Abstract
Purpose:
To evaluate the efficacy and safety of orally administered grape seed proanthocyanidin extract (GSPE) in patients with non-proliferative diabetic retinopathy (NPDR).
Methods:
In this randomized (1:2:2), multicentre, double-blind trial, patients (n = 124; age: 40–78 years) were administered placebo, calcium dobesilate (CD; 750 mg/d), or GSPE (150 mg/d) orally for up to 12 months. All patients had retinal thickening with hard exudates (HEs) that met predefined criteria; the median best-corrected visual acuity was 0.8, as assessed using the Snellen visual acuity card. The main outcome measure was an improvement in HEs by at least 1 grade on a 10-grade severity scale. This was evaluated using fundus photography over 1 year.
Results:
The rate of improvement in the HE severity was higher in the GSPE group than in the CD group. No statistically significant difference existed among the study groups in optical coherence tomography parameters, such as central subfield macular thickness and total macular volume (TMV). However, in the GSPE group, TMV after 9 months of treatment was significantly decreased compared with that at baseline. The GSPE group showed a significantly greater improvement in HE severity than did the placebo or CD group. Four cases in the GSPE group and 2 in the CD group were determined to have developed potential treatment-related adverse reactions, which were all gastrointestinal in nature.
Conclusions:
Oral GSPE therapy for 1 year improved HEs in patients with NPDR. The efficacy of GSPE for HEs was higher than that of oral CD in the study patients.
Keywords: diabetic macular edema, diabetic retinopathy, hard exudates, proanthocyanidin extract
1. Introduction
Diabetic macular edema (DME) is the leading cause of visual impairment in patients with diabetes and affects approximately 1.4% to 12.8% of patients with type 1 or type 2 diabetes.[1] Moreover, a common feature of DME is the presence of hard exudates (HEs), which are closely related to systemic abnormalities such as hyperlipidemia.[2,3] Previous reports have suggested that HEs can affect vision in diabetic patients. Especially, subfoveal deposits of HEs can lead to worse visual outcomes by preventing interaction between the retinal pigment epithelium and outer retinal layer.[9] In addition, severe prolonged subfoveal HEs can cause subretinal fibrosis, resulting in permanent visual loss. Therefore, rapid HEs resorption is required in cases with centrally involved HEs.
Many treatment options, such as macular photocoagulation,[4] intravitreal injections of anti-vascular endothelial antibodies,[5] and intravitreal injections of steroids,[6,7] have been shown to be effective in the treatment of HEs associated with diabetic retinopathy (DR). However, these local treatment modalities are quite invasive, have a short duration of action, and can sometimes cause serious complications such as endophthalmitis or increased intraocular pressure requiring surgery.[8,9] Therefore, there exists an unmet need of sustainable medications for HEs associated with DR.
Proanthocyanidins (also referred to as “procyanidins”) are a type of plant flavonoids. The most active proanthocyanidins are those bound to other proanthocyanidins. A mixture of proanthocyanidins dimers, trimers, and larger molecules is referred to as procyanidolic oligomers (PCOs) or oligomeric proanthocyanidins. There are 2 commercially available sources of PCOs: grape (Vitis vinifera) seed skin extracts and the bark of the maritime pine (Pinus pinaster) extracts.[10] PCO extracted from the bark of the maritime pine is also known as pycnogenols, and PCO-rich extracts from the grape seeds are called “‘grape seed proanthocyanidins extracts (GSPE).” Various pharmacological effects of PCOs have been reported, such as antioxidant and radical scavenging activities, reduction of capillary permeability and fragility, inhibition of destruction of collagen, and reduction of inflammation. Clinical studies have demonstrated the beneficial effects of PCO on the progression of diabetic retinopathy.[11,12] Notably, it has also been reported that GSPE inhibits the vascular endothelial growth factor signaling pathway, which is the main pathway responsible for the progression of diabetic retinopathy.[13–15] Moreover, some clinical studies have reported the beneficial effects of GSPE on metabolic conditions associated with type 2 diabetes, such as arterial hypertension, hyperlipidemia, oxidative stress, and hyperglycemia. The results of one clinical study showed that GSPE significantly improved the markers of inflammation levels of glucose, total cholesterol, and oxidative stress in patients with type 2 diabetic.[16]
Based on results of these experimental and clinical studies, we decided to evaluate the effects of GSPE on hard exudates in subjects with non-proliferative diabetic retinopathy (NPDR) in a double-masked, randomized placebo-controlled multicenter trial. We also compared the effectiveness of GSPE with that of calcium dobesilate (CD)—which is the most widely accepted oral medication for diabetic retinopathy—to estimate more precisely the efficacy of GSPE.[17] The aim of this study was to assess the effectiveness of GSPE in the treatment of HEs associated with NPDR in patients with type 2 diabetes mellitus.
