Abstract
The flowering phenology of early-blooming plants is largely determined by snowmelt timing in high-latitude and high-altitude ecosystems. When the synchrony of flowering and pollinator emergence is disturbed by climate change, seed production may be restricted due to insufficient pollination success. We revealed the mechanism of phenological mismatch between a spring ephemeral (Corydalis ambigua) and its pollinator (overwintered bumblebees), and its impact on plant reproduction, based on 19 years of monitoring and a snow removal experiment in a cool-temperate forest in northern Japan. Early snowmelt increased the risk of phenological mismatch under natural conditions. Seed production was limited by pollination success over the 3 years of the pollination experiment and decreased when flowering occurred prior to bee emergence. Similar trends were detected on modification of flowering phenology through snow removal. Following snowmelt, the length of the pre-flowering period strongly depended on the ambient surface temperature, ranging from 4 days (at greater than 7°C) to 26 days (at 2.5°C). Flowering onset was explained with an accumulated surface degree-day model. Bumblebees emerged when soil temperature reached 6°C, which was predictable by an accumulated soil degree-day model, although foraging activity after emergence might depend on air temperature. These results indicate that phenological mismatch tends to occur when snow melts early but subsequent soil warming progresses slowly. Thus, modification of the snowmelt regime could be a major driver disturbing spring phenology in northern ecosystems.
Keywords: Bombus, global warming, phenological mismatch, pollinator, snowmelt, spring ephemeral
1. Background
The phenology of diverse organisms has changed in response to ongoing global warming [1–3]. If the environmental cues determining phenological events differ or the sensitivity to environmental cues varies among species, phenological synchrony between interacting species may be disturbed by climate change [4,5]. Plant–pollinator interactions are a key mutualism in terrestrial ecosystems. Phenological mismatch disrupts these mutualistic relationships when the temporal overlap of flowering and pollinator activity is decreased by phenological modifications, and it may result in population declines in plants and/or insects [4]. The possibility of plant–pollinator phenological mismatch with changing climate is widely discussed. Significant phenological mismatch was reported between specific plants and pollinators in some studies [6,7], while less significant or unclear trends were found in other studies that examined assemblages of interacting species [3,8,9]. This discrepancy suggests that phenological mismatch can occur between particular interacting species but broader assemblages are more robust [9,10]. Although phenological shifts in response to climatic change are well known, our knowledge about the mechanism and ecological impacts of phenological mismatch is more limited [10–12].
In addition to the analyses of historical records and long-term monitoring of phenologies of interacting species, experimental regulations of phenologies are effective approaches to test the occurrence of phenological mismatch [10,12,13]. Several experimental studies investigated this using artificial regulation of flowering phenology [14–16], while experimental studies controlling the timing of pollinator emergence are limited [17]. Furthermore, the ecological significance of phenological mismatch in terms of fitness of interacting species is rarely evaluated [7,14]. To better understand the prevalence and impact of phenological mismatch given ongoing environmental change, it is crucial to clarify the factors governing the phenological responses of interacting species and evaluate the effect of mismatch on fitness.
Synchrony of interacting species is sensitive to climate fluctuations, especially when development occurs rapidly during short growing seasons, and so even small differences in phenological responses may cause significant mismatch. Flowering phenology in arctic, alpine and boreal ecosystems is strongly influenced by warming [16,18]. Furthermore, the vulnerability of phenological events varies temporally, and spring phenologies are most susceptible to climate fluctuations [1,2,14,19]. Spring ephemerals, which have a short growing period between snowmelt and canopy closure of overstorey vegetation, grow fast and have potentially high reproductive activity [20], but their pollination success is a primary factor limiting seed production [20,21]. They are therefore most at risk from such a phenological mismatch.
Bumblebees (Bombus spp., Apidae) are important pollinators for many plant species in temperate, alpine and subarctic ecosystems [22]. In early spring, overwintered queens visit spring ephemerals for nectar before establishing the colony, and the timing of queen bee emergence can strongly affect the pollination success of early-blooming plants [6,7]. Subsequent colony development determines the amount of floral resources (pollen and nectar) required, and the availability of floral resources during the colony development influences the number of workers and production of new queen and male bees [22]. This cascade effect forms the link between flowering phenology and plant and pollinator populations [21]. Any degradation of phenological matching between spring ephemerals (as a nectar resource) and queen bees may therefore have negative impacts, not only on the pollination success of spring ephemerals, but also on colony development and its subsequent pollination service to late-blooming, bumblebee-pollinated plants.
Our previous study [7] conducted in natural cool-temperate forests of Japan reported that flowering onset of a spring ephemeral (Corydalis ambigua) and emergence of queen bees were related in different ways to the timing of snowmelt. The phenological mismatch between them increased with earlier snowmelt time when flowering onset was accelerated more rapidly than queen bee emergence, resulting in lower pollination success in early springs [7]. Since that study was based on the observation of natural populations without any experimental treatment, the determinants of flowering phenology and emergence timing of queen bees were not clearly defined, and any generalization regarding the impacts of phenological mismatch on pollination service to spring ephemerals was limited.
In the present study, in addition to long-term monitoring of natural conditions (19 years), we conducted a snow removal experiment to manipulate flowering phenology of C. ambigua for 3 years in order to reveal the mechanism of phenological mismatch and its ecological impacts on pollination success. The aims of this study were as follows. (1) Record the spring phenology of C. ambigua and its queen bee pollinator and describe the relationship between the snowmelt timing, degree of phenological mismatch and seed production, using (a) long-term monitoring data and (b) experimental manipulation of snowmelt. (2) Clarify the environmental cues that determine flowering onset and queen bee emergence and the mechanism of phenological mismatch. We hypothesized that the flowering phenology of the spring ephemeral is determined by the combination of snowmelt timing and subsequent ambient surface temperature, while the emergence of bumblebees from hibernation may be determined by the soil temperature that overwintering bees experience [23].
2. Methods
(a). Study site and system
This study was conducted in a natural deciduous forest in Nopporo (43°25′ N, 143°32′ E), Hokkaido, northern Japan. This forest is located on a flat area at 50–75 m elevation (electronic supplementary material, figure S1). Snow usually covers the ground from early December to early April, and the soil does not freeze at this time due to the insulating layer of snow; maximum winter snow depth is 80–100 cm. Annual mean air temperature is 7.1°C, ranging from −6.3°C (January) to 20.6°C (August), and annual precipitation is 930 mm. Leaf emergence of canopy trees usually occurs in mid-May, and the understorey is shaded by closed canopy until mid-October. From the snowmelt in April to canopy closure in late May, flowering of spring bloomers progresses sequentially among species, including Adonis ramose, Petasites japonicus var. giganteus, Corydalis ambigua, Trillium apetalon and Anemone flaccida, in that order.
