 25 patients, 6 patients age <40 years, 55 patients (NIHSS) >20, 36 patients occlusion in the posterior circulation, 7 patients neurological recovery or recanalization before endovascular therapy, 46 patients mRS score ≥4 and 8 patients’ contraindications for intravenous thrombolysis. The final sample size consisted of 84 patients. Moreover, 4 patients were also excluded after surgery due to incomplete clinical data. Eighty patients ultimately met inclusion criteria for this trial and were divided into 2 groups (45 patients from Group P, 35 patients from Group D, Fig. 1). Patients’ characteristics were insignificantly different between the 2 groups (P > .05, Table 1). There were insignificant differences between the 2 groups with respect to stroke characteristics (P > .05, Table 2).
Figure 1.
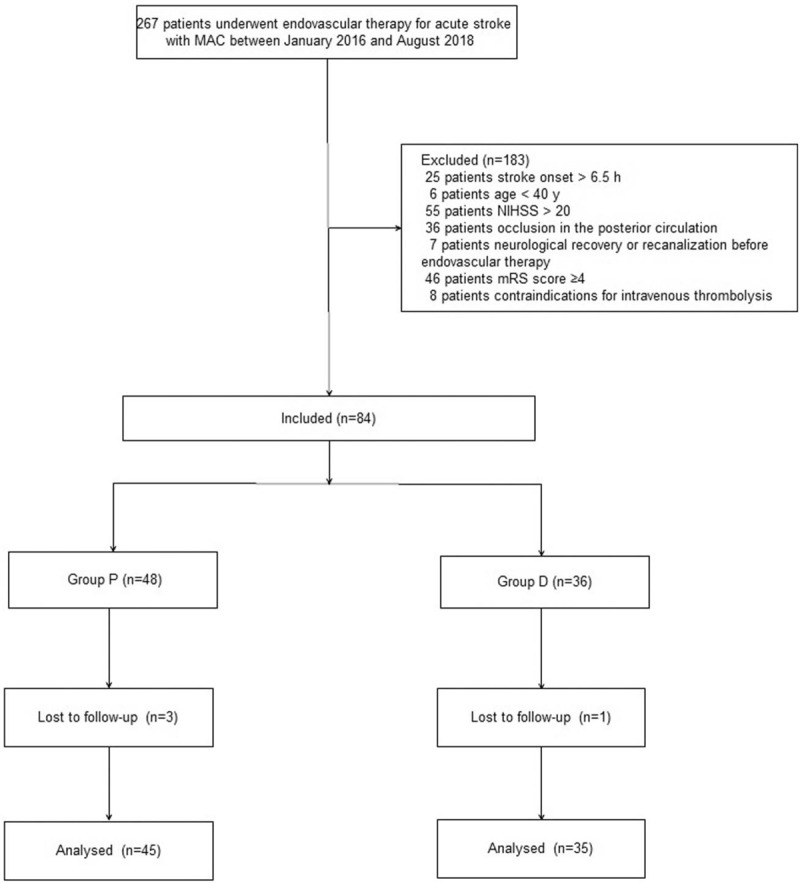
Patient enrollment diagram. This illustrates the flow of all patients screened and excluded.
Table 1.
Patient characteristics.
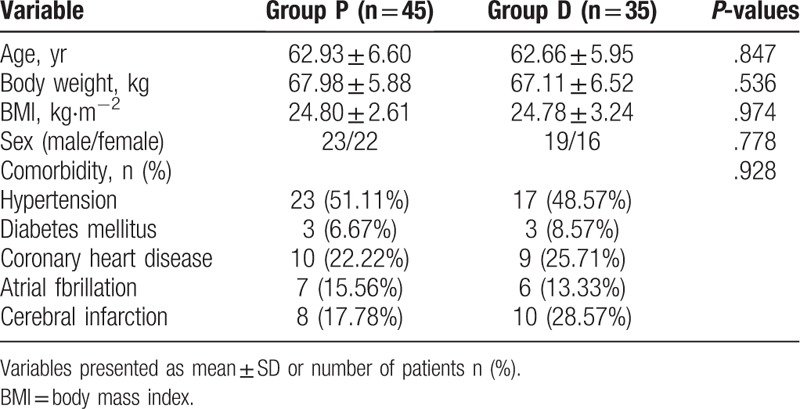
Table 2.
Stroke characteristics.