2. Materials and methods
2.1. Study design
This was a multicentre, randomized, double-blind controlled study that compared the efficacy and safety of Vitis vinifera extract, calcium dobesilate (CD), and placebo in subjects with DME. Patients made 6 clinic visits, namely the screening visit; baseline visit (T0); and follow-up visits at 3 (T3), 6 (T6), 9 (T9), and 12 (T12) months. This study was approved by the institutional review board (IRB) and/or ethics committee of each participating center. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study and was approved by the relevant IRB.
2.2. Study population
From November 2012 through January 2015, we enrolled 153 patients at 12 tertiary hospital centers in South Korea. Table 1 shows the selection criteria for the study population. At baseline, the ocular lesions were graded using color fundus photography and fluorescein angiography (FA). The photographs and FA images were subsequently sent to a review committee comprising off-site assessors who were unaware of the initial investigators’ assessment. This committee was nominated to confirm the quality of the images and the grade of the lesions.
Table 1.
Selection criteria of study population.

2.3. Treatment
Eligible patients were randomized to 1 of the 3 study groups in a 1:2:2 ratios (placebo: GSPE: CD group). The randomization schedule was generated and prepared using cubeIWRS solution (CRScube Inc., Seoul, South Korea, HQ). Randomization was performed using a complete randomization algorithm according to the order of the baseline visit (Fig. 1). Subjects took 3 tablets of a masked study medication 3 times daily for 12 months; the first dose was taken in the morning of the baseline visit (T0) after baseline assessments were performed, and the last dose was taken in the evening before the month 12 visit (T12). Three daily oral doses of 50-mg tablets of GSPE (Entelon, Hanlim Pharm, Seoul, South Korea) were administered to patients in the GSPE group. Placebo tablets lacked GSPE, but their appearance was identical to that of the study group tablets. Commercially available 250-mg CD tablets (Doxium, Ilsung Pharm, Seoul, South Korea) were used in this study. The identity of the masked study medications was concealed by storing the medications in individually sealed envelopes at the study sites.
Figure 1.

Schematic diagram of participants’ disposition. CD = calcium dobesilate, GSPE = grape seed proanthocyanidin extract, ITT = intension-to-treat, PP = per protocol.
2.4. Efficacy assessment
A comprehensive ophthalmic examination, including the assessment of the best-corrected visual acuity (BCVA) using the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol, intraocular pressure (IOP) measurement, slit-lamp biomicroscopy, indirect ophthalmoscopy, and optical coherence tomography (OCT), was performed during every visit. The OCT examination was performed using a 6-radial scan protocol or cube scan protocol according to local guidelines of each center; the ETDRS style map was used to calculate the central subfield mean thickness (CSMT) and total macular volume (TMV). FA was performed at the screening visit and T12 visit.
