Abstract
Rationale:
Mixed adenoneuroendocrine carcinoma (MANEC) is a rare neoplasm, and consensus on the treatment is unavailable.
Patient concern:
A 60-year-old Chinese man presented with obstructive symptoms while eating and paroxysmal stomach pain for more than a month.
Diagnosis:
MANEC was diagnosed based on clinical manifestations, imaging findings, and pathological examinations.
Interventions:
The patient underwent radical gastrectomy and received XELOX adjuvant chemotherapy (oxaliplatin 200 mg day 1 + capecitabine 1.5 g twice a day) after surgery.
Outcomes:
After 4 cycles of XELOX adjuvant chemotherapy were administered, abdominal computerized tomography and liver magnetic resonance showed liver metastasis.
Lessons:
The therapy of gastric MANEC is based on surgical operation, and adjuvant chemotherapy program has an important influence on its prognosis. Therefore, further studying the effectiveness of XELOX adjuvant chemotherapy for gastric MANEC is necessary.
Keywords: adjuvant chemotherapy, mixed adenoneuroendocrine carcinoma, stomach, XELOX
1. Introduction
In 2010, the World Health Organization proposed the term “mixed adenoneuroendocrine carcinoma (MANEC),” which refers to a tumor with adenocarcinoma and neuroendocrine differentiation; each component accounts for at least 30% of the tumor,[1] and the disease often occurs in the gastrointestine, testicles, and pancreas. MANEC is a biphasic tumor that was reported as a literature case due to its rarity and heterogeneity. The pathogenesis and clinical features of MANEC are different from those of pure adenocarcinoma and neuroendocrine carcinoma (NEC). Whether or not the biological behavior and characteristics of MANEC are similar to those of NEC or adenocarcinoma remains unclear. No uniform standard is currently available for adjuvant chemotherapy for MANEC. We presented a rare case of a gastric MANEC patient who received XELOX adjuvant chemotherapy (oxaliplatin 200 mg day 1 + capecitabine 1.5 g twice a day [bid]) for 4 cycles after radical gastrectomy.
Informed written consent was obtained from the patient for the publication of this case report and accompanying images. No ethical approval was obtained because this study is a retrospective case report and did not involve a prospective evaluation.
2. Case report
A 60-year-old man was admitted to the hospital because of experiencing obstructive symptoms when eating accompanied by paroxysmal stomach pain for more than a month. No abnormality was found during clinical examination. Past medical history indicates that he had suffered from gallbladder polyps for more than 5 years. Personal history shows that he has been drinking for more than 30 years. In a routine gastroscopy (Fig. 1A), an irregular protrusion was found in the posterior wall of the cardia with a deep ulcer in the center, and the lesions extended to the lesser curvature of the fundus and the posterior wall. Endoscopic biopsy (Fig. 1B) suggests the following findings: poorly differentiated adenocarcinoma of the cardia, moderate chronic atrophic gastritis (active) with moderate intestinal metaplasia, and lymphoid follicle formation in the gastric antrum. In addition, immunohistochemistry was positive for CK7, CK8, CAM5.2, and P53. Enhanced computerized tomography (CT) (Fig. 2A) showed irregular