3.2. Intraoperative variables
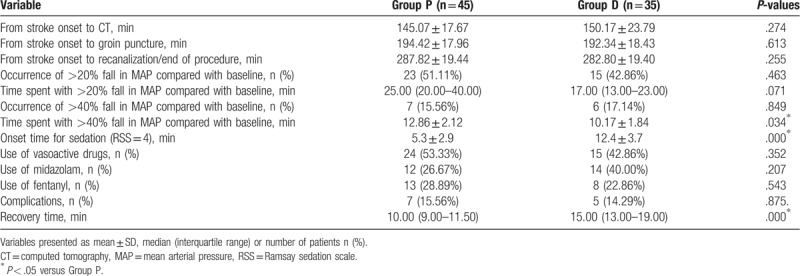
There were insignificant differences between the 2 groups with respect to HR, RR, and SPO2 during the procedure. MAP was significantly lower in the propofol group from T2 to T4 (P < .05, Fig. 2). Although there were no differences between the 2 groups with respect to the incidence of fall in MAP >20%, MAP >40% and time spent with MAP fall >20% from baseline MAP, the time spent with MAP fall >40% from baseline MAP was significantly longer in the propofol group (12.86 ± 2.12 minutes vs 10.17 ± 1.84 minutes; P = .034, Table 3). Midazolam and fentanyl were similar in the patients who used vasoactive drugs between the 2 groups (P > .05, Table 3). The time interval from stroke onset to CT room, from stroke onset to groin puncture, and from stroke onset to recanalization/end of the procedure, also, were insignificantly different between the 2 groups (P > .05, Table 3). The mean onset time for sedation (RSS = 4) was 12.4 ± 3.7 minutes in the dexmedetomidine group and 5.3 ± 2.9 minutes in the propofol group (P < .01, Table 3). The recovery time was longer in the dexmedetomidine group (10.00 [9.00–11.50] minutes vs 15.00 [13.00–19.00] minutes; P < .01, Table 3). There were no differences between the groups to complications such as respiratory depression, nausea, vomiting, and bradycardia (Table 3).
Figure 2.
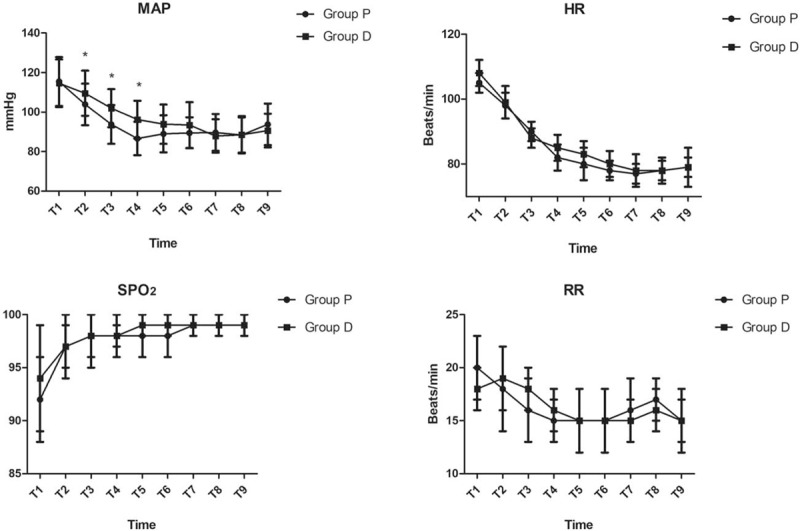
Intraoperative hemodynamic data monitored between the 2 groups at the following time points: arrival at the operating room (T1), 5 min (T2), 10 min (T3), 15 min (T4), 20 min (T5), 25 min (T6), 30 min (T7), 35 min (T8), 40 min (T9) during the procedure. ∗P < .05 versus Group P.
Table 3.
Intraoperative variables.