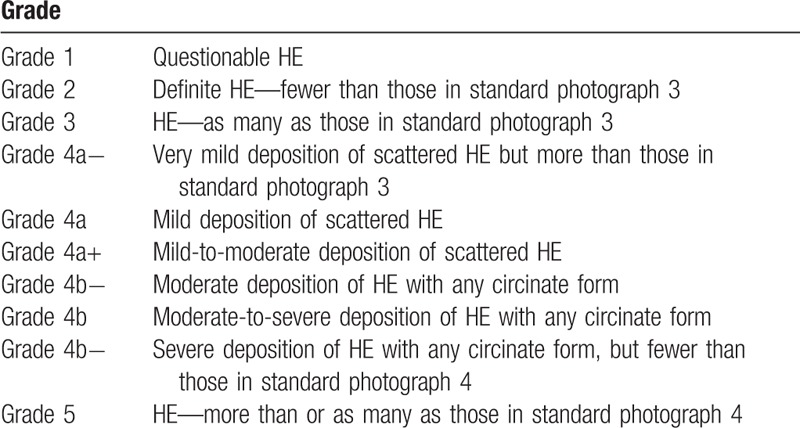
The primary efficacy endpoint of this study was an improvement in the HE severity at T12 visit. The improvement of HE was defined as a decrease in the HE severity by at least 2 categories of severity at T12 compared with the baseline visit. Fundus photography was performed on F2 fields (ETDRS standard), and the HE severity was graded according to a specifically designed grading system that extended the Airlie House classification.[18] This grading system for HE severity has been used in a previously published clinical trial from our group.[19] We used this grading system without modification. Briefly, this grading system was based on the area of retina involved by HE and the amount of HE observed; the ETDRS standard photographs 3 and 4 were used for comparison. In our grading system for HE severity, grade 4 fundus photographs were divided into grade 4a and 4b, which consequently were divided into “−,” “0,” and “+” grades. Thus, the fundus photographs yielded a grading of HE severity that spanned 10 grades overall (1, 2, 3, 4a-, 4a, 4a+, 4b-, 4b, 4b+, and 5; Fig. 2 and Table 2). Each fundus photograph was graded by 2 trained graders (HC and YUS). The 2 graders were masked to the clinical data and performed the grading independently without knowing the other grader's score. The final grade of HE severity was determined by a third grader (HKK) if there was a disagreement.
Figure 2.

Representative examples of fundus photographs in each grade A Grade 2. B Grade 3. C Grade 4a-. D Grade 4a. E Grade 4a+. F Grade 4b-. G Grade 4b. H Grade 4b+.
Table 2.
Grading of hard exudates.
The secondary efficacy end points were
-
(1)
changes in retinal thickness (CSMT; in micrometers) and volume (TMV; in cubic millimeters) (assessed using OCT) at each follow-up visit compared to the baseline visit (T0),
-
(2)
change in the grade of diabetic retinopathy on FA imaging, and
-
(3)
change in BCVA from baseline to T12 visit.
Efficacy assessments were performed on both eyes; however, if both eyes met the inclusion criteria, the investigator designated the study eye at baseline.
2.5. Safety endpoints
Vital signs were measured during each visit, whereas laboratory tests were performed at T0 and T12 visits. The laboratory safety tests included tests for the following: glycosylated hemoglobin (HbA1c), serum creatinine, total cholesterol, triglyceride, HDL-cholesterol, and LDL-cholesterol. The investigators also monitored for possible adverse events by questioning the patients during each visit and on the final day of assessment.
2.6. Statistical analysis
This study was designated as a comparison of treatment groups (GSPE and CD groups) versus placebo group at 12 months after treatment. Chi-squared and Fisher exact test were used to compare the improvement in the HE severity grade. Changes in OCT parameters, FA parameters, and BCVA between baseline visit (T0) and each follow-up visit within each treatment group were analyzed using paired t test or signed rank test. Repeated measure analysis of variance (ANOVA) was used to compare the changes of parameters among treatment groups. The safety analysis was performed on the safety group by monitored the following: the incidence of adverse events, laboratory data, vital signs, and changes in physical examination findings. All analyses were performed using the Statistical Package for the Social Science version 12.0 for Windows (SPSS Inc., Chicago, IL).
3. Results
3.1. Disposition and baseline characteristics
A total of 124 patients from 12 centers were randomized (51 patients in the GSPE group, 47 patients in the CD group, and 26 patients in the placebo group). All but 1 patient (GSPE group) received at least 1 dose of the study drug; this patient was not included in the efficacy analysis. Most subjects in each treatment group completed the study; however, 24 (19.35%) of 124 subjects were withdrawn.
Analyses were performed on the basis of the intention-to-treat (ITT) principle. All randomized subjects who received at least 1 dose of the study drug were included in the safety analyses (n = 123). Fifteen of these patients withdrew with no efficacy visits after baseline; therefore, 108 subjects were included in the ITT efficacy group (41 in the GSPE group, 42 in the CD group, and 25 in the placebo group). Among the ITT efficacy group, 22 subjects violated the protocol, and a total of 86 subjects (69.35%) were included in the per-protocol (PP) efficacy group (32 in the GSPE group, 35 in the CD group, and 19 in the placebo group). Treatment groups were similar with regard to the demographic and baseline characteristics (Table 3).
Table 3.
Baseline characteristics of enrolled participants.

