Abstract
Complex landscapes including semi-natural habitats are expected to favour natural enemies thereby enhancing natural pest biocontrol in crops. However, when considering a large number of situations, the response of natural biocontrol to landscape properties is globally inconsistent, a possible explanation being that local agricultural practices counteract landscape effects. In this study, along a crossed gradient of pesticide use intensity and landscape simplification, we analysed the interactive effects of landscape characteristics and local pesticide use intensity on natural biocontrol. During 3 years, using a set of sentinel prey (weed seeds, aphids and Lepidoptera eggs), biocontrol was estimated in 80 commercial fields located in four contrasted regions in France. For all types of prey excepted weed seeds, the predation rate was influenced by interactions between landscape characteristics and local pesticide use intensity. Proportion of meadow and length of interface between woods and crops had a positive effect on biocontrol of aphids where local pesticide use intensity was low but had a negative effect elsewhere. Moreover, the landscape proportion of suitable habitats for crop pests decreased the predation of sentinel prey, irrespectively of the local pesticide use intensity for weed seeds, but only in fields with low pesticide use for Lepidoptera eggs. These results show that high local pesticide use can counteract the positive expected effects of semi-natural habitats, but also that the necessary pesticide use reduction should be associated with semi-natural habitat enhancement to guarantee an effective natural biocontrol.
Keywords: biocontrol, landscape simplification, natural enemies, predation, semi-natural habitats, sentinel prey
1. Introduction
The biological control of crop pests by their natural enemies provides agriculture with a valuable, but poorly quantified ecosystem service [1,2]. Increasing the intensity and stability of pest control over time and space is crucial to promote this ecosystem function in agroecological systems [3]. Management to maximize natural biocontrol should be designed at multiple spatial scales because both local-field and landscape-scale factors may contribute to maintaining healthy communities of natural enemies that can regulate crop pests [4,5].
Recent meta-analyses have, however, concluded that natural pest biocontrol exhibits inconsistent responses to surrounding landscape characteristics in terms of semi-natural habitat area and/or crop area [6–8]. One possible explanation of this high variability of responses arises from the diversity of natural enemies that can act as biocontrol agents of different crop pests, each differing in traits that influence their sensitivity to landscape structure. Alternatively, landscape effects could be present, but masked by effects of local farm management [8,9]. Some recent studies support this view, evidencing that landscape effects on natural biocontrol can be conditional on local management at the field/farm scale, such as when landscape effects on aphid or weed suppression are modulated by local within-field plant diversity [10] or local soil tillage regime [11], respectively. Moreover, considered landscape variables are often restricted to the sole quantification of land cover types that do not always reflect the actual functional values of various landscape elements for the target organisms, e.g. insect pests [6].
Among farm-management factors, the intensity of pesticide use is likely to be a major driver of variation in the diversity and abundance of biocontrol agents and in their pest regulating activity. This question has been addressed through comparisons of biocontrol intensity between organic systems (i.e. without synthetic pesticides but often using organic-approved crop protection products) and conventional ones [12], but these farming systems also vary in many other ways than pesticide use [13], including agricultural practices likely to impact pests and natural enemies. Furthermore, this dichotomy assumes that all farmers practicing conventional management use the same pesticide strategy which is not a realistic assumption, especially given recent shifts in policy development aiming at practices that reduce reliance on agrichemicals including pesticides [14]. A few studies have addressed the impact of local pesticide use intensity per se on natural biocontrol: for example, the level of pesticide use was inversely related to invertebrate predation of weed seeds in cereal crops [15] and of aphids [16,17]. The negative impact of insecticide pressure on natural biocontrol can be mitigated by a positive effect of semi-natural habitats in the landscape [18]. Local pesticide use can also modulate responses of natural pest control agents to landscape-scale factors, as seen in enhanced occurrence of a codling moth predator in orchards [19]. Overall, the literature highlights the context dependence of natural pest biocontrol responses to the effects of land management at both local (field to farm) and landscape scales and calls for additional large-scale empirical studies over a wide range of climates and agroecosystems that simultaneously evaluate local management factors alongside landscape effects.
