Supplemental Digital Content is available in the text
Keywords: blood pressure, cardiovascular risk factors, randomized interventional study, type 2 diabetes, wine
Abstract
Background:
Previous studies identified conflicting results about the effects of wine intake on glucose parameters and the risk of cardiovascular diseases in type 2 diabetes mellitus (T2DM). The present study further investigated the association between wine digestion and these outcomes in T2DM patients.
Material and methods:
A search of PubMed, Embase, and Scopus databases (up to November 2018) was performed for randomized interventional trials which evaluated the effect of wine on blood pressure (BP), glucose parameters and lipid profiles in T2DM people. We used a variety of tests: fixed and random effects models, Q Cochrane test and I2 index, Egger and Begg tests, forest plots, and sensitivity analysis in our study.
Results:
A total of 9 randomized interventional studies were included in this meta-analysis. Overall, significant association between wine intake with diastolic BP (weighted mean difference [WMD] = 0.10; 95% confidence interval [95% CI]: −0.01 to 0.20, P = .03 I2 = 13%) and total cholesterol (TC) (WMD = 0.16, 95% CI: 0.02–0.31, P = .03, I2 = 6%), whereas no noticeable differences in glucose parameters, systolic BP, low-density lipoprotein cholesterol (LDLC), triglyceride (TG) and high-density lipoprotein cholesterol (HDLC) were identified between wine and controls groups (fasting glucose [FG],WMD = −0.00, 95% CI: −0.58 to 0.58; fasting insulin [FI], −0.22, −2.09 to 1.65; HbAc1%, −0.16, −0.40 to 0.07; systolic blood pressure, 0.12, −0.05 to 0.28; LDLC, −0.02, −0.25 to 0.21; TG, −0.34, −1.31 to 0.64; HDLC, 0.22, −0.08 to 0.53].
Conclusion:
This meta-analysis revealed that moderate wine consumption among T2DM patients could reduce the level of diastolic blood pressure and TC, but not glucose parameters and other cardiovascular risk factors.
1. Introduction
The disease burden of diabetes mellitus (DM) has become a global public health issue over the last decades, and its prevalence is projected to further rise given the obesity epidemic, sedentarity, and ageing of the population.[1] Compared with the general population, DM is associated with a higher mortality, mainly attributable to cardiovascular causes.[2,3] Presumed contributors to the increased cardiovascular disease risk in DM include associated dyslipidemia, hypertension, insulin resistance, and hypercoagulability.[2,4] Since the early ‘90s, a number of experimental and clinical studies have been published, describing the protective effect of red or white wine on different pathways of the pathogenesis of a variety of disorders including cardiovascular diseases.[5,6] Antioxidants, flavonoids, and polyphenols become the first substances contained in red/white wine with proven beneficial effects in various diseases, such as inhibition of low-density lipoprotein (LDL) oxidation or attenuation of ischemia-reperfusion injury.[7]
Better controlled blood pressure (BP), especially diastolic blood pressure (DBP), glucose parameters and the lipid profile can effectively lower the risk of cardiovascular diseases in type 2 diabetes mellitus (T2DM) patients.[8] However, in previous studies, the association of wine consumption with cardiovascular disease risk demonstrated contradictory results in those outcomes of T2DM patients. Some studies reported that wine had no significant effects on BP, glucose parameters, or lipid profile,[9–11] whereas, some organization drew opposite conclusions.[12–14] For instance, Gepner et al found that after 6 months of intervention, the average 24-hours BP did not differ between the wine and water groups, but a transient decrease in BP was detected in the red wine group at midnight and the following morning.[10] On the contrary, Mori and his colleagues reported that in well-controlled T2DM individuals moderate red wine raises awake BP and lowers asleep BP but does not otherwise favorably adversely modify cardiovascular factors.[15] Similarly, some studies observed that moderate wine consumption for 30 days did not alter the level of fasting glucose (FG), total cholesterol (TC), low-density lipoprotein cholesterol (LDLC), triglyceride (TG), and high-density lipoprotein cholesterol (HDLC), but serum insulin significantly grew in T2DM patients.[16] However, a multicenter, randomized clinical intervention trial observed that among patients with T2DM who had previously abstained from alcohol, initiation of moderate daily wine consumption reduced FG but not postprandial glucose.[17]
In the present article we include 9 randomized controlled study (RCT) and randomized intervention crossover study, and carried out a meta-analysis of effects of wine intake on BP, glucose parameters, and lipid profile in T2DM people.
2. Materials and methods
2.1. Data sources and search strategies
A system literature search was performed using PubMed, EMBASE, and Scopus for articles published up to November 2018. The following MeSH or Emtree terms and their combination were searched in the title and abstract: “type 2 diabetes” or “type 1 diabetes mellitus” or “diabetes” and “cardiovascular disease” or “myocardial infarction” or “heart failure” or “cardiovascular death” or “blood pressure” or “blood glucose” and “red wine” or “wine” or “fortified wine.” References cited in relevant articles produced from the search were also manually searched for possible misses in our inclusion. To avoid any potential publication bias, we did not place any limitations on date, publication type, sample origin, country, or language. As this study is a meta-analysis of previously published clinical trials, the ethical approval and patient consent are not required.
