Supplemental Digital Content is available in the text
Keywords: coronary atherosclerotic heart disease, drug-coated balloon, drug-eluting stent, percutaneous coronary intervention, small coronary artery vessel disease
Abstract
Background:
Drug-coated balloon as a novel therapeutic strategy has been used to treat restenosis in cases of bare metal and drug-eluting stents. However, evidence of its safety and efficacy is scarce in de novo small coronary artery vessel disease. This meta-analysis aimed to compare the safety and efficacy of the drug-coated balloon and the drug-eluting stent.
Methods:
The PubMed, EMBASE, Web of Science, and Cochrane library databases were searched for studies published up to October 17, 2018. Studies comparing the drug-coated balloon with the drug-eluting stent strategy in patients with de novo small coronary artery vessel disease (reference diameter, <3 mm) were identified. The clinical outcomes were nonfatal myocardial infarction, cardiac death, all-cause death, target lesion revascularization, and target-vessel revascularization. Data were analyzed using the statistical software RevMan (version 5.3). Fixed effects models were performed to calculate the pooled odds ratios (ORs) and 95% confidence intervals (95% CIs). Sensitivity analyses were used to detect potential sources of heterogeneity, while subgroup analyses were implemented to assess the differential effects.
Results:
Three randomized controlled trials and 3 nonrandomized controlled studies were identified. Six studies including a total of 1800 patients compared the differences between the drug-coated balloon and the drug-eluting stent strategies in patients with de novo small coronary artery vessel disease. The results indicated that the drug-coated balloon strategy was associated with a significant reduction in nonfatal myocardial infarction (OR 0.53, 95% CI 0.31–0.90, P = .02) compared with the drug-eluting stent strategy, while insignificant inter-strategy differences were observed in cardiac death (OR 1.56, 95% CI 0.73–3.33, P = .25), all-cause death (OR 0.56, 95% CI 0.25–1.23, P = .15), target lesion revascularization (OR 1.24, 95% CI 0.73–2.1, P = .43), and target-vessel revascularization (OR 0.95, 95% CI 0.59–1.52, P = .84).
Conclusions:
This meta-analysis suggests that the drug-coated balloon strategy is noninferior to the drug-eluting stent strategy, delivering a good outcome in nonfatal myocardial infarction, and can be recommended as an optimal treatment strategy in patients with de novo small coronary artery vessel disease. Larger randomized controlled studies with longer follow-up periods are needed to further confirm the benefits of the drug-coated balloon strategy.
1. Introduction
Severe coronary artery disease in the small coronary vessels is a common discovery during coronary angiography.[1] The incidence of percutaneous coronary intervention for small coronary artery vessels is 30% to 40%, which is a real challenge for contemporary interventional cardiology due to the high restenosis rate and increased risk of adverse outcomes despite the use of drug-eluting stents.[2] Up to now, guidelines for the optimal treatment strategy are not available for patients with de novo small coronary artery vessel disease. Therefore, it is advisable that a suitable alternative be chosen to treat small coronary artery vessel disease.
The drug-coated balloon, as a novel therapeutic strategy, was initially introduced to overcome the restenosis rates of bare-metal and drug-eluting stents, and recommended in the European Society of Cardiology guidelines (class I, level of evidence A).[3,4] The drug-coated balloon strategy is currently an option in patients with de novo small coronary lesions. Its theoretical advantages are as follows: the drug-coated balloon is a semi-compliant angioplasty balloon covered with lipophilic drugs that are rapidly delivered to the vessel wall after balloon inflation by a specific matrix, which avoids the implantation of foreign bodies and reduces the late inflammatory response, restenosis, and thrombosis.[5] In addition, the absence of a metallic stent and long-term polymer ensures the integrity of the vascular anatomy, which decreases hemodynamic abnormalities and shortens the duration of dual antiplatelet therapy.[6,7]
Despite the promising characteristics of the drug-coated balloon strategy, its usefulness in patients with de novo small coronary lesions remains unclear. This study was initially designed to summarize existing evidence to directly compare the differences between the 2 strategies in patients with de novo small coronary artery vessel disease. The results of the statistical analyses yielded a major finding that the drug-coated balloon therapy was superior to drug-eluting stent in nonfatal myocardial infarction.