thickening of the cardia–fundus wall, small lymph nodes in the liver and stomach gap, and no distant metastasis. The patient underwent laparoscopic radical gastrectomy (total gastrectomy + D2 lymph node dissection + Roux-en-Y anastomosis) + cholecystectomy + lysis of adhesion + hyperthermic intraperitoneal chemotherapy. Postoperative histopathology report (Fig. 1C) indicates MANEC of the cardial part and lesser curvature (poorly differentiated adenocarcinoma, part of mucous adenocarcinoma with neuroendocrine adenocarcinoma) and lymph node metastasis. The upwelling mass in the cardial part and lesser curvature had a size of 4.5 × 3.3 × 0.8 cm and invaded the entire layer of the gastric wall, tunica serosa, and lower esophagus. However, no invasion was observed in the greater omentum. Tumor emboli were also found in the vessels. One of the 13 groups of regional lymph nodes had metastasis (pT4bN1M0). The upper and lower cutting edges were negative. Immunohistochemistry shows the following results: P53(90%+), Ki-67(70%+), C-erB-2(−), CKpan(+), CAM5.2(+), P63(−), P40(−), Syn(partial+), CgA(focal+), CD56(weak+), MLH1(+), PMS2(+), MSH2(+), MSH6(+), CD34(vascular+), D2-40(lymphatic vessels+), and S-100(neural+). Postoperative abdominal CT (Fig. 2B and C) shows postoperative changes in gastric malignant tumors and the absence of distant metastasis. The patient was given XELOX adjuvant chemotherapy (oxaliplatin 200 mg day 1 + capecitabine 1.5 g bid). Diarrhea occurred during chemotherapy, and evaluation of the curative effect after 4 cycles revealed the disease as progressive (Fig. 2D, E, and F). The patient underwent radiofrequency ablation of a metastatic liver tumor, and the disease progressed postoperatively. TP (paclitaxel 250 mg day 1 + Cisplatin 40 mg day 1-3) was administered for 6 cycles, and grade IV myelosuppression occurred during chemotherapy. Re-examination after chemotherapy indicated disease progression. The patient is currently taking Apatinib.
Figure 1.
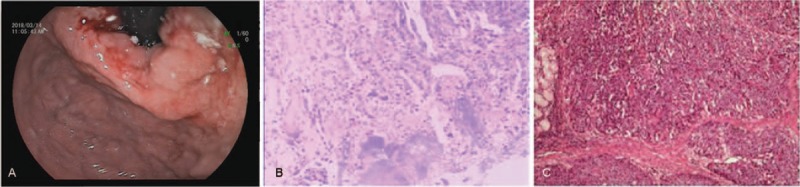
(A) Gastroscopy showing an ulcerating malignant-looking mass in the cardia–fundus of the stomach. (B) Endoscopic biopsy showing poorly differentiated adenocarcinoma of the cardia; moderate chronic atrophic gastritis (active) with moderate intestinal metaplasia and lymphoid follicle formation in the gastric antrum. (C) Postoperative histopathology showing MANEC of the cardial part and less curvature (poorly differentiated adenocarcinoma, part of mucous adenocarcinoma with neuroendocrine adenocarcinoma) and lymph node metastasis. MANEC = mixed adenoneuroendocrine carcinoma.
Figure 2.
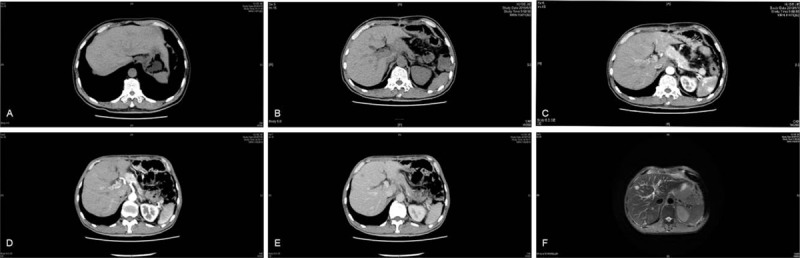
(A) CT scan showing probable gastric cancer because of irregular thickening of the wall of cardia with no liver metastasis. (B and C) CT scan showing postoperative changes in gastric malignant tumors and the absence of liver metastasis, respectively. (D and E) CT scan showing a nodule in the right lobe of the liver. Enhancement degree of the liver nodule significantly increased in the arterial phase (D) and rapidly decreased in the venous phase (E). (F) Liver MRI showing a nodule of the right lobe of the liver (approximately 11 mm in diameter), and metastasis was considered. CT = computerized tomography, MRI = magnetic resonance imaging.