2.2. Inclusion criteria
-
(1)
The study compared wine with a control (such as water, wine grape juice, or abstainer) in patients who were diagnosed with T2DM.
-
(2)
The study was an RCT or randomized intervention (crossover study) only.
-
(3)
The major outcomes included the FG, fasting insulin (FI), HbA1C%, DBP, systolic blood pressure (SBP), TC, LDLC, TG, and HDLC.
2.3. Exclusion criteria
-
(1)
Articles were from the same institution, duplicated publications or included the same data sets.
-
(2)
No outcomes of interest were reported.
-
(3)
Evidence of severe diabetic complications (such as proliferative retinopathy or diabetic nephropathy); subjects had a cardiac surgery or angioplasty during the past 6 months; non-ischemic cardiomyopathy, implanted pacemaker, or permanent tachyarrhythmias; non-cardiac diseases such as inflammatory disorders, malignancy, or infection.
-
(4)
Animal studies, case reports, meta-analysis, and non-original research (such as editorials, review articles, and letter to the editor).
2.4. Data extraction and analysis
Data extraction was conducted from each included study independently by 2 investigators (Jianhua Ye and Ligang Bao). The following data were extracted by reviewing all the included studies: name of the first author, publication year, location, age and number of participants, male/female ratio, study period, intervention, and study design. Also, all outcomes as mentioned above were extracted for meta-analysis. Any disagreement in the data extraction was resolved through discussion with the third reviewer (Xufeng Chen) until a consensus was reached.
2.5. Risk of bias
The Cochrane Collaboration's tool for assessing risk of bias (ROB) was used to assess the methodological quality of each included study (https://training.cochrane.org/handbook). In this tool, the following 7 items were assessed in all included studies:
-
(1)
the randomization process,
-
(2)
allocation concealment,
-
(3)
blinding of participants and personnel,
-
(4)
blinding of outcome assessments,
-
(5)
completeness of the outcome data,
-
(6)
reporting of results, and
-
(7)
other sources of bias.
Each item was assigned a judgment of “a high risk of bias,” “an unclear risk,” or “a low risk of bias (LRB)” based on the data presented in the article. Namely, the judgment was “high risk” if the item was reported incorrectly. The judgment was “low risk” if the item possessed sufficient and correct information. If the item possessed insufficient or unmentioned information, the judgment was “an unclear risk.” An “unclear risk” judgment was also assigned if the item was reported, but the ROB was unknown. Disagreements were addressed by obtaining a consensus between experienced reviewers (Jianhua Ye and Ligang Bao).
2.6. Data synthesis and analysis
The desired outcomes were SBP, DBP, FG, FI, HbAc1%, TC, LDLC, TG, and HDLC. The statistical analysis was performed with RevMan 5.3.5 program (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) by 2 reviewers (Jianhua Ye and Xufeng Chen) and STATA software version 14 for Mac (StataCorp LP, College Station, TX). The weighted mean difference (WMD), reported with 95% confidence intervals (CIs) was adopted to evaluate all outcomes. Between-study heterogeneity was assessed through the Q test and I2 statistics. When P value less than .05 or I2 more than 30% represented the existence of significant heterogeneity, the random-effects model was selected to evaluate the overall WMD. Publication bias was detected by the Begg and Egger tests. Sensitivity analyses were conducted to evaluate the influence of individual studies on the final conclusion, by dropping the included studies individually and then calculating the modified effects. A P < .05 was considered statistically significant.
3. Results
3.1. Study selection
The initial search retrieved 1175 records (citations) from PubMed, Embase, and Scupos. After removing of the duplicates, the unique 712 articles remained. Of these, 669 articles were excluded after first screening based on the abstracts or titles. The remaining 43 articles were screened for further full-text reviewing. In the second screening. We excluded a total 34 articles that did not fulfill the inclusion criteria (2 articles for no full text, 23 for not providing data of wine and risk of cardiovascular diseases, 7 for not being randomized intervention, 2 for not having eligible data), ultimately leaving 9 randomized intervention studies for inclusion in the comprehensive meta-analysis (Fig. 1).
Figure 1.