3.2. Efficacy
The treatment success rate was the highest in the GSPE group (43.90%), followed by the CD group (14.29%), and the placebo group (8%) (P = .0007) (Table 4). The GSPE group showed higher success not only when compared with the placebo group (chi-squared test, P = .0021) but also when compared with the CD group (chi-squared test, P = .0029). However, the treatment success rate in the CD group was not statistically significantly different compared with that in the placebo group (P = .7) (Fig. 3).
Table 4.
Comparison of treatment success rate, which was defined as a decrease in hard exudates severity of more than 2 grades at 1 year, among treatment groups (grape seed proanthocyanidin extract and calcium dobesilate) and placebo group.
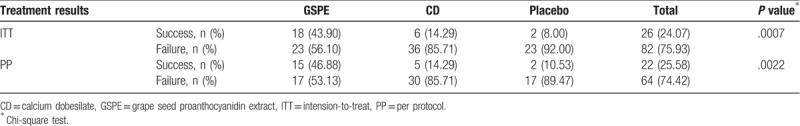
Figure 3.

Comparison of treatment success rate among the 3 groups. CD = calcium dobesilate, GSPE = grape seed proanthocyanidin extract. Chi-squared test was used for statistical analyses.
With respect to secondary efficacy assessment, there was no significant difference among the groups with regard to the amount of CSMT changes from baseline during all visit times. However, the GSPE group showed a tendency of having a large decrease at the final visit (−3.19 ± 9.34 in the GSPE group, −1.50 ± 24.39 in the CD group, +3.26 ± 16.44 in the placebo group). With respect to the TMV changes from baseline, a significantly larger decrease was observed in the GSPE group at visit T9 and visit T12. In contrast, there was no significant change in TMV from baseline to each visit, including T9 and T12, in the CD or placebo group (Table 5). With respect to progression of diabetic retinopathy grade as assessed by FA, no significance difference was observed both in intragroup and intergroup comparisons. There were no significant changes in the BCVA among the treatment groups at all visit time points (Table 5).
Table 5.
Changes (difference between visit 1 and visit 5) in central subfield macular thickness, total macular volume, and best-corrected visual acuity.

3.3. Safety
As a result of monitoring the potential adverse events, overall, 35 of 123 patients in the safety assessment population (28.45%) reported adverse events (Table 6). Eighteen of 50 patients in the GSPE group (36.00%) reported 27 reactions, 13 of 47 patients in the CD group (27.66%) reported 19 reactions, and 4 of 26 patients in the placebo group (15.38%) reported 7 reactions.
Table 6.
Safety report in current study.

Infection-related events were observed in 8 patients [4 (8%) in the GSPE group, 2 (4.3%) in the CD group, and 2 (7.7%) in the placebo group]. Gastrointestinal disorders were observed in 11 patients [5 (10%) in the GSPE group, 4 (8.5%) in the CD group, and 2 (7.7%) in the placebo group]. Eye disorders were observed in 9 patients [5 (10%) in the GSPE group, 3 (6.4%) in the CD group, and 1 (3.9%) in the placebo group]. Nervous system disorders were observed in 4 patients [(3 (6%) in the GSPE group, 1 (2.1%) in the CD group, and 0 (0%) in the placebo group]. However, among them, 4 patients in the GSPE group and 2 in the CD group were determined to have potential treatment-related gastrointestinal adverse reactions. One patient in the GSPE group, 2 in the CD group, and 1 in the placebo group reported adverse events, which were of moderate severity and classified as definitely not associated with the assigned treatments. Four patients in the GSPE group and 1 in the CD group were instructed to discontinue the drug.
There was no statistically or clinically relevant evidence of difference among the treatment groups with regard to vital signs and laboratory results. However, some participants showed abnormal laboratory results. Two patients in the GSPE group, 1 in the CD group, and 2 in the placebo group showed a high HbA1c level (>7.0%) at the final visit. One patient in the placebo group showed a high level of HbA1c (7.6%) and serum triglyceride (253 mg/dL).