2. Methods
2.1. Data sources and search strategy
The PubMed, EMBASE, Web of Science, and Cochrane library databases were searched from inception until October 17, 2018 using the following terms: “drug-coated balloon,” “drug eluting balloon,” “drug-eluting stent,” “drug coated stent,” “small coronary artery disease,” “small coronary vessel,” “small-vessel coronary artery disease,” and “small vessel disease” without language restriction. The electronic search strategy was complemented by manual review of the reference list of each included article. References of recent reviews, editorials, and meta-analyses were also examined. The report of the methods in this article was in accord with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[8] The protocol of the review was not registered. All analyses were based on previous published some studies, thus, no ethical approval or patient consent was required.
2.2. Study selection
Studies included as eligible followed the patient, intervention, comparison, and outcome model:
-
(1)
all patients with de novo small coronary artery vessel disease;
-
(2)
complete reporting of clinical outcomes (cardiac death, all-cause death, nonfatal myocardial infarction, target lesion revascularization, and target-vessel revascularization);
-
(3)
comparison of the drug-coated balloon and the drug-eluting stent strategies;
-
(4)
randomized controlled trials or nonrandomized studies.
Studies regarding the drug-coated balloon combined with the bare metal stent versus the drug-eluting stent, lacking the comparison or control group, and for which data concerning the above outcomes were incomplete were excluded.
2.3. Study endpoints and definitions
The clinical endpoints included cardiac death, all-cause death, non-fatal myocardial infarction, target lesion revascularization, and target-vessel revascularization. Cardiac death was defined as death due to cardiac causes. Death attributed to various causes was defined as all-cause death. The definition of nonfatal myocardial infarction was consistent with the guidelines of the third universal definition of myocardial infarction.[9] Target lesion revascularization was defined as any repetitive revascularization within the segment treated with the stent or drug-coated balloon, while target-vessel revascularization was defined as any repetitive revascularization of the target vessel.
2.4. Data extraction
The titles, abstracts and full-text articles of the relevant publications were manually reviewed after removal of duplicates, and studies with irrelevant details were sequentially excluded according to the eligibility criteria. Related data were centrally checked to ensure integrity and relevance before they were tabulated for the convenience analysis. Four independent authors (M Li, Ch Guo, MB Zhang, and YH Lv) played an important role in extracting the data. To reduce bias, these extractors were blinded to information that may influence their judgment (eg, title, author names, and journal influence) during this process. The extracted data were examined by the last author (ZL Wang). Any contradictions were resolved by discussion among the authors. The analyzed studies’ principal investigators were contacted in cases of missing information.
2.5. Quality assessment
The eligible studies’ risk of bias was assessed using the Cochrane Collaboration's tool for randomized controlled trials.[10] The tool consists of 7 points: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Trial with >2 high-risk components were considered as having a moderate risk of bias, and trial with >4 high-risk components was considered to have a high risk of bias. The Newcastle–Ottawa scale was used to assess the quality of non-randomized studies.[11] Discrepancies were resolved by consensus after discussion among the authors. The quality evaluation of each study was also performed independently. Study quality did not change their weight in this analysis, but it did function as an indicator of validity.
2.6. Statistical analysis
Fixed-effect models were used to analyze outcomes based on the Mantel–Haenszel method unless there was evidence of heterogeneity (I2 > 50%), while random effects models were used. Studies without outcome events or in which only a few events resulting in an infinite odds ratio (OR) were weighted as 0. ODs and the corresponding 95% confidence intervals (CIs) were calculated for categorical variables. Study heterogeneity was assessed by the Higgins I2 test, and the bound of I2 > 50% indicated significant heterogeneity. Publication bias was not assessed, if fewer than 10 studies were enrolled in an analysis. Sensitivity analyses were performed by omitting each study in a step-by-step manner and recalculating the pooled OR to detect whether any single study was primarily responsible for the final results. Subgroup analyses were used to explore the reason for the heterogeneity. Two main factors were analyzed as the objects of subgroup analyses: difference of study design (randomized controlled studies and nonrandomized controlled studies), and discrepancy of device type (first-generation drug-eluting stent and second-generation drug-eluting stent). All statistical tests were 2-sided, and P values ≤.05 were considered statistically significant. Review Manager version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark) was used for the statistical analyses.