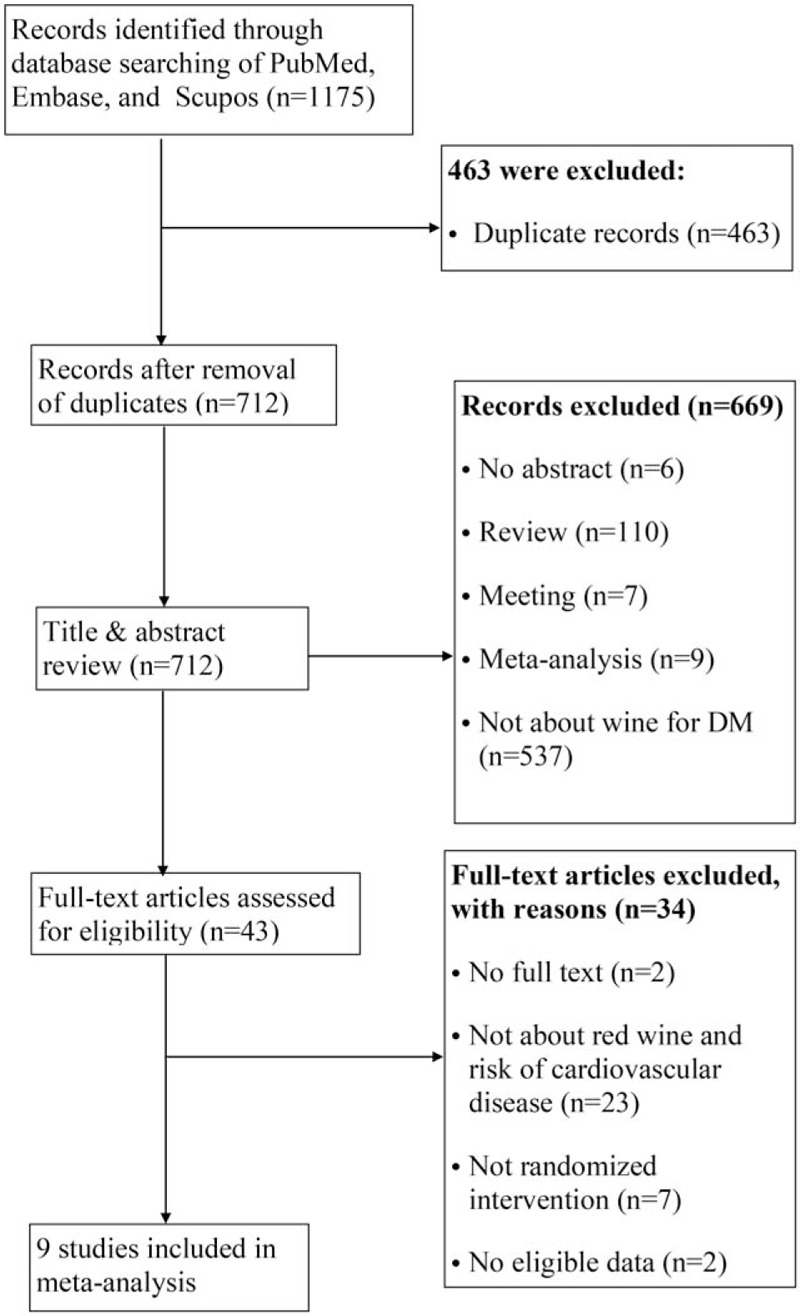
Flow diagram of the literature search and selection process.
3.2. Characteristics of studies
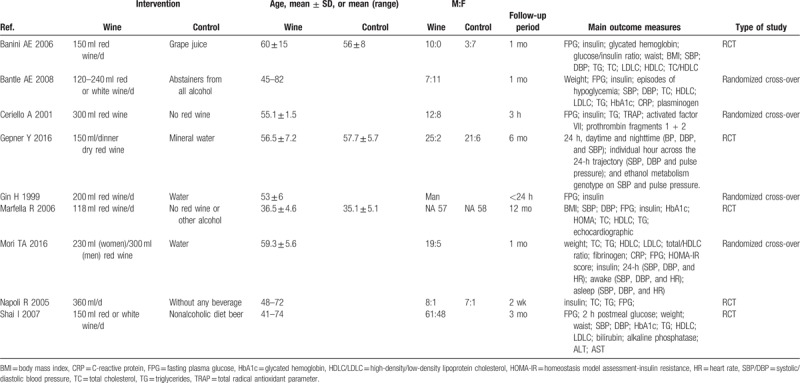
Table 1 shows the characteristics of all the included 9 studies in this analysis. Among these randomized trials were RCTs (n = 5) and crossover studies (n = 4). One study included only men and the age ranged from 19 to 82 years old. Regarding follow-up period, most studies followed at least 2 weeks (n = 7), while 2 studies examined the short-term effect (<24 hours) of wine in T2DM patients. Seven studies performed by consuming red wine, except 2 studies (Shai I 2007 and Bantle AE 2008) in which patients were treated with red or white wine. Intervention in the control group varied among the different studies, including no red wine or other alcohol (n = 5),[12–14,16,17] water (n = 3),[10,11,15] and grape juice (n = 1).[9]
Table 1.
Included studies and their characteristics.
3.3. SBP and DBP
All pooled analyses were conducted using a fixed effects model for SBP and DBP. A total of 5 trials comprising 190 experimental patients and 155 controls provided data on the changes of SBP and DBP caused by wine consumption. As shown in Figure 2, no significant differences in SBP were observed between the 2 groups (WMD = 0.12, 95% CI: −0.05 to 0.28, P = .17, I2 = 29%), while wine intake could reduce the level of DBP in T2DM patients (WMD = 0.10, 95% CI: 0.01–0.20, P = .03, I2 = 13%).
