Abstract
Few prospective studies have reported the cumulative incidence of venous thromboembolism (VTE) in the intensive care unit (ICU), especially for patients receiving guideline-recommended VTE prophylaxis. We aimed to design a prospective observational study to investigate the cumulative incidence and risk factors of ICU-acquired VTE for those populations.
We prospectively studied 281 consecutively included patients in the ICU at a single center. All patients provided informed consent. Patients received ultrasound evaluation and were followed for VTE before ICU discharge or within 28 days of ICU stay. The type of VTE thromboprophylaxis was also recorded for all patients. Variables from univariate analyses that were associated with VTE were included in the binary logistic regression analysis to determine VTE predictors. The cumulative VTE incidence with 95% confidence interval (CI) was estimated using Kaplan–Meier methods.
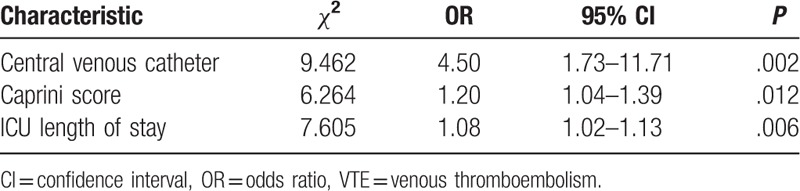
Patients had a median age of 60 years (range, 18–89) and an acute physiology and chronic health evaluation II score of 17 (range, 4–36). Despite all patients receiving guideline-recommended thromboprophylaxis, the cumulative incidence of VTE at 7, 14, 21, and 28 days was 4.45% (95% CI 2.55–7.71), 7.14% (95% CI 4.61–10.97), 7.53% (95% CI 4.92–11.43), and 9.55% (95% CI 6.55–13.81), respectively. Central venous catheter use (P = .002, odds ratio [OR] = 4.50), Caprini score (P = .012, OR = 1.20), and ICU length of stay (P = .006, OR = 1.08) were independent risk factors related to the incidence of VTE for patients admitted to the ICU.
Our prospective observational study found that the 28-day cumulative incidence of VTE was relatively high for patients admitted to the ICU, despite the use of guideline-recommended thromboprophylaxis. Patients with femoral central venous catheter, prolonged ICU length of stay, or a high Caprini score may have an increased risk of developing VTE.
Keywords: caprini score, incidence, intensive care unit, prophylaxis, venous thromboembolism
1. Introduction
Venous thromboembolism (VTE), which includes upper and lower extremity deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious complication for patients in the intensive care unit (ICU).[1] When patients are admitted to the ICU, they are at high risk of VTE, especially with sedation and mechanical ventilation, compared with others not in the ICU.[2] Previous studies showed that effective prophylaxis may be associated with a low incidence of hospital-acquired VTEs.[3,4] Indeed, earlier evidence showed that patients receiving thromboprophylaxis have a lower incidence of VTE, ranging from 5% to 37.2%, as compared with those not receiving thromboprophylaxis in the ICU.[5–7] Currently, prevention of VTE has been included in hospital accreditation and quality of care indicators around the world. Recently, a multicentre observational cross-sectional study showed that 98.3% patients received prophylaxis in the ICU.[8] According to the guideline, both mechanical prophylaxis and pharmacological prophylaxis can be treatment measures for preventing VTEs.[1] As patients in the ICU have a high risk of major bleeding, the balance between VTE and hemorrhagic risk should be estimated daily when these individuals are given pharmacological prophylaxis.
The incidence of VTE varies widely in the literature, depending on whether the diagnosis is made by screening protocols or simply by evaluating symptomatic patients. Other causes include the time of the observational study, which is inconsistent in prospective or retrospective studies. To our knowledge, few studies have reported the cumulative incidence of VTE for ICU patients receiving guideline-recommended VTE prophylaxis. Hence, the purpose of this study was to assess the cumulative incidence and identify the risk factors of VTE within 28 days of ICU stay among patients receiving guideline-recommended VTE prophylaxis.
2. Methods
2.1. Patients
This prospective, single-center, observational study was performed between April 2018 and November 2018. Patients were aged ≥18 years admitted to the ICU of the First Affiliated Hospital of Chongqing Medical University.