3. Results
3.1. Search results
The search results are illustrated in Figure 1. The initial search retrieved 1252 articles, of which 122 were duplicates. After browsing titles, abstracts and full texts, the final 6 studies contained 1800 patients with de novo small coronary artery vessel disease were included in the meta-analysis.
Figure 1.
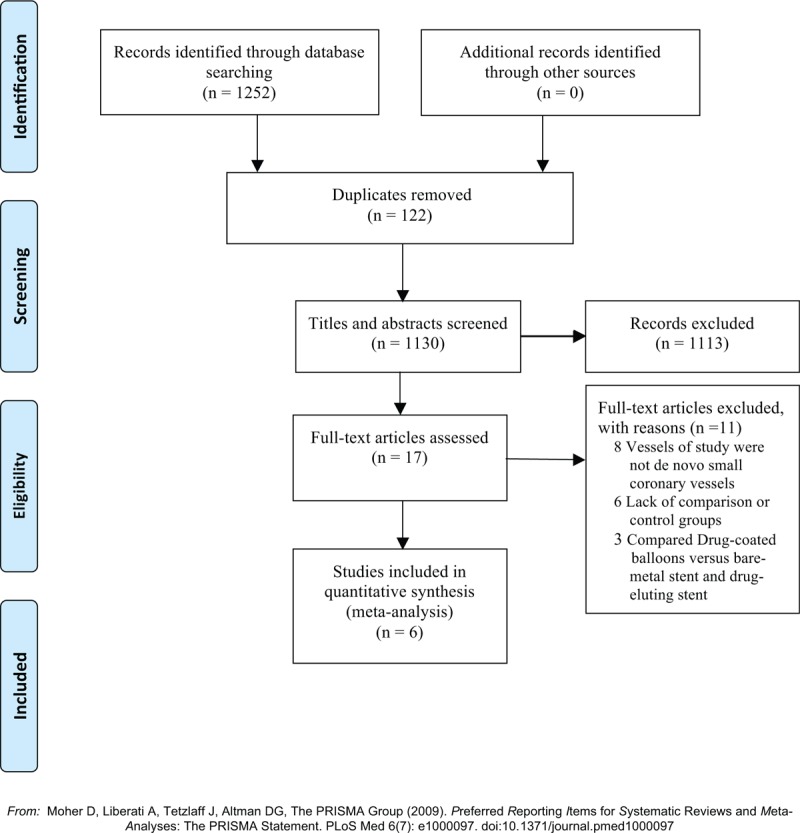
Flow chart about the searching process.
3.2. Study characteristics
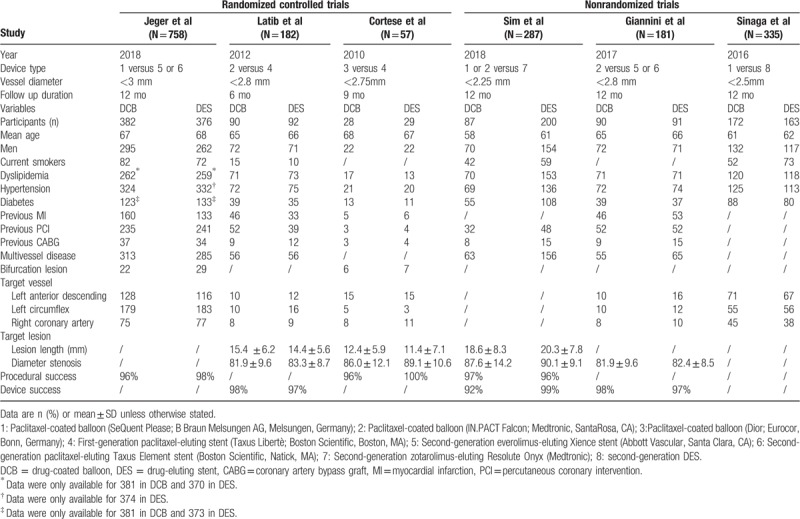
The baseline characteristics of the included studies are presented in Table 1. Three randomized controlled trials,[12–14] and 3 nonrandomized studies[15–17] containing 1800 patients with de novo small coronary artery vessel disease were eligible for inclusion in the meta-analysis; among them, 849 (47%) patients underwent the drug-coated balloon and 951 (53%) received the drug-eluting stent. Patients in the drug-coated balloon strategy group received SeQuent Please, IN.PACT Falcon, or Dior paclitaxel-coated balloon, while those in the drug-eluting stent strategy group received the first or second-generation stents from various manufacturers. The mean follow-up period of the different studies was from 6 to 12 months. Most participants were males, which accounted for 76%. The mean age of patients receiving the drug-coated balloon was from 58 to 68 years, while that of patients adopting the drug-eluting stent was from 61 to 68 years. Risk factors for cardiovascular disease including hypertension, diabetes, dyslipidemia, and smoking were revealed in most of the studies. Data for hypertension, diabetes, and dyslipidemia were reported completely in 6 studies. The proportion of patients with hypertension, diabetes, and dyslipidemia was 80%, 42%, and 72%, respectively.
