Abstract
Background:
Although the prognostic significance of base excess (BE) in patients with paraquat (PQ) poisoning has been investigated for several years, the results remain controversial. Thus, we performed for the first time a comprehensive meta-analysis to explore the value of BE in predicting the prognosis of patients with PQ poisoning.
Methods:
We searched PubMed, EMBase, Web of Science, ScienceDirect, Cochrane Library, and the Chinese National Knowledge Infrastructure to identify all relevant papers that were published up to August 2018. The data were extracted for pooled analysis, heterogeneity testing, sensitivity analysis, publication bias analysis, and subgroup analysis.
Results:
Pooled analysis revealed that a decreased BE is correlated with poor mortality (pooled OR = 21.358, 95% CI: 12.716–35.873, P < .001). Pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were 78% (95% CI: 0.66–0.86), 88% (95% CI: 0.66–0.97), 6.6 (95% CI: 2.2–19.9), 0.25 (95% CI: 0.18–0.36), and 26 (10–69), respectively. No publication bias was detected by Egger test (P = .263) and Begg test (P = .462). Sensitivity analyses indicated no important differences among the estimates of effects.
Conclusion:
Our findings show that BE is useful for predicting the prognosis of PQ poisoning.
Keywords: base excess, paraquat, prognosis
1. Introduction
Paraquat (N,N-dimethyl-4,4-lipoprotein dichloride; PQ) is a non-selective contact herbicide that has been extensively used worldwide for many years, particularly in developing agricultural countries. PQ is sold in around 130 countries for use on large and small farms, plantations, estates, and nonagricultural weed control. Although previous studies have investigated various treatment modalities for acute PQ poisoning, the fatality rate remains high, with affected individuals presenting a mortality rate of 50% to 90%.[1–3] Thus, biomarkers for the prognosis and clinical monitoring of acute PQ poisoning must be urgently developed for the formulation of appropriate treatment plans and develop future PQ antidotes.
The base excess (BE) level has been recently applied as a prognostic marker in patients with acute PQ poisoning.[4–8] Multiple logistic regression has associated decreased BE levels with high risk of mortality. However, the sample sizes of the previous studies were small, and many studies lacked sufficient information to evaluate the relationship between BE level and mortality. Therefore, we conducted the present meta-analysis to overcome these limitations.
2. Materials and methods
Our meta-analysis was performed in accordance with the meta-analysis of observational studies in the epidemiology checklist.[9] Our study did not involve ethical issues as the data were based on published studies. The review protocol was previously registered in the International Prospective Register of Systematic Reviews (Protocol number: PROSPERO 2018:CRD42018110526).
2.1. Search strategy
We conducted a systematic search of scientific databases, including PubMed, EMBase, Web of Science, ScienceDirect, Cochrane Library, and the Chinese National Knowledge Infrastructure, to find all relevant papers published from inception to August 2018. The following terms were used: “paraquat” and “base excess”. The reference lists of the included studies were searched for identification of additional studies.
2.2. Inclusion and exclusion criteria
Studies that satisfied the following criteria were eligible for the meta-analysis: retrospective or prospective cohort studies that reported on the effects of BE on patients with PQ poisoning. The following studies or data were excluded: abstracts, comments, case reports, reviews, meta-analyses, conference papers, letters, and editorials. In cases of overlap between authors or centers among different studies, the higher-quality and/or more recent study was selected. Studies from the same authors or centers but with different patient cohorts were included.
2.3. Data collection
Two authors (FWZ and SLZ) independently assessed the quality of the selected studies and extracted the data by using data extraction forms. The following information was extracted from each study: first author's name, year of publication, sample size, mortality percentage, BE level and study period.
2.4. Quality assessment of the selected articles
Two reviewers (CPW and JG) independently assessed the methodological quality of all included studies by using the Newcastle–Ottawa scale (NOS).[10,11] scale with a maximum of nine stars was adopted for the evaluation of the selection, comparability, exposure, and outcome of each study. Studies with a NOS scores of >7 were considered high-quality works.[12] Any discrepancy in data extraction and quality assessment was resolved through discussion.
2.5. Statistical analysis
The pooled HR with its 95% CI was utilized for the quantitative assessment of the effects of hypothermia on patients of cardiac arrest. Cochrane Q and I2 tests were used to evaluate the heterogeneity among the studies. The heterogeneity was considered statistical significant for if P < .05 or I2 > 50%. The fixed-effects model was applied when P > .05 and I2 < 50%; otherwise, the random-effects model was selected.[13] Additionally, we conducted sensitivity analyses by removing one study each time and recalculating the pooled effects. Potential publications bias (considered present if P ≤ .1) was assessed by conducting statistical tests for funnel plot asymmetry and conducting Egger and Begg tests. All these analyses were conducted on STATA 14.0 software (Stata Corporation, College Station, TX).
3. Results
3.1. Search strategy
Approximately 153 publications, of which 65 were duplicated studies, were extracted from the database search by using the described searching strategy (Fig. 1). After the titles and the abstracts were screened, 48 studies were assessed for eligibility by full-text screening. Then, 25 studies that satisfied the exclusion criteria were removed after the full-text review. Finally, 5 studies encompassing 528 patients with PQ poisoning were included in this meta-analysis.
Figure 1.

