Abstract
The objective of this study was to examine whether Magnetic resonance imaging (MRI) features of knee osteoarthritis (OA) had an association with the level of serum uric acid (SUA). The MRI of the OA patients from June 2015 to July 2017 were studied. The patients fulfilled the following inclusion criteria: 1) meet American College of Rheumatology (ACR) radiological and clinical criteria for OA of the knee, 2) age ≤ 65years old, 3) Body mass index (BMI) < 25 kg/m2. Patients with OA were categorized into two groups based on the level of SUA. Patients with SUA level lower than 360 umol/L were recruited into the first group and the others were the second group. Odds ratios (OR) and 95% confidence intervals (CI) for SUA level and different MRI patterns were estimated with multivariable logistic regression.71 patients were included in this research. The mean age of the first group was 54.5 ± 8.4 and the second group was 55.6 ± 6.4. The Body Mass Index (BMI) of two groups was 22.7 ± 1.3 and 23.23 ± 1.9 separately. The mean SUA and creatinine (CR) level of the second group were 433.8 ± 70.6 umol/L and 80.1 ± 23.9 umol/L. There were statistically more focal erosions, osteophytes, bone marrow lesions and synovitis in the MRIs of the second group. A positive association between SUA level and synovitis as well as soft tissue swelling in MRIs was observed in patients with knee OA (OR = 1.017; 1.008, 95% CI: 1.007–1.028; 1.000–1.016). In conclusion, subjects with higher SUA level were more likely to have MRI abnormalities. OA patients need to lower their SUA level in order to keep the disease from progressing.
Keywords: MRI, osteoarthritis, serum uric acid, synovitis
1. Introduction
Osteoarthritis (OA) which defined as a degenerative joint disease affects almost all joint structures. It causes pain, disability, and economic burden to more than 33% of persons aged 60 years and older.[1] As 1 of the 5 leading causes of disability among noninstitutionalized adults, knee OA is usually a slowly progressing disease, whereas some patients show more rapid progression leading to severe joint damage.[2] Although OA is initiated by mechanical stress on cartilage, its progression depends on cellular and biochemical factors, including both localized factors and systemic factors.[3] The diagnosis of OA is currently based on radiographic criteria and clinical symptoms.[4] In the past decade, crucial progress has been made in the development of the radiographic technology, especially magnetic resonance imaging (MRI). MRI is a noninvasive technology that can generate images of the structural changes that occur in the joint tissues, including the progressive degradation of cartilage, menisci and ligaments, synovial inflammation and changes to the subchondral bone cartilage.[5]
Elevated serum uric acid (SUA) which is thought to be the product of human purine metabolism mirroring supersaturation of the extracellular fluid with urate is regarded as a marker of impaired metabolism nowadays.[6] A large body of evidence has accumulated suggests that hyperuricemia may play a role in the development and pathogenesis of metabolic syndromes. In recent years there has been a renewed interest in hyperuricemia and its association with a number of clinical disorders other than gout, including hypertension, atherosclerosis, cardiovascular disease, and chronic kidney disease.[7] However, its relationship with OA is still unclear. Some studies inferred that OA is not simply a disease related to aging or mechanical stress of joints but rather a metabolic disorder in which various interrelated humoral mediators contribute to the initiation and progression of the disease process.[8,9] Knee OA and metabolic syndrome share age and obesity as risk factors. Many investigators had studied the association of OA and other components of metabolic syndrome including hypertension, dyslipidemia, and impaired glucose tolerance.[10,11] However, to our knowledge, hardly had any studies put their focus on investigating the relationship between SUA and knee OA. In the meantime, for the purpose of optimizing the management of OA, it is important to discover the possible predictors of progression of OA. The certain prognostic factors can be used to identify high-risk groups, which may have implications for patient information and management.
Therefore, we hypothesized that there was a latent association of the elevated SUA levels and the MRI features of knee OA. The objective of this study was to investigate the relationship between them in order to provide a possible predictor of progression of OA.
2. Materials and methods
2.1. Patients selection
To address the proposed hypothesis, potential subjects who were in accordance with the following criteria were enrolled:
-
1)
meet American College of Rheumatology (ACR) radiological and clinical criteria for OA of the knee;
-
2)
age< = 65years old;
-
3)
Body mass index (BMI) <25kg/m2.[4]
Exclusion criteria:
-
1)
attacked by gout previously;
-
2)
had surgery of the detected knee joint previously;
-
3)
had other rheumatoid conditions;
-
4)
had trauma of the detected joint previously.