In this paper, we evaluated the natural biocontrol of crop pests, based on a set of sentinel prey, along a crossed gradient of pesticide use intensity (field-scale intensification of agricultural practices) and landscape simplification (landscape-scale intensification accounting for an increase of the area of crops, a decrease of semi-natural habitats and of length of interfaces between crops and semi-natural habitats). The analysis is based on data collected during three consecutive years in 80 fields of perennial and annual crops located in four French regions. Specifically, we tested three hypotheses: (i) biocontrol decreases as local in-field pesticide use intensity increases, (ii) biocontrol decreases as landscape simplification increases, and (iii) pesticide use intensity modulates landscape-scale effects on pest biocontrol, i.e. there are interactive, conditional effects of pesticide use and landscape characteristics on the intensity of biocontrol.
2. Material and methods
(a). Study regions and monitored fields selection
Natural biocontrol was monitored in 80 commercial agricultural fields from 2014 to 2016, with 20 fields located in each of four climatically distinct regions of France: the ‘Basse-Durance’ valley near Avignon (AVI), the agricultural plain of Côte d'Or near Dijon (DIJ), the LTSER ‘Zone Atelier Armorique’ near Rennes (REN) and the LTSER ‘Zone Atelier Pyrénées-Garonne’ near Toulouse (TOU, electronic supplementary material, figure S1). The AVI landscape was dominated by apple production while the primary land use in the other three regions was rotational arable production.
Fields were selected in order to simultaneously cover a crossed gradient of landscape simplification and of local pesticide use intensity. Using a land cover map (10 m2 resolution) combining the Corine Land Cover, the Land Parcel Identification System (official European database for payments in the framework of the Common Agricultural Policy) and the French National Institute of Geographic and Forest Information (IGN) databases, the landscape simplification gradient was defined using three metrics: (i) the proportion of annual crops (proportion of perennial crops for AVI), (ii) the proportion of semi-natural habitats (forest, hedgerows, fallows), and (iii) landscape heterogeneity calculated with the Shannon index considering nine land cover types (urban areas, forests, fallows, wetlands, water courses, annual crops, perennial crops, permanent meadows and temporary meadows). To cover a large range of local pesticide use intensity, the fields were selected into three types of agricultural systems: organic farmers, non-organic farmers involved in a national network with a pesticide use reduction goal (French DEPHY network) and conventional farmers. The mean size of the selected fields was 1.32 ha (±s.d. 1.30), 8.24 ha (±s.d. 3.71), 4.26 ha (±s.d. 2.03) and 7.92 ha (±s.d. 5.86) in the AVI, DIJ, REN and TOU regions, respectively. Within each region, the distance between fields ranged from 1 to 55 km (mean = 14 km ± s.d. 8 km). The 80 fields were managed by 74 different farmers. The crops grown in sampled fields were classified into eight categories: cereals (wheat, barley, oat and triticale); summer crops (maize, sunflower, soya bean and sorghum); spring and winter oleaginous (oilseed rape, mustard and flax); legumes (faba bean, peas, lentil, alfalfa and clover); apple orchards; grass–legume mixtures; meadows and fallows (electronic supplementary material, table S1). Because the selection of fields was based on their position along the crossed gradient of landscape simplification and of local pesticide use intensity, and because the same fields were monitored for several years, it was not possible to simultaneously control the distribution of the type of crop grown.
(b). Estimation of potential pest biocontrol with sentinel prey cards
The natural biocontrol of weeds and invertebrate pests in each field was estimated using predation intensity assays comprising sentinel prey items affixed to cards (5 × 5 cm sandpaper) with glue. To monitor a range of diet and microhabitat used by diverse predators, we exposed four types of sentinel prey (electronic supplementary material, figure S2): (i) ‘weed seed’ [15,20] with 10 glued Viola arvensis seeds exposed for 96 h on the ground with a nail (glue: SADER® WOOD PRO D3 diluted with two-thirds of water); (ii) ‘Ephestia’ containing egg clusters of Ephestia kuehniella (Lepidoptera) fixed to the crop plant with a stapler and exposed for 96 h (eggs are too small to allow for a precise enumeration so a 5 mm-wide cluster was glued to the card; glue: SADER® All-purpose solvent-free) [21]; (iii) ‘ground-level aphid’ with three glued adult pea aphids Acyrthosiphon pisum exposed for 24 h on the ground [22]; and (iv) ‘crop-level aphid’ with three glued adult pea aphids A. pisum fixed to the crop plant and exposed for 24 h (glue: UHU® Twist&Glue solvent-free). Glue types were chosen within a set of low toxic ones after practical tests ensuring that prey were just fixed but not mired, and that they did not come off during the exposition time period.