4. Discussion
Retinal hard exudates (HEs), which are primarily deposited in the outer plexiform layer, are frequently observed along with DME; HEs are composed of lipids and lipoproteins. HEs develop in the early stages of DR, and it is known that an increase in the number of HEs is associated with an increased risk of visual loss and subretinal fibrosis in DME.[3,20] In particular, visual outcomes are worse when HEs are deposited beneath the fovea, which may block the interaction between the neurosensory retina and the retinal pigment epithelium.[21] Therefore, the severity of HEs can be considered an important target for the treatment of DME and 1 of surrogates for the assessment of treatment efficacy. In this study, 43.9% of patients treated with GSPE showed a decrease in HE severity that was lower than 2 grades after 1-year treatment. The treatment success rate was significantly higher in the GSPE group than in the CD group (14.29%, P = .0029) and placebo group (8%, P = .0021). A similar tendency was also observed in the PP analysis group (46.88% in the GSPE group, 14.29% in the CD group, and 10.53% in the placebo group). The beneficial effects of GSPE in the management of HEs can be inferred from the significantly higher proportion of subjects in the GSPE group who showed improvements in HE severity compared to both, CD and placebo groups.
In terms of safety, the GSPE group (36%) showed more adverse events than the CD group (27.66%). However, this difference did not reach statistical significance. In the GSPE group, most of the adverse events (66.67%) were mild and did not require any further treatment. Furthermore, among them, only 5 cases (18.52%) were possibly associated with the assigned treatment, which again was not certain. There are 2 types of dosage forms of Entelon (GSPE) in the actual market. One is a 50 mg tablet used in this study and the other one is a 150 mg tablet. Entelon 300 mg per day (150 mg tablet twice daily) is used for the treatment of veno-lymphatic insufficiency and this dose is approved by the Korea Food and Drug Administration. This daily dosage is 2 times higher than that used in our study (300 mg vs 150 mg). A previous study has reported the efficacy of GSPE (300 mg/day for 3 months) in 4729 patients with veno-lymphatic insufficiency.[22] Among these patients, gastrointestinal adverse events were observed in 3.7% of the patients after 45 days of taking the drug and it was decreased to 1.42% at the final visit (90 days). The differences in sample size, study protocol, and criteria for adverse events may result in the difference in the safety results between the 2 studies. Therefore, we believe that the dose of GSPE used in this study did not exceed the therapeutic window that could have caused adverse events, because it was relatively low as compared to the dosage for veno-lymphatic insufficiency.
Several research groups have reported to reduce HEs associated with DR. Intravitreal injections of steroids such as triamcinolone and dexamethasone implant have been suggested for the treatment of DME.[7] They can decrease macular thickness and HEs, however, can also cause adverse effects such as cataract and increased intraocular pressure.[9,23] Although there have been conflicting results, Domalpally et al suggested that HEs were decreased in patients who had monthly anti-VEGF injections for over 1 year.[5] The purpose of these local treatments is mainly to recover vision by reducing macular thickness rather than reducing HEs. Therefore, they are not suitable for the treatment of early stage DR patients without vision-threatening DME.
In this study, we included subjects with mild to moderate NPDR who had macular thickness of less than 300 μm because intravitreal drug injection is currently the first-line of treatment for visual improvement in patients with significant ME, and the patients could not be treated alone with oral drugs such as GSPE. Song et al (DRESS study group) conducted a clinical trial with a similar design to our study and suggested that oral sulodexide, which is a highly purified glycosaminoglycan (GAG), showed a significant decrease in HEs in patients with mild to moderate NPDR, with central foveal thickness ≤300 μm.[19] Based on previous animal study,[24] they presumed that oral sulodexide may repair endothelial damage due to diabetes and normalize the vascular permeability and GAG metabolism, resulting in resorption of HEs. They reported that oral sulodexide did not show significant differences in central retinal thickness compared to the placebo group.[19] Similarly, in our study, in the analysis of OCT parameters (including CSMT and TMV), the GSPE group was not significantly different compared to either the DC or the placebo group. However, significant improvement was observed in GSPE patients at month 9 and 12 compared to the TMV at baseline. The mean decrease from the baseline measurement could be interpreted as a meaningful difference compared to the repeated TMV measurements at both 9-month and 12-month visits.[25] However, baseline macular thickness in both the studies was not large, it was difficult to identify changes in central retinal thickness by oral drugs, and further studies are warranted. The changes in the visual acuity in our study were not significantly different among the study groups at 12 months’ final visits. Previous clinical studies have demonstrated a significantly improved visual performance in patients with diabetic retinopathy.[11] However, in this study, we did not observe a significant improvement in the visual acuity in the GSPE group patients.