3. Discussion
Gastric MANEC is a rare malignant neuroendocrine tumor of the digestive system, particularly in the stomach. The histogenesis of adenocarcinoma with NEC remains unclear. The following 2 hypotheses are presented:
-
(1)
adenocarcinoma and NEC originated from 2 different cell lines. The adenocarcinoma cells originated from multipotent stem cells whereas NEC cells were from embryonic neural cells. Two distinct precursor cells were produced synchronously.
-
(2)
Adenocarcinoma and NEC originated from single endoderm multipotent stem cells. Tumor development is affected by hormones, local microenvironment, and unstable genome, which eventually lead to bidirectional or multi-directional differentiation.[2,3,10]
Marinos et al[7] reported a case of MANEC and found squamous cell carcinoma in addition to the 2 components of adenocarcinoma and NEC. Therefore, we believe that the pathogenesis of mixed cancer belongs to the latter.
Endoscopy and imaging examination are the main diagnostic methods of gastrointestinal tumors. However, distinguishing preoperative gastroscopy from adenocarcinoma is occasionally difficult due to the few samples and the heterogeneity of tumor tissues. In the case presented, preoperative gastroscopy and biopsy were diagnosed as adenocarcinoma. However, postoperative pathology was confirmed as MANEC, indicating that preoperative endoscopic biopsy has definite limitations on the diagnosis of MANEC. Postoperative histopathology and immunohistochemistry are the gold standards for the diagnosis of MANEC.
Given the rare occurrence of gastric MANEC, its biological behavior remains controversial at present. The biological behavior of MANEC largely depends on the cell types with evident heterogeneity in tumor tissues.[4] Kim et al[6] believed that NEC often invades lymphatic vessels and vascular lumen due to its invasive biological behavior and metastasizes to the lymph nodes and liver during the early stage. Moreover, the biological behavior of MANEC is similar to that of NEC. Lee et al[5] showed that the characteristics of adenocarcinoma components affect the well-differentiated neuroendocrine components.
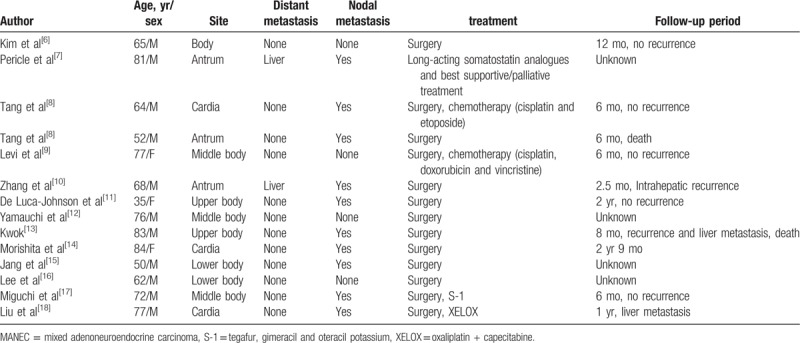
According to the review of relevant literature (Table 1),[6–18] 14 patients were diagnosed with gastric MANEC based on surgical operation, which can be combined with chemotherapy and targeted therapy. However, their adjuvant chemotherapy options are different. Lee et al[16] proposed that the treatment should focus on the invasive histological components due to the mixed components of the tumor. For neuroendocrine components with good differentiation or low malignancy, chemotherapy should be concentrated on the adenocarcinoma. In the case of small or large cell NEC, the neuroendocrine component should be the primary target of treatment. A study[19] reported that cisplatin combined with irinotecan can cover adenocarcinoma and NEC. Kwok[13] recommended the use of cisplatin combined with etoposide for the treatment of patients with distant metastasis of gastric MANEC and believed that platinum-based chemotherapy should be the first-line treatment for advanced diseases. According to the NCCN guidelines, XELOX (capecitabine + oxaliplatin) is the preferred adjuvant chemotherapy for patients with gastric cancer after R0-D2 resection (Class 1 recommended). In the present case, the patient underwent XELOX chemotherapy for 4 cycles after receiving radical gastrectomy, and liver metastasis occurred after 4 