Exclusion criteria were as follows:
-
(1)
VTE found at admission or within 1 day of admission in the ICU,
-
(2)
patients died within 48 hours after ICU admission, and
-
(3)
patients with missing or incomplete data during the study period.
All patients without risk of bleeding received low-molecular-weight heparin (LMWH; 4000 IU) anticoagulation therapy twice daily. Ethics approval was obtained before the survey, and written informed consent was obtained from included patients or their authorized family member.
2.2. Outcomes
The primary outcome was the cumulative incidence of VTE, including DVT and PE, during 28 days in the ICU. The second outcome was the risk factors associated with VTE. Demographic characteristics, Caprini score, and other risk factors for VTE were collected. Caprini score was a valid and convenient way to stratify VTE risk[9] and was used to evaluate each patient on the first day and every 3 days thereafter until VTE was found. The maximum Caprini score was selected for analysis. Information on sex, age, body mass index, maximum muscle strength, continuous renal replacement therapy (CRRT), platelet transfusion, ICU length of stay, acute physiology and chronic health evaluation (APACHE) II score, sedation, mechanical ventilation, central venous catheter (CVC; femoral vein), and abdominal hypertension was also collected. Finally, all-cause 28-day mortality was also recorded.
2.3. Evaluation of VTE
Duplex scan was used to examine patients for DVT with a broadband width linear array transducer L 4 to 12 MHz by B-mode ultrasound combined D and CDFI modes. The trained researchers assessed patients for the following signs and symptoms:
-
(1)
localized tenderness,
-
(2)
pitting edema, and/or
-
(3)
swelling in each lower extremity.
If patients had 1 or more of the above signs and symptoms, they were indicated to have signs or symptoms consistent with DVT. Duplex scan was immediately conducted on the first day of ICU admission for all patients in a standardized fashion.[10] In addition, duplex scan was performed on days 7, 14, 21, and 28 and at any time DVT was clinical suspected. PE was defined by computed tomography pulmonary angiography (CTPA). CTPA was not examined for all patients, but only for those with suspected PE who already had DVT or respiratory dysfunction. The VTE screening was terminated when patients were discharged from the ICU or the ICU stay was >28 days. According to the results, patients were divided into VTE and non-VTE groups.
In our previous studies, distal DVT was defined as thrombus involving only the popliteal vein or (and) calf vein. Proximal DVT was defined as the thrombus involving at least the femoral vein and above; meanwhile, the thrombus may also involve the popliteal vein or (and) calf vein.[11–13] VTE was defined as proximal DVT (both symptomatic and asymptomatic), symptomatic distal DVT, and PE.[14,15]
2.4. The definition about risk of bleeding
If the patient answered “yes” to 1 or more of the following 5 questions, the patient was deemed to have a risk of bleeding, and mechanical prophylaxis was administered only for those patients:
-
(1)
Is the patient experiencing any active bleeding?
-
(2)
Does the patient have (or has the patient had a history of) heparin-induced thrombocytopenia?
-
(3)
Is the patient's platelet count <100,000/mm3?
-
(4)
Is the patient taking oral anticoagulants, platelet inhibitors (eg, nonsteroidal anti-inflammatory drugs)?
-
(5)
Is the patient's creatinine clearance abnormal?[9]
2.5. Statistical analysis
The sample size was calculated according to Serrano's study.[16] To achieve a desired precision of 95% confidence interval (CI) width of 0.1 around the estimated VTE rate at 1 month, 140 patients were required assuming the incidence of VTE was 7% at 1 month. All data were analyzed using SPSS version 21.0 statistical package. Univariate analysis was performed using the chi-squared test or t test. Binary logistic regression was used in the multivariate analysis: backward selection based on likelihood ratio. Univariate and multivariate analyses were conducted to determine the risk factors of VTE. The Kaplan–Meier method was used to test the cumulative incidence of VTE. The odds ratio (OR) for VTE substratified by Caprini score was estimated using chi-squared test. All P-values were 2-sided, with values <.05 were considered statistically significant.