In each field, we exposed the four card types in 10 plots evenly positioned along two parallel transects, 10 m away from each other (electronic supplementary material, figure S2). Transects were perpendicular to the field border, with the first position at 50 m from the border and the last one at 100 m (150 m in orchards owing to their more elongated shape). The number of remaining prey on cards after exposure was counted in the field, except for Ephestia cards, which because of their small size required visual inspection with a magnifying binocular in the laboratory and were assigned to two classes: unconsumed (less than 5% of the eggs consumed) or consumed (greater than 5%). Predation intensity on each prey type in each field (n = 10 cards) was assessed by summation of: (i) the number of consumed prey (predation success events, or consumed Ephestia cards) and (ii) the number of unconsumed prey (predation failure events, or unconsumed Ephestia cards). Within each sampling year, two sessions of prey cards exposure were conducted: during the crop vegetative growth period and then during fruit, ears or pods maturation (see the electronic supplementary material, table S2 for the session dates in each region). The same protocol was conducted in 2014, 2015 and 2016.
The theoretical size of the dataset was 480 statistical individuals (20 fields, four regions, two sessions and 3 years). However, because of some missing data concerning pesticide use or sentinel prey cards, the actual size of the dataset was 420 for Ephestia cards, 451 for ground-level aphid cards, 449 for crop-level aphid cards and 443 for weed seed cards (electronic supplementary material, table S3).
(c). Local pesticide use intensity
The type and the applied doses of each pesticide (insecticides, fungicides and herbicides) used in each field each year of the survey was recorded through annual farmers' interviews. Pesticide use intensity was evaluated with the treatment frequency index (TFI, number of reference doses applied per hectare) [23] and calculated for each field each year as
where Di is the applied dose, Si the treated surface area, Dri the reference dose obtained from the French Ministry of Agriculture online database [24] and S the total area of the field for each spraying operation i. As expected, the TFI was highly variable between crops and considerably higher in apple orchards than in annual crops. It was mostly owing to the difference in the number of insecticide treatments (mean TFI in apple orchards: 21.2 ± s.d. = 8.0; oleaginous: 5.6 ± s.d. = 2.4; summer crops: 2.8 ± s.d. = 2.5; cereal: 2.9 ± s.d. = 2.2; legume: 1.1 ± s.d. = 2.5; electronic supplementary material, figure S3). No treatment was applied in fallows, grass–legume mixtures or meadows. Because we were interested in the effect of pesticide use intensity relative to a standard for each focal crop, the TFI variable was crop-normalized per crop type subtracting the mean value over the 3 years from the raw TFI value and dividing by its standard deviation (hereafter relative TFI).
(d). Landscape variables
All land cover types were mapped in a 1 km2 buffer circle centred on each monitored field, in ArcGIS 10.3 (ESRI©ArcMAP™10.3.1, 1995–2015), combining the Land Parcel Identification System and field observations. The scale of 1 km is often used in pest control studies and was shown to be a relevant spatial extent to reveal the response of specialist and generalist predators and pests to landscape factors [6,8]. Land cover was described with the seven following categories: urban areas, woods, fallows, water, annual crops, perennial crops and meadows. Using the free software Chloe 4.0 [25] on 10 m rasterized maps, we calculated eight landscape variables within each buffer (electronic supplementary material, table S4): (i) the Shannon diversity index of land cover types SHDI; semi-natural habitat cover variables: the proportion of land area covered (ii) by woody elements (pWood), (iii) by meadows (pMeadow), and (iv) by semi-natural habitats (pSNH = pWood + pMeadow); and crop and semi-natural habitat connectivity: the length (m) of interfaces (v) between crops and woods (iWood), (vi) between crops and meadows (iMeadow), and (vii) between crops and semi-natural habitats (iSNH = iWood + iMeadow). Using a refined map specifying the cover of each crop type following the classification chosen for the monitored fields (cereals, summer crops, oleaginous, legumes, apple, grass–legume mixtures, meadows), we also calculated (viii) the proportion of crops similar to that of the monitored field (i.e. corresponding to the same crop type using the above-mentioned eight-crop types classification), thereafter called ‘proportion of target crop’ (pTargetCrop). Here we assume that crops belonging to the same crop type class are equally suitable for the same range of pests and natural enemies, pTargetCrop thus representing a measure of the proportion of suitable habitat for pests of the monitored fields and associated enemies [6,26].