months. Combined with Table 1, the analysis in this study suggests that the distant metastasis of gastric MANEC often occurs in the liver. This phenomenon is related to the hematogenous metastasis of tumor cells. Moreover, postoperative histopathology indicates that patients with lymph node metastasis and tumor emboli in vessels may have a considerable risk of liver metastasis. Adjuvant chemotherapy may be an important factor affecting the prognosis of gastric MANEC, which includes adenocarcinoma and NEC. Lymph nodes and liver metastasis are usually invaded by the NEC rather than the adenocarcinoma component.[10,19,20] Whether or not XELOX can cover both components remains unknown. Nie et al[21] concluded that the differentiation of NEC and adenocarcinoma cells in gastric MANEC can affect the prognosis of patients; a low degree of cell differentiation results in poor prognosis of patients. Lee et al[22] reported that histopathological features and tumor staging of neuroendocrine components are the factors influencing prognosis. Volante et al[23] indicated that when neuroendocrine tumor components are well differentiated, the prognosis of mixed carcinoma is determined by the adenocarcinoma component. By contrast, when neuroendocrine components are poorly differentiated, the prognosis of mixed carcinoma depends on NEC. Furthermore, different patients may have varied sensitivities to XELOX chemotherapy. Rug dosage, preoperative treatment, and nutritional status of patients may also have an effect on the prognosis. Therefore, when clinical workers select first-line adjuvant chemotherapy for patients with gastric MANEC, they should maintain a relatively cautious attitude toward XELOX regimen. Owing to the absence of statistical significance of the case report and the failure to provide differentiation degree for the 2 tumor components, the suitability of XELOX regimen for postoperative first-line adjuvant chemotherapy in patients with gastric MANEC needs further confirmation.
Table 1.
An overview of 14 cases of gastric MANEC in the literature.
In conclusion, a case of liver metastases in patients with gastric MANEC who received 4 cycles of XELOX adjuvant chemotherapy is presented, and relevant literature is reviewed to provide information on this treatment for clinical workers. Gastric MANEC is a rare malignant tumor, and its biological behavior, pathological features, clinical treatment, and prognosis must be further studied.
Author contributions
Zhixian Lin: contributed to the conception of the article and wrote the manuscript; Jiangfeng Chen: contributed significantly to analysis and manuscript preparation; Yong Guo: contributed significantly to modify the article.
Conceptualization: Zhixian Lin.
Data curation: Jiangfeng Chen.
Validation: Yong Guo.
Writing – original draft: Zhixian Lin, Jiangfeng Chen.
Writing – review and editing: Yong Guo.
Footnotes
Abbreviations: bid = twice a day, CT = computerized tomography, MANEC = mixed adenoneuroendocrine carcinoma, MRI = magnetic resonance imaging, NEC = neuroendocrine carcinoma, WHO = World Health Organization.
The study was funded “The 13th Five-Year plan” Zhejiang Provincial Key Discipline Construction Project of Traditional Chinese Medicine (Integrated traditional Chinese and Western Medicine) (2017-XK-A09) and Zhejiang Famous Traditional Chinese Medicine Guo Yong Academic Experience Inheritance and Specialist Construction Fund Project (Zhejiang TCM 201728).
The authors have no conflicts of interest to declare.