Figure 2.
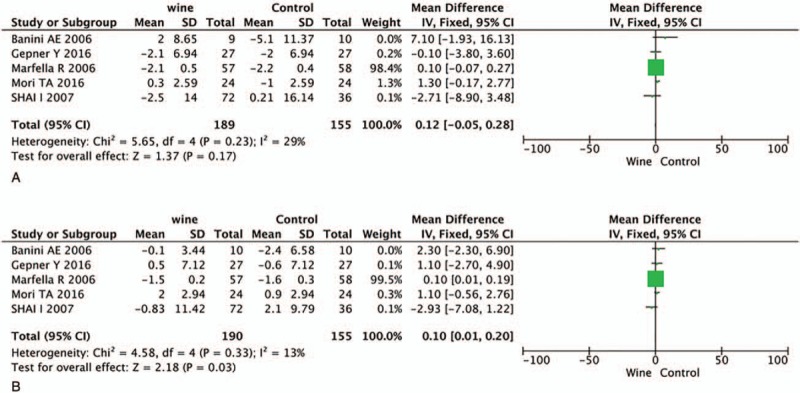
Forest plot of SDP (A) and DBP (B) when comparing red wine with control. DBP = diastolic blood pressure, SDP = systolic blood pressure.
3.4. FG, FI, and HbAc1%
The number of trials that reported FG, FI, and HbAc1% was 6, 3, and 5, respectively. No significant differences in these 3 outcomes were observed between the 2 groups (FG, WMD = −0.00, 95% CI: −0.58 to 0.59, P = 1.00, I2 = 97%, Fig. 3A; FI, WMD = −0.22, 95% CI: −2.09 to 1.65, P = .82, I2 = 93%, Fig. 3B; HbAc1%, WMD = −0.16, 95% CI: −0.40 to 0.07, P = .17, I2 = 78%, Fig. 3C).
Figure 3.
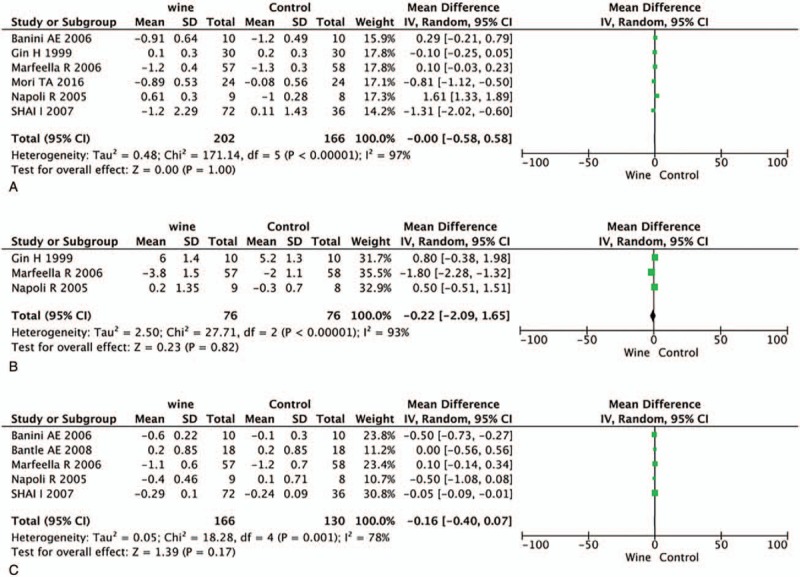
Forest plot of fasting glucose (A), insulin (B) and HbAc1% (C), when comparing red wine with control. HbA1c = glycated hemoglobin.
3.5. The lipid profiles
There are 4 trials provided data on TC and LDLC, 6 on TG and 5 on HDLC. The level of TC was significantly decreased after drinking wine in T2DM patients (WMD = −0.16, 95% CI: 0.02–0.31, P = .03, I2 = 6%, Fig. 4A). However, no significant differences in the other 3 lipid-associated measurements and the results are as follows: LDLC, WMD = −0.02, 95% CI: −0.25 to 0.21, P = .86, I2 = 54%, Figure 4B; TG, WMD = −0.34, 95% CI: −1.31 to 0.64, P = .50, I2 = 99%, Figure 4C; HDLC, WMD = 0.22, 95% CI: −0.08 to 0.53, P = .15, I2 = 95%, Figure 5.
Figure 4.