Although its exact mechanisms have not been proved yet, GSPE can play a role in the management of metabolic conditions in patients with diabetic mellitus. PCO extracts have been shown to reduce blood cholesterol levels and the size of cholesterol deposits in arteries in animal and human studies.[26] In this study, we investigated the lipid profiles, including total cholesterol, LDL, and HDL levels, at the baseline and last visit. However, we did not observe significant differences in lipid profiles among the groups. Therefore, it is difficult to explain the observed effects of GSPE decreasing the HE severity in this study with the ability of GSPE to alter lipid profiles. Additionally, in a double-blind study, GSPE at dosages of 150 and 300 mg/day was shown to produce a mild hypotensive effect (−11 mm Hg for both systolic and diastolic readings) in patients with the metabolic syndrome.[27] It has also been reported that PCOs may reduce the fasting glucose level. In a study of patients with type 2 diabetes, there was a dose-dependent reduction in the fasting blood glucose level of those who received PCOs. These effects were confirmed in a double-blind placebo-controlled study in patients with type 2 diabetes. After 8 weeks of treatment, the median drop in the fasting blood glucose in the PCO-administered group was 35.28 mg/dL.[28]
It has been suggested that PCOs are particularly useful in addressing the microvascular pathology of diabetes. In addition to the antioxidant effects, which are the most investigated effects of GSPE, PCOs can increase erythrocyte membrane fluidity,[29] increase platelet aggregation,[30] and enhance endothelial nitric oxide synthase activity to increase the nitric oxide level.[31] In 1 study in type 2 diabetic patients, PCO administration improved patients’ microangiopathic symptoms, such as increase in skin flux at rest, increase in capillary filtration, and deterioration of venoarteriolar response.[28] Significant improvements in microcirculation, retinal edema, and visual acuity were observed in patients with diabetic retinopathy who received pycnogenol, which is another type of PCOs.[12]
Relatively small sample size was one of the limitations of this study. Another limitation was that we enrolled patients who had DME with HEs but excluded those whose CSMT exceeded 300 μm, which was a criterion designed to prevent a high loss to follow-up over the 12-month period. Therefore, the population involved in this study can be summarized as patients with non-center involving DME with HEs. The relatively low baseline value of CSMT may account for the discrepancy between the results of CSMT and those of TMV. Further studies are needed for identifying the effect of oral GSPE in patients with significant macular edema with decreased vision. Third, while measuring the amount of HEs, we used a subjective grading system. Recent researches regarding DME with HEs have reported various quantitative measurements using image software.[7,32] However, at the beginning of this trial, we did not discuss the objective method for measuring HEs. Additionally, we did not estimate the blood concentration of GSPE. Because GSPE is an oral drug, it is important to evaluate its pharmacokinetic parameters to determine its bioavailability and relevance of the blood concentration in patients undergoing treatment. However, we did not measure the blood concentration of GSPE, because there is no established pharmacokinetics (PK) data on this drug. The drug used in this study (EntelonR) was developed by Sanofi, France. The original developer Sanofi has a document confirming the excretion of isotopically labeled Vitis vinifera in rats in the early stage of development.[33] However, Sanofi does not have any PK data in humans.
In conclusion, the present findings indicate that patients NPDR who used GSPE showed significantly more reduction with respect to HEs compared to those who did not use GSPE. HEs not involving the fovea do not affect vision; therefore, they do not necessarily have to be reduced. However, oral GSPE can be considered as an adjunctive therapy in patients with increasing number of HEs in the center, or in patients refusing intravitreal injections. Although further long-term studies with large samples are necessary, oral GSPE seems to be a promising adjunctive treatment for early-stage DME with HEs.
Acknowledgments
The authors thank the MOGEN study group for supplying data for this study.
Mogen study group (except authors)
Byung Ro Lee, Hanyang University Hospital
Hyeong Gon Yu, Seoul National University Hospital
Il Han Yun, Nunevit Eye Clinic
Ji Hun Song, Ajou University Hospital
Se Woong Kang, Samsung Medical Center
Si Dong Kim, Daegu Catholic University Medical Center
Sung Chul LEE, Yonsei University Hospital
Tae Gon Lee, Dr. Lee's Eye Clinic
Young-Gyoon Kim, Dabom Eye Clinic
Author contributions
Conceptualization: Hakyoung Kim.
Data curation: Yong Un Shin, Heeyoon Cho, So Hyun Bae, Hakyoung Kim.
Formal analysis: Yong Un Shin, Sang Woong Moon, Heeyoon Cho, So Hyun Bae, Hakyoung Kim.
