Abstract
This study aimed to explore the diagnostic performance of the ratio of renal resistive index (RRI) to semiquantitative power Doppler ultrasound (PDU) score in predicting acute kidney injury (AKI) 3 in critically ill patients.
This study was a prospective, observational study that included 101 critically ill patients. RRI and semiquantitative PDU score were measured within 6 hours following admission to the intensive care unit (ICU). The ratio of RRI to PDU (RRI/PDU) was calculated as follows: RRI / PDU. If PDU score was 0, the RRI/PDU was 1. Meanwhile, AKI was defined according to the Kidney Disease Improving Global Outcomes criteria.
Median RRI/PDU was 0.234 (0.190, 0.335) in patients with AKI 0–2 and 0.636 (0.411, 0.738) in patients with AKI 3 (P < .001). As assessed by the area under the receiver operator characteristic curves (AUC), RRI/PDU performed best in diagnosing AKI 3 [AUC = 0.935 (95% CI: 0.868–0.974)]. Optimal cuto for RRI/PDU was > 0.37, and the sensitivity and specificity were 90.5% and 90.0%, respectively. In 93 patients, except for 8 patients with a PDU score of 0, the AUC of RRI/PDU [0.938 (95% CI: 0.868–0.977)] was superior to the PDU score (0.905 [95% CI: 0.826–0.956], P = .133), RRI [0.782 (95% CI: 0.684–0.861), P = .016], serum creatinine [0.801 (95% CI: 0.705–0.877), P = .017], or 6 hours AKI stage (0.876 [95% CI: 0.791–0.935], P = .110) in predicting AKI 3 on D5.
In our study, RRI, PDU score, RRI/PDU, and 6 hours AKI stage were useful in predicting AKI 3. Furthermore, RRI/PDU may be a better predictor of AKI 3.
Keywords: acute kidney injury, renal resistive index, semiquantitative power Doppler ultrasound score
1. Introduction
Acute kidney injury (AKI) is common in patients confined in intensive care units (ICU) and remains to be associated with poor outcomes.[1] According to the current diagnostic criteria, serum creatinine (SCr) and urine volume are used as diagnostic and staging criteria for AKI.[2] However, oliguria is not specific to renal dysfunction, and SCr elevation is usually delayed for several hours after the renal insult and occurs only when the glomerular filtration rate (GFR) is severely diminished.[3] Moreover, Zarbock et al[4] found that early renal replacement therapy (RRT) (RRT within 8 hours of diagnosis of Kidney Disease Improving Global Outcomes [KDIGO] stage 2) reduced mortality over the first 90 days compared with the delayed initiation of RRT (RRT within 12 hours of KDIGO stage 3 or no RRT). Additionally, patients with KDIGO stage 3 always need RRT. However, the diagnosis of KDIGO stage 3 would usually take 12 to 24 hours. Hence, if an indicator is available for predicting KDIGO stage 3 within 6 hours of admission, the patients may receive early RRT and obtain improved outcomes.
Many biomarkers such as serum cystatin C,[5] neutrophil gelatinase-associated lipocalin,[6] urinary kidney injury molecule [7] and so on are potentially useful to predict AKI in critical care. However, most of these biomarkers have not been widely used in clinical practice, and the detection is time-consuming and cannot be performed at any time. Doppler-based renal resistive index (RRI) calculation and semiquantitative power Doppler ultrasound (PDU) score provide rapid, noninvasive, and repeatable investigations, possibly granting early AKI detection in patients confined in the ICU,[8,9] but its diagnostic performance remains insufficiently evaluated. Acute tubular necrosis is the main mechanism of AKI in intensive care settings and persists even when the hemodynamic status has been restored. Three preliminary human studies have shown that RRI is useful in distinguishing the condition from prerenal azotemia.[10–12] Meanwhile, semiquantitative PDU assesses renal perfusion; prolonged renal hypoperfusion may lead to kidney injury. Thus, we combined these 2 indicators to RRI/PDU, which may have a better performance than RRI or PDU score in predicting KDIGO stage 3 in critically ill patients.