Following these considerations, consecutive patients between June 2015 and July 2017 were recruited in Hainan general hospital. Subjects were informed of and provided written and verbal consent to the experimental protocol and procedures. This study was approved by the Ethics Committee of Hainan general hospital (Ethical approval No.:Med-Eth[2018]78).
2.2. Clinical data and laboratory measures
For each patient, we collected demographic data covering age, gender, height, and weight. As to the laboratory measures, blood samples were obtained from an antecubital vein in the morning after a requested 12 hours overnight fast. SUA and creatinine (CR) were recorded.
2.3. MRI data
One radiologist who was not aware of the clinical history and laboratory results read the MRI results. Scans were made using a 3.0 T MRI Scanner (GE Signa Horizon Echospeed, LX9.0, General Electric Medical Systems, Milwaukee). The MRI sequences were chosen in close collaboration with an MRI technician and musculoskeletal radiologist. Coronal, sagittal and axial T1-weighted (T1w) fat-suppressed (fs) pre/postintravenous gadolinium (Gd) (0.1 mmol Gd/kg body weight; Magnevist, Bayer Schering Pharma AG, Leverkusen, Germany) images were acquired from a 3-dimensional dual-echo technique. Patients were in supine position with the knee joint extension placed in a dedicated extremity coil centrally in the magnetic field. Inflammatory and structural changes were graded as present or absent (score 0 or 1) and a lesion by lesion comparison was made. One reader scored all the MRI results including the presence of synovitis, focal erosions, joint effusion, osteophytes, bone marrow lesions, soft tissue (including periarticular muscle, ligament, tendon) swelling, collateral ligament discontinuity, and meniscus injury.
2.4. Statistical analysis
Results for quantitative clinical and demographic variables were reported as the mean ± standard deviation. MRI diagnosed results were considered as dichotomous data depend on absent/present. The comparison of the quantitative values was performed with Student t test for paired samples and the McNemar test for qualitative values. The association between the SUA and the patterns of MRIs was analyzed by multivariable logistic regression. P <.05 was considered significant. Tests were performed using statistical software SPSS 20.0.
3. Results
3.1. Demographic characteristics of patients
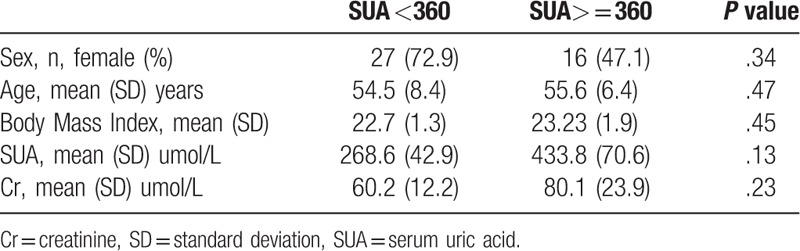
In order to make the results more intuitional, we artificially divided the patients into 2 groups based on the level of SUA. Patients with the SUA level lower than 360umol/L were recruited into the first group. Those with higher than or equal to 360umol/L were included in the second group.71 patients (28 male: 43female) fulfilled the inclusion criteria. The demographic data were summarized in Table 1. The mean age, BMI of the 2 groups were not significantly different. The SUA level of the first group was 268.6 ± 42.9umol/L, and the second group was 433.8 ± 70.6umol/L. The CR level of the second group was higher, however, without dramatically different. The normal laboratory values of the SUA and CR levels in our institution are 155 to 357umol/L and 41 to 73umol/L separately.
Table 1.
Demographics and clinical characteristics of patients.
3.2. MRI findings of the 2 groups
The different MRI changes in 2 groups were demonstrated in Fig. 1. The 8 most common patterns were listed in it. As shown in Fig. 1, we included the focal erosions, osteophytes, bone marrow lesions, joint effusion, synovitis, meniscus injury, collateral ligament discontinuity, soft tissue swelling into our analysis. The percentage of patients had the different lesions of the first group were 40.5%, 29.7%, 21.6%, 71.8%, 10.8%, 75.7%, 45.9%, and 24.3% separately. As to the second group, the number changed to 79.4%, 58.8%, 47.1%, 82.4%, 67.6%, 50%, 32.3%, and 44.1%. There were statistically more focal erosions, osteophytes, bone marrow lesions, and synovitis in the MRIs of the second group.