Corydalis ambigua Chem. Et Schlecht (Papaveraceae) is a common spring ephemeral species in northern Japan. Each plant produces one or two inflorescences and each of the three to 20 zygomorphic flowers has a spur in which nectar collects. There are some variations in flower colour but it is commonly mauve or purple. This species is self-incompatible and dominantly visited by bumblebees [24]. Shoots emerge soon after snowmelt, flowering season is usually from mid-April to early May, and aboveground parts die after seed dispersal in late May. Thus, it has a typical life history of spring ephemerals. It is a perennial, non-clonal species.
Queens of the bumblebee Bombus hypocrita sapporoensis Cockerell, a major pollinator of C. ambigua, usually emerge from hibernation coincident with flowering of this plant [7,20]. Due to high nectar production and formation of dense populations, C. ambigua is the most important nectar resource for queen bees soon after emergence [21]. Queen bees usually suck nectar by perforating spurs of flowers and seldom visit legitimately but they are an available pollinator owing to accidental pollen removal and deposition during nectar robbing [24]. It has been shown that B. hypocrita carried out about 90% of pollinator visits to C. ambigua flowers, and the remaining 10% of visitors were queens of B. ardens sakagamii and B. diversus tersatus [25].
(b). Monitoring of plants and pollinators
Monitoring of the flowering period of C. ambigua and date of first emergence of queen bees was conducted during 1999–2017. Flowering phenology was observed within a 20 m × 20 m area in the central part of a large population (greater than 1 ha). At the same time, seed-set rates under natural pollination were recorded for 30–60 plants randomly selected every year except for 2004. During the flowering period, the number of flowers of tagged plants was recorded and all fruits (pods) were harvested before seed dispersal. Sampled pods were carefully opened in the laboratory, and the number of mature seeds and undeveloped ovules were counted. Seed-set rate at the inflorescence level was calculated as a ratio of matured seed number to total ovule number. Individual flowers have 9.1 ovules on average, ranging from 4 to 14. Ovule production of aborted flowers was estimated from the mean number of ovules per pod of the same inflorescence.
The emergence of queen bees was observed by walking along a 1.2 km trail in the forest providing access to the study site (electronic supplementary material, figure S1). Searching for bee emergence started when snow melted at the trail, and normally we carried out a survey every other day, but not when it was rainy, snowy or cool (less than 5°C). Observation was conducted by 1–3 people (including G.K.), and observation periods were continued until the first queen bee was observed along the trail. We used 1–3 h each time to search for flower visitation or flying queen bees and for foraging scars on C. ambigua flowers along the trail. Since C. ambigua is the earliest major nectar resource for overwintered bees, the first detection of nectar robbing scars reflects the time of emergence from hibernation when flowering occurred ahead of bee emergence. There may be some time lag between the time of emergence and the start of nectar robbing. However, we assumed that the time-lag effect would be small because we commonly detected first flying and robbing scars on the same day or robbing scars prior to flying, but seldom flying prior to robbing scars when flowering of C. ambigua had started. This suggests quick learning of nectar robbing soon after emergence. Before the flowering in the study site, we carefully checked C. ambigua flowers blooming at the forest edges, where, due to earlier snowmelt, flowering progresses earlier than in the central part of the forest. Before the onset of flowering of C. ambigua even in the forest edges, only Petasites japonicus var. giganteus (Compositae) is available as a floral resource for queen bees, although visits of queen bees to this species are occasional. Thus, we also carefully checked flowers of this species for bee presence before the flowering of C. ambigua.
Air temperature (at 1.5 m) and soil temperature (at 5 cm depth) were recorded at the automatic weather station (see electronic supplementary material, figure S1) at 1 h intervals since 2010 using a datalogger (Hobo, Onset Co., USA). The air temperature sensor was shielded from direct solar radiation.
(c). Snow removal experiment
We conducted a 3-year snow removal experiment from 2014 to 2016. In November 2013, we randomly selected three locations within a 50 m × 50 m site in a large C. ambigua population, and marked a pair of fixed plots at each location (i.e. six plots in total; electronic supplementary material, figure S1). Within each pair these were randomly allocated to control (C1 to C3) and snow removal treatments (R1 to R3). These treatments were conducted at exactly the same plots throughout the experimental period. The plot size was 5 m × 5 m, which is fairly large for manipulative experiments. Plot size was decided to be as large as practically possible to avoid the strong edge effect common with smaller plots [26], such as limitations of the effect of snow removal (e.g. wind-enhanced refilling of snow back onto the plot; or flooding from melting of surrounding snow [27], with potential subsequent freezing thus creating an ice layer; or insufficient area to enable adequate soil response to exposure to subsequent air temperature), insufficient number of flowering plants to study and limited pollinator attraction (small floral patch size may not be attractive to bees [28]). The plots in each pair were approximately 3 m apart at their closest edge, and the pairs were approximately 35 m from each other. Since overwintering buds of C. ambigua are located around soil-surface at the time of snowmelt, development of shoots after snowmelt may be influenced by surface temperatures. A data logger (Tidbit V2, Onset Co., USA) was therefore fixed at the centre of each plot to record hourly soil-surface temperature. The logger sensors were set under the litter layer to shield from solar radiation.
Snow was removed from the plots (electronic supplementary material, figure S2) by manual shovelling with a spade, leaving 10 cm remaining to protect plants under snow, and removing an area 50 cm wider than the plot border, to avoid potential edge effects. In 2014 snow was removed in mid-February, but subsequent snowfall refilled the plots and it was necessary to remove snow again in mid-March. In 2015 and 2016, snow removal was therefore carried out only once a year in mid-March, which was sufficient. Surface temperature under snow was continuously kept around 0–1°C throughout the winter irrespective of snow depth in this site. The snow removal treatment of this study therefore did not influence the thermal conditions during the snow-covered period. Snowmelt timing was determined for each plot as the date when the surface temperature suddenly rose above 0–2°C and began to fluctuate (see electronic supplementary material, figure S3).
To test whether the timing of flowering in the removal plots was purely dependent on snowmelt timing, we did not apply the removal treatment in 2017, but conducted all plant and bee observations as described below. Since there were no significant differences in the control and removal plots for any of the measured variables in 2017 (see descriptions below), all six plots were thus treated statistically as intact controls in that year.
After snowmelt, we counted the number of inflorescences during the flowering period in each plot at 1–4-day intervals. We randomly selected 20 plants producing inflorescences in each plot before flowering and marked them with numbered tags, recorded the number of flowers opening at 1–4-day intervals, and harvested pods at fruiting before seed dispersal. Seed-set rates were measured as mentioned above.