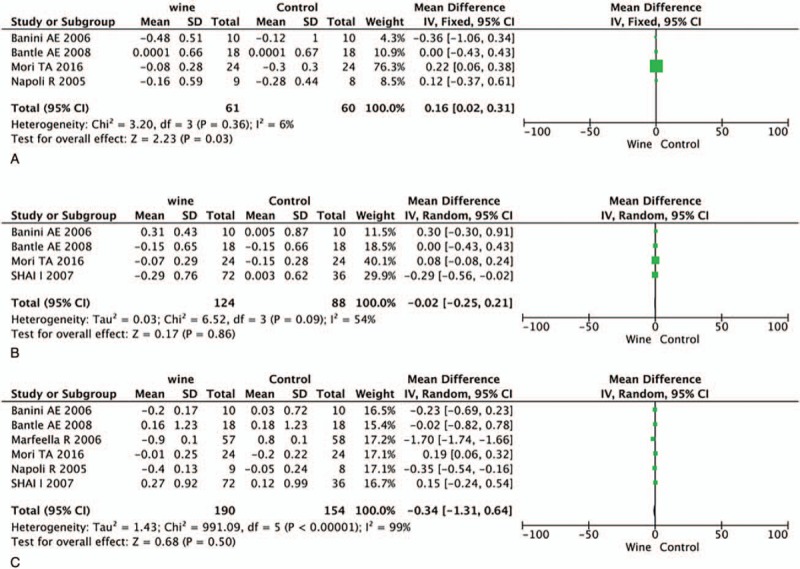
Forest plot of TC (A), LDLC (B), and TG (C). LDLC = low-density lipoprotein cholesterol, TC = total cholesterol, TG = triglyceride.
Figure 5.
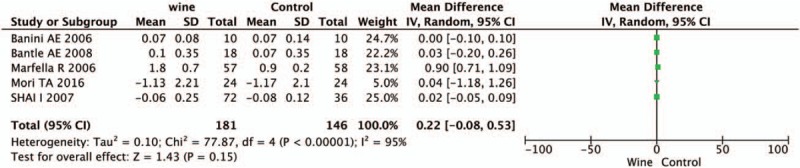
Forest plot of HDLC. HDLC = high-density lipoprotein cholesterol.
3.6. Assessment of the ROB
The methodological quality of all studies was assessed by Cochrane ROB criteria. The evaluation of ROB is shown in Figures 6 and 7. Randomly generated sequences were judged as a LRB in 6 interventional trials. Three studies mentioned that the clinical trial was randomized, but did not report further details. Blinding of treating doctors, subjects, and outcome assessors were judged as a LRB in 1 trials, 7 trials, and 0 trials, respectively. No evidence for any trial that had a high risk of incomplete outcome data and selective reporting bias. We did not identify other obvious sources of bias in the trials, either.
Figure 6.
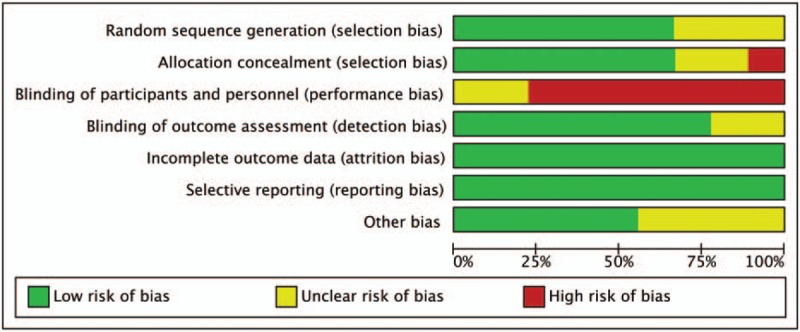
Risk of bias summary.
Figure 7.
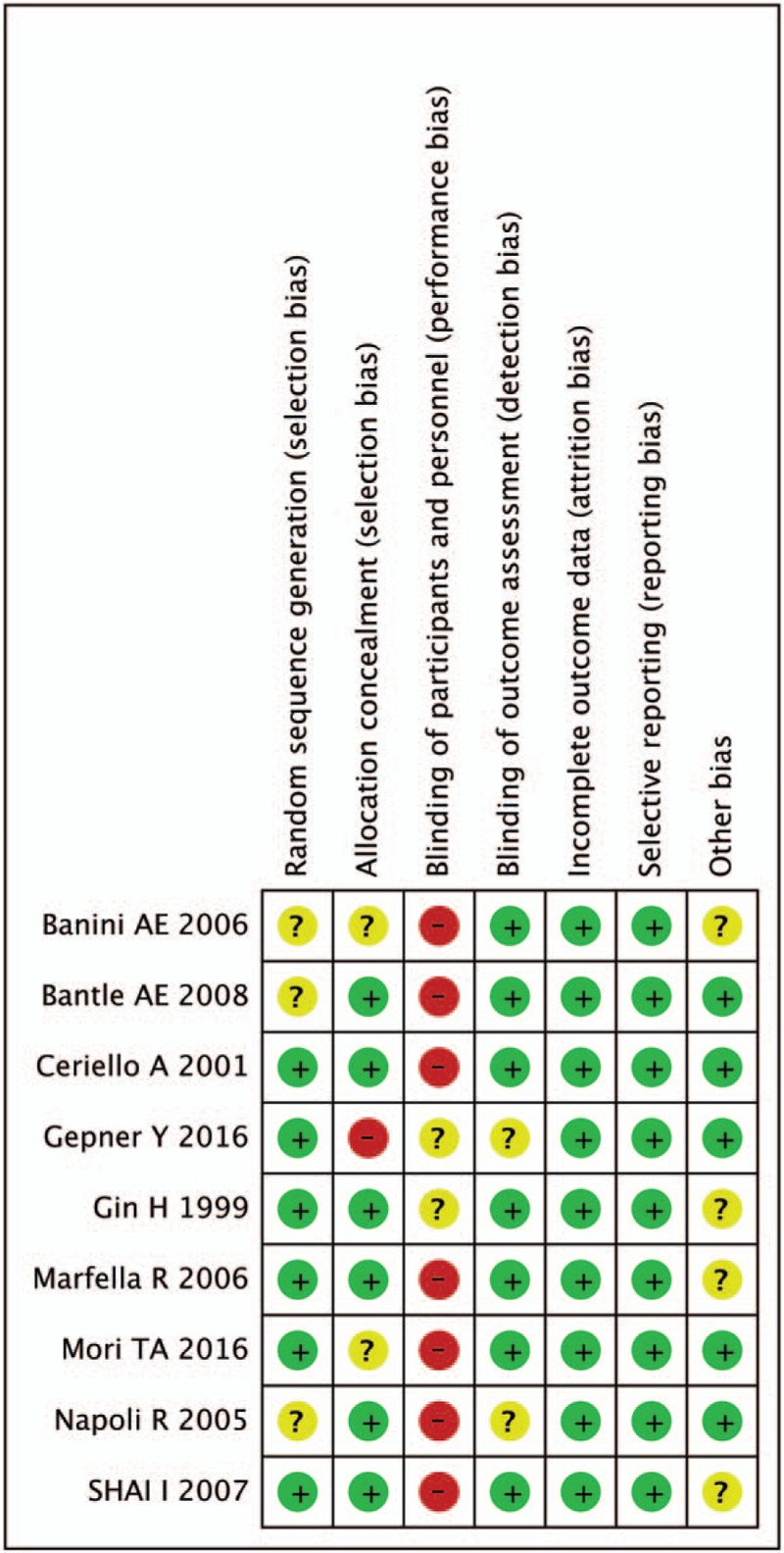
Risk of bias assessment. Legend: (+) indicates high risk of bias; (?) indicates unclear risk of bias; (−) indicates low risk of bias.
3.7. Sensitivity analysis
A sensitivity analysis was performed when I2 > 30%, by individually removing each study to determine whether the pooled results changed. It was found that the outcome was not significantly changed when any study was excluded from this meta-analysis (Supplementary Table S1).
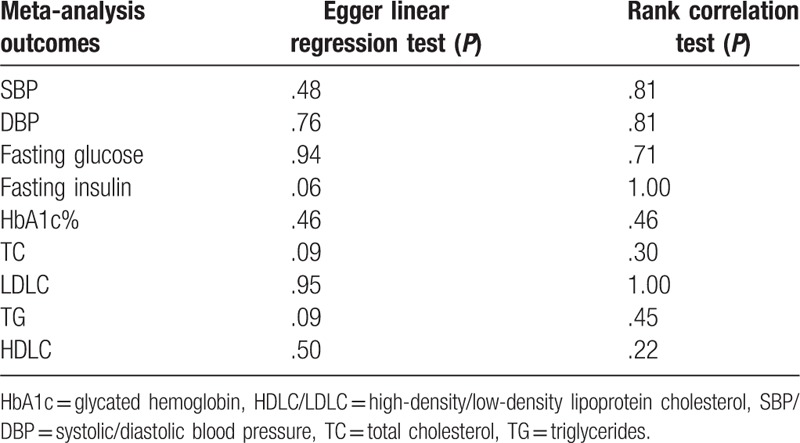
3.8. Publication bias assessment
There was no publication bias according to the Begg and Egger tests in evaluating the effect of wine consumption in the glucose parameters and cardiovascular risk factors. Nonstatistically significant P values (P > .06) were obtained using Egger's test and rank correlation test for each of the data set analysis, thus reflecting the lack of publication bias in the meta-analysis. Table 2 lists the publication bias assessment method with its respective P value for each test.
Table 2.
Assessment of publication bias.
4. Discussion
This meta-analysis showed that moderate consumption of red/white wine could effectively reduce the DBP and TC in T2DM patients, but the SBP, glucose parameters and other lipid-associated measurements did not present significant differences in these individuals. Although Koppes et al reported that alcohol consumption is associated with a lower risk of mortality and coronary heart disease in type 2 diabetic population in their meta-analysis concerning observational studies,[18] we found different results. To our best knowledge, this is the first meta-analysis to estimate the relationship between wine consumption with glucose parameters and cardiovascular risk factors, by combining all available randomized interventional trials up to date. Further ROB evaluation, sensitivity, and publication bias tests provided additional support for the robustness of our results. Although heterogeneity is prevalent in the analysis, results from sensitivity indicate that the overall effect size evaluations did not vary substantially during exclusion of each study.