Figure 1.
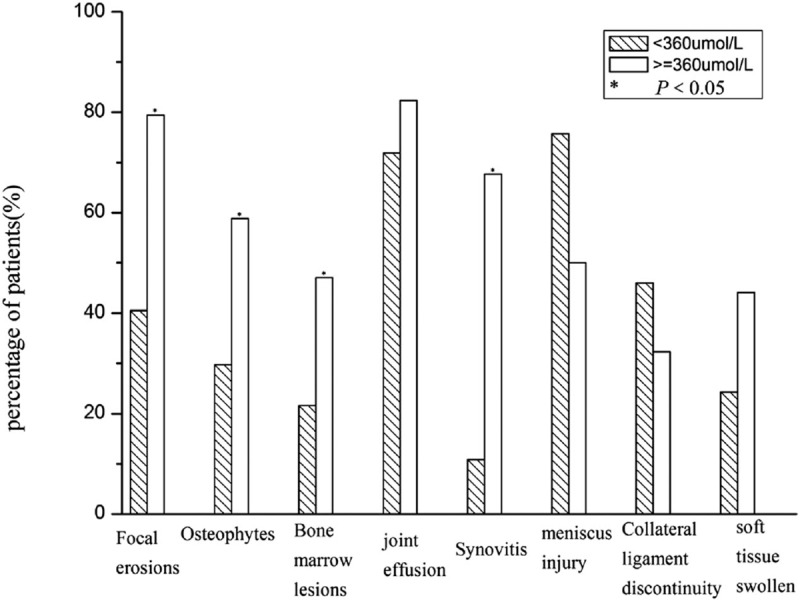
MRI findings of patients with different SUA level. MRI = Magnetic resonance imaging, SUA = serum uric acid.
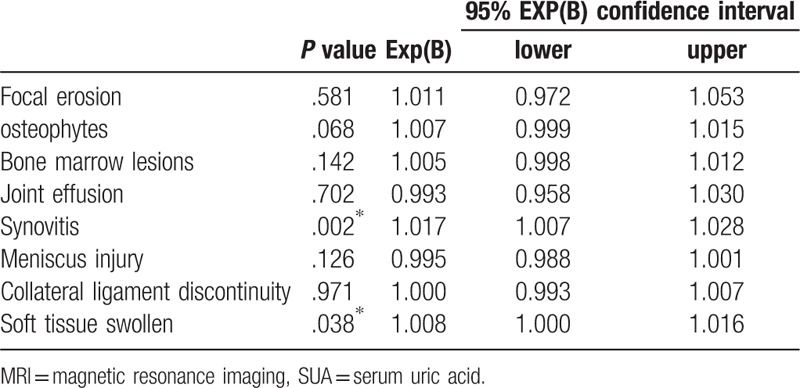
3.3. The level of SUA and patterns of MRI changes
In order to certify the relationship between the level of SUA and the MRI changes, we use multivariable logistic regression. The results were shown in Table 2. In the analysis process, statistically significant associations were found between the level of SUA and 2 MRI features including synovitis (odds ratio [OR] = 1.017, 95% confidence interval [CI] 1.007–1.028; P = .02) and soft tissue swelling (OR = 1.008, 95% CI 1.000–1.016; P = .038).
Table 2.
The association between MRI for the detection of various knee lesions and the SUA level, including odds ratios and 95% confidence intervals.