Flowchart of the literature search and study selection for this meta-analysis.
3.2. Study characteristics and quality of included studies
The characteristics of the included articles are presented in Table 1. The 5 included studies were published between 2007 and 2015, and all studies were conducted in China. Five studies were published in English, and 2 studies were published in Chinese. The scores of these studies ranged from 7 to 8. Therefore, all eligible articles were considered.
Table 1.
Study characteristics.

3.3. Association of BE on mortality
Pooled analysis showed that decreased BE was correlated with poor mortality (pooled OR = 21.358, 95% CI: 12.716–35.873, P < .001; Fig. 2), with moderate heterogeneity (I2 = 42.3%, P = .134). The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were 78% (95% CI: 0.66–0.86), 88% (95% CI: 0.66–0.97), 6.6 (95% CI: 2.2–19.9), 0.25 (95% CI: 0.18–0.36), and 26 (10–69), respectively. The area under the curve (AUC) of the BE tests was 0.87 (95% CI: 0.83–0.89), thereby implying that the diagnostic accuracy was relatively high (Fig. 3).
Figure 2.

Forest plot of the association between BE and mortality.
Figure 3.

Summary ROC (SROC) curve for the 5 included studies. Numbers in brackets are 95% CIs. AUC = area under ROC curve, ROC = receiver operating characteristic, SENS = sensitivity, SPEC = specificity.
3.4. Heterogeneity and publication bias assessment
Meta-regression analyses were performed on the basis of mortality percentage (≥50% vs <50%), sample size (≥100 vs <100), and publication year (before 2013 vs after 2013). However, these factors did not account for the source of heterogeneity (Table 2). No publication bias was detected by Egger test (P = .263) and Begg test (P = .462) (Fig. 4).
Table 2.
Meta-regression analysis of potential sources of heterogeneity.

Figure 4.