Funding acquisition: Hakyoung Kim.
Investigation: Yong Un Shin, So Hyun Bae.
Methodology: Heeyoon Cho, So Hyun Bae.
Project administration: Heeyoon Cho, So Hyun Bae.
Resources: Mogen study group.
Supervision: Heeyoon Cho, So Hyun Bae, Hakyoung Kim.
Validation: So Hyun Bae, Hakyoung Kim.
Visualization: Sang Woong Moon, Yong Un Shin.
Writing – original draft: Sang Woong Moon, Yong Un Shin.
Writing – review & editing: Sang Woong Moon, Yong Un Shin, Heeyoon Cho, Hakyoung Kim.
Hakyoung Kim orcid: 0000-0002-5581-0057.
Footnotes
Abbreviations: BCVA = best-corrected visual acuity, CD = calcium dobesilate, CSMT = central subfield mean thickness, DME = Diabetic macular edema, DR = diabetic retinopathy, ETDRS = Early Treatment Diabetic Retinopathy Study, FA = fluorescein angiography, GSPE = grape seed proanthocyanidin extract, HEs = hard exudates, ITT = intention-to-treat, NPDR = non-proliferative diabetic retinopathy, OCT = optical coherence tomography, PCO = procyanidolic oligomers, TMV = total macular volume.
SWM and YUS are contributed equally to this work as cofirst authors.
This research was financially supported by Hanlim Pharm. Co. Ltd. Seoul, Republic of Korea
The authors declare no conflicts of interest.
Contributor Information
Collaborators: and for the Mogen Study Group
References
- [1].Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Waller S, Thyagarajan S, Kaplan F, et al. Dramatic resolution of massive retinal hard exudates after correction of extreme dyslipidaemia. Eye (Lond) 2009;23:738. [DOI] [PubMed] [Google Scholar]
- [3].Chew EY, Klein ML, Ferris FL, 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol 1996;114:1079–84. [DOI] [PubMed] [Google Scholar]
- [4].Deák GG, Bolz M, Kriechbaum K, et al. Effect of retinal photocoagulation on intraretinal lipid exudates in diabetic macular edema documented by optical coherence tomography. Ophthalmology 2010;117:773–9. [DOI] [PubMed] [Google Scholar]
- [5].Domalpally A, Ip MS, Ehrlich JS. Effects of intravitreal ranibizumab on retinal hard exudate in diabetic macular edema: findings from the RIDE and RISE phase III clinical trials. Ophthalmology 2015;122:779–86. [DOI] [PubMed] [Google Scholar]
- [6].Cekic O, Bardak Y, Tig US, et al. Quantitative evaluation of reduction of plaque-like hard exudates in diabetic macular edema after intravitreal triamcinolone injection. Int Ophthalmol 2008;28:95–9. [DOI] [PubMed] [Google Scholar]
- [7].Shin YU, Hong EH, Lim HW, et al. Quantitative evaluation of hard exudates in diabetic macular edema after short-term intravitreal triamcinolone, dexamethasone implant or bevacizumab injections. BMC Ophthalmol 2017;17:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baudin F, Benzenine E, Mariet AS, et al. Association of acute endophthalmitis with intravitreal injections of corticosteroids or anti-vascular growth factor agents in a nationwide study in France. JAMA Ophthalmol 2018;136:1352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aref AA, Scott IU, Oden NL, et al. Incidence, risk factors, and timing of elevated intraocular pressure after intravitreal triamcinolone acetonide injection for macular edema secondary to retinal vein occlusion: SCORE study report 15. JAMA Ophthalmol 2015;133:1022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther 2002;40:158–68. [DOI] [PubMed] [Google Scholar]
- [11].Spadea L, Balestrazzi E. Treatment of vascular retinopathies with Pycnogenol. Phytother Res 2001;15:219–23. [DOI] [PubMed] [Google Scholar]
- [12].Steigerwalt R, Belcaro G, Cesarone MR, et al. Pycnogenol improves microcirculation, retinal edema, and visual acuity in early diabetic retinopathy. J Ocul Pharmacol Ther 2009;25:537–40. [DOI] [PubMed] [Google Scholar]
- [13].Huang S, Yang N, Liu Y, et al. Grape seed proanthocyanidins inhibit angiogenesis via the downregulation of both vascular endothelial growth factor and angiopoietin signaling. Nutr Res 2012;32:530–6. [DOI] [PubMed] [Google Scholar]