3. Resuts
3.1. The general information of eligible patients
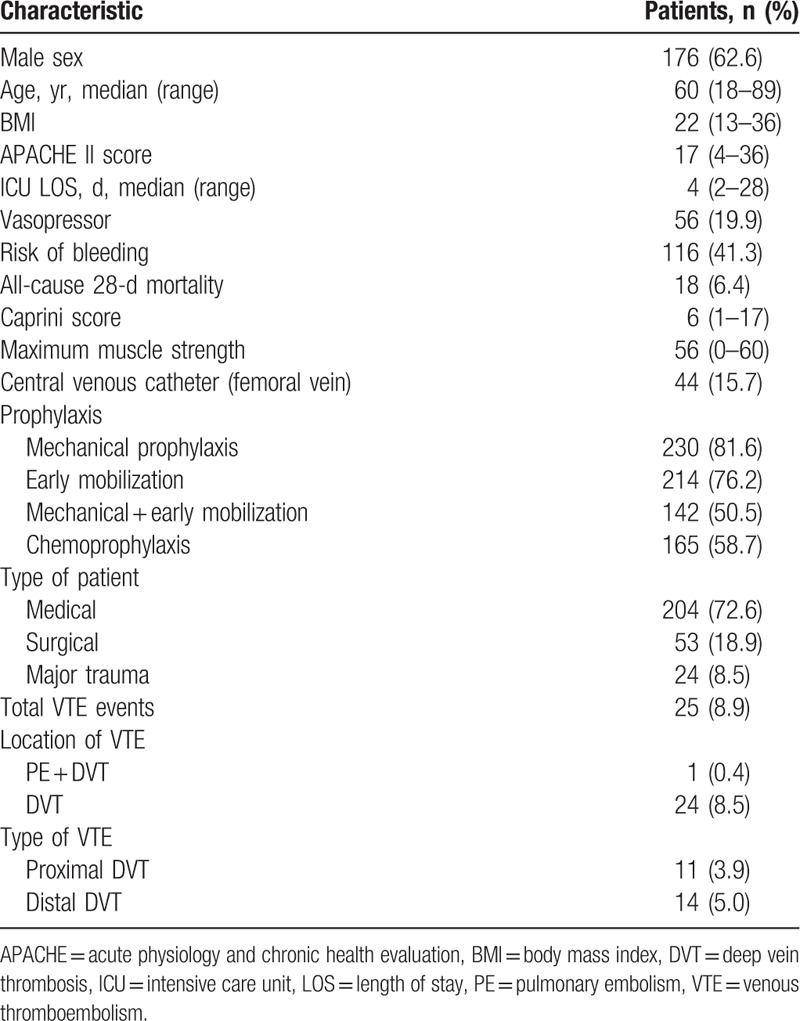
From April 2018 to November 2018, 324 eligible patients were screened, of whom 281 were consecutively included in the study. Exclusion criteria included ICU length of stay <2 days (n = 30), development of acute VTE before ICU admission (n = 11), and age <18 years (n = 2) (Fig. 1). The baseline characteristics of the enrolled patients are presented in Table 1. On admission, 72.6% presented medical, 18.9% surgical, and 8.5% major trauma pathology. Patients had a median age of 60 years (range, 18–89), with an APACHE II score of 17 (range, 4–36), body mass index of 22 (range, 13–36), and maximum muscle strength of 56 (range, 0–60). The median ICU length of stay was 4 days (range, 2–28), and the Caprini score was 6 (range, 1–17). A total of 15.7% of patients had a CVC (femoral vein), 19.9% patients were given a vasopressor, and 41.3% patients had a risk of bleeding before developing VTE. A total of 18 patients died within 28 days and the all-cause, 28-day mortality rate was higher in patients with VTE, but the difference was not significant (8.0% vs 6.3%, P = .667). All patients received prophylaxis: 81.6% mechanical, 76.2% early mobilization, 50.5% both mechanical and early mobilization, and 58.7% LMWH.
Figure 1.