3.3. Postoperative variables
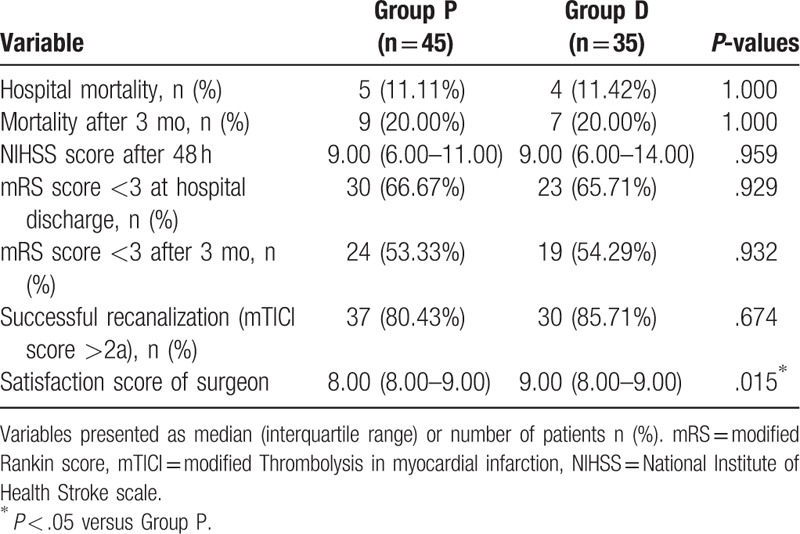
There were insignificant differences between the 2 groups to good neurological outcome (mRS score <3, P > .05, Fig. 3) and mortality both at hospital discharge and 3 months later, successful recanalization and NIHSS score after 48 hours (P > .05, Table 4). However, the satisfaction score of the surgeon was higher in the dexmedetomidine group (8.00 [8.00–9.00] vs 9.00 [8.00–9.00]; P < .01, Table 4).
Figure 3.
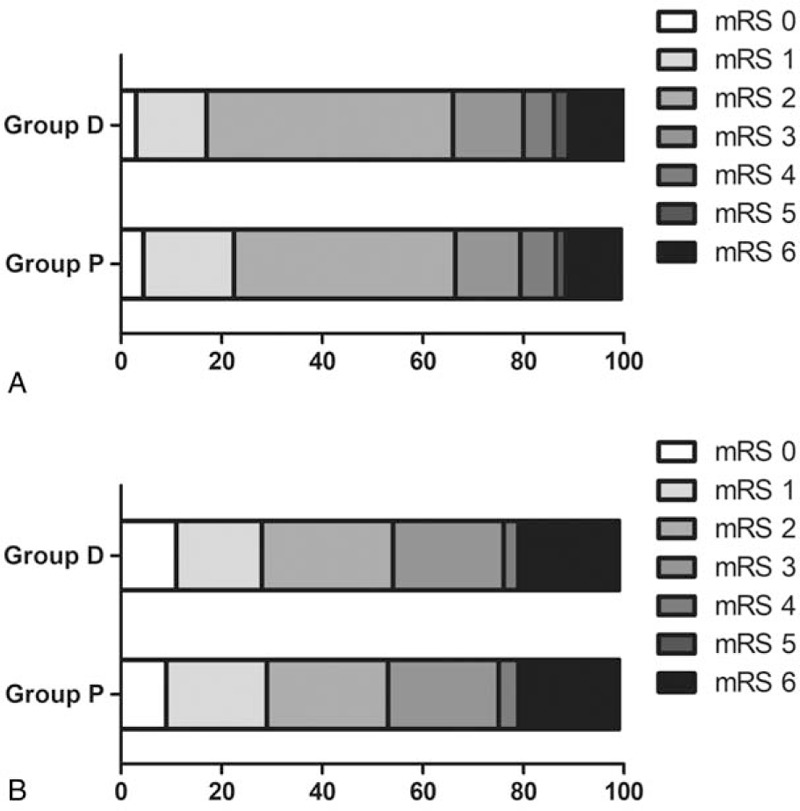
Neurological outcome expressed as mRS score both at hospital discharge (A) and 3 months later (B). mRS range, 0 to 6 (0, no symptoms; 1, no clinically relevant disability; 2, slight disability [able to look after own affairs without assistance but not to the full extent]; 3, moderate disability [requires some help but able to walk unassisted]; 4, moderately severe disability [requires assistance and unable to walk unassisted]; 5, severe disability [requires constant nursing care]; 6, dead). mRS = modified Rankin scale.
Table 4.
Postoperative variables.
4. Discussion
This study showed that MAP was significantly lower in the propofol group until 15 minutes after anesthesia was induced and the time spent with MAP fall >40% from baseline MAP was significantly longer in the propofol group. Though the mean onset time for sedation (RSS = 4) and recovery time were longer in the dexmedetomidine group, the satisfaction score of the surgeon was higher in the dexmedetomidine group. There were insignificant differences between the 2 groups with respect to HR, RR, and SPO2 during the procedure, good neurological outcome, and mortality both at discharge and 3 months later, successful recanalization, NIHSS score after 48 hours and complications.
