Abstract
Since the early 1990s, ocean temperatures have increased and blooms of the icthyotoxic dinoflagellate Cochlodinium polykrikoides (a.k.a. Margalefidinium polykrikoides) have become more widespread across the Northern Hemisphere. This study used high-resolution (1–30 km), satellite-based sea surface temperature records since 1982 to model trends in growth and bloom season length for strains of C. polykrikoides inhabiting North American and East Asian coastlines to understand how warming has altered blooms in these regions. Methods provided approximately 180× greater spatial resolution than previous studies of the impacts of warming on harmful algae, providing novel insight into near shore, coastal environments. Along the US East Coast, significant increases in potential growth rates and bloom season length for North American ribotypes were observed with bloom-favourable conditions becoming established earlier and persisting longer from Chesapeake Bay through Cape Cod, areas where blooms have become newly established and/or intensified this century. Within the Sea of Japan, modelled mean potential growth rates and bloom season length of East Asian ribotypes displayed a significant positive correlation with rising sea surface temperatures since 1982, a period during which observed maximal cell densities of C. polykrikoides blooms have significantly increased. Results suggest that warming has contributed, in part, to altering the phenology of C. polykrikoides populations, potentially expanding its realized niche in temperate zones of the Northern Hemisphere.
Keywords: climate change, Margalefidinium polykrikoides, Cochlodinium polykrikoides, harmful algae, sea surface temperature, ocean warming
1. Introduction
The combustion of fossil fuels has increased carbon dioxide (CO2) in the Earth's atmosphere, promoting an increase in ocean temperature [1]. One consequence of rising temperatures is the transformation of ecosystems, as they become more or less hospitable for individual organisms [1,2]. While investigations predicting the effects of rising temperature on marine life have been relatively common, several unknowns remain regarding the impacts of recent ocean warming on ocean organisms.
Many harmful algal blooms (HABs) have increased in their frequency, distribution and intensity in recent decades [3,4]. While such trends are due, in part, to enhanced observation, awareness and accelerated nutrient loading within coastal zones [5], the contributions of climate change to these phenomena remain poorly understood. Warming temperatures are predicted to have a large influence on HABs, in particular, their cellular growth rate [3,4]. The effects of temperature on HABs can be complex, however, altering the growing season [3,4], community composition [6], predator–prey interactions [7], and the uptake and utilization of nutrients [7,8]. Warmer sea surface temperatures in some locations have been associated with increases in growth and extended growing seasons for certain HABs [3,4,9]. While there is growing interest regarding the impacts of future climate change on HABs, few studies have assessed high-resolution, decadal trends in sea surface temperature that may be altering the phenology and growth of HABs.
The impacts of climate change on HABs are highly strain- and species-specific, whereby such changes may expand the realized niche of some phytoplankton while simultaneously contracting the suitable habitat of others [3,4,9]. For example, warmer temperatures that promote the growth and expansion of some toxic cyanobacteria inhibit the growth of other, non-toxic, eukaryotic algae [9]. In addition, some toxic diatom blooms (Pseudo-nitzschia spp.) have been attributed to cooler upwelled, nutrient-enriched water [10], but have also been associated with warmer, stratified conditions [11]. Beyond impacts on bloom growth, the harmful effects elicited by HABs are likely to change in warmer oceans [12,13]. Identification of strain- and species-specific trends of HABs is required to better predict their behavior and ecology in the future, climate-changed, environments.
First identified in 1895, blooms of Cochlodinium spp. (a.k.a Margalefidinium [14]) have been observed within the Northern Hemisphere for decades [15]. Blooms can wreak significant damage within coastal ecosystems, being highly lethal to a variety of marine life including finfish, shellfish and coral [15–18]. Widespread marine animal die-offs often follow dense blooms [17,19] that can contribute to significant economic losses [16]. While some controversy exists regarding the toxic mechanism associated with C. polykrikoides, the majority of studies indicate that the production of reactive oxygen species (ROS) by this alga cause some of the harmful effects [13,15,16]. Since the 1990s, C. polykrikoides blooms have become more widespread and increasingly intense [15]. Blooms within the southern reaches of the Chesapeake Bay have progressively intensified since the early 1990s [20] and blooms once non-existent along coastal New York (i.e. Long Island) are now annual occurrences [17,21]. Similarly, in East Asia, C. polykrikoides blooms have become increasingly widespread and problematic since the late 1980s and early 1990s [19]. While nutrient loading [17,20], transoceanic transport of cysts [22] and increased monitoring have all probably contributed to these trends [15], the effects of changing ocean temperatures on these events are unclear. Multiple studies have linked warmer temperatures to higher growth rates for this alga [15], but an understanding of how regional changes in sea surface temperatures may alter C. polykrikoides bloom phenology has been elusive.
The objective of this study was to identify how recent warming of coastal oceans has contributed to altering the potential growth rate and phenology of C. polykrikoides blooms. High-resolution temperature records (e.g. 1–30 km) were used to model trends in the growth rate and the bloom season length for C. polykrikoides along temperate regions of the Northern Hemisphere where blooms have recently intensified and expanded. Multiple radiometer records were used to create temperature-growth models for two strains of C. polykrikoides. Models were groundtruthed with long-term records of cell densities and bloom occurrences as well as in situ measurements of ocean temperatures.
2. Material and methods
(a). Temperature records
The National Oceanic and Atmospheric Administration's (NOAA) Optimum Interpolation Sea Surface Temperature (OISST) version 2 was used as one proxy for near-surface ocean temperature over the period 1982 to 2016. OISST v. 2 includes buoy, ship and satellite observations that are bias-corrected and then interpolated to supplement potential gaps and/or observations from missing regions. The resolution of this long-term record (¼ degree = 20×; approx. 30 km in mid-latitudes) is ideal for resolving regional trends, but may not be representative of local-scale trends. As HABs frequently occur on meso- to micro-scales, we also used a second high-resolution SST product capable of resolving more complex and fine-scale marine environments. The GHRSST Level 4 MUR Global Foundation Sea Surface Temperature Analysis v. 4.1 provides ultra-high (1 km) resolution, daily temperature records from 2002 to 2016. This 15-year record allows for observed trends and relationships to be compared to decadal or multi-year oscillations.