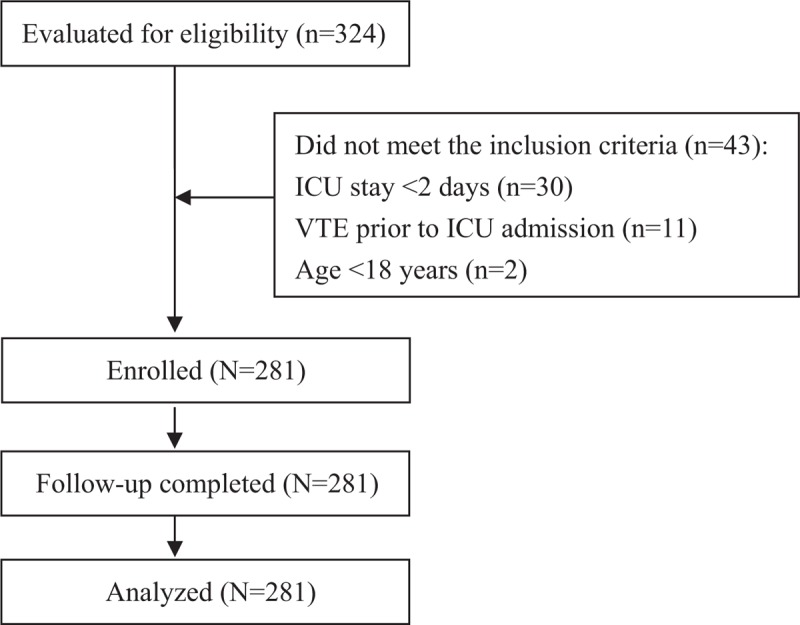
Consort flow diagram of this study procedure.
Table 1.
Demographic and clinical characteristics of enrolled patients (N = 281).
3.2. The cumulative incidence of VTE within 28 days
Over the 28 days, 25 patients developed VTE: 1 patient with both PE and lower extremity proximal DVT, 11 patients with proximal DVT, and 13 patients with distal DVT. The cumulative incidence of VTE at 7, 14, and 21 days was 4.45% (95% CI, 2.55–7.71), 7.14% (4.61–10.97), and 7.53% (range, 4.92–11.43), respectively, while the cumulative incidence at 28 days was 9.55% (95% CI, 6.55–13.81). The hazard rates for the time intervals of 1 to 7 days, 8 to 14 days, 15 to 21 days, and 22 to 28 days were 0.0056 (12 VTEs), 0.0036 (7 VTEs), 0.0005 (1 VTEs), and 0.0054 (5 VTEs), respectively (Fig. 2).
Figure 2.
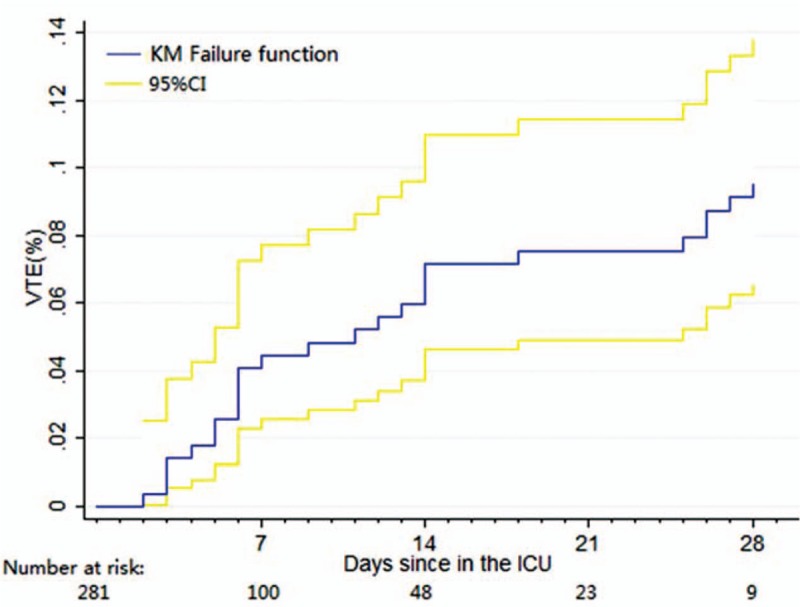
Kaplan–Meier failure function with 95% confidence interval. CI = confidence interval, KM = Kaplan–Meier, VTE = venous thromboembolism.
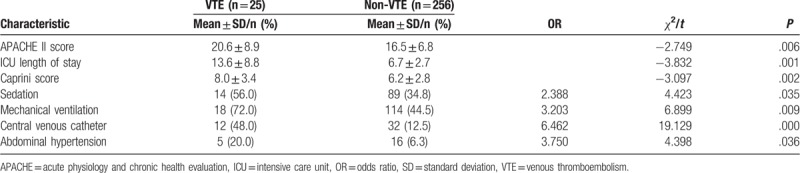
3.3. Univariate analysis of risk factors for patients with VTE
The statistical analysis is shown in Table 2. There were significant differences between patients with or without VTE in ICU length of stay (13.6 ± 8.8 vs 6.7 ± 2.7 days, P < .01). Significant differences in the other factors, such as APACHE II score, Caprini score, sedation, mechanical ventilation, CVC, and abdominal hypertension, were also found between patients with and without VTE (P < .05). However, other factors of age, sex, body mass index, maximum strength muscle, CRRT, and platelet transfusion had no influence on the occurrence of VTE (P > .05).