4. Discussion
OA is thought to be the most prevalent chronic joint disease in the world.[1] The incidence of OA is rising because of the aging population and the epidemic of obesity.[2] Therefore, it is profound to demonstrate the possible predictors of OA, which may define the implications for patient information in order to make the suitable treatment plan. The aim of this study was to assess the association between SUA level and MRI-based assessments of knee OA. Our study suggested a positive association between SUA level and 2 MRI features including synovitis and soft tissue swelling. Our finding was consistent with the recent result achieved by Oshinsky et al discovering that patients with hyperuricemia were more likely to experience a more rapid rate of OA progression, and to have a higher rate of synovitis as seen on MRI.[12]
Knee OA and metabolic syndrome share many risk factors together.[13] Meanwhile, hyperuricemia is regarded as a new marker for metabolic syndrome.[14] However, hardly had any studies put their focus on investigating the potential relationship between changes in the MRI findings of knee OA and the SUA level until now. We enrolled patients in accordance with the following criteria:
-
1)
meet ACR radiological and clinical criteria for OA of the knee;
-
2)
age< = 65years old;
-
3)
BMI<25kg/m2.[4]
As prior studies implied that unit increase in BMI was related to the increased odds of OA meeting ACR criteria clinically/radiographically estimated with logistic regression models independent of other serum indexes.[15–17] These data indicated that the impact of BMI may obscure the impact of gout on OA among obese populations. Therefore, we only included non-overweight subjects in this study. We divided the patients into 2 groups as we had mentioned above. The most common radiographic change of the first group was meniscus injury. 75.7% patients of the group had the alteration. As to the second group, joint effusion was the most familiar. There were statistically more focal erosions, osteophytes, bone marrow lesions and synovitis in the images of the second group. A potential mechanism underlying the observed result may be angiogenesis of subchondral bone caused by uric acid. As we all know, OA was no longer deemed to be a non-inflammatory disease driven by articular cartilage “wear and tear”. Recent evidence has led to a new view that OA pathophysiology should be perceived in the context of the entire joint with involvement of several tissues in which the synovium, meniscus and soft tissue also plays a pivotal role.[4,18,19] We detected that there were statistically positive associations between the level of SUA and 2 MRI features including synovitis and soft tissue swelling. Synovitis was thought to be a significant predictor for all measures of radiographic progression including development of erosions.[20] As a result, patients with relatively higher SUA level may have a more rapid radiographic progression. In other word, OA patients may need to lower their SUA level to keep the disease from progressing. As to soft tissue swelling, according to the traditional view of it, cartilage matrix macromolecules are released into the joint fluid during degradation, triggering a secondary inflammation reaction, in the meantime, causing pain to the patients.[18] Consequently, it resulted in acute inflammation process in the joint area. Anna E. and her colleagues substantiated that uric acid may be a factor promoting the pathological process of OA through activation of the inflammasome, which may explain why the higher SUA level was related to the MRI soft tissue swelling.[21] There had been several studies put their effort on discovering the association between uric acid and patterns of OA. One research concluded that there was a possible role of elevated SUA in the multifactorial etiology of generalized OA. Nonetheless, they observed negative relationship between SUA and OA of the knee joint.[22] However, they did not probe into the different changes in radiographic images. Meanwhile, they use X-ray as radiographic assessment tool which may make them ignore some early changes during disease course. In 2015, one report regarding the relationship of gout and OA gave the conclusion that the presence of asymptomatic hyperuricemia was associated with increased knee OA prevalence and severity.[17] This result was consistent with ours, at the same time, gave our conclusion a reasonable explanation.
In the interpretation of our results, the following limitations require careful discussion. First, our study only included 71 patients. The small sample size of this study may have increased the possibility of type II error. In the meantime, these participants do not represent the entire general population because they were recruited from only one area. Second, as our study was retrospective, it was difficult to know the exact pain degree of the outpatients at that moment. As a result, a more precise designed and larger-population prospective study is needed to further assess these potentially promising findings.
In conclusion, several important findings of this study might have implications in clinical practice or future research should be paying attention to. Our study determined that the SUA seemed to play a role in the radiographic progression of OA. Subjects with higher SUA were more likely to have MRI abnormalities. Thus, OA patients need to lower their SUA level compared with normal people in order to keep the disease from processing. Further studies could focus on the underlying factors that may play a role during the radiographic progression in OA patients with higher SUA level.
Acknowledgments
We are very thankful to all participants for their cooperation in this study. We thank all the staff for their dedication.
Author contributions
Data curation: Lu Xiao, Feng Zhan, Shudian Lin.
Formal analysis: Lu Xiao, Feng Zhan, Shudian Lin.
Investigation: Lu Xiao, Feng Zhan, Shudian Lin.
Methodology: Lu Xiao, Feng Zhan, Shudian Lin.
Supervision: Feng Zhan, Shudian Lin.
Writing – original draft: Lu Xiao.
Writing – review & editing: Lu Xiao.