Hypertension is a prominent preventable cause of premature morbidity and mortality and its prevalence increased in DM. People with hypertension and established cardiovascular diseases are at particular high risk in general population.[19,20] Therefore, the management of elevated BP is a critical component of the comprehensive clinical management of diabetes. Previous study had revealed that in well-controlled type 2 diabetic individuals moderate red wine drinking could raise awake BP and lower asleep BP.[15] Among our findings, the association between wine intake and DBP reduction was significant, while the SBP showed no significant differences in wine group and controls. Although the present finding did not show significant decrease in SBP, which is consistent with the general trend from various wine consumption trials.[12,17] This inconsistency might be attributed to the fact that we just pooled the average BP in our analysis, but the wine drinking affects BP differently between individual hours across the 24-hours trajectory.[10] Owing to insufficient data we could not do pooled analysis on BP changes based specific hours.
We observed no impact of wine on glycemic control by monitoring FG, FI, and HbAc1% level. Some prior studies reported the same results with ours, such as Napoli et al and Mori et al who also did not found differences between wine group and controls in FG, FI, and HbAc1%.[9,13] However, other organizations drew different conclusion by reporting that the level of FI decreased after 1-year red wine intervention, otherwise no significant differences were observed in FG, HbAc1%.[12,16] The discrepancy could come from the differences in study participants, study design, the beverage type (red or white wine) and approaches to measurement of insulin sensitivity.[15,21,22]
Individuals with T2DM have coexistent dyslipidemia characterized by increased TGs and reduced HDLC.[23,24] In contrast to the well-documented effects of alcohol in healthy individuals to increase HDL cholesterol. We observed no changes in serum HDL cholesterol with red wine relative to water or no drinking in diabetic patients. In a multicenter randomized clinical intervention trial, diabetic subjects consuming red wine that diabetic subjects consuming 13 g alcohol containing 13 g alcohol daily for 3 months showed no increase in HDL cholesterol and decrease in LDL cholesterol and TG.[17] Whereas the level of TC was reduced in diabetic subjects drinking wine in our meta-analysis. The likely explanation for inconsistency among studies is related to the wine dose or duration or to unique characteristics of the study population.
4.1. Limitation
As with all meta-analysis, several limitations merit consideration in our study. First of all, there is a lack of wider coverage of RCT studies included in the analysis, which may limit the credibility for all outcomes calculated in this meta-analysis. Second, according to the ROB evaluation, most of studies included in this meta-analysis could not be double-blind. In addition, we enrolled some crossover studies, rather than all parallel designed RCTs. The final limitation may relate to the heterogeneity of some outcomes, which may have been caused by a number of factors, including different intervention of control groups, the baseline characteristics of study population and the wine drinking regimen like drinking with meal and fasting drinking. These factors have been proved to influence some outcomes in diabetic subjects.[9,11,12,25]
5. Conclusion
This meta-analysis identified that wine consumption in short-term (<24 hours) or long-term (2 years) could reduce DBP and TC in diabetic subjects, while no significant differences were observed in the values of SBP, glucose parameters, LDLC, TG, and HDLC. Further investigation including more longer intervention RCTs and more homogeneity of participants are needed to affirm the efficacy and safety of wine intake and its association with the risk of cardiovascular diseases among type 2 diabetes.
Author contributions
Conceptualization: Jianhua Ye.
Data curation: Jianhua Ye, Xufeng Chen.
Formal analysis: Ligang Bao.
Investigation: Xufeng Chen, Ligang Bao.
Methodology: Xufeng Chen, Ligang Bao.
Software: Xufeng Chen, Ligang Bao.
Supervision: Jianhua Ye.
Validation: Xufeng Chen.
Writing – original draft: Jianhua Ye.
Writing – review and editing: Jianhua Ye, Ligang Bao.