Table 1.
Characteristics of included studies.
3.3. Quality assessment and risk of bias
The quality of most randomized controlled trials was higher according to the Cochrane quality assessment criteria (Supplementary Fig. 1). Three randomized controlled trials revealed a high risk of bias at blinding of participants and personnel. Two trials reported by Jeger et al and Cortese et al showed high risk of bias at blinding of outcome assessment and allocation concealment, respectively. The quality of the nonrandomized studies was also assessed (Supplementary Table 1). All nonrandomized studies met at least 17 variables from the STROBE checklist.[18]
3.4. Clinical outcomes
Six studies provided data on nonfatal myocardial infarction. The results indicated that the drug-coated balloon strategy was associated with a significant reduction in nonfatal myocardial infarction (OR 0.53, 95% CI 0.31–0.90, P = .02) compared with the drug-eluting stent strategy. However, there was no significant difference in cardiac death (OR 1.56, 95% CI 0.73–3.33, P = .25), all-cause death (OR 0.56, 95% CI 0.25–1.23, P = .15), target lesion revascularization (OR 1.24, 95% CI 0.73–2.1, P = .43), and target-vessel revascularization (OR 0.95, 95% CI 0.59–1.52, P = .84) between 2 strategies (Fig. 2). Statistical heterogeneity was not evident in the clinical outcomes.
Figure 2.
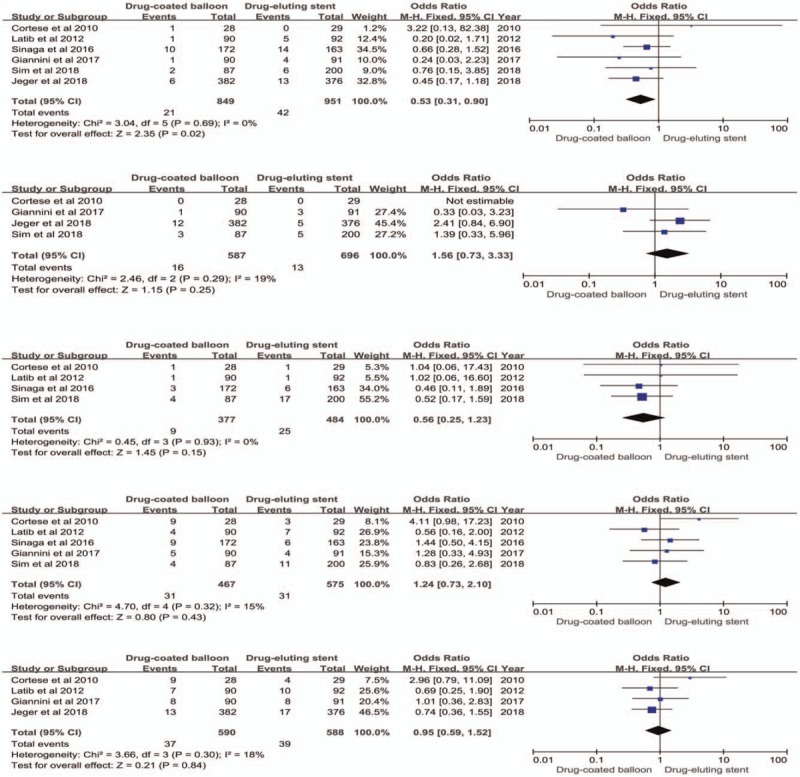
Forest plots comparing drug-coated balloon with drug-eluting stent in nonfatal myocardial infarction, cardiac death, all-cause death, target lesion revascularization, and target-vessel revascularization.