Using a high-resolution product allows for resolution of most, but not all, coastal and estuarine features. Hence, the GHRSST temperature product uses multiple sources of sea surface temperature data but relies primarily upon night-time retrievals of surface temperature from multiple, concurrent satellite instruments. Dependence upon night-time observations may induce a cold bias, leading to a slight underestimation of peak daily temperatures. Accordingly, station-based water temperature observations from United States Geological Survey (USGS) water-quality monitoring stations and the National Data Buoy Center (NOAA) were compared with GHRSST-based temperature records. In shallow, tidally mixed tributaries, GHRSST-based temperature records slightly underestimated maximum daily temperature during the growing season (electronic supplementary material, figures S1 and S2). Hence, while trends in overall growth remain unaffected, growth rates derived via GHRSST-based temperatures are likely to be conservative estimates of in situ growth within these habitat types. Still, the GHRSST provided approximately 180 times greater resolution than the OISST dataset at 40°N latitude, the approximate mid-point of our study latitudes, marking a significant improvement over prior approaches. For offshore and well-mixed estuarine locations, areas where C. polykrikoides blooms are common, temperature records closely matched station-based observations (electronic supplementary material, figures S1 and S2).
(b). Estimation of growth rates
Two distinct strains of C. polykrikoides were included in our analyses: the East Asian ribotype, indigenous to regions of Korea, Japan and China; and the American/Malaysian ribotype, indigenous to the Atlantic coast of the US, Malaysia and other regions [15]. Laboratory- and field-derived growth rates for both ribotypes of C. polykrikoides were obtained from previously published datasets [13,23–27] and used to generate temperature-growth curves. Experimental data points were re-sampled 10 000 times and each re-sampled experimental dataset was fitted with a fourth-order polynomial as per Moore et al. [3]. The curves were sorted and ranked by growth rate. Daily growth rates during the blooming season (e.g. June–October [15]) within the known species distribution for both ribotypes were calculated using each SST product to assess trends in growth over time. Days in which modelled daily growth exceeded 75% of maximum were considered to have the potential for bloom formation and were counted as days in the blooming season. A threshold of 75% of maximal growth was chosen as the threshold above which growth is most likely to offset cellular loss processes such as grazing pressure and other losses thus leading to an HAB [28]. In addition, the first and last date at which growth exceeded 75% of maximum rates were considered to be the start and termination of the bloom season.
The magnitude of trends in growth, blooming season and the timing of bloom initiation/termination were determined using the non-parametric Theil–Sen magnitude estimation method [29]. A corresponding Mann–Kendall test determined significance (S) of the observed trend [30]. Non-parametric methods were implemented to reduce the influence of non-normality, outliers and asymmetry (skewness) in the SST products and growth rate response data.
(c). Historical records of C. polykrikoides blooms
Records of cell densities from C. polykrikoides blooms along the South Korean peninsula were obtained from the Korean National Institute of Fisheries Sciences (NIFS; http://www.nifs.go.kr/red/news_2.red). Observations of maximum cell densities during annual blooms (1962, 1982–2016) were grouped by Korean province and/or major metropolitan area (e.g. Busan) and plotted versus time. Trend strength as well as significance was determined using Thiel–Sen magnitude estimation and Mann–Kendall tests respectively (see above).
3. Results
(a). US east coast
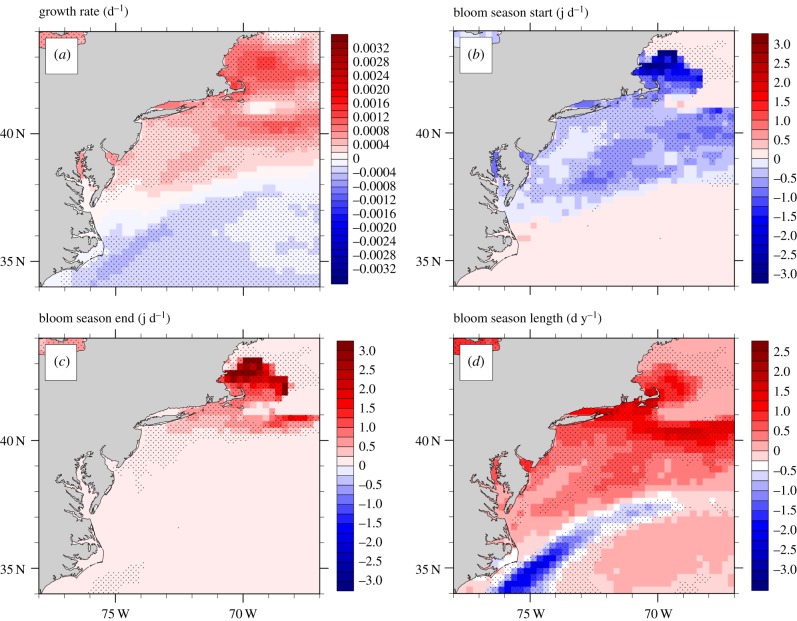
Model simulations using ¼ degree resolution temperature observations (1982–2016) revealed significant (p < 0.05; Mann–Kendall) increases in the potential growth rate of American/Malaysian ribotypes of C. polykrikoides across large expanses of the US East Coast (figure 1a) as sea surface temperatures have warmed. Specifically, along coastal areas of Massachusetts (MA), Rhode Island (RI), Connecticut (CT) and New York (NY), bloom season temperatures have increased by 0.68–1.36°C since 1982 and C. polykrikoides potential growth rates have exhibited significant (p < 0.05; Mann–Kendall) increases of up to 0.001–0.002 doublings day−1 year−1 (figure 1a). Less intense but significant (p < 0.05; Mann–Kendall) increasing trends in growth (0.0004–0.0008 doublings day−1 year−1) were also observed along New Jersey (NJ), within Delaware (DE), Maryland (MD) and as far south as Virginia (VA; figure 1a).