Table 2.
Univariate analysis of risk factors for VTE.
3.4. Multivariate analysis of risk factors for VTE
In multivariable analysis, 3 factors were found to have an influence on the incidence of VTE. Table 3 shows that CVC (P = .002, OR = 4.50), Caprini score (P = .012, OR = 1.20), and ICU length of stay (P = .006, OR = 1.08) were independent risk factors related to the occurrence of VTE.
Table 3.
Multivariate analysis of risk factors for VTE.
3.5. Odds for VTE substratified by caprini score
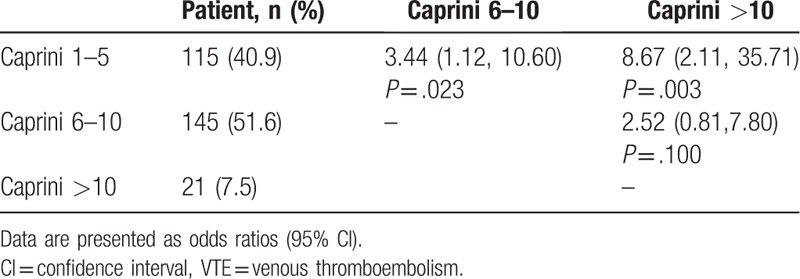
Table 4 suggests the proportions of patients with a Caprini score of 1 to 5, 6 to 10, and greater than 10 were 40.9%, 51.6%, and 7.5%, respectively. Patients with a Caprini score of 1 to 5 were significantly less likely to develop VTE events compared with those with a Caprini score of 6 to 10 (OR = 3.44; 95% CI, 1.12–10.60) and greater than 10 (OR = 8.67; 95% CI, 2.11–35.71). However, there was no significance between the group with Caprini scores of 6 to 10 and the group with a score greater than 10.
Table 4.
Odds for VTE substratified by Caprini score.
4. Discussion
VTE is a life-threatening complication, and its incidence is a sign of quality care for patients in the ICU. We designed a prospective observational study to investigate the cumulative VTE incidence, risk factors, and outcomes for those patients. VTE prophylaxis was an effective way to decrease the occurrence of VTE. In our study, all patients received guideline-recommended VTE prophylaxis. The cumulative VTE incidence within 7 days after ICU admission was as high as 4.45%, and the incidence increased between 14 and 28 days. In a prospective review of 261 patients with critical medical-surgical illness, 9.6% patients developed VTE during their ICU stay.[17] Similarly, in other studies, the rate of VTE ranged from 5% to 15% for patients admitted to the ICU.[18–20] However, in contrast to another study reported by Jia et al, our incidence was higher than the 3.1% of patients with symptomatic VTE in the ICU after surgery.[21] We believe that the following reasons may have contributed to the high cumulative VTE incidence. First and foremost, in the prior study, only proximal DVT was included. In our study, both proximal DVT and symptomatic distal DVT were recorded when we counted the number of VTEs. Moreover, our study was a cumulative VTE incidence study, whereas the former study was a cross-sectional retrospective survey study. Another prospective study by Kaplan et al of 113 patients with severe sepsis and septic shock showed a VTE incidence of 37.5%.[7] This high VTE rate might be attributed to special observation patients and the very small sample size. Indeed, in our study, the incidence of thrombosis was higher in patients with sepsis than those without sepsis.