References
- [1].Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 4th ed.2010;Lyon: International Agency for Research on Cancer, 417. [Google Scholar]
- [2].Paniz Mondolfi MA, Slova D, Fan W, et al. Mixed adenoneuroendocrine carcinoma (MANEC) of the gallbladder: a possible stem cell tumor? Pathol Int 2011;61:608–14. [DOI] [PubMed] [Google Scholar]
- [3].Scardoni M, Vittoria E, Volante M, et al. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology 2014;100:310–6. [DOI] [PubMed] [Google Scholar]
- [4].Capella C, La Rosa S, Uccella S, et al. Mixed endocrine exocrine tumors of the gastrointestinal tract. Semin Diagn Pathol 2000;17:91–103. [PubMed] [Google Scholar]
- [5].Lee JH, Kim HW, Kang DH, et al. A gastric composite tumor with an adenocarcinoma and a neuroendocrine carcinoma: a case report. Clin Endosc 2013;46:280–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim KH, Lee HJ, Lee SH, et al. Mixed adenoneuroendocrine carcinoma in the stomach: a case report with a literature review. Ann Surg Treat Res 2018;94:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pericle M, Toumpanakis C, Lumgair H, et al. Gastric mixed adenoneuro- endocrine carcinoma with a trilineage cell differentiation: case report and review of the literature. Case Rep Oncol 2012;5:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tang Q, Zhou Z, Chen J, et al. Correlation of metastasis characteristics with prognosis in gastric mixed adenoneuroendocrine carcinoma: two case reports. Medicine (Baltimore) 2017;96:e9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Levi SG, Carboni F, Valle M, et al. Mixed adenoneuroendocrine gastric carcinoma: a case report and review of the literature. J Gastric Cancer 2014;14:63–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang W, Xiao W, Ma H, et al. Neuroendocrine liver metastasis in gastric mixed adenoneuroendocrine carcinoma with trilineage cell differentiation: a case report. Int J Clin Exp Pathol 2014;7:6333–8. [PMC free article] [PubMed] [Google Scholar]
- [11].De Luca-Johnson J, Zenali M. A previously undescribed presentation of mixed adenoneuroendocrine carcinoma. Case Rep Pathol 2016;2016:9063634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yamauchi H, Sakurai S, Nakazawa N, et al. A case of mixed adenoneuroendocrine carcinoma of the stomach with focal intestinal metaplasia and hypergastrinemia. Int Surg 2015;100:562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kwok CM. Mixed adenoneuroendocrine carcinoma of the stomach. Case Rep Gastroenterol 2015;9:241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Morishita Y, Sugitani M, Sheikh A, et al. Collision tumor of the stomach: a rare case of an adenocarcinoma and carcinoid tumor. Arch Pathol Lab Med 2005;129:407–9. [DOI] [PubMed] [Google Scholar]
- [15].Jang KY, Moon WS, Lee H, et al. Gastric collision tumor of large cell neuroendocrine carcinoma and adenocarcinoma – a case report. Pathol Res Pract 2010;206:387–90. [DOI] [PubMed] [Google Scholar]
- [16].Lee HH, Jung CK, Jung ES, et al. Mixed exocrine and endocrine carcinoma in the stomach: a case report. J Gastric Cancer 2011;11:122–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Miguchi M, Iseki M, Shimatani K. Advanced gastric neuroendocrine carcinoma with an adenocarcinoma component. Case Rep Gastroenterol 2012;6:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu JY, Yang XL, Zhang JP. A case of apatinib in the treatment of gastric mixed sex gland neuroendocrine carcinoma. Chinese tumor clinic 2017;44:1005. [Google Scholar]
- [19].Strosberg JR, Coppola D, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas 2010;39:799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Okita NT, Kato K, Takahari D, et al. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric Cancer 2011;14:161–5. [DOI] [PubMed] [Google Scholar]
- [21].Nie L, Li M, He X, et al. Gastric mixed adenoneuroendocrine carcino-ma: correlation of histologic characteristics with prognosis. Ann Diagn Pathol 2016;25:48–53. [DOI] [PubMed] [Google Scholar]
- [22].Lee EJ, Park SM, Maeng L, et al. Composite glandular-endocrine cell carcinomas of the stomach: clinicopathologic and methylation study. APMIS 2005;113:569–76. [DOI] [PubMed] [Google Scholar]
- [23].Volante M, Rindi G, Papotti M. The grey zone between pure(neuro) endocrine and non-(neuro) endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch 2006;449:449–506. [DOI] [PubMed] [Google Scholar]