Figure 1.
Trends (1982–2016) in the (a) growth rate, (b) beginning of bloom-favourable conditions, (c) termination of bloom-favourable conditions and (d) bloom season length for C. polykrikoides (American/Malaysian ribotype) along the Eastern US using a standard resolution (¼ degree) data product. Stippling indicates regions where trends are statistically significant (p < 0.05; Mann–Kendall test). (Online version in colour.)
Beyond growth rate, the potential bloom phenology of C. polykrikoides has also changed along the US East Coast. Warming along coastal areas of MA, CT, RI and NY was found to establish bloom-favourable conditions earlier (figure 1b) and maintain them for longer in the season (figure 1c), resulting in increases of 1–2.25 days of the bloom season per year, corresponding to a season up to two months longer today compared with 1982 (figure 1d). A lesser, albeit significant (p < 0.05; Mann–Kendall), lengthening of the bloom season was found along coastal regions of NJ, DE, MD and VA with blooms occurring earlier, persisting later and the complete bloom season expanding by up to a month since 1982 (figure 1b–d). Along lower latitudes (e.g. less than 35°N) of the eastern US, bloom conditions were found to persist significantly (p < 0.05; Mann–Kendall) longer in some locations. Conversely, near to and within the Gulf Stream (approx. 35°N, 75°W–38°N, 70°W), significant (p < 0.05; Mann–Kendall) declines to the overall growing season of C. polykrikoides were observed whereby growth-favourable conditions were shortened by as much as 2.5 days year−1 (figure 1d). South of the Gulf Stream (less than 35°N), growth-favourable conditions were found to significantly increase (p < 0.05; Mann–Kendall; figure 1d), but for the same areas, significant (p < 0.05; Mann–Kendall) declines in annual potential growth rates were observed (figure 1a).
More recent (2002–2016), higher-resolution (1 km) temperature records confirmed the significant (p < 0.05; Mann–Kendall), positive effects of warming on potential cellular growth rates of American/Malaysian strains of C. polykrikoides across large regions of the northeast US (electronic supplementary material, figure S3a). Along coastal areas of RI, MA and NY including Narragansett Bay, Buzzards Bay, Vineyard Sound, Nantucket Sound, Long Island Sound, the Peconic Estuary and the southern lagoons of Long Island, bloom season temperatures have increased by 0.77–1.54°C since 2002 and simulated growth rates have increased by 0.001–0.002 doublings day−1 year−1 (p < 0.05; Mann–Kendall; electronic supplementary material, figure S3a). Higher-resolution temperature records also revealed that bloom-favourable conditions are now present for significantly (p < 0.05; Mann–Kendall) longer periods within multiple areas along southern New England, expanding by two weeks (southern and eastern bays of Long Island) to over a month (e.g. 35 days; Buzzard's Bay, MA) since 2002 (electronic supplementary material, figure S3b).
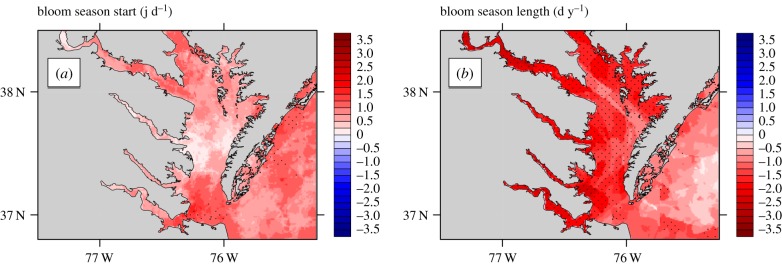
In the vicinity of Chesapeake Bay, high-resolution temperature records indicated potential growth rates within the James River and the southern-most extent of the estuary were maximal with an increasing trend since 2002 (figure 2a,b). Within the southern reaches of Chesapeake Bay (e.g. York River, James River) warming has advanced the potential bloom season by as much as 2.5 days year−1 (p < 0.05; Mann–Kendall; figure 3a). This has led to a significant (p < 0.05; Mann–Kendall) extension of bloom-favourable conditions of up to two and four weeks since 2002 (figure 3a,b).
Figure 2.
(a) Mean growth rate and (b) growth rate trend 2002–2016 within Chesapeake Bay using high-resolution (1 km) data product. Stippling indicates regions where trends are statistically significant (p < 0.05; Mann–Kendall test). (Online version in colour.)
Figure 3.
Trends (2002–2016) in (a) bloom season start and (b) bloom season length within Chesapeake Bay using high-resolution (1 km) data product. Stippling indicates regions where trends are statistically significant (p < 0.05; Mann–Kendall test). (Online version in colour.)
(b). Sea of Japan
Warming along northeast Asia has led to a predicted increase in growth rate and habitat range for East Asian strains of C. polykrikoides. For areas along the eastern coast of South Korea, simulated growth rates have significantly increased (p < 0.05; Mann–Kendall) by 0.001–0.004 doubling day−1 year−1 (figure 4a). Similarly, potential growth rates along the northern coastline of Japan (Kitakyushu to the island of Hokkaido) and into the Strait of Tartary and along the east coast of China were predicted to have increased by 0.0004–0.0016 doublings day−1 year−1 (p < 0.05; figure 4a). Warming temperatures along the South Korean peninsula (eastern coast) have the potential to result in significant seasonal advances of the bloom season (∼ 0.75 days year−1) with similar increases (p < 0.05; Mann–Kendall) observed along the northern coastlines of Japan and into the Strait of Tartary (figure 4b). In addition, significant (p < 0.05; Mann–Kendall) delays to the end of the potential growing season were predicted along the northern Sea of Japan and into the Strait of Tartary (figure 4c). Given these temporal shifts of the potential growing season, significant (p < 0.05; Mann–Kendall) increases in the total number of blooming days along both the west and east coasts of the Korean peninsula were observed with bloom conditions remaining present for 0.50–0.75 days longer year−1 or two to four weeks since 1982 (figure 4d). The bloom season within large regions of the Sea of Japan, western reaches of the Yellow Sea, the Bohai Sea and the East China Sea were also found to significantly (p < 0.05; Mann–Kendall) increase by 0.25–1.25 days year−1 or two to six weeks since 1982 (figure 4d).