In our prospective study, nearly half of the events occurred within 7 days after ICU admission. This may be because a higher proportion of patients received mechanical ventilation within first 7 days. In our study, 75 of 132 patients received mechanical ventilation in the first 7 days. In addition, we also found that the rate of CVC in the location of the femoral vein was higher in the 7 days after ICU admission as compared with other times. To our knowledge, mechanical ventilation and CVC were the risk factor of VTE.[7,22]
Factors in the dynamic Caprini score were found to associate with occurrence of VTE in ICU patients. The Caprini score is widely used in the risk assessment of VTE for medical, surgical, and trauma patients, by which patients are divided into different risks and corresponding measures implemented.[7,16,23] Use of the Caprini score is recommended in the guidelines of VTE prevention for Western surgical patients.[24] To our knowledge, this is the first prospective study that dynamically recorded patents’ Caprini scores, and the maximum Caprini score was used in the analysis. It may increase the accuracy of the assessment compared with other retrospective studies. Our findings identified those patients with a Caprini score >5 had a higher risk of developing VTE than those with a Caprini score of 1 to 5. However, we also found that the Caprini scores of patients with VTE were higher than in those patients without VTE (8.0 vs 6.2). This means that many non-VTE patients were classified into the highest group (Caprini score ≥5). This limited the ability to distinguish VTE risk from ICU patients. Therefore, increasing the cut-off point for classifying patients might be a solution. Bahl et al[25] reported that this changed the Caprini risk assessment model and added a classification of a “super high risk” group (>8) on the basis of the highest risk group. Similarly, a Caprini score >10 may be more effective in distinguishing VTE risk among cancer patients admitted to the ICU for postoperative care.[21] The other solution was the combination of the Caprini score and thrombotic biomarkers for assessment of VTE risk in ICU patients. Fu et al[26] reported that the combination of plasma markers (D-dimer and thrombomodulin) and Caprini score could increase the predictive value.
Our findings also identified that ICU length of stay and CVC in the location of the femoral vein were the independent risk factors of VTE. We found that patients had a longer ICU stay with VTE compared with those without VTE. Similarly, Kumar et al[27] and Malato et al[28] reported that a prolonged ICU stay was a risk factor for DVT. This may be because patients usually rest in bed and are subject to long-term immobilization, especially patients with mechanical ventilation. Long-term immobilization could lead to venous reflux obstruction. What is worse, if the patient had VTE, the ICU stay would be prolonged. However, other studies also showed that differences in ICU stay were not significant between the VTE and non-VTE groups.[29,30] CVC use was an important risk factor for VTE, especially if it was inserted in the femoral vein. The incidence of VTE ranged from 10% to 69% with a femoral catheter.[2,31] In our study, 12 of 44 patients with a femoral catheter had developed VTE. The majority cause of a high rate of femoral catheter use was that 25 of 44 patients had received CRRT. Similarly, many studies showed that CVC was an independent risk factor for VTE, and the incidence of CVC-related VTE ranged from 1.81% to 26%.[32–35] Our findings emphasize the importance of removing CVCs as soon as possible when patients are no longer receiving CRRT.
Among the other factors, APACHE II, sedation, mechanical ventilation, and abdominal hypertension, no significant differences were found between patients with or without VTE in the multivariate analysis. The duration of mechanical ventilation was associated with occurrence of VTE, as identified in other studies.[2,7] This may be because we recorded only whether the patient was on mechanical ventilation. This means that mechanical ventilation itself does not increase the incidence of VTE but rather the prolongation of mechanical ventilation. At the same time, other studies reported sedation and abdominal hypertension to be risk factors of VTE.[2,6] To our knowledge, the major influence of sedation is its result of immobilization for ICU patients. However, our study showed that 76.2% patients received early mobilization. Therefore, sedation may not be a risk factor for ICU patients who have received prophylaxis of early mobilization. In terms of abdominal hypertension, the lack of effect may be due to the small sample size. In our study, only 21 of 281 patients had abdominal hypertension. Similarly, APACHE II was also found to have no influence on the development of VTE.[36]
Our findings have several clinical implications for ICU medical staff treating and caring for patients. First, since nearly half of the events occurred within 7 days after ICU admission, medical staff should maintain a high suspicion and strengthen the prevention and screening of VTE for this population. Moreover, VTE risk may be overestimated when the Caprini score alone is used to assess ICU patients’ risk of developing VTE. Therefore, the Caprini score should be combined with biochemical indicators, such as D-dimer. Finally, if there are no contraindications, early mobilization should be performed, especially for patients receiving sedation.