2. Materials and methods
2.1. Patients
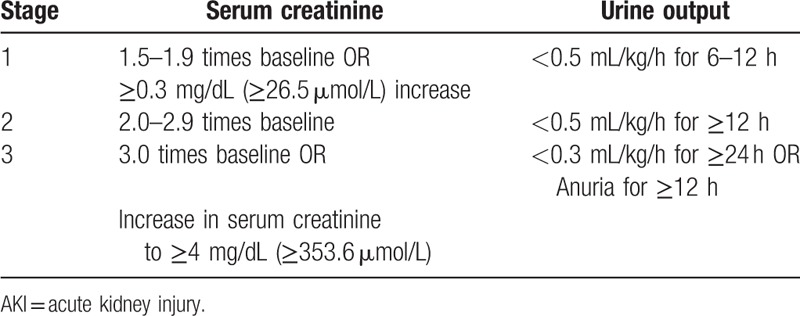
The study was approved by the ethics committee of the Cangzhou Central Hospital in Cangzhou City, Hebei Province, China (ethical approval number: 2017-078-01), and every patient or next of kin was informed that the collected data could be used for research purposes. We studied critically ill patients admitted in the Emergency ICU of Cangzhou Central Hospital from January 2018 to July 2018 but only those who met the following criteria: admission for sepsis (as defined by the sepsis-3 criteria[13]), polytrauma (as defined by an Injury Scaling Severity score ≥ 25[14]), cardiac failure (as defined by Killip classification grade IV in patients with acute myocardial infarction or by New York Heart Association functional class IV in patients with acute heart failure), and critical conditions due to other causes. Noninclusion criteria included the age younger than 18 years, survival time of less than 24 hours, pregnancy, intraperitoneal pressure of more than 15 mm Hg, suspected or confirmed obstructive renal failure, arrhythmia, and known renal artery stenosis. Additionally, we did not include patients recovering from previously diagnosed AKI at the time of inclusion and those with severe chronic renal failure with a basal creatinine clearance value of lower than 30 mL/min. AKI was defined according to KDIGO criteria (Table 1). Baseline creatinine was estimated by the Modification of Diet in Renal Disease equation, assuming a low normal value for baseline GFR (75 mL/min /1.73 m2).[15] The equation is as follows (Creatinine is in mg/dL).
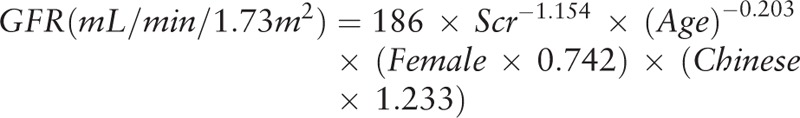 |
Table 1.
Staging of AKI.
2.2. Study protocol and data collection
In addition to the demographic data, height, weight, type of admission (sepsis, cardiac failure, polytrauma, or other causes), and accompanying diseases, the following data were collected within 6 hours from admission: SCr level, 6 hours urine output, arterial lactate concentration, use of mechanical ventilation, use of vasoactive drugs, and 6 hours KDIGO stage. APACHE II score and SOFA score were evaluated 24 hours after admission. Renal function was assessed on D5 according to the KDIGO criteria. The mortality and use of continuous renal replacement therapy (CRRT) were collected on day 28.
2.3. RRI and semiquantitative PDU score measurements
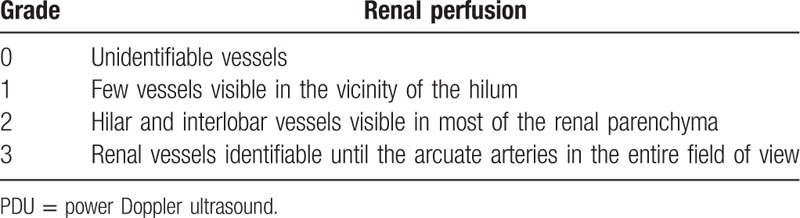
Renal echography was performed by an intensivist with sufficient experience in this technique within the first 6 hours of admission and after mean arterial pressure (MAP) ≥ 65 mm Hg. The operators were aware of the results of the other tests and other available clinical information. RRI was calculated from the right kidney in most patients. The ultrasound machines used were CX30 (Philips) and HD15 (Philips). Renal Doppler was performed on the interlobar arteries by using a convex array probe. The Doppler gain was set to obtain a clear outline of flow waves with minimal background noise. The Doppler spectrum was considered optimal when at least 3 similar consecutive waveforms were visualized. The RRI was calculated as follows: (peak systolic velocity − end diastolic velocity)/peak systolic velocity. The RRI value is independent of the angle between the ultrasound beam and blood flow. Three measurements were performed and averaged to obtain the mean RRI value. Renal perfusion was assessed by semiquantitative PDU score (Table 2).[16] The ratio of RRI to PDU (RRI/PDU) was calculated as follows: RRI/PDU. If the PDU score was 0, the RRI/PDU was 1. MAP, heart rate (HR), type and dose of catecholamine infusion, and oxygenation index were recorded during the renal ultrasound examination.