In 2014, the Society of Neuroscience in Anesthesiology and Critical Care recommended that the choice of anesthetic technique and agents should be based on the clinical characteristics of patients and decided jointly by the anesthesiologist, the stroke neurologist and the neurointerventionalist.[22] Despite limited data, 2015 American Heart Association/American Stroke Association Guidelines recommended that conscious sedation is preferable than general anesthesia during endovascular therapy for acute stroke (Class IIb; Level of Evidence C).[23] As a result, most of the moderately-severely cooperated patients (NIHSS score <20) undergoing endovascular therapies for acute stroke adopted MAC in our hospital since 2016.
The result is consistent with prior studies that the incidence of a good neurological outcome (mRS score <3) 3 months later was 30% to 60% in the CS group.[24,25] Previous studies imply that independent predictors of a better outcome (mRS score <3) include age, NIHSS score, Alberta stroke program early CT score, successful reperfusion, lower baseline systolic blood pressure, and higher blood pressures during the procedure.[24,26] As all the above factors were comparable between the 2 groups, insignificant differences were found between the 2 groups with respect to good neurological outcome and mortality both at hospital discharge and 3 months later in our study.
Recent studies infer that systolic blood pressure beyond 140 to 180 mm Hg before recanalization was an independent predictor of poor neurologic outcome in patients undergoing endovascular therapy for acute stroke.[27,28] Previous studies also imply higher blood pressures were associated with higher incidences of death whereas lower blood pressures correlated with coronary artery disease.[29,30] As a result, it is very important to maintain both, the adequate perfusion pressure and reduce the time of hypotension, especially before recanalization. A previous study has reported that more than 70% of stroke patients had blood pressure greater than 170/110 mm Hg.[31] Hence, we took effective intervention measures to maintain MAP within 100 to 130 mm Hg before the endovascular therapy. In this trial, we adopted the MAP measured before sedation as the baseline though the timing of “baseline” values were inconsistent. The goal of the treatment was to maintain systolic blood pressure between 140 and 180 mm Hg and MAP >80 mm Hg before recanalization as the previous study has reported that MAP greater than 80 mm Hg was an independent predictor of a good neurological outcome.[24] Inconsistent with the previous study, we found that the time spent with MAP fall >40% from baseline MAP was significantly different. However, mRS score <3 at 3 months, there was no difference between the 2 groups.[12] One explanation for this result could be the neuroprotective effect of propofol such as a decrease in the cerebral metabolic rate of oxygen.
In this study, an anaesthesiologist discreetly made the choice of the vasopressor. Phenylephrine and ephedrine are the most commonly used drugs to treat intraoperative hypotension in order to maintain blood pressure and cerebral perfusion pressure. However, phenylephrine and ephedrine have very different pharmacological effects on the brain oxygenation: phenylephrine is a pure α1-agonist, while ephedrine is a mixed-acting agent with positive inotropic and chronotropic effects.[32] The prior study had stated that the cerebral tissue oxygen saturation (SctO2) was significantly decreased after phenylephrine bolus treatment and preserved after ephedrine bolus treatment even though MAP in both the cases significantly increased.[33] Another study also demonstrated the negative impact of norepinephrine infusion on cerebral oxygenation.[34] As a result, we adopt ephedrine as the first-line treatment.
Although patients in both groups had satisfactory sedation and recovery, we observed unacceptable SPO2 values in some patients in both the groups. This does not seem to be important in patients with lower NIHSS scores (NIHSS <20), as we assume that may cause disastrous consequences for patients with hemodynamic instability. A previous study has found that propofol may be more effective on cerebral metabolic rate than dexmedetomidine. Besides, the most remarkable decrease in SctO2 happened at 5th and 10th minutes compared to a baseline without any hemodynamic and respiratory changes.[35] We also did not find any significant difference between the 2 groups in terms of RR during surgery. Previous studies synopsize hyperventilation may lead to vasoconstriction of the cerebral microcirculation and reduce oxygenation in the ischemic penumbra, although the optimal end-tidal carbon dioxide tension in ischemic stroke patients remains unknown.[36,37]
Dexmedetomidine has been safely and effectively used to facilitate neurological evaluations during awake craniotomy and deep brain stimulator implantation.[38,39] We did not record any increase in the procedural related complications in this study. The previous study reported onset time in the dexmedetomidine group may be longer; however, the relevant time intervals during this study were similar between the 2 groups.[40] The reason may be due to more frequent interruptions and less working conditions in the propofol group and analgesic characteristic and synergistic effects of opioids of dexmedetomidine. Given the comorbidities of patients in our study, a lower propofol dose (2–4 mg·kg−1·h−1) was adopted than the previous studies to reduce the adverse events. However, more remedial steps (patients used vasoactive drugs, midazolam and fentanyl) were taken to complete the endovascular therapy in this study than the previous one.[41] One important measure of the success of sedation protocol is the rate of conversion to general anesthesia. No patient needed to adopt general anesthesia to complete the surgery in this trial.