3.5. Subgroup and sensitivity analyses
The subgroup analyses showed differential effects caused by the study design (randomized controlled trials vs nonrandomized controlled studies) and device type (first vs second-generation drug-eluting stent). There was no significant difference in the rate of cardiac death, all-cause death, target lesion revascularization, and target-vessel revascularization between the strategies (Supplementary Figs. 2 and 3). However, when both strategies were regrouped according to discrepancies in study design, the result demonstrated no difference in the prognostic indicator of nonfatal myocardial infarction in the randomized controlled trial (Supplementary Fig. 2). In addition, regrouping upon device type, the drug-coated balloon was associated with a significant reduction in non-fatal myocardial infarction (OR 0.54, 95% CI 0.31–0.95, P = .03) compared with the second-generation drug-eluting stent, while no significant difference was found between the drug-coated balloon and the first-generation drug-eluting stent (OR 0.46, 95% CI 0.10–2.07, P = .31) (Supplementary Fig. 3).
There was moderate statistical heterogeneity in the randomized controlled group in term of target lesion revascularization (I2 = 76%) (Supplementary Fig. 2), while moderate statistical heterogeneity was also yielded in the first-generation drug-eluting stent group in terms of nonfatal myocardial infarction (I2 = 50%), target lesion revascularization (I2 = 76%), and target-vessel revascularization (I2 = 66%) (Supplementary Fig. 3). The sensitivity analysis revealed the PICCOLETO study was the main source of heterogeneity (Supplementary Fig. 4).
4. Discussion
This is the first meta-analysis carried out to directly compare the efficacy of drug-coated balloon and drug-eluting stent strategies in patients with de novo small coronary artery vessel disease. The principal finding is that the drug-coated balloon strategy was associated with a significant reduction in the clinical outcomes of nonfatal myocardial infarction, while there were no statistical differences in the remaining clinical outcomes.
Vessel diameter is a directly predictive parameter of restenosis.[19] Percutaneous coronary intervention for patients with small coronary artery vessel has intrinsic drawbacks such as acute vessel closure due to vessel recoil after plain old balloon angioplasty and neointimal proliferation after stenting.[16] Although newer-generation drug-eluting stents are used for interventional therapy, restenosis remains unavoidable.[19] The optimal selection based on the current guidelines and consensus are un-available in patients with de novo small coronary artery vessel disease.
The drug-coated balloon as a novel treatment approach seems to demonstrate promising results. However, it has similar disadvantages to plain old balloon angioplasty following elastic recoil or dissections, and bailout stenting is sometimes required.[20] Furthermore, due to the effects of a shorted balloon inflation time and scour of blood flow, it is questioned whether a sufficient amount of drug is delivered to the vessel wall and whether its ability to sustain drug concentration is equivalent to drug-eluting stent.[21]
A number of studies have demonstrated the benefit of drug-coated balloon in patients with small coronary artery vessel disease.[22–24] However, the consequence is less clearly defined due to the lack of comparison or control groups. Previous meta-analyses showed that the drug-coated balloon after the bare-metal stent was associated with a higher rate of target lesion revascularization than the drug-eluting stent, and no statistically significant difference was found in nonfatal myocardial infarction between the 2 groups.[25] In the present meta-analysis, the drug-coated balloon revealed an overt advantage over drug-eluting stent in nonfatal myocardial infarction, and was comparable to the drug-eluting stent in target lesion revascularization. In contrast to previous meta-analyses, small-caliber vessels were precisely defined in our study, which was a powerful predictor of restenosis with a 0.50 mm reduction in reference vessel diameter and a 60% increased risk of restenosis.[26] The differences between the drug-eluting balloon and drug-eluting stent strategies were directly compared to reduce heterogeneity. In the latest network meta-analysis, the available evidence was summarized to discuss the safety and efficacy of different interventions for patients with small coronary vessel disease.[27] The investigation suggested that the sirolimus-eluting stent translates a significant decrease in risk of target lesion revascularization compared with other treatments (paclitaxel-eluting stent, drug-coated balloon, bare-metal stent, and balloon angioplasty) as the most effective treatment for small-vessel disease, which was inconsistent with our research finding. First, previous studies reported that the sirolimus-eluting stent appeared to be superior to the paclitaxel-eluting stent in reducing the risk of target lesion revascularization.[28,29] In addition, this discrepancy in term of target lesion revascularization is related to sampling-size and various device types. In the network meta-analysis, only 2 of the 19 randomized trials compared differences between the drug-coated balloon and the drug-eluting stent strategies, and 2 different paclitaxel coating technologies (urea and shellac) were used in the drug-coated balloon strategy. Moreover, the indirect evidence originates from open network without closed loops, which has never been directly compared between 2 intervention groups.