- [14].Lu J, Zhang K, Chen S, et al. Grape seed extract inhibits VEGF expression via reducing HIF-1alpha protein expression. Carcinogenesis 2009;30:636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Campochiaro PA, Group CPS. Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest Ophthalmol Vis Sci 2004;45:922–31. [DOI] [PubMed] [Google Scholar]
- [16].Kar P, Laight D, Rooprai HK, et al. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: a double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet Med 2009;26:526–31. [DOI] [PubMed] [Google Scholar]
- [17].Benarroch IS, de Salama Benarroch AR, Nano H, et al. Calcium dobesilate as a treatment for capillary fragility in diabetic retinopathy. Ophthalmologica 1974;168:370–5. [DOI] [PubMed] [Google Scholar]
- [18].Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98suppl 5:786–806. [PubMed] [Google Scholar]
- [19].Song JH, Chin HS, Kwon OW, et al. Effect of sulodexide in patients with non-proliferative diabetic retinopathy: diabetic retinopathy sulodexide study (DRESS). Graefes Arch Clin Exp Ophthalmol 2015;253:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lammer J, Bolz M, Baumann B, et al. Detection and analysis of hard exudates by polarization-sensitive optical coherence tomography in patients with diabetic maculopathy. Invest Ophthalmol Vis Sci 2014;55:1564–71. [DOI] [PubMed] [Google Scholar]
- [21].Fong DS, Segal PP, Myers F, et al. Subretinal fibrosis in diabetic macular edema. ETDRS report 23. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol 1997;115:873–7. [DOI] [PubMed] [Google Scholar]
- [22].Henriet JP. Veno-lymphatic insufficiency. 4,729 patients undergoing hormonal and procyanidol oligomer therapy. Phlebologie 1993;46:313–25. [PubMed] [Google Scholar]
- [23].Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 2014;121:2473–81. [DOI] [PubMed] [Google Scholar]
- [24].Kristova V, Kriska M, Vojtko R, et al. Trends in vascular pharmacology research in the Department of Pharmacology and Clinical Pharmacology, Faculty of Medicine, Comenius University. Bratislava Interdiscip Toxicol 2011;4:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Comyn O, Heng LZ, Ikeji F, et al. Repeatability of Spectralis OCT measurements of macular thickness and volume in diabetic macular edema. Invest Ophthalmol Vis Sci 2012;53:7754–9. [DOI] [PubMed] [Google Scholar]
- [26].Devaraj S, Vega-Lopez S, Kaul N, et al. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids 2002;37:931–4. [DOI] [PubMed] [Google Scholar]
- [27].Sivaprakasapillai B, Edirisinghe I, Randolph J, et al. Effect of grape seed extract on blood pressure in subjects with the metabolic syndrome. Metabolism 2009;58:1743–6. [DOI] [PubMed] [Google Scholar]
- [28].Cesarone MR, Belcaro G, Rohdewald P, et al. Improvement of diabetic microangiopathy with pycnogenol: a prospective, controlled study. Angiology 2006;57:431–6. [DOI] [PubMed] [Google Scholar]
- [29].Sivonova M, Waczulikova I, Kilanczyk E, et al. The effect of Pycnogenol on the erythrocyte membrane fluidity. Gen Physiol Biophys 2004;23:39–51. [PubMed] [Google Scholar]
- [30].Chang WC, Hsu FL. Inhibition of platelet aggregation and arachidonate metabolism in platelets by procyanidins. Prostaglandins Leukot Essent Fatty Acids 1989;38:181–8. [DOI] [PubMed] [Google Scholar]
- [31].Fitzpatrick DF, Bing B, Rohdewald P. Endothelium-dependent vascular effects of Pycnogenol. J Cardiovasc Pharmacol 1998;32:509–15. [DOI] [PubMed] [Google Scholar]
- [32].Sasaki M, Kawasaki R, Noonan JE, et al. Quantitative measurement of hard exudates in patients with diabetes and their associations with serum lipid levels. Invest Ophthalmol Vis Sci 2013;54:5544–50. [DOI] [PubMed] [Google Scholar]
- [33].Harmand MF, Blanquet P. The fate of total Flavanolic Oligomers (OFT) extracted from “Vitis vinifera L.” in the rat. European Journal of Drug Metabolism and Pharmacokinetics 1978;3:15–30. [Google Scholar]