Figure 4.
Trends (1982–2016) in the (a) growth rate, (b) beginning of bloom-favourable conditions, (c) termination of bloom-favourable conditions, and (d) bloom season length for C. polykrikoides (East Asian ribotype) in East Asia using standard resolution (¼ degree) data product. Stippling indicates regions where trends are statistically significant (p < 0.05; Mann–Kendall test). (Online version in colour.)
For tropical latitudes along Eastern Asia (e.g. less than 30°N) simulated growing conditions were predicted to be established later (e.g. 0.15–0.25 days year−1) and subside earlier in the season (figure 4a,b), although trends were not statistically significant (p > 0.05; Mann–Kendall). Slight, but significant (p < 0.05; Mann–Kendall) increases in bloom season length occurred off the east coast of China (figure 4c, south of 30°N). For large regions south of Japan, however, significant declines in potential growth rates were observed (p < 0.05; Mann–Kendall; figure 4d).
(c). Trends in C. polykrikoides densities from South Korea
Bloom records (1962, 1982–2016) from South Korea demonstrate that over the past several decades, peak cell densities during blooms of C. polykrikoides within major metropolitan areas and three Korean provinces have undergone significant increases (all p < 0.05; Mann–Kendall) since observations began (electronic supplementary material, figure S4). Coastal waters around the city of Busan and the neighbouring province of Gyeongbuk have exhibited significant (τ = 0.536, n = 14, p < 0.001 and τ = 0.396, n = 12, p < 0.001, respectively) increases in bloom intensity with maximum cell densities now exceeding 3 × 104 and 1.5 × 104 cells ml−1, respectively (electronic supplementary material, figure S4a,b). While maximum densities reported from the southeastern province of Gyeongnam peaked at a few thousand cells ml−1 during the early 1990s, densities exceeding 3 × 104 cells ml−1 have become more common recently, a significant increase (τ = 0.301, n = 29, p < 0.01; electronic supplementary material, figure S4c). Similar patterns were observed for southern South Korean provinces (e.g. Jeonnam; n = 21, τ = 0.34, p < 0.01) whereby maximum densities during the early 1990s were consistently only a few thousand cells ml−1 whereas more recent events have exhibited an order of magnitude higher densities (e.g. 2 × 104 cells ml−1; electronic supplementary material, figure S4d).
4. Discussion
Coastal oceans along temperate, western boundaries of the North Atlantic and North Pacific have warmed and expanded the realized niche of two ribotypes of C. polykrikoides. High- and low-resolution temperature-based model simulations predict that decadal warming trends have increased growth rates of this alga across large expanses of the Northern Hemisphere. In addition to growing faster, simulations indicate that conditions favourable for C. polykrikoides blooms are now established earlier and persist longer, resulting in an up to two-month increase in bloom season length since 1982 across large expanses of the northwest Atlantic and northwest Pacific Oceans.
Like temperature, nutrients can play a central role in the occurrence of phytoplankton blooms [5] and, thus, coastal eutrophication may be a contributing factor to C. polykrikoides blooms in our study areas. Improvements in the handling of wastewater and agricultural runoff during our study period (1982–2016) have led to stable or, in some cases, decreasing nutrient loads in many of our regions of interest [31–33]. Despite these trends, C. polykrikoides blooms have progressively intensified since the 1980s. Hence eutrophication alone is probably not the primary driver of enhanced and more frequent blooms of C. polykrikoides in our study regions.
This study is supportive of several others that collectively suggest warming temperatures have and will support an expansion of some types of HABs [12]. For example, Moore et al. [3] suggested that as sea surface temperatures warm within Puget Sound (WA, USA), favourable conditions for the saxitoxin-producing dinoflagellate, Alexandrium catenella, will occur earlier and subside later in the season as critical temperature thresholds (e.g. 13°C) are breached sooner and sustained longer. Similarly, Gobler et al. [4] reported, that for large regions of the North Pacific and North Atlantic Oceans, decadal warming trends have made these regions conducive for the toxin-producing dinoflagellates, A. catenella and Dinophysis acuminata. The GHRSST approach pioneered to examine HABs in this study provided approximately 180 times greater resolution than the prior assessments of HABs at mid-latitudes, marking a substantial improvement over prior approaches [4]. The global expansion and increased intensities of these HABs represent substantial human-health risks, as biotoxins produced by these dinoflagellates can be harmful to individuals who come into direct contact with toxins or inadvertently consume them via contaminated seafood [34]. While toxicants produced by C. polykrikoides have no known adverse human-health effects, the expansion of these blooms may have large disruptive effects within coastal zones [17].
Ocean warming has not and, of course, will not lead to an increase in the distribution or intensity of all HABs in all places and a careful examination of the results presented here and in prior studies help emphasize this point. For example, in the current study, regions of the US. East Coast and Asia south of 35°N displayed declining growth rates for C. polykrikoides since 1982. Moreover, there are some regions, such as Chesapeake Bay, where the bloom season for C. polykrikoides has expanded since 1982 but has retracted for other HABs (e.g. A. catenella and D. acuminata; [4]). This highlights another important aspect of climate change and HABs, specifically that ocean warming will change the threat of HABs in specific ecosystems, diminishing the likelihood of some HABs, but increasing the likelihood of others. These changes can be a challenge to both medical communities that may face new human health challenges [35,36] and marine organisms and ecosystems that will be naive to prior exposure to newly occurring HABs, and thus potentially more vulnerable to these new events [37].