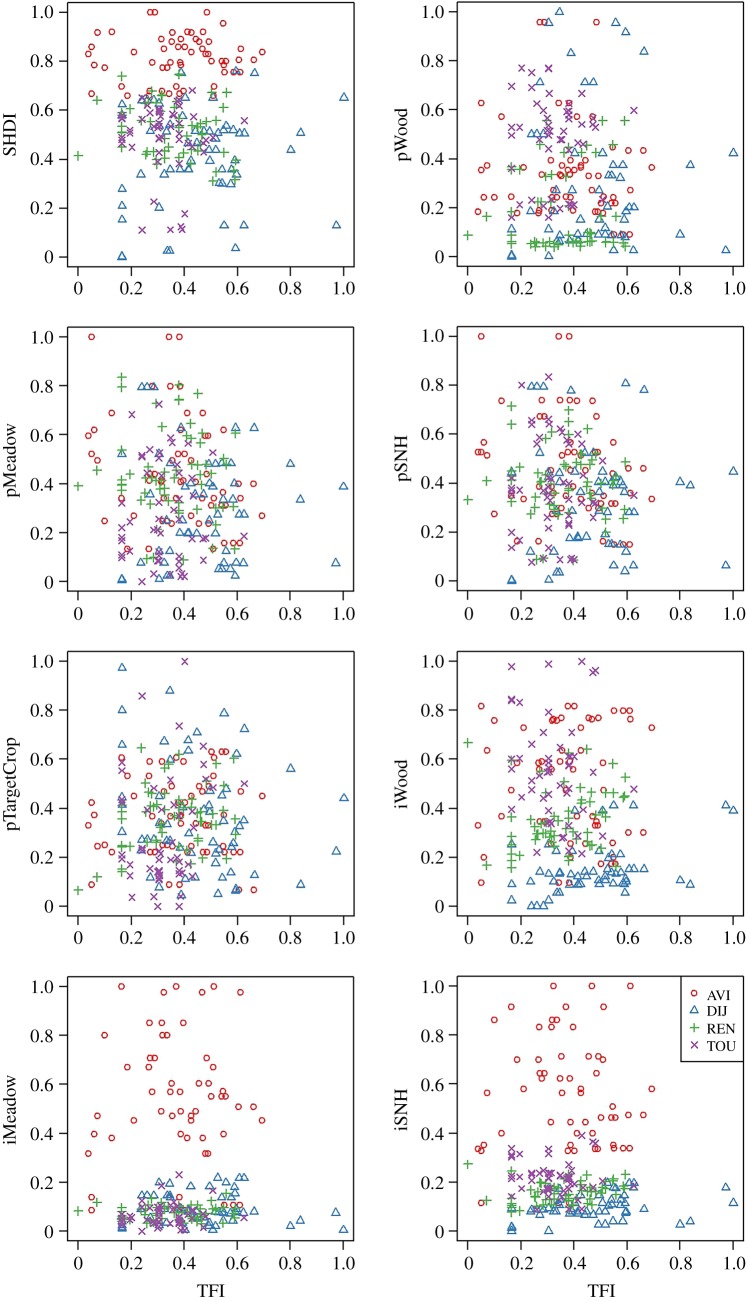
The landscape gradient covered by the 80 fields was important, e.g. with SHDI ranging from 0.3 to 1.82, pSNH from 1 to 85%, and pTargetCrop from 0 to 78% (electronic supplementary material, table S5). Fields were distributed along a gradient of increasing landscape simplification, characterized by a decrease in the proportion of area covered by semi-natural habitats and in the length of interfaces between semi-natural habitats and crops, and an increase in the proportion of target crop. For most landscape variables, the gradient of variation covered within a single region was narrower than that covered by the full 80-fields dataset (figure 1), i.e. some combinations of landscape characteristics were specific to some regions. Similarly, the range of pesticide use intensity differed between regions. The correlation matrices between explanatory variables are presented in the electronic supplementary material, figure S4 and table S6.