Supplementary Material
Footnotes
Abbreviations: BP = blood pressure, CI = confidence interval, FG = fasting glucose, FI = fasting insulin, HDLC = high-density lipoprotein cholesterol, LDLC = low-density lipoprotein cholesterol, ROB = risk of bias, TG = triglyceride, T2DM = type 2 diabetes mellitus, WMD = weighted mean difference.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Nanditha A, Ma RC, Ramachandran A, et al. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care 2016;39:472–85. [DOI] [PubMed] [Google Scholar]
- [2].Bhanpuri NH, Hallberg SJ, Williams PT, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiovasc Diabetol 2018;17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jansen van Vuren E, Malan L, von Kanel R, et al. Prospective associations between cardiac stress, glucose dysregulation and executive cognitive function in Black men: the sympathetic activity and ambulatory blood pressure in Africans study. Diab Vasc Dis Res 2018;1479164118816221. [DOI] [PubMed] [Google Scholar]
- [4].Li X, Wang J, Shen X, et al. Higher blood pressure predicts diabetes and enhances long-term risk of CVD events in individuals with impaired glucose tolerance- 23-year follow-up of the Daqing Diabetes Prevention Study. J Diabetes 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barden A, Shinde S, Phillips M, et al. The effects of alcohol on plasma lipid mediators of inflammation resolution in patients with Type 2 diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids 2018;133:29–34. [DOI] [PubMed] [Google Scholar]
- [6].Golan R, Shai I, Gepner Y, et al. Effect of wine on carotid atherosclerosis in type 2 diabetes: a 2-year randomized controlled trial. Eur J Clin Nutr 2018;72:871–8. [DOI] [PubMed] [Google Scholar]
- [7].Rasines-Perea Z, Teissedre PL. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017;22: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cryer MJ, Horani T, DiPette DJ. Diabetes and hypertension: a comparative review of current guidelines. J Clin Hypertens (Greenwich) 2016;18:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Banini AE, Boyd LC, Allen JC, et al. Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition 2006;22:1137–45. [DOI] [PubMed] [Google Scholar]
- [10].Gepner Y, Henkin Y, Schwarzfuchs D, et al. Differential effect of initiating moderate red wine consumption on 24-h blood pressure by alcohol dehydrogenase genotypes: randomized trial in type 2 diabetes. Am J Hypertens 2016;29:476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gin H, Rigalleau V, Caubet O, et al. Effects of red wine, tannic acid, or ethanol on glucose tolerance in non-insulin-dependent diabetic patients and on starch digestibility in vitro. Metabolism 1999;48:1179–83. [DOI] [PubMed] [Google Scholar]
- [12].Marfella R, Cacciapuoti F, Siniscalchi M, et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with Type 2 diabetes mellitus. Diabet Med 2006;23:974–81. [DOI] [PubMed] [Google Scholar]
- [13].Napoli R, Cozzolino D, Guardasole V, et al. Red wine consumption improves insulin resistance but not endothelial function in type 2 diabetic patients. Metabolism 2005;54:306–13. [DOI] [PubMed] [Google Scholar]
- [14].Ceriello A, Bortolotti N, Motz E, et al. Red wine protects diabetic patients from meal-induced oxidative stress and thrombosis activation: a pleasant approach to the prevention of cardiovascular disease in diabetes. Eur J Clin Invest 2001;31:322–8. [DOI] [PubMed] [Google Scholar]
- [15].Mori TA, Burke V, Zilkens RR, et al. The effects of alcohol on ambulatory blood pressure and other cardiovascular risk factors in type 2 diabetes: a randomized intervention. J Hypertens 2016;34:421–8. [DOI] [PubMed] [Google Scholar]
- [16].Bantle AE, Thomas W, Bantle JP. Metabolic effects of alcohol in the form of wine in persons with type 2 diabetes mellitus. Metabolism 2008;57:241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shai I, Wainstein J, Harman-Boehm I, et al. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: a multicenter, randomized, clinical intervention trial. Diabetes Care 2007;30:3011–6. [DOI] [PubMed] [Google Scholar]
- [18].Koppes LL, Dekker JM, Hendriks HF, et al. Meta-analysis of the relationship between alcohol consumption and coronary heart disease and mortality in type 2 diabetic patients. Diabetologia 2006;49:648–52. [DOI] [PubMed] [Google Scholar]
- [19].Saiz LC, Gorricho J, Garjon J, et al. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database Syst Rev 2018;7:CD010315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li Y, Wei FF, Wang S, et al. Cardiovascular risks associated with diastolic blood pressure and isolated diastolic hypertension. Curr Hypertens Rep 2014;16:489. [DOI] [PubMed] [Google Scholar]
- [21].Woerdeman J, Del Rio D, Calani L, et al. Red wine polyphenols do not improve obesity-associated insulin resistance: a randomized controlled trial. Diabetes Obes Metab 2018;20:206–10. [DOI] [PubMed] [Google Scholar]
- [22].Urquiaga I, Troncoso D, Mackenna MJ, et al. The consumption of beef burgers prepared with wine grape pomace flour improves fasting glucose, plasma antioxidant levels, and oxidative damage markers in humans: a controlled trial. Nutrients 2018;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Webb D, Dales J, Zaccardi F, et al. Intensive versus standard multifactorial cardiovascular risk factor control in screen-detected type 2 diabetes: 5 year and longer-term modelled outcomes of the ADDITION-Leicester study. Diabetes Metab Res Rev 2018;e3111. [DOI] [PubMed] [Google Scholar]
- [24].Haddad JA, Haddad AN. The past decade in type 2 diabetes and future challenges. Hormones (Athens) 2018;17:451–9. [DOI] [PubMed] [Google Scholar]
- [25].Fenwick EK, Xie J, Man RE, et al. Moderate consumption of white and fortified wine is associated with reduced odds of diabetic retinopathy. J Diabetes Complications 2015;29:1009–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