This study is not without limitations. First, all the patients recruited in this study had lower NIHSS scores (NIHSS <20). Further studies will be needed to identify the results of patients with higher NIHSS scores. Second, we adopt RSS and VAS to assess the level of sedation and pain during surgery. It could be more precise in combination with objective monitoring indicators such as the Bispectral index, spectral entropy and evoked potential. Third, though previous studies have reported that the brain may also be exposed to hypoxia even if there are no changes with the routine monitor, we still did not adopt cerebral oximetry monitor in this study to assess the cerebral tissue perfusion due to technological and economic limitations.[42] Fourth, we only selected 1 dose instead of designing a dose-response study for the large number of patients in each group. Finally, this study was restricted to single center, and the results cannot be transposed to other centers.
In summary, dexmedetomidine was not superior to propofol as a sedative agent during endovascular therapy in patients with acute stroke for a long-term functional outcome, though the satisfaction score of the surgeon was higher in the dexmedetomidine group.
Author contributions
Bin Wu, Hongping Hu, and Shengjie Liu conceived and designed the trail. Bin Wu, Ailan Cai, and Shengjie Liu collected the data. Chunguang Ren analyzed the data. Bin Wu, Hongping Hu, and Shengjie Liu wrote this paper.
Conceptualization: Bin Wu, Shengjie Liu.
Data curation: Hongping Hu, Ailan Cai, Chunguang Ren.
Methodology: Hongping Hu, Ailan Cai, Shengjie Liu.
Project administration: Ailan Cai, Chunguang Ren, Shengjie Liu.
Software: Bin Wu, Hongping Hu, Chunguang Ren.
Writing – original draft: Bin Wu, Hongping Hu, Ailan Cai, Shengjie Liu.
Writing – review and editing: Bin Wu, Shengjie Liu.
Footnotes
Abbreviations: BMI = body mass index, CA = cerebral angiography, DSA = digital subtraction angiography, HR = heart rate, ICA = internal carotid artery, ICA-T = internal carotid artery terminus, MAC = monitored anesthesia care, MAP = mean arterial pressure, MCA = middle cerebral artery, mRS = modified Rankin scale, mTICI = modified thrombolysis in myocardial infarction, NIHSS = National Institute of Health Stroke scale, RR = respiratory rate, RSS = Ramsay sedation scale, SctO2 = cerebral tissue oxygen saturation, VAS = visual analog scale.
The data used to support the findings of this study are available from the corresponding author upon request.
The authors have no funding disclosures.
The authors declared that the research was conducted in the absence of any commercial or financial relationships.