The reduced risk of nonfatal myocardial infarction in the drug-coated balloon strategy was exposed in our analysis. A possible explanation for the finding is that the benefit of drug-eluting stent is counterbalanced by the absence of the metal framework in the drug-coated balloon, which reduces late inflammation, permits positive vessel remodeling, and shortens the dual antiplatelet therapy duration.[30] Furthermore, provisional stenting as one of bailout strategies may hold important values in suboptimal angiography results, significant dissection, or acute elastic recoil after drug-coated balloon, which reduces unnecessary stents implantation and is more easily accepted by patients with small vessel disease.[31]
There were insignificant disparities in respect of cardiac death, all-cause death, and target-vessel revascularization between 2 strategies in our analysis. Similar to this analysis, the earlier study reported by Luca et al[32] also discovered that routine stenting did not improve the above outcomes in patients with de novo small coronary artery vessel disease compared with balloon angioplasty. There are no absolute advantages of stent implantation in patients with small coronary artery vessel disease, possibly due to vessel diameter, a vital parameter. Small vessels are usually subjected to a high thrombotic burden, which may predispose an individual to acute or subacute thrombosis and worse outcomes after stents implantation.[19,32] In addition, some studies reported higher target-vessel revascularization rates with the drug-eluting balloon versus the drug-eluting stent in patients with small coronary artery vessel disease during the 6-months follow-up period, which was inconsistent with our study finding.[16,33,34] This phenomenon of higher target-vessel revascularization rates in the drug-eluting balloon can be explained by the “catch-up” phenomenon. The gradually increasing revascularization rates of the drug-eluting stent will not appear until later (6-months to 1-year).[16,33–35]
Interestingly, there was no significant difference in the outcome indicator of nonfatal myocardial infarction between strategies in the randomized control study, which was contradictory with the meta-analysis finding. However, the subgroup analyses showed that significant trend favored the drug-coated balloon over the second-generation drug-eluting stent in term of nonfatal myocardial infarction, while no significant difference was demonstrated between the drug-coated balloon and the first-generation drug-eluting stent strategies in the same prognostic indicator. This difference in the outcome indicator may be explained by the heterogeneity between studies and the small sample sizes included in them. After regrouping, moderate statistical heterogeneity was detected between the different studies. The sensitivity analysis revealed that the heterogeneity was caused by the PICCOLETO study.[14] One primary factor also derived from the small registered population. Only 57 patients were included and there was insufficient evidence to state the clinical conclusions.
It is worth mentioning that our clinical outcomes must be interpreted cautiously. Studies included in this analysis had different baseline demographics and comorbidities such as more male patients and a high proportion of people with hypertension and dyslipidemia (80% and 72%, respectively). Additionally, the success of interventional treatment for small coronary vessels mainly depended on the operator's discretion or expertise and patient characteristics, which should be considered.
5. Limitations
The limitations of the present study should be acknowledged. First, the studies included in the meta-analysis had varied in clinical and methodological features without standardized criteria that could be used to distribute patients into the drug-coated balloon or drug-eluting stent group. Second, this analysis was based on study-level data. No individual patient data allowed us to identify potential differences in the available strategies in a specific patient subgroup. Third, the use of different device types may be an important source of heterogeneity. Fourth, the follow-up periods of the included studies were short. Fifth, some important prognostic indicators such as stent thrombosis, major bleeding, and restenosis were not evaluated due to the limited number of studies included to assess these. Finally, use of the drug-coated balloon in other complex anatomic scenarios, such as native lesions of chronic total occlusion or diffuse lesions, was also not addressed.