(a). Observational records of blooms
There are multiple reports from around the world of C. polykrikoides blooms becoming newly established and/or intensifying within locations identified in the current study as areas where warming has enabled an expansion of bloom season and growth since 1982. For example, C. polykrikoides in Narragansett Bay (RI) was first observed in the 1980s but has become progressively more intense (dem.ri.gov) [38] with the most widespread and dense bloom (e.g. 103–104 cells ml−1), to date, occurring in 2016 (dem.ri.gov). C. polykrikoides blooms with cell densities exceeding 104 cells ml−1 spread to nearby Judith Pond (RI) in the late 1990s [39]. Within Buzzards Bay (MA), C. polykrikoides blooms were not observed until 2005, but now are reported annually (buzzards.bay.org) [40]. Reports of C. polykrikoides have also recently (since around 2010) emerged within both Martha's Vineyard and Nantucket (MA) [41]. In NY, C. polykrikoides was never observed prior to 2004 despite robust monitoring (Suffolk County Department of Health Services, suffolkcountyny.gov, 1976–2016) but now blooms annually within multiple Long Island estuaries (e.g. Great South Bay, Shinnecock Bay, the Peconic Estuary, Long Island Sound) where densities annually exceed 104 cells ml−1 [13,17,22].
Reports of C. polykrikoides are now more common within the lower tributaries of Chesapeake Bay. C. polykrikoides was first documented in the York River in the 1960s, but since 1992 blooms have been observed in more locations (e.g. Lafayette, James and Elizabeth Rivers) where they have become frequent and intense [20]. Blooms historically peaking at only one or two thousand cells ml−1 [42] now exceed 2.8 × 104 cells ml−1, span several hundred km2, persist for over a month [20] and originate from the regions of Chesapeake Bay where modelled growth rates of C. polykrikoides were maximal and bloom seasons have significantly increased.
In South Korea, dinoflagellate blooms have been reported since 161 A.D., but C. polykrikoides and its associated fish-killing behaviour only became apparent during and after the 1990s [16,19]. Blooms are now frequent along much of the South Korean peninsula occurring in numerous estuaries [43,44]. South Korean bloom records since the 1980s reveal that maximum bloom densities have concurrently risen significantly within multiple Korean coastlines (p < 0.05; electronic supplementary material, figure S4). Blooms that had reached only a few thousand cells ml−1 now exceed 3 × 104 cells ml−1 (electronic supplementary material, figure S4). To the southwest, C. polykrikoides populations also appear to have expanded into coastal regions along the South China Sea [45]. Recent findings by Park et al. [46] reveal intra-specific succession of C. polykrikoides blooms driven, in part, by changing temperatures. Our findings suggest that continued warming in these regions may selectively promote strains that respond positively to warmer sea surfaces.
In southwestern Japan (e.g. Yatsushiro Sea), C. polykrikoides was first reported in 1977 and blooms have been common in the region since [44]. Cells originating from southeast Korea and/or southwestern Japanese estuaries (e.g. Western Kyushu, Japan) can be transported north in warm ocean currents (Tsushima) to eastern Korean provinces (e.g. Gyeongbuk, etc.) and eastward into the Western Sea of Japan where they recently have begun to form dense blooms exceeding thousands of cells ml−1 [43,44]. While controversy exists regarding the ultimate source of blooms occurring along the northern shoreline of Honshu, Japan, C. polykrikoides was first observed in the Oki Islands and along the Tottori and Hyogo prefectures in 2002 with blooms now frequently occurring in both locales [44]. Recently, and for the first time, C. polykrikoides blooms were also identified in northern Japanese waters (e.g. [47]). While blooms have not been reported north of Japan, C. polykrikoides cysts have recently been recovered from the northern Sea of Japan where potential growth rates and bloom seasons have displayed the largest expansion in the region [48].
Within subtropical zones (e.g. 25–35°N) included in this study, bloom phenology was not found to change but significant declines in mean potential growth rates were observed, presumably as temperatures have risen to beyond levels optimum for growth (figures 1 and 4). Within these locales, there have not been reports of C. polykrikoides blooms and model simulations suggest that as warming continues, these areas will become increasingly less hospitable for C. polykrikoides. While there are reports of C. polykrikoides blooms from equatorial latitudes including the Philippines [49], Malaysia [50] and the Gulf of Oman [18], temperature-growth rates of C. polykrikoides strains from tropical or subtropical regions are presently unavailable. The formation of devastating blooms in these regions have yet to be fully understood and are likely driven by multiple factors beyond just temperature.
The mechanisms underlying the recent emergence of C. polykrikoides blooms within the Northern Hemisphere, to date, are not yet fully understood. While increased awareness of blooms, nutrient loading within coastal zones and translocation of cysts have all likely contributed to these phenomena [5,22], our findings suggest that recent warming can contribute to accelerated growth rates and expanded growing seasons, with both processes capable of facilitating the increasing bloom densities that have been reported in the literature [17,20,40]. For large areas within both the northeast US (e.g. Georges Bank, Cape Cod Bay, Hudson Shelf Valley, etc.) and East Asia (e.g. Bohai Sea, Northern Sea of Japan, Strait of Tartary, etc.) temperatures favourable for C. polykrikoides blooms have expanded its potential growth and/or growing season, but blooms have not been recorded. Multiple factors control phytoplankton blooms including temperature, light, nutrients, grazing, minimal advective loss and the presence of seed populations [28]. If one or more of these parameters are not within ideal ranges, blooms of C. polykrikoides are unlikely to occur. For example, while warming temperatures may promote a poleward expansion of C. polykrikoides blooms, a reduced photoperiod at higher latitudes may nonetheless limit growth [51]. Our findings suggest, however, that in areas where these conditions are favourable, blooms of C. polykrikoides could grow faster and persist longer today than they could have three decades ago.