Figure 1.
Distribution of the data for each region (AVI, Avignon; DIJ, Dijon; REN, Rennes; TOU, Toulouse). SHDI, Shannon diversity index of land cover types; pWood, proportion of woody elements; pMeadow, proportion of meadows; pSNH, proportion of semi-natural habitats; pTargetCrop, proportion of crops similar to that of the monitored field; iWood, length of crops/woods interfaces; iMeadow, length of crops/meadows interfaces; iSNH, length of crops/semi-natural habitats interfaces (TFI normalized per crop type and then scaled between 0 and 1 over the whole dataset; landscape variables scaled between 0 and 1 over the whole dataset). (Online version in colour.)
(e). Meteorological covariables
We hypothesized that the meteorological conditions occurring during the days the cards were exposed in the field could have influenced the predation rates. Consequently, daily meteorological data were gathered (INRA, CLIMATIK database) to describe the conditions prevailing during each session of exposure for each region and year. These data were region-specific, assuming that the 20 fields of each region shared the same meteorological conditions at a given session, a given year, which is reasonable given that the within-region two-by-two distances between fields were very small compared with the two-by-two distances between regions (electronic supplementary material, figure S1). Included variables were daily mean (TM), minimum (TN) and maximum (TX) temperature (°C), mean (V) and maximum (VX) wind speed (m.s−1), rainfall (RR, mm), and mean (UN), minimum (UM) and maximum (UX) humidity (%). These variables were aggregated as the minimum (for TN, UN), maximum (for TX, VX, UX), mean (for TM, V, UM) or sum (for RR) over the 2 days covering the 24 h exposure of the aphid cards (24 h meteorological dataset) and over the 5 days covering the 96 h of exposure of the Ephestia and weed seed cards (96 h meteo dataset). A normed principal component analysis (PCA) was performed for the 24 h dataset and the 96 h dataset (R package ade4, R software version 3.4.4) to provide synthetic descriptors of meteorological conditions.
For the 24 h meteorological dataset, the first three principal components (PCs) captured 37%, 27% and 15% of the total variance, respectively (electronic supplementary material, table S7). PC1 was positively correlated with humidity and negatively with temperature; PC2 was negatively correlated with temperature and PC3 was negatively correlated with wind speed (electronic supplementary material, figure S5). For the 96 h meteorological dataset, the first three components captured 43%, 29% and 10% of the total variance, respectively (electronic supplementary material, table S7). PC1 was positively correlated with wind speed and negatively with humidity; PC2 was negatively correlated with temperature and PC3 was negatively correlated with rainfall (electronic supplementary material, figure S6). The underlying contributions of the regions, the years and the sessions to the PCs was assessed by projecting these illustrative factors on the two first factorial planes (see the electronic supplementary material, figures S5 and S6 and detailed description below table S7). The first three components (PC1, PC2, PC3) were used as synthetic meteorological covariables in subsequent analyses.
(f). Statistical analyses
First, the pairwise Spearman rank correlations between the predation rates on each type of sentinel prey cards were calculated over the whole dataset to identify possible redundancies between the four types of cards.