6. Conclusions
This meta-analysis supports the use of drug-coated balloons for de novo small coronary lesions, which accompany a good outcome in non-fatal myocardial infarction. Further large randomized controlled trials are required to assess the benefits of the drug-coated balloon.
Author contributions
Investigation: Zhi-Lu Wang.
Supervision: Zhi-Lu Wang, Chen Guo, Yong-Hui Lv, Ming-Bo Zhang.
Writing – original draft: Min Li.
Writing – review and editing: Min Li.
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence interval, OR = odds ratio.
ML, CG, and Y-HL contributed equally to this work.
The study received no funding from the public, commercial, or non-profit sectors.
All authors declare no conflicts of interest regarding the submitted article.
Supplemental Digital Content is available for this article.
References
- [1].van der Heijden LC, Kok MM, Danse PW, et al. Small-vessel treatment with contemporary newer-generation drug-eluting coronary stents in all-comers: insights from 2-year DUTCH PEERS (TWENTE II) randomized trial. Am Heart J 2016;176:28–35. [DOI] [PubMed] [Google Scholar]
- [2].Alfonso F, Garcia-Guimaraes M. Optimal coronary interventions in small vessels: is size all that matters? JACC Cardiovasc Interv 2016;9:1335–7. [DOI] [PubMed] [Google Scholar]
- [3].Harada Y, Colleran R, Pinieck S, et al. Angiographic and clinical outcomes of patients treated with drug-coated balloon angioplasty for in-stent restenosis after coronary bifurcation stenting with a two-stent technique. EuroIntervention 2017;12:2132–9. [DOI] [PubMed] [Google Scholar]
- [4].Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention 2015;10:1024–94. [DOI] [PubMed] [Google Scholar]
- [5].Alfonso F, Scheller B. State of the art: balloon catheter technologies-drug-coated balloon. EuroIntervention 2017;13:680–95. [DOI] [PubMed] [Google Scholar]
- [6].Richelsen RK, Overvad TF, Jensen SE. Drug-eluting balloons in the treatment of coronary de novo lesions: a comprehensive review. Cardiol Ther 2016;5:133–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Iijima R, Kougame N, Hara H, et al. Clinical outcomes of drug-coated balloons in coronary artery disease unsuitable for drug-eluting stent implantation. Circ J 2018;82:2025–31. [DOI] [PubMed] [Google Scholar]
- [8].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [9].Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–67. [DOI] [PubMed] [Google Scholar]
- [10].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [12].Jeger RV, Farah A, Ohlow MA, et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet (London, England) 2018;392:849–56. [DOI] [PubMed] [Google Scholar]
- [13].Latib A, Colombo A, Castriota F, et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the BELLO (Balloon Elution and Late Loss Optimization) study. J Am Coll Cardiol 2012;60:2473–80. [DOI] [PubMed] [Google Scholar]
- [14].Cortese B, Micheli A, Picchi A, et al. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart 2010;96:1291–6. [DOI] [PubMed] [Google Scholar]
- [15].Sim HW, Ananthakrishna R, Chan SP, et al. Treatment of very small de novo coronary artery disease with 2.0 mm drug-coated balloons showed 1-year clinical outcome comparable with 2.0 mm drug-eluting stents. J Invasive Cardiol 2018;30:256–61. [PubMed] [Google Scholar]
- [16].Giannini F, Latib A, Ancona MB, et al. A propensity score matched comparative study between paclitaxel-coated balloon and everolimus-eluting stents for the treatment of small coronary vessels. Catheter Cardiovasc Interv 2017;90:380–6. [DOI] [PubMed] [Google Scholar]
- [17].Sinaga DA, Ho HH, Watson TJ, et al. Drug-coated balloons: a safe and effective alternative to drug-eluting stents in small vessel coronary artery disease. J Interv Cardiol 2016;29:454–60. [DOI] [PubMed] [Google Scholar]
- [18].von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. [DOI] [PubMed] [Google Scholar]