To clarify the potential seed-set ability of plants without pollen limitation, we conducted a hand-pollination treatment in 2014–2016 for plants growing outside of the experimental plots in order to minimize the artificial disturbance of the experimental plots. We selected 20 plants arbitrarily at flowering within a fixed 5 m × 5 m area (HP plot, electronic supplementary material, figure S1), and hand-pollinated all flowers using pollen from multiple (3–5) plants more than 5 m from the recipient plants (and not from the control or removal plots). Then, the seed-set rates were measured as mentioned above.
In 2016, we observed the bumblebee visitation frequency during flowering for 1–3 h on clear days, for 11 days in total, between 5 April and 9 May. We selected the plot with the densest inflorescences in each observation day (R1 and R2 in the early flowering season, and subsequently C1 and C2), and counted the bumblebee visits to the plot per hour.
(d). Analysis
Linear regressions were used to analyse the relationship between date (as day of year, DoY) of snowmelt and flowering onset or bee emergence, or phenological mismatch in the long-term dataset (1999–2017). Mismatch (in number of days) was calculated as the date of flowering onset in the study area minus that of bee emergence in the forest (negative value when flowering occurred prior to bee emergence). Variation in naturally pollinated seed-set (seed/ovule ratio per inflorescence) in response to mismatch was analysed with a generalized linear model (GLM) with a binomial error distribution and logit-link function in which mismatch was the explanatory variable.
Flowering progress within the experimental plots was fitted to a unimodal function of DoY using a GLM with a Poisson error distribution and log-link function, in which the number of open inflorescences was the response variable and DoY with a quadratic term was the explanatory variable. Based on this function, we defined (1) the flowering onset as the DoY on which the number of open inflorescences reached 10% of that plot's maximum inflorescence number for that year, and (2) the flowering period as the length of time (in days) that the inflorescence number was greater than 10% of the total inflorescence number of a plot. The end of flowering was therefore defined as the DoY when the number of flowers decreased to 10% of the maximum plot value. We used these estimated values of flowering properties for the analyses instead of observed values since our observation frequency was not consistent within and across flowering seasons. The relationship between flowering onset and flowering period within plots was analysed by the comparison of determination coefficient (R2) across plots and years.
We analysed the effects of snow removal on flowering onset, mismatch, and seed-set using generalized linear mixed-effect models (GLMMs). We set two random intercepts in the GLMMs; the first term is location of each pair of control and removal plots (electronic supplementary material, figure S1) and the second term is year (2014–2017) in which treatment (control, snow removal) is nested. We incorporated the nested random effect because our experimental design was not balanced throughout the years (i.e. snow removal treatment was not performed in 2017 and all of the six plots were used as a control treatment in that year), after checking that there were no differences between the control and removal plots in any measured parameter for 2017. In the pre-analysis for 2017 data, we conducted a GLMM for each of flowering onset, mismatch, and seed-set to test that there were no differences between the values for the control and removal plots in 2017. However, there were potential limitations in our experimental design in terms of the small number of levels for random effects and unbalanced allocation of treatments to the experimental plots over years. These limitations might reduce the statistical power, but results obtained in our analyses seemed to adequately reflect the patterns that we detected in the experiment. Variation in the flowering onset of individual plants and mismatch were explored using a GLMM with a gamma error distribution and log-link function in which treatment was the explanatory variable. Since mismatch varied from −9 to 11 days among plants, observed values of mismatch were transformed into positive values by adding 10 for fitting to a gamma distribution model. Variation in seed set was analysed by GLMMs with a binomial error distribution in which effects of treatment and mismatch were separately analysed because these variables are collinear. First, the effect of treatment on seed set was analysed. Then, the effect of mismatch on seed set was analysed for each treatment.
The extent that seed-set was pollen limited was tested by comparing seed-set of hand-pollinated (n = 16–19) and naturally pollinated plants (in control plots, n = 20 per plot) using a GLMM with a binomial error distribution in which treatment (hand pollination, control) and year (2014–2016) were explanatory variables, and plot (HP, C1, C2, C3) was incorporated as a random factor.
The temperature dependence of the pre-flowering period (i.e. the number of days between snowmelt and flowering onset) in the experimental plots was determined with a linear regression between the pre-flowering period and mean daily surface temperature during the pre-flowering period. Furthermore, we calculated the accumulated degree-days (DD) for flowering onset from snowmelt day to flowering onset day in every plot, using a threshold value of 1°C, since the surface was maintained around 0–1°C before snowmelt (electronic supplementary material, figure S3).
Similarly, we evaluated the relationship between the date at which the soil attained a given temperature (within the range of 5–7°C) and the date of first bee observation during 2010–2017. The temperature giving the smallest mean deviation from observed emergence dates was selected as the determinant for bee emergence (i.e. threshold mean temperature estimator). We also calculated accumulated DD for emergence from soil data using a 2°C threshold temperature since soil was maintained below 2°C before snowmelt (electronic supplementary material, figure S3). Using the mean accumulated DD over 8 years, we calculated the expected bee emergence day with reference to the soil temperature record in each year. Comparing the deviation between observed bee emergence day and estimated emergence day by the threshold mean temperature or accumulated DD estimator, we evaluated which estimator best fitted the emergence date.
After hibernation, however, the foraging activity of bumblebees is likely to be weather dependent, and that may also affect the timing of first observation. We therefore tested the temperature dependence and seasonal progress of bee activity using 2016 flower visitation data, with a GLM with a Poisson error distribution, where number of bee visits per plot per hour was the response variable, and air temperature and DoY were explanatory variables.
All statistical analyses were performed using an open source system, R version 3.4.4 (R Development Core Team, 2018, https://www.r-project.org). We conducted GLMs using the R function ‘glm’, and GLMMs using the R function ‘glmer’ in the library of ‘lme4’ for the analyses. Wald test (binomial and Poisson distribution) or t-test (Gamma distribution) was performed to test for significance in the GLMs and GLMMs.