Footnotes
Abbreviations: ACR = American College of Rheumatology, BMI = body mass index, CI = confidence intervals, CR = creatinine, Gd = gadolinium, MRI = magnetic resonance imaging, OA = osteoarthritis, OR = odds ratios, SUA = serum uric acid.
This work was funded by the Department of Rheumatology, Hainan General Hospital.
Our manuscript is a unique submission and is not being considered for publication by any other source in any medium. Further, the manuscript has not been published, in part or in full, in any form.
The authors have no conflicts of interest to disclose.
References
- [1].McDonough CM, Jette AM. The contribution of osteoarthritis to functional limitations and disability. Clin Geriatr Med 2010;26:387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science 1996;274:740–3. [DOI] [PubMed] [Google Scholar]
- [3].Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011;7:33–42. [DOI] [PubMed] [Google Scholar]
- [4].Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–26. [DOI] [PubMed] [Google Scholar]
- [5].Pelletier JP, Raynauld JP, Abram F, et al. A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthritis Cartilage 2008;16suppl 3:S8–13. [DOI] [PubMed] [Google Scholar]
- [6].Terkeltaub R. Gout. Novel therapies for treatment of gout and hyperuricemia. Arthritis Res Ther 2009;11:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin SD, Tsai DH, Hsu SR. Association between serum uric acid level and components of the metabolic syndrome. J Chin Med Assoc JCMA 2006;69:512–6. [DOI] [PubMed] [Google Scholar]
- [8].Velasquez MT, Katz JD. Osteoarthritis: another component of metabolic syndrome. Metab Syndr Relat Disord 2010;8:295–305. [DOI] [PubMed] [Google Scholar]
- [9].Zhuo Q, Yang W, Chen J, et al. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol 2012;8:729–37. [DOI] [PubMed] [Google Scholar]
- [10].Sturmer T, Brenner H, Brenner RE, et al. Non-insulin dependent diabetes mellitus (NIDDM) and patterns of osteoarthritis. The Ulm osteoarthritis study. Scand J Rheumatol 2001;30:169–71. [DOI] [PubMed] [Google Scholar]
- [11].Day C. Metabolic syndrome, or what you will: definitions and epidemiology. Diab Vasc Dis Res 2007;4:32–8. [DOI] [PubMed] [Google Scholar]
- [12].Krasnokutsky S, Oshinsky C, Attur M, et al. Serum urate levels predict joint space narrowing in non-gout patients with medial knee osteoarthritis. Arthritis Rheumatol 2017;69:1213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Magliano M. Obesity and arthritis. Menopause Int 2008;14:149–54. [DOI] [PubMed] [Google Scholar]
- [14].Billiet L, Doaty S, Katz JD, et al. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol 2014;2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Toivanen AT, Heliovaara M, Impivaara O, et al. Obesity, physically demanding work and traumatic knee injury are major risk factors for knee osteoarthritis--a population-based study with a follow-up of 22 years. Rheumatology (Oxford) 2010;49:308–14. [DOI] [PubMed] [Google Scholar]
- [16].Martin KR, Kuh D, Harris TB, et al. Body mass index, occupational activity, and leisure-time physical activity: an exploration of risk factors and modifiers for knee osteoarthritis in the 1946 British birth cohort. BMC Musculoskelet Disord 2013;14:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Howard RG, Samuels J, Gyftopoulos S, et al. Presence of gout is associated with increased prevalence and severity of knee osteoarthritis among older men: results of a pilot study. J Clin Rheumatol 2015;21:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 2013;21:16–21. [DOI] [PubMed] [Google Scholar]
- [19].Roemer FW, Crema MD, Trattnig S, et al. Advances in imaging of osteoarthritis and cartilage. Radiology 2011;260:332–54. [DOI] [PubMed] [Google Scholar]
- [20].Haugen IK, Slatkowsky-Christensen B, Boyesen P, et al. MRI findings predict radiographic progression and development of erosions in hand osteoarthritis. Ann Rheum Dis 2016;75:117–23. [DOI] [PubMed] [Google Scholar]
- [21].Denoble AE, Huffman KM, Stabler TV, et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Nat Acad Sci USA 2011;108:2088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sun Y, Brenner H, Sauerland S, et al. Serum uric acid and patterns of radiographic osteoarthritis--the Ulm Osteoarthritis Study. Scand J Rheumatol 2000;29:380–6. [DOI] [PubMed] [Google Scholar]