Table 2.
Semiquantitative PDU score for evaluating intrarenal perfusion.
2.4. Statistical analysis
Results were described as median and interquartile ranges, mean, and standard deviation, or numbers and percentages (%), as appropriate. Kolmogorov–Smirnov test was used to examine the normality of all numeric continuous variables. Nonparametric tests (Mann–Whitney U test) were used to examine the difference in variables without a normal distribution, whereas independent sample t tests were used if with a normal distribution. Categorical data including gender accompanying diseases, use of mechanical ventilation, use of vasoactive drugs, mortality, and use of CRRT were compared between AKI 3 group and AKI 0 to 2 groups by χ2 test. When there was only less than 5 observations in an group-outcome combination, the Fisher test was used.[17] Receiver operator characteristic (ROC) curves were plotted to examine the RRI, PDU score, RRI/PDU, and 6 hours AKI stage in predicting AKI 3. A binary logistic regression was performed to identify the independent predictors of AKI 3. First, univariable analysis was used to explore the unadjusted association between variables and outcome. Continuous variables were checked for their linearity in relation to the logit of the outcome by examining the smoothed scatter plot.[18] Correlations between RRI/PDU and some parameters were evaluated using Pearson correlation coefficient. Statistical tests were performed using SPSS 19. ROC curves were performed using MedCalc. Delong test was used to compare AUROCs between each predictor. All tests were 2-sided, and P values < .05 were considered statistically significant.
3. Results
3.1. General characteristics of patients
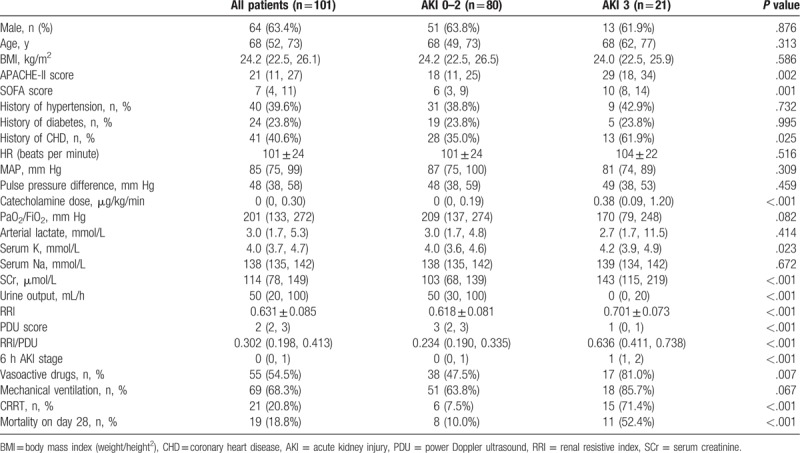
During the study period, 124 patients were included. Among these patients, 10 died within 24 hours, 5 abandoned treatment during hospitalization, 3 unsuitable for RRI due to arrhythmia or abdominal hypertension, and 5 patients progressed to AKI 3 stage within 6 hours from admission. Therefore, 101 patients (31 with sepsis, 40 with cardiac failure, 6 with polytrauma, and 24 with other causes) were included in the study. According to KDIGO stage assessed on D5, 48 patients (48/101, 47.5%) had no AKI. Of the 53 patients with AKI, 15 (15/101, 14.9%) had AKI 1, 17 (17/101, 16.8%) had AKI 2, and 21 (21/101, 20.8%) had AKI 3. The patients’ characteristics are shown in Table 3. APACHE II score, SOFA score, complicated coronary heart disease (CHD), serum K, SCr, RRI, PDU score, RRI/PDU, 6 hours AKI stage, urine output, use of vasoactive drugs, use of CRRT, mortality on day 28, were significantly different in the AKI 3 group compared with those in AKI 0 to 2 groups (P < .05).