3. Results
(a). Phenological mismatch under natural conditions
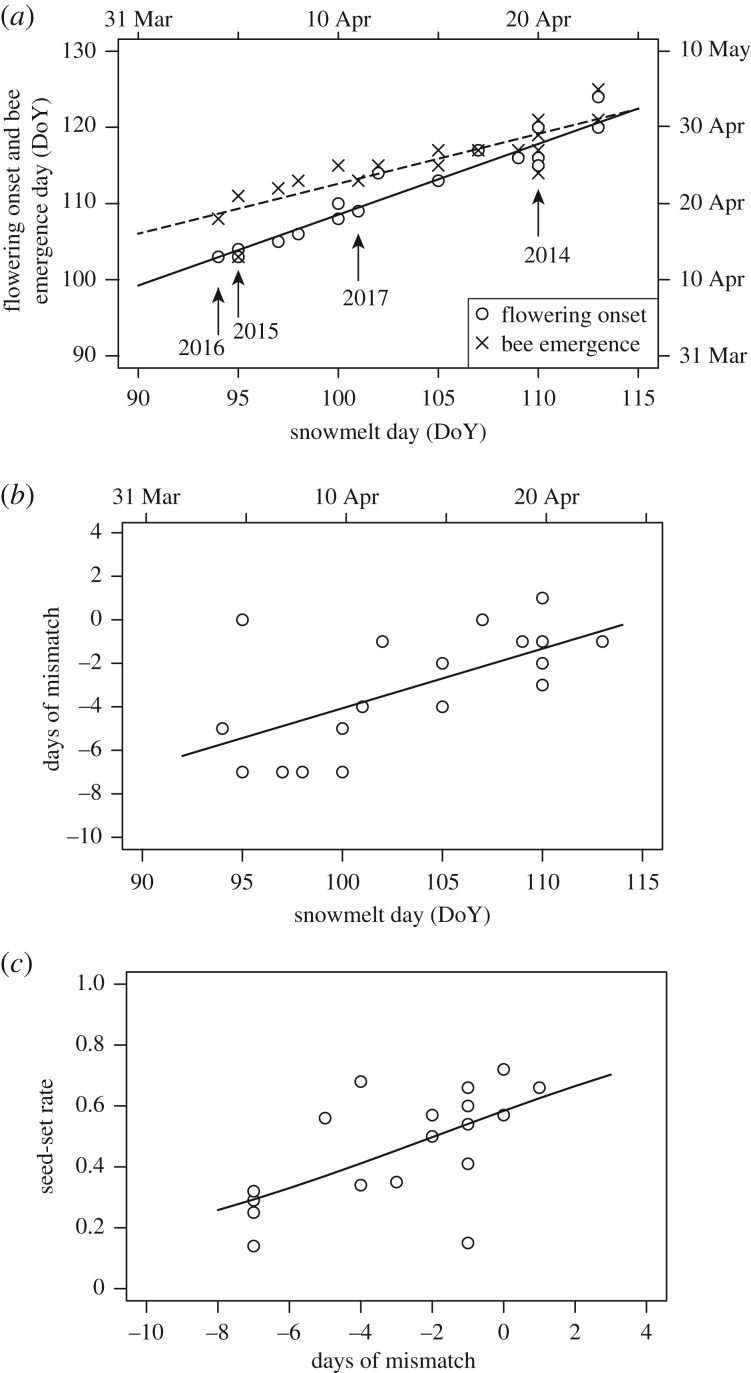
The 19-year monitoring dataset revealed that both flowering onset of C. ambigua and first emergence day of bumblebees occurred earlier when snow melted earlier (R2 = 0.91 and 0.72, d.f. = 17, p < 0.001, respectively; figure 1a; electronic supplementary material, figure S4). However, the slope of the regression line was steeper for flowering onset. As a result, phenological mismatch was larger in early snowmelt years (R2 = 0.39, d.f. = 17, p = 0.002; figure 1b) in which flowering of C. ambigua started up to one week earlier than bee emergence. Seed-set with natural pollination varied depending on the extent of mismatch (d.f. = 17, z = 9.22, p < 0.001 by GLM; figure 1c), and was about 60% when mismatch was small, but decreased to around 30% with 7-day mismatch.
Figure 1.
(a) The relationship between date of snowmelt (day of year) and flowering onset of Corydalis ambigua (solid line and circles) and bumblebee emergence (dashed line and crosses), (b) the relationship between snowmelt and phenological mismatch between flowering onset and bee emergence, and (c) the relationship between phenological mismatch and seed-set rate for 19 years (1999–2017). Linear regression lines (a,b) and a logistic regression curve obtained by GLM (c) are shown. R2 = 0.91, p < 0.001 for flowering onset and R2 = 0.72, p < 0.0001 for bee emergence in (a); R2 = 0.39, p = 0.002 in (b); z = 9.22, p < 0.001 in (c).
(b). Responses of flowering phenology and reproduction to snow removal
During the experimental period (2014–2017), snowmelt timing in control plots varied from year to year; ranging from 30 March to 24 April (electronic supplementary material, table S1). Following manual removal, snowmelt was advanced by 12–28 days (electronic supplementary material, table S1 and figure S4).
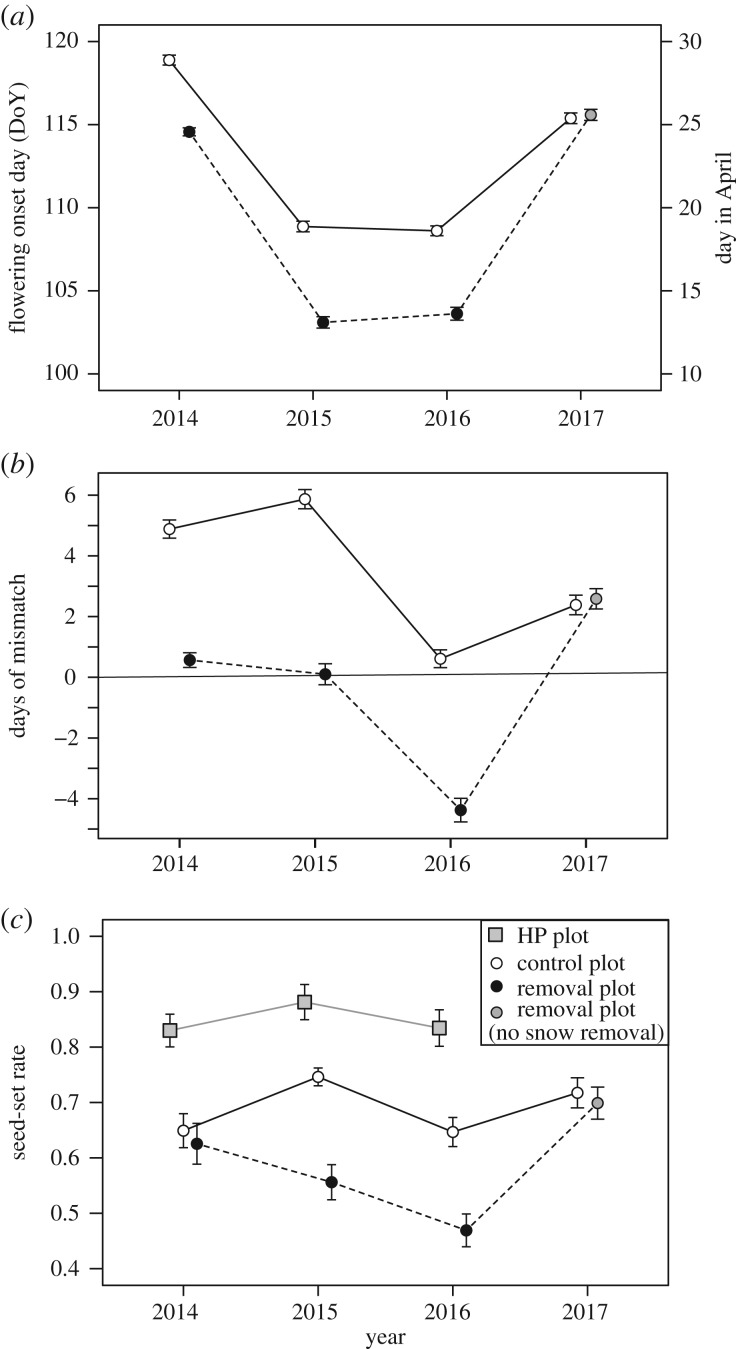
In the pre-analysis for 2017 data in which the snow-removal treatment was not performed, GLMMs of flowering onset, mismatch and seed-set revealed that all of the variables did not differ significantly between the control and removal plots, although mean seed-set rates tended to be larger in the control plots (d.f. = 114, t = 0.4, p = 0.66 for flowering onset, d.f. = 114, t = 0.42, p = 0.67 for mismatch days, and d.f. = 115, z = −1.87, p = 0.06 for seed-set rate). Thus, data from all plots in 2017 were considered as controls for subsequent analyses. Flowering onset in the control plots varied from 12 April to 27 April among years (electronic supplementary material, table S1). Snow removal advanced flowering onset by 5.1 days on average, ranging from 3 to 8 days (figure 2a; electronic supplementary material, figure S5). Flowering onset varied significantly between the treatments (p < 0.0001; electronic supplementary material, table S2a). The length of flowering periods varied from 15 to 24 days across plots and years, and was extended when flowering started early in the season (flowering period length in relation to flowering onset, R2 = 0.72; see electronic supplementary material, figure S6 for details). In the controls, flowering started after bee emergence in 2014, 2015, and 2017, but concurrently with emergence in 2016 (figure 2b; see also electronic supplementary material, figure S5). In the removal treatment, however, flowering started concurrently with bee emergence in 2014 and 2015, but before emergence in 2016, and so mismatch varied significantly between treatments (p < 0.0001; electronic supplementary material, table S2b).