(b). Impacts of climate change on marine phenology
While the ecological impacts of early warming have yet to be fully understood, there is evidence to suggest that differential responses among organisms may disrupt trophic interactions in some marine food webs [7]. Warming within the North Sea has led to an earlier seasonal peak in phytoplankton, while the emergence of copepod grazers has been less affected, an asynchrony that has led to decreases in size for some calanoid copepods, a key food source for commercially important cod fisheries [7]. Similar mismatch scenarios have been observed within the California Current and the eastern Wadden Sea where larval fish and shellfish are now spawned before seasonal increases in their primary food sources [52,53]. The impacts of an earlier C. polykrikoides bloom season have yet to be characterized, but vulnerable larval stages of many marine fish, bivalves and crustaceans are spawned during late spring to early summer and remain as larvae into mid-summer months [54]. Hence, earlier and longer-lasting blooms of C. polykrikoides may jeopardize vulnerable early life-stage organisms by making encounters with C. polykrikoides more likely [13]. For example, prolonged bloom seasons may increase the risk of exposure for Argopecten irradians (bay scallop) that are typically spawned in early summer and autumn [54].
(c). Impacts of warming on C. polykrikoides
Numerous investigations with multiple C. polykrikoides strains have demonstrated that as temperatures increase to an optimal level, growth rates become more rapid [13,23–26]. This study has identified specific geographical locales where warming temperatures correspond to increases in growth of C. polykrikoides. The ecosystem level impacts of such increases are not fully understood although recently it has been reported that the toxic effects of American/Malaysian ribotypes of C. polykrikoides on finfish and shellfish were less severe at higher temperatures (e.g. 24–28°C [13]). However, temperature-enhanced growth may lead to blooms that are increasingly intense and nonetheless lethal [13]. Given the lengthening bloom season, C. polykrikoides is now persisting longer into the autumn, a period of time during which water temperatures decline and lethal effects may become more severe [13].
Beyond growth and lethality, temperatures also influence chain-forming behavior of C. polykrikoides, with warmer temperatures (e.g. 24–27°C) promoting longer (e.g. more than four cells) chains and lower temperatures favouring singular cells [13,55]. Chain formation is common among many dinoflagellates and can enable enhanced vertical migration as well as protection from grazers [55]. For C. polykrikoides, studies have demonstrated the ingestion rates of four-cell chains by copepods are substantially lower than those of two- and single-cell populations [55]. Thus, warming trends that favour C. polykrikoides growth and chain formation may also lead to a suppression of top-down controls (i.e. grazing) during blooms that may, in turn, further promote their persistence and intensity.
Altogether, multiple temperature records used to simulate trends in the growth and phenology of C. polykrikoides blooms during the past several decades reveal that ocean warming has contributed to their expansion within multiple regions of the Northern Hemisphere. For both the American/Malaysian ribotype in northwest Atlantic and the East Asian ribotype in the northwest Pacific, growth rates have increased and bloom seasons have expanded. Blooms are now common in areas where they had not been observed previously and maximum recorded bloom densities have increased significantly. As climate change continues, C. polykrikoides blooms within temperate zones of the Northern Hemisphere are likely to continue to expand geographically, grow faster and persist for longer, representing a heightened threat to marine life.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.5s8k1pr [56].
Authors' contributions
C.J.G., O.M.D. and A.W.G. conceived and designed work. O.M.D. and A.W.G. gathered datasets and analysed data. All authors contributed equally to the preparation of the manuscript.
Competing interests
The authors have no competing interests.
Funding
The authors were supported by the Simons Foundation, New York Sea Grant (grant no. R-CMB-40), the Chicago Community Foundation and the Rauch Foundation.
References
- 1.Doney SC, et al. 2012. Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 4, 11–37. ( 10.1146/annurev-marine-041911-111611) [DOI] [PubMed] [Google Scholar]
- 2.Walther GR, et al. 2002. Ecological responses to recent climate change. Nature 416, 389–395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 3.Moore SK, Trainer VL, Mantua NJ, Parker MS, Laws EA, Backer LC, Fleming LE. 2008. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 7(Suppl. 2), S4 ( 10.1186/1476-069X-7-S2-S4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gobler CJ, Doherty OM, Hattenrath-Lehmann TK, Griffith AW, Kang Y, Litaker RW. 2017. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl Acad. Sci. USA 114, 4975–4980. ( 10.1073/pnas.1619575114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heisler J, et al. 2008. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8, 3–13. ( 10.1016/j.hal.2008.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinder SL, Hays GC, Edwards M, Roberts EC, Walne AW, Gravenor MB. 2012. Changes in marine dinoflagellate and diatom abundance under climate change. Nat. Clim. Change 2, 271–275. ( 10.1038/nclimate1388) [DOI] [Google Scholar]