- [19].Claessen BE, Smits PC, Kereiakes DJ, et al. Impact of lesion length and vessel size on clinical outcomes after percutaneous coronary intervention with everolimus- versus paclitaxel-eluting stents pooled analysis from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice) Randomized Trials. JACC Cardiovasc Interv 2011;4:1209–15. [DOI] [PubMed] [Google Scholar]
- [20].Li J, Tzafriri R, Patel SM, et al. Mechanisms underlying drug delivery to peripheral arteries. Interv Cardiol Clin 2017;6:197–216. [DOI] [PubMed] [Google Scholar]
- [21].Schorn I, Malinoff H, Anderson S, et al. The Lutonix(R) drug-coated balloon: a novel drug delivery technology for the treatment of vascular disease. Adv Drug Deliv Rev 2017;112:78–87. [DOI] [PubMed] [Google Scholar]
- [22].Vaquerizo B, Miranda-Guardiola F, Fernandez E, et al. Treatment of small vessel disease with the paclitaxel drug-eluting balloon: 6-month angiographic and 1-year clinical outcomes of the Spanish multicenter registry. J Interv Cardiol 2015;28:430–8. [DOI] [PubMed] [Google Scholar]
- [23].Unverdorben M, Kleber FX, Heuer H, et al. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter in the PEPCAD I study: are lesions clinically stable from 12 to 36 months? EuroIntervention 2013;9:620–8. [DOI] [PubMed] [Google Scholar]
- [24].Abellas-Sequeiros RA, Benezet J, Agarrado Luna A, et al. Percutaneous coronary intervention for treating de-novo lesions in small coronary vessels: initial experience with the Essential paclitaxel-coated balloon. Coron Artery Dis 2018;29:477–81. [DOI] [PubMed] [Google Scholar]
- [25].Cui K, Lyu S, Song X, et al. Drug-eluting balloon versus bare-mental stent and drug-eluting stent for de novo coronary artery disease: a systematic review and meta-analysis of 14 randomized controlled trials. PLoS One 2017;12:e0176365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cassese S, Byrne RA, Tada T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 2014;100:153–9. [DOI] [PubMed] [Google Scholar]
- [27].Siontis GC, Piccolo R, Praz F, et al. Percutaneous coronary interventions for the treatment of stenoses in small coronary arteries: a network meta-analysis. JACC Cardiovasc Interv 2016;9:1324–34. [DOI] [PubMed] [Google Scholar]
- [28].Kong J, Liu P, Fan X, et al. Long-term outcomes of paclitaxel-eluting versus sirolimus-eluting stent for percutaneous coronary intervention: a meta-analysis. J Coll Physicians Surg–Pak 2017;27:432–9. [PubMed] [Google Scholar]
- [29].Zhang X, Xie J, Li G, et al. Head-to-head comparison of sirolimus-eluting stents versus paclitaxel-eluting stents in patients undergoing percutaneous coronary intervention: a meta-analysis of 76 studies. PLoS One 2014;9:e97934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Picard F, Doucet S, Asgar AW. Contemporary use of drug-coated balloons in coronary artery disease: where are we now? Arch Cardiovasc Dis 2017;110:259–72. [DOI] [PubMed] [Google Scholar]
- [31].Moreno R, Fernandez C, Alfonso F, et al. Coronary stenting versus balloon angioplasty in small vessels: a meta-analysis from 11 randomized studies. J Am Coll Cardiol 2004;43:1964–72. [DOI] [PubMed] [Google Scholar]
- [32].De Luca G, Suryapranata H, van’t Hof AW, et al. Comparison between stenting and balloon angioplasty in patients undergoing primary angioplasty of small coronary vessels. Am Heart J 2006;152:915–20. [DOI] [PubMed] [Google Scholar]
- [33].Mehilli J, Dibra A, Kastrati A, et al. Randomized trial of paclitaxel- and sirolimus-eluting stents in small coronary vessels. Eur Heart J 2006;27:260–6. [DOI] [PubMed] [Google Scholar]
- [34].Godino C, Furuichi S, Latib A, et al. Clinical and angiographic follow-up of small vessel lesions treated with paclitaxel-eluting stents (from the TRUE Registry). Am J Cardiol 2008;102:1002–8. [DOI] [PubMed] [Google Scholar]
- [35].Hermiller JB, Fergus T, Pierson W, et al. Clinical and angiographic comparison of everolimus-eluting and paclitaxel-eluting stents in small coronary arteries: a post hoc analysis of the SPIRIT III randomized trial. Am Heart J 2009;158:1005–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