Figure 2.
(a) Flowering onset (day of year), (b) phenological mismatch (days) between flowering onset and bee emergence, and (c) seed-set rate of the hand-pollinated plants (HP), control plots and snow removal plots during the experimental period (2014–2017). The snow removal treatment was conducted during 2014–2016, and all plots were used as control in 2017. Mean ± s.e. See electronic supplementary material, table S2 for statistical results.
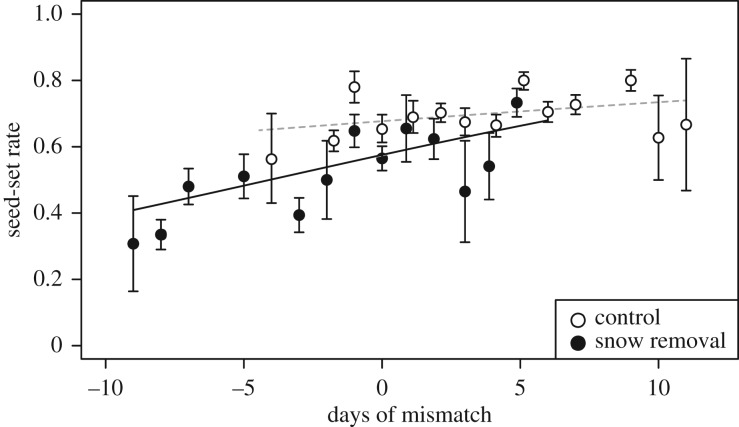
Seed-set in hand-pollinated plants was 83–88% (figure 2c), indicating a high potential seed-set in C. ambigua. Seed-set with natural pollination was 65–74%, and therefore 16–23% lower than that of hand-pollinated plants (p < 0.0001; electronic supplementary material, table S3); both varied among years. The GLMM revealed that seed-set success with natural pollination was significantly lower in the removal treatment than control (p = 0.013; electronic supplementary material, table S2c). The effect of mismatch on seed-set was apparent when flowering occurred prior to bee emergence in both of the treatments as shown in figure 3 (p < 0.0001; electronic supplementary material, table S2c).
Figure 3.
The relationship between phenological mismatch and seed-set rates of individual plants in the control (open circles and dashed line) and snow removal treatments (closed circles and solid line). Mean ± s.e. Data during the experimental period (2014–2017) are pooled. A logistic regression curve is indicated separately for each treatment.
(c). Environmental cues for flowering phenology and bee emergence
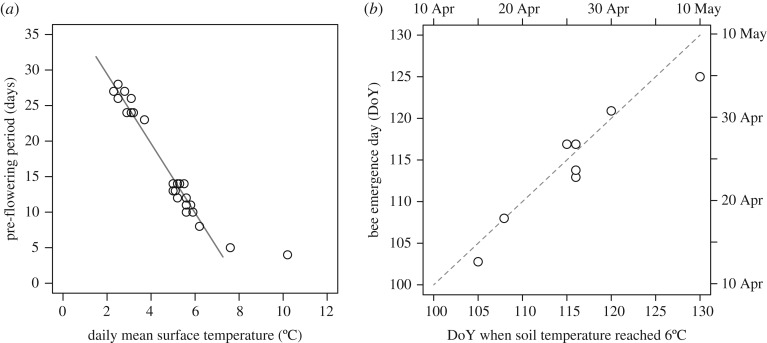
The length of pre-flowering period of C. ambigua was highly correlated with surface temperature within the range 2–7°C (R2 = 0.97, d.f. = 21, p < 0.0001; figure 4a). Pre-flowering period was shortened by 4.9 days per 1°C warming; it took 26 days at 2.5°C and 15 days at 5°C, while it took only 4–5 days at greater than 7°C. Therefore, flowering onset was strongly determined by the timing of snowmelt and subsequent ambient temperature. Accumulated surface DD for flowering onset calculated for every plot was 49.4 ± 7.7 degree-days (mean ± s.d.; electronic supplementary material, table S4). We also calculated accumulated soil DD for control plots (because soil temperature was measured under snow-intact condition); it was 31.2 ± 10.5 degree-days and more variable than that using surface temperature.
Figure 4.
(a) The relationship between daily mean surface temperature and pre-flowering days after snowmelt in the experimental plots, and (b) the relationship between the date on which soil temperature attained 6°C and first observation date of bumblebees during 2010–2017 in the study forest. In (a) a linear regression between surface temperature and pre-flowering period was performed for the range of 2–7°C; y = −4.9x + 39.2, R2 = 0.96, p < 0.0001. In (b) the dashed relationship represents the 1 : 1 line.