- 7.Edwards M, Richardson AJ. 2004. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884. ( 10.1038/nature02808) [DOI] [PubMed] [Google Scholar]
- 8.Goldman JC, Carpenter EJ. 1974. A kinetic approach to the effect of temperature on algal growth. Limnol. Oceanogr. 19, 756–766. ( 10.4319/lo.1974.19.5.0756) [DOI] [Google Scholar]
- 9.Paerl HW, Huisman J. 2008. Blooms like it hot. Science 320, 57–58. ( 10.1126/science.1155398) [DOI] [PubMed] [Google Scholar]
- 10.Trainer VL, Hickey BM, Horner RA. 2002. Biological and physical dynamics of domoic acid production off the Washington coast. Limnol. Oceanogr. 47, 1438–1446. ( 10.4319/lo.2002.47.5.1438) [DOI] [Google Scholar]
- 11.McCabe RM, et al. 2016. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res. Lett. 43, 10 366–10 376. ( 10.1002/2016GL070023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu FX, Tatters AO, Hutchins DA. 2012. Global change and the future of harmful algal blooms in the ocean. Mar. Ecol. Progr. Ser. 470, 207–233. ( 10.3354/meps10047) [DOI] [Google Scholar]
- 13.Griffith AW, Gobler CJ. 2016. Temperature controls the toxicity of the icthyotoxic dinoflagellate, Cochlodinium polykrikoides. Mar. Ecol. Progr. Ser. 545, 63–76. ( 10.3354/meps11590) [DOI] [Google Scholar]
- 14.Gómez F, Richlen ML, Anderson DM. 2017. Molecular characterization and morphology of Cochlodinium strangulatum, the type species of Cochlodinium, and Margalefidinium gen. nov. for C. polykrikoides and allied species (Gymnodiniales, Dinophyceae). Harmful Algae 63, 32–44. ( 10.1016/j.hal.2017.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudela RM, Gobler CJ. 2012. Harmful dinoflagellate blooms caused by Cochlodinium sp.: global expansion and ecological strategies facilitating bloom formation. Harmful Algae 14, 71–86. ( 10.1016/j.hal.2011.10.015) [DOI] [Google Scholar]
- 16.Kim CS, Lee SG, Lee CK, Kim HG, Jung J. 1999. Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. J. Plankt. Res. 21, 2105–2115. ( 10.1093/plankt/21.11.2105) [DOI] [Google Scholar]
- 17.Gobler C, et al. 2008. Characterization, dynamics, and ecological impacts of harmful Cochlodinium polykrikoides blooms on eastern Long Island, NY, USA. Harmful Algae 7, 293–307. ( 10.1016/j.hal.2007.12.006) [DOI] [Google Scholar]
- 18.Richlen ML, Morton SL, Jamali EA, Rajan A, Anderson DM. 2010. The catastrophic 2008–2009 red tide in the Arabian gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 9, 163–172. ( 10.1016/j.hal.2009.08.013) [DOI] [Google Scholar]
- 19.Kim HG. 1998. Harmful algal bloom in Korean coastal waters focused on three fish-killing dinoflagellates. In Harmful algal blooms in Korea and China (eds Kim HG, Lee SG, Lee CK), pp. 1–20. Pusan, Republic of Korea: Pusan National Fisheries Research and Development Institute. [Google Scholar]
- 20.Mulholland MR. et al. 2009. Understanding causes and impacts of the dinoflagellate, Cochlodinium polykrikoides, blooms in the Chesapeake Bay. Estuaries Coasts 32, 734–747. ( 10.1007/s12237-009-9169-5) [DOI] [Google Scholar]
- 21.Griffith AW, Shumway SE, Gobler CJ. 2019. Differential mortality of North Atlantic bivalve molluscs during harmful algal blooms caused by the dinoflagellate, Cochlodinium (a.k.a. Margalefidinium) polykrikoides. Estuaries Coasts 42, 190–203. ( 10.1007/s12237-018-0445-0) [DOI] [Google Scholar]
- 22.Tang YZ, Gobler CJ. 2012. The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae 20, 71–80. ( 10.1016/j.hal.2012.08.001) [DOI] [Google Scholar]
- 23.Kim HC, Lee CK, Lee SG, Kim HG, Park CK. 2001. Physico-chemical factors on the growth of Cochlodinium polykrikoides and nutrient utilization. J. Korean Fish. Soc. 34, 445–456. [Google Scholar]
- 24.Lee CK, Kim HC, Lee SG, Jung CS, Kim HG, Lim WA. 2001. Abundance of harmful algae, Cochlodinium polykrikoides, Gyrodinium impudicum and Gymnodinium catenatum in the coastal area of South Sea of Korea and their effects of temperature, salinity irradiance, and nutrient on the growth in culture. J. Korean Fish. Soc. 34, 536–544. [Google Scholar]
- 25.Kim DI. 2004. Effects of temperature, salinity and irradiance on the growth of the harmful red tide dinoflagellate Cochlodinium polykrikoides Margalef (Dinophyceae). J. Plankt. Res. 26, 61–66. ( 10.1093/plankt/fbh001) [DOI] [Google Scholar]
- 26.Yamatogi T, Sakaguchi M, Iwataki M, Matsuoka K. 2006. Effects of temperature and salinity on the growth of four harmful red tide flagellates occurring in Ishaya Bay in Ariake Sound, Japan. Ippon Suisan Gakkaishi 72, 160–168. ( 10.2331/suisan.72.160) [DOI] [Google Scholar]
- 27.Doinsing JW. 2010. Effects of salinity, temperature and irradiance on the growth of Cochlodinium polykrikoides. Masters, Universiti Malaysia Sabah. [Google Scholar]
- 28.Smayda TJ. 2008. Complexity in the eutrophication–harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae 8, 140–151. ( 10.1016/j.hal.2008.08.018) [DOI] [Google Scholar]
- 29.Theil H. 1992. A rank-invariant method of linear and polynomial regression analysis. In Henri Theil's contributions to economics and econometrics, vol. 23 (eds Raj B, Koerts J), pp. 345–381. Dordrecht, Netherlands: Springer. [Google Scholar]
- 30.Mann HB. 1945. Nonparametric tests against trend. Econometrica 13, 245–259. [Google Scholar]
- 31.EPA. 1994. Long Island sound study: comprehensive conservation and management plan. Stamford, CT: US Environmental Protection Agency. [Google Scholar]
- 32.Boesch DF, Brinsfield RB, Magnien RE. 2001. Chesapeake Bay eutrophication. J. Environ. Qual. 30, 303–320. ( 10.2134/jeq2001.302303x) [DOI] [PubMed] [Google Scholar]