Table 3.
Main patient characteristics according to KDIGO stage assessed on D5.
3.2. Comparison of predictive value for AKI 3
ROC curves were plotted to examine the values of SCr, RRI, PDU score, RRI/PDU, and 6 hours AKI stage in predicting AKI 3. The ROC curves of these indicators are shown in Tables 4 and 5. The area under the ROC curves (AUC) of SCr, RRI, PDU score, RRI/PDU, and 6 hours AKI stage were 0.756, 0.782, 0.912, 0.935, and 0.850, respectively. As assessed by the AUC, RI/PDU performed best in diagnosing AKI 3 [AUC = 0.935 (95% CI: 0.868–0.974)]. Optimal cuto for RI/PDU was > 0.37, and the sensitivity and specificity were 90.5% and 90.0%, respectively.
Table 4.
The best cutoff value analysis for the prediction of AKI 3.
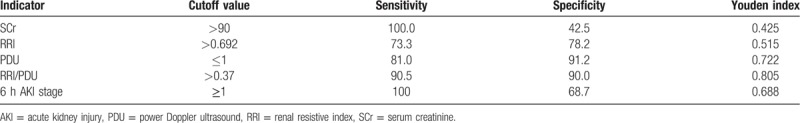
Table 5.
AUC for SCr, RRI, PDU, RI/PDU, and 6 h AKI stage as predictors of AKI 3 on D5.

We compared the AUC of SCr, RRI, PDU score, RRI/PDU, and 6 hours AKI stage in predicting AKI 3 in 93 patients, except for 8 patients with a PDU score of 0 (Fig. 1). The AUC of RRI/PDU [0.938 (95% CI: 0.868–0.977)] was superior to the PDU score (0.905 [95% CI: 0.826–0.956], P = .133), RRI [0.782 (95% CI: 0.684–0.861), P = .016], SCr [0.801 (95% CI: 0.705–0.877), P = .017], or 6 hours AKI stage (0.876 [95% CI: 0.791–0.935], P = .110) in predicting AKI 3 on D5.
Figure 1.
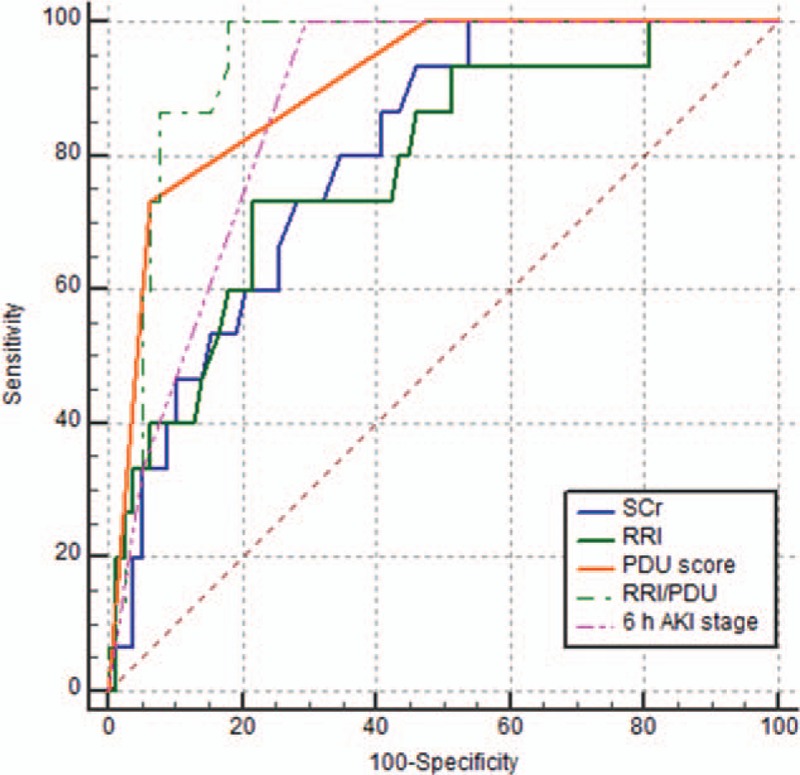
ROC curves for SCr, RRI, PDU score, RRI/PDU, and 6 hours AKI stage as predictors of AKI 3. AKI = acute kidney injury, PDU = power Doppler ultrasound, RRI = renal resistive index, ROC = receiver operator characteristic, SCr = serum creatinine.