In the analysis of threshold mean temperature for bee emergence, the date when soil temperature reached 6°C best described the first observation date of bumblebees (R2 = 0.88; figure 4b), with a deviation of 1.0 ± 2.4 (mean ± s.d.) days between expected and observed values (electronic supplementary material, table S5). Accumulated DD for bee emergence calculated from the soil temperature was 29.1 ± 10.8 degree-days. Correlation of the accumulated soil DD and the observed bee emergence date (R2 = 0.89) had an even lower deviation, i.e. 0.0 ± 3.0 days (electronic supplementary material, table S5), indicating that the bee emergence was best predicted by accumulated soil temperature. We also estimated bee emergence date using accumulated air temperature (with 2°C threshold value): the deviation from the observed emergence date was −1.25 ± 3.3 days. This indicates that accumulated soil temperature is a better estimator of bee emergence date than accumulated air temperature.
During the observation of bumblebee foraging activity in 2016, the first bee was sighted on 18 April in the forest, but on that date, no bees were observed visiting the plots despite flowering onset in all plots (electronic supplementary material, figure S7). Visitation frequency at the plots peaked 4 days later on 22 April, and continuous visits were observed after that. Bee visitation frequency significantly increased with ambient air temperature (d.f. = 8, z = 5.19, p < 0.0001) and seasonal progress (d.f. = 8, z = 2.05, p = 0.041).
4. Discussion
Our long-term monitoring of the flowering onset of a spring ephemeral, emergence of queen bees and seed-set success clearly indicate that the phenological events were strongly related to the timing of snowmelt. These trends were confirmed by the snow removal experiment. The flowering phenology of C. ambigua was determined by snowmelt date and subsequent ambient temperature, while bee emergence seemed to depend on belowground temperature although foraging activity was influenced by air temperature. The phenological mismatch between the spring ephemerals and their pollinators might occur when soil warming progresses slowly after snowmelt due to cooler ambient temperatures.
(a). Importance of snowmelt timing as a trigger of phenological mismatch
Timing of snowmelt is an important predictor of spring events in high-latitude and high-altitude environments both for plants and insects [9]. This is because spring phenologies are strongly determined by the thermal requirements of various organisms and snow creates a specific thermal environment at the local scale. Due to the snow's insulation, the soil and surface at our site was maintained constantly at 0–2°C throughout the winter (electronic supplementary material, figure S3). After spring snowmelt, the surface is abruptly exposed to fluctuating air temperature and quickly warms, while the soil gradually warms with smaller daily fluctuations. Thus, there is a time lag for soil warming after snowmelt (electronic supplementary material, figure S8), and this difference between the rate of warming of the surface and soil appears to be the driving factor behind the phenological mismatch. During the experimental period in this study, 30 accumulated degree-days were attained 7 days later in the soil than at the surface when snow melted early in April (2015–2017), while there were only 3 days difference when snow melted after mid-April (2014). This indicates that the time lag for soil warming would be larger in spring with early snowmelt.
Phenological mismatch between interacting species may occur when the species use different environmental cues as a determinant of phenological events or when responsiveness to a specific cue is different between species [5,29]. Although the spring emergence of pollinators may shift earlier in response to warmer spring temperatures and earlier snowmelt in high-latitude and high-altitude ecosystems [8,9], little is known regarding the environmental determinants of their emergence after hibernation. Overwintered queen bees are known to emerge when soils reached 5–9°C, depending on species [23]. In this study, the date when soil attained 6°C was closely related to the bee emergence date in the forest, although accumulated soil temperature was a more reliable predictor of bumblebee emergence rather than a single soil temperature (see electronic supplementary material, table S5), similar to what has been reported for trap-nesting bee emergence [17].
Since soils gradually warm after snowmelt (electronic supplementary material, figure S3), bee emergence timing may be more synchronous than that of flowering. The threshold temperature and/or effective degree-days may be species-specific; B. hypocrita sapporoensis is the earliest bumblebee species in this region, and may have a lower thermal requirement to break diapause than other bumblebee species. Even after emergence, however, foraging activity of bumblebees is influenced by the weather, and cool conditions decrease flower visitation frequency. In 2016, bee emergence occurred 4 days later than expected from the accumulated soil DD estimator (electronic supplementary material, figure S8). This might be explained by cool air for several days (less than 6°C) before emergence and so activity of bees that had ended their hibernation might have been lower than normal. Thus, both the timing of diapause termination and the weather at that time (and shortly after) affect the availability of pollinators in early spring. Furthermore, thermal conditions of the soil may also vary with micro-topography, snowmelt date, and depth of soil in which bumblebees are overwintering. This variation may also explain some of the discrepancies between predicted and observed bee emergence dates. We need more information on the overwintering ecology of bumblebees for a greater understanding of the determinants of emergence.
Pre-flowering period of C. ambigua is highly air temperature dependent, and ranged from 4 days at greater than 7°C to 26 days at 2.5°C. Similarly, earlier flowering onset than pollinator emergence is reported in a subalpine meadow of the Rocky Mountains, since higher threshold temperature for diapause termination of bees was required than that for development of early bloomers [17]. As air temperature generally increases as the season progresses in spring, the pre-flowering period becomes shorter when snowmelt is delayed, and this may buffer the yearly variation in flowering time caused by the fluctuation of snowmelt date [16]. However, spring temperatures often vary daily and only a few warm days can rapidly advance plant phenology. Therefore, both snowmelt timing and the subsequent air temperature are important environmental cues for flowering phenology of spring ephemerals.
(b). Ecological significance of phenological mismatch between plant and pollinators
Despite many studies of phenological shifts with a warmer climate, there are only a few studies examining the effects of mismatch on plant reproduction [7,14,15]. As hand-pollinated plants in our study had continuously high seed-set, any variation in seed-set with natural pollination reflected pollination failure. Our study clearly demonstrated that phenological mismatch between flowering onset and bee emergence strongly related to the seed-set success of C. ambigua, and indicates that risk of mismatch is higher in years with earlier snowmelt. The strong impact on fitness seen here may be more apparent in specialist relationships than generalist relationships between interacting species [12]. Overwintering bumblebee queens are specialist pollinators for C. ambigua, which is self-incompatible and relies on visitation by queen bees for seed production. These specific biological situations make the pollination relationship between C. ambigua and bumblebees sensitive to phenological variation.
Our experiment, however, may have overestimated the negative effect of mismatch if the early appearance of relatively small flowering patches (i.e. in the snow removal plots) occurred earlier than flowering in the general area, making them unapparent or less attractive for bumblebees. As shown in figure 3, plants of the snow removal plots tended to have lower seed-set success than control plants even with the same number of days of mismatch. This might reflect the negative effect of isolated patches (i.e. Allee effect; a positive effect of density). This bias in the snow removal experiment (i.e. small flowering patches available in the snow removal treatment) may be more important in determining the plant seed-set results rather than mismatch per se. Such an intrinsic limitation in the experimental control of flowering phenology is outlined by Forrest [10]. Even though our experiment involves some artificial bias, however, its results clearly reflect the pattern observed in natural conditions (figure 1c).