Funnel plot of the potential publication bias in the prognosis prediction value of BE for mortality. (A) Begg test; and (B) Egger test.
3.5. Sensitivity analysis
We evaluated the effect of each study on the summary results by sequentially excluding a single study. Sensitivity analyses indicated no important differences in the estimates of the effects (Fig. 5).
Figure 5.
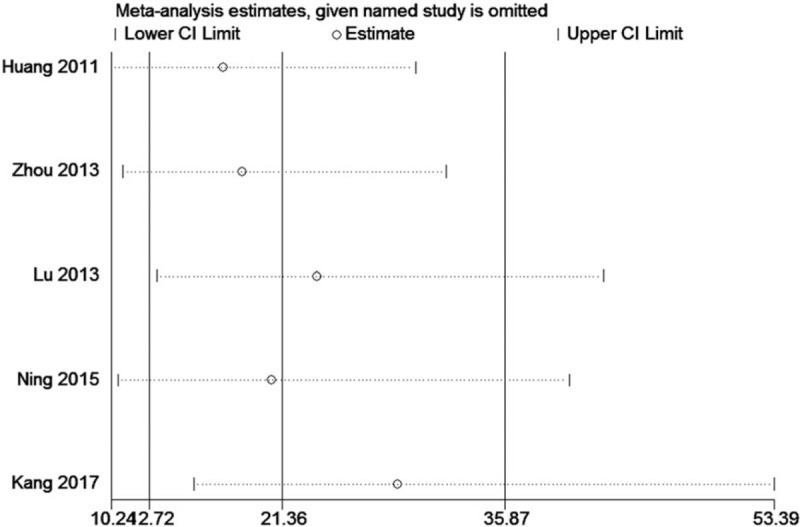
Sensitivity analysis of the correlation between BE and mortality.
4. Discussion
This meta-analysis summarized the existing evidence regarding the association between BE level and clinical outcomes in patients with PQ poisoning. The main findings of our meta-analysis indicated that patients with PQ poisoning who had elevated BE levels presented significantly increased risks of mortality.
The correlation between the BE level and predictive power of mortality in patients with PQ poisoning was due to several reasons. First, PQ directly or indirectly induced the production of free oxygen radicals and the superoxidation of lipids. Consequently, the energy metabolism in the human body was blocked, aerobic metabolism was reduced, whereas anaerobic metabolism was increased. Thus, numerous acidic metabolites were accumulated, ultimately inducing metabolic acidosis and increasing the negative BE value.[4] Second, the BE level was negatively correlated with the PQ concentration in the plasma, as indicated by the Pearson correlative analysis results (γ = −0.400, P < .01).[8] Third, early alkalization treatment can reduce organ injury and mortality.[14]
The current systematic review and network meta-analysis has some limitations. First, most of the original studies used in our meta-analysis had a case-control study design, which is particularly vulnerable to potential biases (including selection bias and information bias). Second, the sample size of several studies was small, which may lead to limited generalizability. Third, all included studies were conducted in China, which may cause publication bias.
Based on the findings from related scientific studies, our meta-analysis integrated the results of individual studies, which revealed that a decreased BE was significantly associated with poor prognosis in patients with PQ poisoning. We proposed that BE should be regarded as a prognostic biomarker. Nevertheless, the clinical utility of BE must be confirmed by well-designed investigations with large sample sizes in the future.
Acknowledgment
The authors would like to thank Wang Li for assistance in statistical analysis.
Author contributions
Conceptualization: Shun Yi Feng.
Data curation: Feng Wei Zhang, Jie Gao, Su Li Zhang, Cheng Pu Wu, Wen Jing Bai.
Methodology: Jie Gao.
Project administration: Yong Li.
Software: Yong Li.
Supervision: Wen Jing Bai.
Writing – original draft: Feng Wei Zhang.
Writing – review & editing: Shun Yi Feng.
Footnotes
Abbreviations: AUC = area under the curve, CI = confidence interval, OR = odds ratio, PQ = paraquat, QUADAS-2 = Quality Assessment of Diagnostic Accuracy Studies 2 tool.
Feng Wei Zhang and Jie Gao were contributed equally to this work.
The authors declare no conflict of interest.
References
- [1].Wu WP, Lai MN, Lin CH, et al. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: a nationwide study. PLoS One 2014;9:e87568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tan JT, Letchuman Ramanathan G, Choy MP, et al. Paraquat poisoning: experience in hospital taiping (year 2008 – October 2011). Med J Malaysia 2013;68:384–8. [PubMed] [Google Scholar]
- [3].Gao J, Feng SY, Wang J, et al. Prolonged methylprednisolone therapy after the pulse treatment for patients with moderate-to-severe paraquat poisoning: a retrospective analysis. Medicine (Baltimore) 2017;96:e7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huang C, Zhang X. Prognostic significance of arterial blood gas analysis in the early evaluation of paraquat poisoning patients. Clin Toxicol (Phila) 2011;49:734–8. [DOI] [PubMed] [Google Scholar]
- [5].Zhou YX, Fang JJ, He XJ. Early base excess and arterial carbon dioxide partial pressure monitoring in predicting the outcome of patients with acute paraquat poisoning. China Mod Dr 2013;51:58–60. [Google Scholar]
- [6].Lu MF, Xia ZF, Wang C, et al. Value of arterial lactic acid and buffer excess in predicting the prognosis of patients with paraquat poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2013;31:667–70. [PubMed] [Google Scholar]
- [7].Ning Z, Bai YL, Lu H, et al. Prognostic value of plasma C-reactive protein in the evaluation of paraquat poisoning patients. Asian Pac J Trop Biomed 2015;5:841–4. [Google Scholar]
- [8].Kang XW, Tong H, Cao KQ, et al. Value of base excess in predicting the prognosis of patients with paraquat poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2017;35:25–9. [DOI] [PubMed] [Google Scholar]
- [9].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Oremus M, Oremus C, Hall GB, et al. Inter-rater and test–retest reliability of quality assessments bynovice student raters using the Jadad and Newcastle–OttawaScales. BMJ Open 2012;2:e001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sun MM, Fan YY, Dang SC. Comparison between uncut Roux-en-Y and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol 2018;24:2628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7:1–73. [DOI] [PubMed] [Google Scholar]
- [13].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [14].Song HQ, Li XM. Observation on the efficacy of comprehensive alkalization therapy in the treatment of acute paraquat poisoning. J Clin Res 2013;30:483–5. [Google Scholar]