A limitation of our prospective study includes the small sample size. This may have decreased the sensitivity of our statistics in the univariate and multivariate analysis of risk factors for VTE. However, based on the sample size calculation, 140 patients were enough for our study. Moreover, in our study, we used only duplex scan to determine whether patients had DVT rather than venography, which may have reduced the sensitivity of screening asymptomatic distal DVT. However, asymptomatic distal DVT was not recorded when we analyzed the incidence of VTE. Furthermore, CTPA was not examined for all patients, but only for those with suspected PE who already had DVT or respiratory dysfunction. Therefore, the incidence of PE may be underestimated. Finally, we collected patient's information only within 28 days of the ICU stay, and do not follow patients for VTE who the length of ICU stay is less than 28 days and discharge from ICU without VTE. Because we are unable to collect risk factors of VTE after patient discharge from the ICU. In addition, it is difficult to determine whether the patient has VTE without ultrasound examination. Therefore, it is unclear whether following patients with an ICU stay <28 days up to the 28th day had any influence on the cumulative incidence of VTE.
5. Conclusions
In conclusion, our prospective observational study found that the 28-day cumulative incidence of VTE is relatively high for patients admitted to the ICU, despite the use of guideline-recommended thromboprophylaxis. Patients with femoral CVC, a prolonged ICU length of stay, or a high Caprini score may have an increased risk of developing VTE.
Author contributions
Conceptualization: Chuanlin Zhang, Jie Mi.
Data collection: Xueqin, Wang; Yujun, Zou; Xinyi, Luo; Xiaoya, Chen.
Data curation: Yujun Zou, Ruiying Gan.
Formal analysis: Ruiying Gan.
Funding acquisition: Chuanlin Zhang, Jie Mi.
Investigation: Zeju Zhang, Xueqin Wang, Xiaoya Chen, Zhi Nie, Xinyi Luo.
Literature search: Zeju Zhang.
Manuscript preparation: Chuanlin Zhang.
Methodology: Zhi Nie.
Statistical analysis: Zhi, Nie; Ruiying, Gan.
Study design: Chuanlin Zhang; Jie Mi.
Writing – original draft: Chuanlin Zhang, Jie Mi.
Writing – review and editing: Xueqin Wang.
Footnotes
Abbreviations: APACHE II = acute physiology and chronic health evaluation II, CI = confidence interval, ICU = intensive critical unit, OR = odds ratio, VTE = venous thromboembolism.
This study was supported by the First Affiliated Hospital of Chongqing Medical University Hospital subject (HLJJ 2016-01).
The authors have no conflicts of interest to disclose.
References
- [1].Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;1336 Suppl:381s–453s. [DOI] [PubMed] [Google Scholar]
- [2].Minet C, Potton L, Bonadona A, et al. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care 2015;19:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006;332:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004;110:874–9. [DOI] [PubMed] [Google Scholar]
- [5].Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;1263 Suppl:338s–400s. [DOI] [PubMed] [Google Scholar]
- [6].Boddi M, Peris A. Deep vein thrombosis in intensive care. Adv Exp Med Biol 2017;906:167–81. [DOI] [PubMed] [Google Scholar]
- [7].Kaplan D, Casper TC, Elliott CG, et al. VTE incidence and risk factors in patients with severe sepsis and septic shock. Chest 2015;148:1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Parikh KC, Oh D, Sittipunt C, et al. Venous thromboembolism prophylaxis in medical ICU patients in Asia (VOICE Asia): a multicenter, observational, cross-sectional study. Thromb Res 2012;129:e152–8. [DOI] [PubMed] [Google Scholar]
- [9].Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg 2010;1991 Suppl:S3–10. [DOI] [PubMed] [Google Scholar]
- [10].Dauzat M, Laroche JP, Deklunder G, et al. Diagnosis of acute lower limb deep venous thrombosis with ultrasound: trends and controversies. J Ultrasound 1997;25:343–58. [DOI] [PubMed] [Google Scholar]
- [11].Zhang C, Fu Q, Zhao Y, et al. Short-term anticoagulant therapy and thrombus location are independent risk factors for delayed recanalization of deep vein thrombosis. Med Sci Monit 2016;22:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang L, Zhang C, Mu S, et al. Safety of catheter-directed thrombolysis for the treatment of acute lower extremity deep vein thrombosis: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7922–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li W, Chuanlin Z, Shaoyu M, et al. Catheter-directed thrombolysis for patients with acute lower extremity deep vein thrombosis: a meta-analysis. Rev Lat Am Enfermagem 2018;26:e2990–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;1412 Suppl:e195S–226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;1412 Suppl:e419S–96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Serrano PE, Parpia S, Linkins LA, et al. Venous thromboembolic events following major pelvic and abdominal surgeries for cancer: a prospective cohort study. Ann Surg Oncol 2018;25:3214–21. [DOI] [PubMed] [Google Scholar]