The length of the flowering period depended on the date of flowering onset; the flowering period was longer when flowering started earlier (electronic supplementary material, figure S6a). This variation might reflect a seasonal trend in pollination success because pollinated inflorescences terminate their flowering quickly, while unpollinated inflorescences extend their flowering period to increase pollination success [30]. Thus, the longer flowering period in early-flowering plots might be caused by low pollination success due to phenological mismatch. The flowering period of C. ambigua lasted two to three weeks, while the extent of mismatch was usually less than 10 days. Nevertheless, only several days' mismatch significantly decreased seed-set when flowering occurred prior to bee emergence; the potential ability of seed production may decrease daily, due to rapid physiological ageing in spring ephemerals [31,32]. If so, the extension of the flowering period cannot fully compensate for seed-set success when flowering occurs earlier than pollinator emergence. Also in our experiment, seed-set tended to decrease with an increase in flowering period at the plot level (electronic supplementary material, figure S6b).
(c). Implications of phenological mismatch in spring ephemerals
Our study predicts that the risk of mismatch may increase if snowmelt starts occurring earlier. Spring ephemerals are particularly vulnerable as their high potential reproduction may be limited by insufficient pollination, thereby reducing seed production [7,25]. Experimentally limited seed supply decreased a C. ambigua population within several years (G.K. 2019, unpublished data) and limits the distributions of several understorey herbs [33]. Thus, a continuous reduction in pollination may decrease seed production, restrict seedling establishment and change population dynamics if the frequency of mismatch increases with earlier springs [34].
Since bumblebees are generalist pollinators, they can select any available plant species suitable for resources [22]. However, spring ephemerals are important floral resources for overwintered queens soon after hibernation [6,21], and early-season floral resources affect the establishment and development of colonies [35,36]. Corydalis ambigua is a very important nectar resource in spring due to its dense populations in the deciduous forest ecosystem as well as its large nectar production [21]. Any degradation of C. ambigua populations in the foraging site would therefore be detrimental for bumblebees.
At the same time, the possibility of adaptive evolution of flowering onset to climatic change should be considered [37]. If seed-set success is related to flowering phenology, selective forces should act on flowering onset to maintain phenological matching with pollinator emergence. The possibility of genetic adaptation of flowering phenology to climate change may depend on the life history of individual species, and it is expected to be high in short-lived species with sufficient genetic variation. Furthermore, phenotypic variation in phenological traits is large in species inhabiting a range of climate conditions, such as along an elevational gradient (reviewed in [38]). Corydalis ambigua is a relatively short-lived perennial plant. It grows in a range of snowmelt conditions and timing of flowering varies among local populations [7]. Thus the sensitivity of mismatch to climate also varies among populations, and local adaptation in flowering phenology may be possible. Evaluation of the selective forces acting on phenological traits and the possibility of evolutionary responses to climate change are therefore important issues in global change biology.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Y. Amagai, M.A. Lorig, Y. Mizunaga, K. Onizawa, and Kai-Hsiu Chen for their help in the field, and to S. Johnson, J. Forrest and three anonymous reviewers for their valuable comments and revisions of the earlier version of this manuscript.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.q4fm37m [39].
Authors' contributions
G.K. and E.J.C. planned this study; G.K. led the field manipulations and survey, data analyses and writing the manuscript; E.J.C. contributed in the field and with writing the manuscript.
Competing interests
We have no competing interests.
Funding
This study was supported by funding from JSPS KAKENHI (15H02641, 17K07551) to G.K. and by a mobility grant by UiT to E.J.C. to visit Japan in 2013–2014.
References
- 1.Menzel A, et al. 2006. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976. ( 10.1111/j.1365-2486.2006.01193.x) [DOI] [Google Scholar]
- 2.Parmesan C. 2007. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 13, 1860–1872. ( 10.1111/j.1365-2486.2007.01404.x) [DOI] [Google Scholar]
- 3.Ovaskainen O, Skorokhodova S, Yakovleva M, Sukhov A, Kutenkov A, Kutenkova N, Shcherbakov A, Meyke E, Delgado MM. 2013. Community-level phenological response to climate change. Proc. Natl Acad. Sci. USA 110, 13 434–13 439. ( 10.1073/pnas.1305533110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Memmott J, Craze P, Waser NM, Price MV. 2007. Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 10, 710–717. ( 10.1111/j.1461-0248.2007.01061.x) [DOI] [PubMed] [Google Scholar]
- 5.Thackeray SJ, et al. 2016. Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. ( 10.1038/nature18608) [DOI] [PubMed] [Google Scholar]
- 6.Thomson JD. 2010. Flowering phenology, fruiting success and progressive deterioration of pollination in an early-flowering geophyte. Phil. Trans. R. Soc. B 365, 3187–3199. ( 10.1098/rstb.2010.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo G, Ida TY. 2013. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 94, 2311–2320. ( 10.1890/12-2003.1) [DOI] [PubMed] [Google Scholar]
- 8.Bartomeus I, Ascher JS, Wagner D, Dandorth BN, Colla S, Kornbluth S, Winfree R. 2011. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc. Natl Acad. Sci. USA 108, 20 645–20 649. ( 10.1073/pnas.1115559108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iler AM, Inouye DW, Høye TT, Miller-Rushing AJ, Burkle LA, Johnston EB. 2013. Maintenance of temporal synchrony between syrphid flies and floral resources despite differential phenological responses to climate. Glob. Change Biol. 19, 2348–2359. ( 10.1111/gcb.12246). [DOI] [PubMed] [Google Scholar]
- 10.Forrest JRK. 2015. Plant–pollinator interactions and phenological change: what can we learn about climate impacts from experiments and observations? Oikos 124, 4–13. ( 10.1111/oik.01386) [DOI] [Google Scholar]