- 33.Anderson DM, Glibert PM, Burkholder JM. 2002. Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries 25, 704–726. ( 10.1007/BF02804901) [DOI] [Google Scholar]
- 34.Shumway SE. 1990. A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquacult. Soc. 21, 65–104. ( 10.1111/j.1749-7345.1990.tb00529.x) [DOI] [Google Scholar]
- 35.Backer LC. 2002. Cyanobacterial harmful algal blooms (CyanoHABs): developing a public health response. Lake Reserv. Manage. 18, 20–31. ( 10.1080/07438140209353926) [DOI] [Google Scholar]
- 36.Metcalf JS, Banack SA, Powell JT, Tymm FJM, Murch SJ, Brand LE, Cox PA. 2018. Public health responses to toxic cyanobacterial blooms: perspectives from the 2016 Florida event. Water Policy 20, 919–932. ( 10.2166/wp.2018.012) [DOI] [Google Scholar]
- 37.Griffith AG, Gobler CJ. In press. Harmful algal blooms: a climate change co-stressor in marine and freshwater ecosystems. Harmful Algae. [DOI] [PubMed]
- 38.Tomas CR, Smayda TJ. 2008. Red tide blooms of Cochlodinium polykrikoides in a coastal cove. Harmful Algae 7, 308–317. ( 10.1016/j.hal.2007.12.005) [DOI] [Google Scholar]
- 39.Hargraves PE, Maranda L. 2002. Potentially toxic or harmful microalgae from the northeast coast. Northeastern Natural. 9, 81–120. ( 10.1656/1092-6194(2002)009[0081:PTOHMF]2.0.CO;2) [DOI] [Google Scholar]
- 40.Rheuban JE, et al. 2016. Spatial and temporal trends in summertime climate and water quality indicators in the coastal embayments of Buzzards Bay, Massachusetts. Biogeosciences 13, 253–265. ( 10.5194/bg-13-253-2016) [DOI] [Google Scholar]
- 41.Leavitt D, Karney R, Surier A. 2010. Biology of the Bay Scallop. Northeastern Reg. Aquacult. Center 213, 1–8. [Google Scholar]
- 42.Friedland KD, Lynch PD, Gobler CJ. 2011. Time series mesoscale response of Atlantic Menhaden Brevoortia tyrannusto variation in plankton abundances. J. Coast. Res. 277, 1148–1158. ( 10.2112/jcoastres-d-10-00171.1) [DOI] [Google Scholar]
- 43.Matsuoka K, Mizuno A, Iwataki M, Takano Y, Yamatogi T, Yoon YH, Lee J-B. 2010. Seed populations of a harmful unarmored dinoflagellate Cochlodinium polykrikoides Margalef in the East China Sea. Harmful Algae 9, 548–556. ( 10.1016/j.hal.2010.04.003) [DOI] [Google Scholar]
- 44.Onitsuka G, et al. 2010. Large-scale transport of Cochlodinium polykrikoides blooms by the Tsushima warm current in the southwest Sea of Japan. Harmful Algae 9, 390–397. ( 10.1016/j.hal.2010.01.006) [DOI] [Google Scholar]
- 45.Huang C, Dong Q. 2000. Taxonomic and biological studies on causative organisms from a large scale red tide occurring in Pearl River estuary in the spring, 1998 II. Oceanol. Limnol. Sin. 31, 233–238. [Google Scholar]
- 46.Park BS, Kim JH, Kim J-H, Baek SH, Han M-S. 2018. Intraspecific bloom succession in the harmful dinoflagellate Cochlodinium polykrikoides (Dinophyceae) extended the blooming period in Korean coastal waters in 2009. Harmful Algae 71, 78–88. ( 10.1016/j.hal.2017.12.004) [DOI] [PubMed] [Google Scholar]
- 47.Shimada H, Sakamoto S, Yamaguchi M, Imai I. 2016. First record of two warm-water HAB species Chattonella marina (Raphidophyceae) and Cochlodinium polykrikoides (Dinophyceae) on the west coast of Hokkaido, northern Japan in summer 2014. Reg. Stud. Mar. Sci. 7, 111–117. ( 10.1016/j.rsma.2016.05.010) [DOI] [Google Scholar]
- 48.Orlova TY, Morozova TV, Gribble KE, Kulis DM, Anderson DM. 2004. Dinoflagellate cysts in recent marine sediments from the east coast of Russia. Bot. Mar. 47, 184–201. [Google Scholar]
- 49.Azanza RV, David LT, Borja RT, Baula IU, Fukuyo Y. 2008. An extensive Cochlodinium bloom along the western coast of Palawan, Philippines. Harmful Algae 7, 324–330. ( 10.1016/j.hal.2007.12.011) [DOI] [Google Scholar]
- 50.Anton A, Teoh PL, Mohd-Shaleh SR, Mohammad-Noor N. 2008. First occurrence of Cochlodinium blooms in Sabah, Malaysia. Harmful Algae 7, 331–336. ( 10.1016/J.Hal.2007.12.013) [DOI] [Google Scholar]
- 51.Cloern JE. 1999. The relative importance of light and nutrient limitation of phytoplankton growth: a simple index of coastal ecosystem sensitivity to nutrient enrichment. Aquatic Ecol. 33, 3–15. ( 10.1023/a:1009952125558) [DOI] [Google Scholar]
- 52.Philippart CJM, van Aken HM, Beukema JJ, Bos OG, Cadee GC, Dekker R. 2003. Climate-related changes in recruitment of the bivalve Macoma balthica. Limnol. Oceanogr. 48, 2171–2185. ( 10.4319/lo.2003.48.6.2171) [DOI] [Google Scholar]
- 53.Asch RG. 2015. Climate change and decadal shifts in the phenology of larval fishes in the California current ecosystem. Proc. Natl Acad. Sci. USA 112, E4065–E4074. ( 10.1073/pnas.1421946112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shumway SE, Parsons GJ. 2011. Scallops: biology, ecology and aquaculture, vol. 40 Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 55.Jiang X, Lonsdale DJ, Gobler CJ. 2010. Grazers and vitamins shape chain formation in a bloom-forming dinoflagellate, Cochlodinium polykrikoides. Oecologia 164, 455–464. ( 10.1007/s00442-010-1695-0) [DOI] [PubMed] [Google Scholar]
- 56.Griffith AW, Doherty OM, Gobler CJ. 2019. Data from: Ocean warming along temperate western boundaries of the Northern Hemisphere promotes an expansion of Cochlodinium polykrikoides blooms Dryad Digital Repository. ( 10.5061/dryad.5s8k1pr) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Griffith AW, Doherty OM, Gobler CJ. 2019. Data from: Ocean warming along temperate western boundaries of the Northern Hemisphere promotes an expansion of Cochlodinium polykrikoides blooms Dryad Digital Repository. ( 10.5061/dryad.5s8k1pr) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.5s8k1pr [56].