3.3. Correlation analysis of RRI/PDU
We analyzed the correlations between RRI/PDU and age, HR, MAP, pulse pressure difference, oxygenation index, catecholamine dose, arterial lactate concentration, complicated CHD, hypertension, or diabetes. Positive correlations were found between RRI/PDU and age (r = 0.276, P = .005), between RRI/PDU and catecholamine dose (r = 0.420, P = <.001), and between RRI/PDU and complicated CHD (r = 0.283, P = .004). Conversely, a negative correlation was found between RRI/PDU and oxygenation index (r = −0.223, P = .025).
We also analyzed the correlations between RRI and age, HR, MAP, pulse pressure difference, oxygenation index, catecholamine drug dosage, arterial lactate concentration, complicated CHD, hypertension, or diabetes, and PDU score. Positive correlations were found between RRI and age (r = 0.374, P < .001), between RRI and catecholamine dose (r = 0.290, P = .005), between RRI and pulse pressure difference (r = 0.206, P = .047), and between RRI and complicated CHD (r = 0.217, P = .037). Conversely, a negative correlation was found between RRI and MAP (r = −0.301, P = .003), and between RRI and PDU score (r = −0.508, P < .001).
4. Discussion
RRI and semiquantitative PDU score are easy to perform, rapid, noninvasive, and repeatable. The normal range for the RRI is 0.50 to 0.70.[8] In a population of patients with septic shock, a high RRI is predictive of AKI.[8,9,19] Meanwhile, arterial RRI correlated not only with intrarenal arterial resistance but also with arterial compliance (i.e., renal interstitial and intra-abdominal pressures), age, and central hemodynamic parameters.[20,21] In our study, a positive correlation was found among RRI and age, catecholamine dose, pulse pressure difference, and complicated CHD, and a negative correlation was found between RRI and MAP.
Semiquantitative PDU assesses renal perfusion. PDU is easier to perform than Doppler-based RRI. Furthermore, power Doppler evaluation of renal perfusion using a semiquantitative scale has been advocated. However, bloated abdomen, intestinal distension, and difficulty in changing body position in patients confined in the ICU are common. These circumstances influence the operation and results of PDU score to some extent. Thus, we combined these 2 indicators to RRI/PDU and compared the diagnostic performance of RRI, PDU score, or RRI/PDU in predicting AKI 3 in critically ill patients.
In our study, RRI/PDU performed best in diagnosing AKI 3 as assessed by the AUC, and a statistically significant difference was observed between RRI/PDU and RRI, and between RRI/PDU and Scr. The AUC of RRI/PDU was superior to PDU score and 6 hours AKI stage, although the differences are not statistically significant.
In conclusion, in our population of critically ill patients, the RRI, PDU score, and RRI/PDU, which were measured within 6 hours from admission to the ICU, and 6 hours AKI stage, were useful in predicting AKI 3. RRI/PDU may be a better predictor of AKI stage 3.
However, this present study has some limitations that must be discussed. Chronic renal lesions may impair the renal vasculature and cause an elevated RRI. We tried to limit this effect by systematically excluding patients with chronic renal dysfunction. However, we cannot exclude the role of early asymptomatic chronic renal lesions in this elevation. Furthermore, hemodynamic conditions could influence the RRI and PDU score. We tried to limit this effect by measuring the RRI and PDU score after the interventions that aimed at restoring hemodynamic status. Moreover, some patients may not survive for day 5 which actually creates a problem called competing risk that mortality is a competing risk for AKI.[22] This was also a limitation of our study. Given these limitations, additional studies are needed in larger populations and in critically ill patients with different diseases, such as sepsis, polytrauma, and cardiac shock.