References
- [1].Ding D. Endovascular mechanical thrombectomy for acute ischemic stroke: a new standard of care. J Stroke 2015;17:123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xian Y, Wu J, O’Brien EC, et al. Real world effectiveness of warfarin among ischemic stroke patients with atrial fibrillation: observational analysis from patient-centered research into outcomes stroke patients prefer and effectiveness research (PROSPER) study. BMJ 2015;351:h3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA 2015;313:1451–62. [DOI] [PubMed] [Google Scholar]
- [4].Mokin M, Rojas H, Levy EI. Randomized trials of endovascular therapy for stroke-impact on stroke care. Nat Rev Neurol 2016;12:86–94. [DOI] [PubMed] [Google Scholar]
- [5].Rodrigues FB, Neves JB, Caldeira D, et al. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta-analysis. BMJ 2016;353:i1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boulouis G, Lauer A, Siddiqui AK, et al. Clinical imaging factors associated with infarct progression in patients with ischemic strokeduring transfer for mechanical thrombectomy. JAMA Neurol 2017;74:1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Strbian D, Mustanoja S, Pekkola J, et al. Intravenous alteplase versus rescue endovascular procedure in patients with proximal middle cerebral artery occlusion. Int J Stroke 2015;10:188–93. [DOI] [PubMed] [Google Scholar]
- [8].Pereira VM, Yilmaz H, Pellaton A, et al. Current status of mechanical thrombectomy for acute stroke treatment. J Neuroradiol 2015;42:12–20. [DOI] [PubMed] [Google Scholar]
- [9].Nickles AV, Roberts S, Shell E, et al. Characteristics and outcomes of stroke patients transferred to hospitals participating in the Michigan Coverdell acute stroke registry. Circ Cardiovasc Qual Outcomes 2016;9:265–74. [DOI] [PubMed] [Google Scholar]
- [10].Brinjikji W, Pasternak J, Murad MH, et al. Anesthesia-related outcomes for endovascular stroke revascularization: a systematic review and meta-analysis. Stroke 2017;48:2784–91. [DOI] [PubMed] [Google Scholar]
- [11].Peng Y, Li Y, Jian M, et al. Choice of anesthesia for endovascular treatment of acute ischemic stroke: protocol for a randomized controlled (CANVAS) trial. Int J Stroke 2017;12:991–7. [DOI] [PubMed] [Google Scholar]
- [12].Löwhagen Hendén P, Rentzos A, Karlsson JE, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the anstroke trial (anesthesia during stroke). Stroke 2017;48:1601–7. [DOI] [PubMed] [Google Scholar]
- [13].Lazar RM, Fitzsimmons BF, Marshall RS, et al. Midazolam challenge reinduces neurological deficits after transient ischemic attack. Stroke 2003;34:794–6. [DOI] [PubMed] [Google Scholar]
- [14].Schönenberger S, Uhlmann L, Ungerer M, et al. Association of blood pressure with short-and long-term functional outcome after stroke thrombectomy: post hoc analysis of the SIESTA trial. Stroke 2018;49:1451–6. [DOI] [PubMed] [Google Scholar]
- [15].Islam MR, Yang L, Lee YS, et al. Enkephalin-fentanyl multifunctional opioids as potential neuroprotectants for ischemic stroke treatment. Curr Pharm Des 2016;22:6459–68. [DOI] [PubMed] [Google Scholar]
- [16].Liu Y, Liang F, Liu X, et al. Dexmedetomine reduces perioperative opioid consumption and postoperative pain intensityin neurosurgery: a meta-analysis. J Neurosurg Anesthesiol 2018;30:146–55. [DOI] [PubMed] [Google Scholar]
- [17].Wang Y, Han R, Zuo Z. Dexmedetomidine post-treatment induces neuroprotection via activation of extracellular signal-regulated kinase in rats with subarachnoid haemorrhage. Br J Anaesth 2016;116:384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shu L, Riedel C, Meyne J, et al. Successful recanalization in acute basilar artery occlusion treated with endovascular therapy is independent of thrombus length. J Neurointerv Surg 2017;9:1047–52. [DOI] [PubMed] [Google Scholar]
- [19].Wang W, Feng L, Bai F, et al. The safety and efficacy of dexmedetomidine vs. sufentanil in monitored anesthesia care during burr-hole surgery for chronic subdural hematoma: a retrospective clinical trial. Front Pharmacol 2016;7:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mistry EA, Mistry AM, Nakawah MO, et al. Systolic blood pressure within 24 hours after thrombectomy for acute ischemic stroke correlates with outcome. J Am Heart Assoc 2017;6:e006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim TJ, Ko SB, Jeong HG, et al. Nocturnal desaturation is associated with neurological deterioration following ischemic stroke: a retrospective observational study. J Clin Sleep Med 2017;13:1273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang A, Abramowicz AE. Endovascular thrombectomy in acute ischemic stroke: new treatment guide. Curr Opin Anaesthesiol 2018;31:473–80. [DOI] [PubMed] [Google Scholar]