Second, for each prey card type, we analysed the predation patterns (described by predation success and failure events counts) with generalized linear mixed models. To reduce overdispersion, we fitted a beta-binomial distribution (R package glmmADMB) [27]. Because of significant correlations among landscape variables (electronic supplementary material, table S7) and to avoid an a priori selection of the explanatory variables included in the analysis, for each type of prey card we chose not to include multiple landscape variables in a given model. We first produced a null model including as predictors the crop type of the focal field and the PCs axes scores (PC1, PC2, PC3) of the meteorological data (24 h for aphids; 96 h for weed seed and Ephestia). Then, we produced eight models including each one the variables of the null model, the TFI; one of the eight landscape variables and the interaction between TFI and the landscape variable. Finally, average coefficient estimates were calculated over the nine models (the null model and the eight models including TFI and landscape variable interaction) using the R package MuMIn. Landscape variables and the relative TFI were scaled between 0 and 1 over the whole dataset (3 years and four regions). To account for inter- and intra-annual dependencies, we included the year and the field identifier as a random effects. We deliberately chose not to include a region random effect in order to account for the full range of the landscape and pesticide use gradients and to investigate general trends rather than to focus on region-specific relationships. Data structure corresponding to regions was accounted for through the synthetic meteorological (PC1, PC2, PC3), all together representing more than 80% of the variability captured with the nine meteorological variables which were region-specific at a given session, a given year. By doing so, we assume that differences between regions that are not described by the landscape descriptors and TFI correspond to meteorological differences that were accounted for through the synthetic variables describing each region (electronic supplementary material, figures S5 and S6). All analyses were performed with R software version 3.4.4.
3. Results