Author contributions
Data curation: Yong Li.
Formal analysis: Hai Jun Zhi.
Investigation: Hai Jun Zhi, Jing Zhao, Shen Nie, Yun Jie Ma, Xiao Ya Cui, Meng Zhang.
Writing – original draft: Hai Jun Zhi.
Writing – review & editing: Yong Li.
Footnotes
Abbreviations: AKI = acute kidney injury, ICU = intensive care unit, PDU = power Doppler ultrasound, RRI = renal resistive index, RRT = renal replacement therapy.
The authors have no conflicts of interest to disclose.
References
- [1].Shi HP, Xu DM, Wang GE. Prognostic indicators of patients with acute kidney injury in intensive care unit. World J Emerg Med 2010;1:209–11. [PMC free article] [PubMed] [Google Scholar]
- [2].Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006;10:R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yohannes S, Chawla LS. Evolving practices in the management of acute kidney injury in the ICU (Intensive Care Unit). Clin Nephrol 2009;71:602–7. [DOI] [PubMed] [Google Scholar]
- [4].Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016;315:2190–9. [DOI] [PubMed] [Google Scholar]
- [5].Zhang Z, Lu B, Sheng X, et al. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis 2011;58:356–65. [DOI] [PubMed] [Google Scholar]
- [6].Albeladi FI, Algethamy HM. Urinary neutrophil gelatinase-associated lipocalin as a predictor of acute kidney injury, severe kidney injury, and the need for renal replacement therapy in the intensive care unit. Nephron Extra 2017;7:62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xie Y, Wang Q, Wang C, et al. High urinary excretion of kidney injury molecule-1 predicts adverse outcomes in acute kidney injury: a case control study. Crit Care 2016;20:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Le Dorze M, Bougle A, Deruddre S, et al. Renal Doppler ultrasound: a new tool to assess renal perfusion in critical illness. Shock 2012;37:360–5. [DOI] [PubMed] [Google Scholar]
- [9].Schnell D, Darmon M. Bedside Doppler ultrasound for the assessment of renal perfusion in the ICU: advantages and limitations of the available techniques. Crit Ultrasound J 2015;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Platt JF, Rubin JM, Ellis JH. Acute renal failure: possible role of duplex Doppler US in distinction between acute prerenal failure and acute tubular necrosis. Radiology 1991;179:419–23. [DOI] [PubMed] [Google Scholar]
- [11].Izumi M, Sugiura T, Nakamura H, et al. Differential diagnosis of prerenal azotemia from acute tubular necrosis and prediction of recovery by Doppler ultrasound. Am J Kidney Dis 2000;35:713–9. [DOI] [PubMed] [Google Scholar]
- [12].Stevens PE, Gwyther SJ, Hanson ME, et al. Noninvasive monitoring of renal blood flow characteristics during acute renal failure in man. Intensive Care Med 1990;16:153–8. [DOI] [PubMed] [Google Scholar]
- [13].Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Baker SP, O’Neill B, Haddon W, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 1974;14:187–96. [PubMed] [Google Scholar]
- [15].Group Cepc. Modification and evaluation of MDRD estimating equation for Chinese patients with chronic kidney disease. Chin J Nephrol 2006;22:589–95. [Google Scholar]
- [16].Barozzi L, Valentino M, Santoro A, et al. Renal ultrasonography in critically ill patients. Criti Care Med 2007;35:S198–205. [DOI] [PubMed] [Google Scholar]
- [17].Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med 2016;4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med 2016;4:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Darmon M, Schortgen F, Vargas F, et al. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med 2011;37:68–76. [DOI] [PubMed] [Google Scholar]
- [20].Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology 1999;211:411–7. [DOI] [PubMed] [Google Scholar]
- [21].Murphy ME, Tublin ME. Understanding the Doppler RI: impact of renal arterial distensibility on the RI in a hydronephrotic ex vivo rabbit kidney model. J Ultrasound Med 2000;19:303–14. [PubMed] [Google Scholar]
- [22].Zhang Z. Survival analysis in the presence of competing risks. Ann Transl Med 2017;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]