- [23].Powers WJ, Derdeyn CP, Biller J, et al. American Heart Association Stroke Council. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:3020–35. [DOI] [PubMed] [Google Scholar]
- [24].Bouslama M, Haussen DC, Aghaebrahim A, et al. Predictors of good outcome after endovascular therapy for vertebrobasilar occlusion stroke. Stroke 2017;48:3252–7. [DOI] [PubMed] [Google Scholar]
- [25].Yarbrough CK, Ong CJ, Beyer AB, et al. Endovascular thrombectomy for anterior circulation stroke: systematic review and meta-analysis. Stroke 2015;46:3177–83. [DOI] [PubMed] [Google Scholar]
- [26].Yoon W, Kim SK, Heo TW, et al. Predictors of good outcome after stent-retriever thrombectomy in acute basilar artery occlusion. Stroke 2015;46:2972–5. [DOI] [PubMed] [Google Scholar]
- [27].Men X, Sun W, Fan F, et al. China stroke primary prevention trial: visit-to-visit systolic blood pressure variability is an independent predictor of primary stroke in hypertensive patients. J Am Heart Assoc 2017;6:e004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Perini F, De Boni A, Marcon M, et al. Systolic blood pressure contributes to intracerebral haemorrhage after thrombolysis for ischemic stroke. J Neurol Sci 2010;297:52–4. [DOI] [PubMed] [Google Scholar]
- [29].Okin PM, Kjeldsen SE, Devereux RB. Systolic blood pressure control and mortality after stroke in hypertensive patients. Stroke 2015;46:2113–8. [DOI] [PubMed] [Google Scholar]
- [30].Penaloza-Ramos MC, Jowett S, Barton P, et al. Cost-effectiveness analysis of different systolic blood pressure targets for people with a history of stroke or transient ischaemic attack: economic analysis of the PAST-BP study. Eur J Prev Cardiol 2016;23:1590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mant J, McManus RJ, Roalfe A, et al. Different systolic blood pressure targets for people with history of stroke or transient ischaemic attack: PAST-BP (prevention after stroke–blood pressure) randomised controlled trial. BMJ 2016;352:i708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dyer RA, Emmanuel A, Adams SC, et al. A randomised comparison of bolus phenylephrine and ephedrine for the management of spinal hypotension in patients with severe preeclampsia and fetal compromise. Int J Obstet Anesth 2018;33:23–31. [DOI] [PubMed] [Google Scholar]
- [33].Meng L, Cannesson M, Alexander BS, et al. Effect of phenylephrine and ephedrine bolus treatment on cerebral oxygenation in anaesthetized patients. Br J Anaesth 2011;107:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brassard P, Seifert T, Secher NH. Is cerebral oxygenation negatively affected by infusion of norepinephrine in healthy subjects? Br J Anaesth 2009;102:800–5. [DOI] [PubMed] [Google Scholar]
- [35].Cetin M, Birbicer H, Hallioglu O, et al. Comparative study between the effects of dexmedetomidine and propofol on cerebral oxygenation during sedation at pediatric cardiac catheterization. Ann Card Anaesth 2016;19:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pavilla A, Arrigo A, Mejdoubi M, et al. Measuring cerebral hypoperfusion induced by hyperventilation challenge with intravoxel incoherent motion magnetic resonance imaging in healthy volunteers. J Comput Assist Tomogr 2018;42:85–91. [DOI] [PubMed] [Google Scholar]
- [37].Curley G, Kavanagh BP, Laffey JG. Hypocapnia and the injured brain: more harm than benefit. Crit Care Med 2010;38:1348–59. [DOI] [PubMed] [Google Scholar]
- [38].Goettel N, Bharadwaj S, Venkatraghavan L, et al. Dexmedetomidine vs propofol-remifentanil conscious sedation for awake craniotomy: a prospective randomized controlled trial. Br J Anaesth 2016;116:811–21. [DOI] [PubMed] [Google Scholar]
- [39].Rozet I, Muangman S, Vavilala MS, et al. Clinical experience with dexmedetomidine for implantation of deep brain stimulators in Parkinson's disease. Anesth Analg 2006;103:1224–8. [DOI] [PubMed] [Google Scholar]
- [40].Whalin MK, Lopian S, Wyatt K, et al. Dexmedetomidine: a safe alternative to general anesthesia for endovascular stroke treatment. J Neurointerv Surg 2014;6:270–5. [DOI] [PubMed] [Google Scholar]
- [41].Wang Z, Kou D, Li Z, et al. Effects of propofol-dexmedetomidine combination on ischemia reperfusion-induced cerebral injury. NeuroRehabilitation 2014;35:825–34. [DOI] [PubMed] [Google Scholar]
- [42].Hametner C, Stanarcevic P, Stampfl S, et al. Noninvasive cerebral oximetry during endovascular therapy for acute ischemic stroke: an observational study. J Cereb Blood Flow Metab 2015;35:1722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