- 11.Hegland SJ, Nielsen A, Lázaro A, Bjerknes A-L, Totland Ø. 2009. How does climate warming affect plant–pollinator interactions? Ecol. Lett. 12, 184–195. ( 10.1111/j.1461-0248.2008.01269.x) [DOI] [PubMed] [Google Scholar]
- 12.Rafferty NE, CaraDonna PJ, Bronstein JL. 2015. Phenological shifts and the fate of mutualisms. Oikos 124, 14–21. ( 10.1111/oik.01523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton EM, Rafferty NE. 2017. Plant-pollinator interactions under climate change: the use of spatial and temporal transplants. Appl. Plant Sci. 5, 1600133 ( 10.3732/apps.1600133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gezon ZJ, Inouye DW, Irwin RE. 2016. Phenological change in a spring ephemeral: implications for pollination and plant reproduction. Glob. Change Biol. 22, 1779–1793. ( 10.1111/gcb.13209) [DOI] [PubMed] [Google Scholar]
- 15.Rafferty NE, Ives AR. 2011. Effects of experimental shifts in flowering phenology on plant–pollinator interaction. Ecol. Lett. 14, 69–74. ( 10.1111/j.1461-0248.2010.01557.x) [DOI] [PubMed] [Google Scholar]
- 16.Gillespie MAK, Baggesen N, Cooper EJ. 2016. High Arctic flowering phenology and plant–pollinator interactions in response to delayed snow melt and simulated warming. Environ. Res. Lett. 11, 115006 ( 10.1008/1748-9326/11/11/115006) [DOI] [Google Scholar]
- 17.Forrest JRK, Thomson JD. 2011. An examination of synchrony between insect emergence and flowering in Rocky Mountain meadows. Ecol. Monogr. 81, 469–491. ( 10.1890/10-1885.1) [DOI] [Google Scholar]
- 18.Inouye DW. 2008. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89, 353–362. ( 10.1890/06-2128.1) [DOI] [PubMed] [Google Scholar]
- 19.Badeck FW, Bondeau A, Böttcher K, Doktor D, Lucht W, Schaber J, Sitch S. 2004. Responses of spring phenology to climate change. New Phytol. 162, 295–309. ( 10.1111/j.1469-8137.2004.01059.x) [DOI] [Google Scholar]
- 20.Kudo G, Ida TY, Tani T. 2008. Linkage between phenology, pollination, photosynthesis, and plant reproduction in deciduous forest understory plants. Ecology 89, 321–331. ( 10.1890/06-2131.1) [DOI] [PubMed] [Google Scholar]
- 21.Inari N, Hiura T, Toda MJ, Kudo G. 2012. Pollination linkage between canopy flowering, bumble bee abundance and seed production of understory plants in a cool temperate forest. J. Ecol. 100, 1534–1543. (doi:10.1111-j.1365-2745.2012.02021.x) [Google Scholar]
- 22.Goulson D. 2010. Bumblebees. Behaviour, ecology, and conservation, 2nd edn New York, NY: Oxford University Press. [Google Scholar]
- 23.Alford DV. 1969. A study of the hibernation of bumblebees (Hymenoptera: Bombidae) in southern England. J. Anim. Ecol. 38, 149–170. ( 10.2307/2743) [DOI] [Google Scholar]
- 24.Higashi S, Ohara M, Arai H, Matsuo K. 1988. Robber-like pollinators: overwintered queen bumblebees foraging on Corydalis ambigua. Ecol. Entomol. 13, 411–418. ( 10.1111/j.1365-2311.1988.tb00373.x) [DOI] [Google Scholar]
- 25.Kudo G, Kasagi T. 2004. Floral sex allocation in Corydalis ambigua populations visited by different pollinators. Ecoscience 11, 218–227. ( 10.1080/11956860.2004.11682827) [DOI] [Google Scholar]
- 26.Kenkel NC, Podani J. 1991. Plot size and estimation efficiency in plant community studies. J. Veg. Sci. 2, 539–544. ( 10.2307/3236036) [DOI] [Google Scholar]
- 27.Rumpf SB, Semenchuk PR, Dullinger S, Cooper EJ. 2014. Idiosyncratic responses of high arctic plants to changing snow regimes. PLoS ONE 9, e86281 ( 10.1371/journal.pone.0086281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegland SJ. 2014. Floral neighbourhood effects on pollination success in red clover are scale-dependent. Funct. Ecol. 28, 561–568. ( 10.1111/1365-2435.12223) [DOI] [Google Scholar]
- 29.Donnelly A, Caffarra A, O'Neill BF. 2011. A review of climate-driven mismatches between interdependent phenophases in terrestrial and aquatic ecosystems. Int. J. Biometeorol. 55, 805–817. ( 10.1007/s00484-011-0426-5) [DOI] [PubMed] [Google Scholar]
- 30.Yasaka M, Nishiwaki Y, Konno Y. 1998. Plasticity of flower longevity in Corydalis ambigua. Ecol. Res. 13, 211–216. ( 10.1046/j.1440-1703.1998.00259.x) [DOI] [Google Scholar]
- 31.Lapointe L. 2001. How phenology influences physiology in deciduous forest spring ephemerals. Physiol. Plant. 113, 151–157. ( 10.1034/j.1399-3054.2001.1130201.x) [DOI] [PubMed] [Google Scholar]
- 32.Rothstein DE, Zak DR. 2001. Photosynthetic adaptation and acclimation to exploit seasonal periods of direct irradiance in three temperate, deciduous-forest herbs. Funct. Ecol. 15, 722–731. ( 10.1046/j.0269-8463.2001.00584.x) [DOI] [Google Scholar]
- 33.Ehrlén J, Münzbergova Z, Diekmann M, Eriksson O. 2006. Long-term assessment of seed limitation in plants: results from an 11-year experiment. J. Ecol. 94, 1224–1232. ( 10.1111/j.1365-2745.2006.01169.x) [DOI] [Google Scholar]
- 34.Miller-Rushing AJ, Høye TT, Inouye DW, Post E. 2010. The effects of phenological mismatches on demography. Phil. Trans. R. Soc. B 365, 3177–3186. ( 10.1098/rstb.2010.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crone EE, Williams NM. 2016. Bumble bee colony dynamics: quantifying the importance of land use and floral resources for colony growth and queen reproduction. Ecol. Lett. 19, 460–468. ( 10.1111/ele.12581) [DOI] [PubMed] [Google Scholar]
- 36.Ogilvie JE, Griffin SR, Gezon ZJ, Inouye BD, Underwood N, Inouye DW, Irwin RE. 2017. Interannual bumble bee abundance is driven by indirect climate effects on floral resource phenology. Ecol. Lett. 20, 1507–1515. ( 10.1111/ele.12854) [DOI] [PubMed] [Google Scholar]
- 37.Anderson JT, Inouye DW, McKinney AM, Colautti RI, Michell-Olds T. 2012. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc. R. Soc. B 279, 3843– 3852. ( 10.1098/rspb.2012.1051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forrest J, Miller-Rushing AJ. 2010. Toward a synthetic understanding of the role of phenology in ecology and evolution. Phil. Trans. R. Soc. B 365, 3101–3112. ( 10.1098/rstb.2010.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudo G, Cooper EJ. 2019. Data from: When spring ephemerals fail to meet pollinators: mechanism of phenological mismatch and its impact on plant reproduction Dryad Digital Repository. ( 10.5061/dryad.q4fm37m) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kudo G, Cooper EJ. 2019. Data from: When spring ephemerals fail to meet pollinators: mechanism of phenological mismatch and its impact on plant reproduction Dryad Digital Repository. ( 10.5061/dryad.q4fm37m) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.q4fm37m [39].