- [17].Cook D, Crowther M, Meade M, et al. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med 2005;33:1565–71. [DOI] [PubMed] [Google Scholar]
- [18].Boddi M, Cecchi A, Bonizzoli M, et al. Follow-up after four-year quality improvement program to prevent inferior limb deep vein thrombosis in intensive care unit. Thromb Res 2014;134:578–83. [DOI] [PubMed] [Google Scholar]
- [19].Boddi M, Barbani F, Abbate R, et al. Reduction in deep vein thrombosis incidence in intensive care after a clinician education program. J Thromb Haemost 2010;8:121–8. [DOI] [PubMed] [Google Scholar]
- [20].Lawall H, Oberacker R, Zemmrich C, et al. Prevalence of deep vein thrombosis in acutely admitted ambulatory non-surgical intensive care unit patients. BMC Res Notes 2014;7:431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu JX, Dong J, Ren H, et al. Incidence and risk assessment of venous thromboembolism in cancer patients admitted to intensive care unit for postoperative care. J BUON 2018;23:500–6. [PubMed] [Google Scholar]
- [22].Ibrahim EH, Iregui M, Prentice D, et al. Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis. Crit Care Med 2002;30:771–4. [DOI] [PubMed] [Google Scholar]
- [23].Bateman DK, Dow RW, Brzezinski A, et al. Correlation of the caprini score and venous thromboembolism incidence following primary total joint arthroplasty-results of a single-institution protocol. J Arthroplasty 2017;32:3735–41. [DOI] [PubMed] [Google Scholar]
- [24].Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients. Chest 2012;141:e227S–77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bahl V, Hu HM, Henke PK, et al. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg 2010;251:344–50. [DOI] [PubMed] [Google Scholar]
- [26].Fu Y, Liu Y, Chen S, et al. The combination of Caprini risk assessment scale and thrombotic biomarkers to evaluate the risk of venous thromboembolism in critically ill patients. Medicine (Baltimore) 2018;97:e13232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kumar A, Mehta Y, Ali T, et al. Deep vein thrombosis in medical and surgical intensive care unit patients in a tertiary care centre in North India: incidence and risk factors. J Anaesthesiol Clin Pharmacol 2017;33:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Malato A, Dentali F, Siragusa S, et al. The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes. Blood Transfus 2015;13:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gibson CD, Colvin MO, Park MJ, et al. Prevalence and predictors of deep vein thrombosis in critically Ill medical patients who underwent diagnostic duplex ultrasonography. J Intensive Care Med 2018;19:1–5. [DOI] [PubMed] [Google Scholar]
- [30].Lyman GH, Culakova E, Poniewierski MS, et al. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb Res 2018;164Suppl 1:S112–8. [DOI] [PubMed] [Google Scholar]
- [31].Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 2001;286:700–7. [DOI] [PubMed] [Google Scholar]
- [32].Malinoski D, Ewing T, Bhakta A, et al. Which central venous catheters have the highest rate of catheter-associated deep venous thrombosis: a prospective analysis of 2,128 catheter days in the surgical intensive care unit. J Trauma Acute Care Surg 2013;74:454–60. [DOI] [PubMed] [Google Scholar]
- [33].Amankwah EK, Atchison CM, Arlikar S, et al. Risk factors for hospital-associated venous thromboembolism in the neonatal intensive care unit. Thromb Res 2014;134:305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hrdy O, Strazevska E, Suk P, et al. Central venous catheter-related thrombosis in intensive care patients - incidence and risk factors: a prospective observational study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:369–73. [DOI] [PubMed] [Google Scholar]
- [35].White D, Woller SC, Stevens SM, et al. Comparative thrombosis risk of vascular access devices among critically ill medical patients. Thromb Res 2018;172:54–60. [DOI] [PubMed] [Google Scholar]
- [36].Miri M, Goharani R, Sistanizad M. Deep vein thrombosis among intensive care unit patients; an epidemiologic study. Emerg (Tehran) 2017;5:e13–7. [PMC free article] [PubMed] [Google Scholar]