The mean predation rates were 0.43 (±s.d. 0.31), 0.56 (±s.d. 0.25), 0.73 (±s.d. 0.26) and 0.25 (±s.d. 0.21) for weed seed, Ephestia cards, ground-level aphid and crop-level aphid, respectively. Correlations between the predation rates on the four types of sentinel prey were consistently positive and low (ranging between 0.13 and 0.29, electronic supplementary material, table S8), still significant except for the weed seeds and the crop-level aphids whose predation rates were unrelated. Electronic supplementary material, table S9 provides the ranking of all models for each type of sentinel prey card.
(a). Weed seed predation
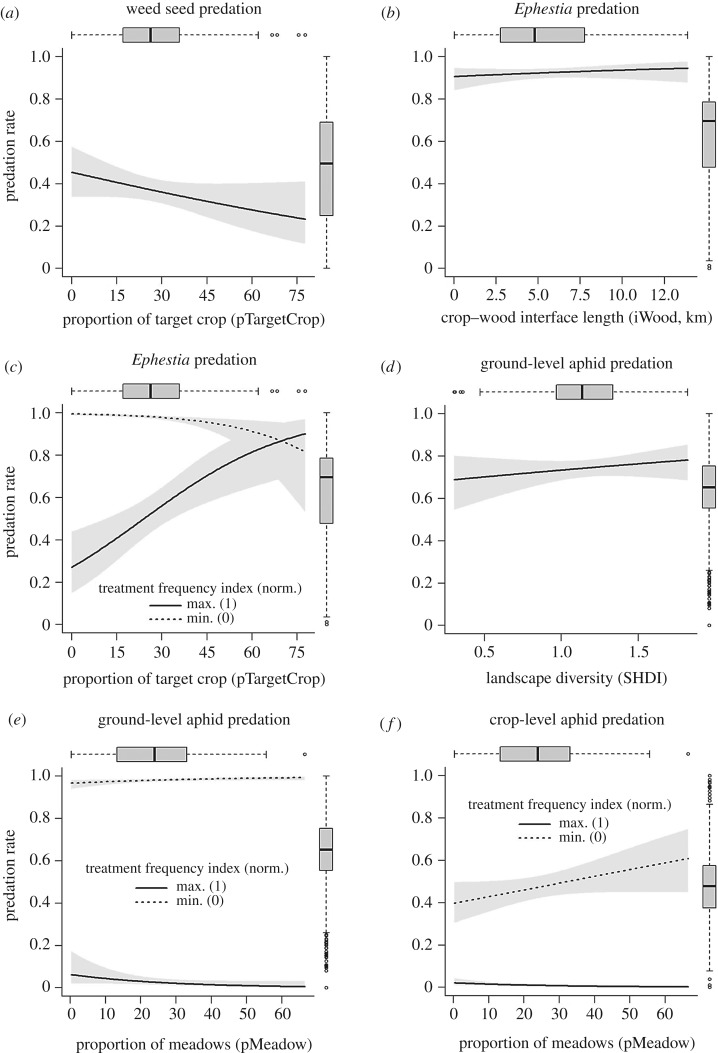
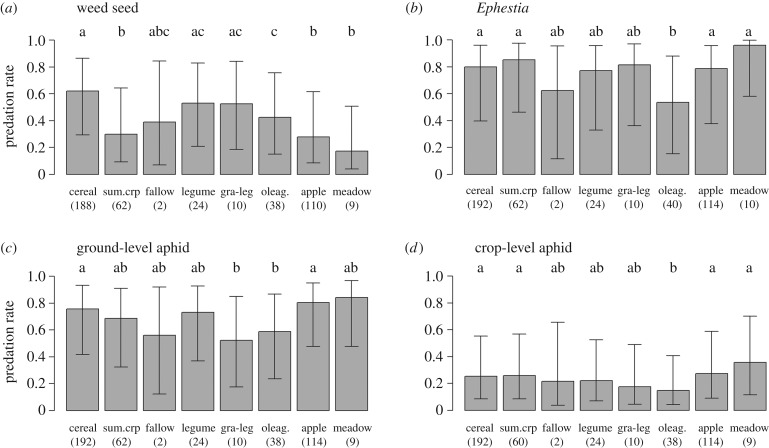
The predation of weed seeds was negatively influenced by the proportion of target crop in the landscape (pTargetCrop), irrespectively of the intensity of pesticide use (table 1 and figure 2a). The predation rate of weed seeds was also significantly lower in summer crops, oleaginous, apple orchards and meadows than in cereals (figure 3). Consumption of weed seeds was greater when wind speed was low and humidity high (table 1, negative effect of PC1), at high temperatures (negative effect of PC2) and under situations of high rainfall (negative effect of PC3).
Table 1.
Averaged estimated effects of the synthetic meteorological variables, pesticide use intensity index, landscape variables and their interaction on the four predation rates. (TFI, treatment frequency index; SHDI, Shannon diversity index of land cover types; pWood, proportion of woody elements, pMeadow, proportion of meadows; pSNH, proportion of semi-natural habitats; pTargetCrop, proportion of target crop (i.e. similar to that of the monitored field); iWood, length of interfaces between crops and woods; iMeadow, length of interfaces between crops and meadows; iSNH, length of interfaces between crops and semi-natural habitats. Values in bold indicate effects. *p < 0.05; **p < 0.01; ***p < 0.001; n.s., not significant.)
| weed seed |
Ephestia |
ground-level aphid |
crop-level aphid |
|||||
|---|---|---|---|---|---|---|---|---|
| effect | estimate | s.e. | estimate | s.e. | estimate | s.e. | estimate | s.e. |
| PC1 | −1.49*** | 0.26 | −1.51*** | 0.33 | 0.23 n.s. | 0.35 | −0.40 n.s. | 0.29 |
| PC2 | −0.68* | 0.27 | −0.56 n.s. | 0.35 | 0.28 n.s. | 0.31 | −0.30 n.s. | 0.26 |
| PC3 | −0.73* | 0.33 | 1.06* | 0.45 | 0.26 n.s. | 0.33 | −0.38 n.s. | 0.26 |
| TFI | −1.16 n.s. | 0.89 | −2.23* | 1.00 | −0.47 n.s. | 1.02 | 0.53 n.s. | 0.73 |
| SHDI | 1.43 n.s. | 0.79 | 1.31 n.s. | 0.94 | 1.67* | 0.82 | 0.06 n.s. | 0.71 |
| pWood | −1.01 n.s. | 0.69 | 1.08 n.s. | 0.88 | 0.68 n.s. | 0.74 | 0.05 n.s. | 0.66 |
| pMeadow | 1.11 n.s. | 0.67 | 1.20 n.s. | 0.88 | 1.58* | 0.76 | 0.85 n.s. | 0.61 |
| pSNH | 0.64 n.s. | 0.72 | 1.53 n.s. | 0.93 | 1.62* | 0.79 | 0.76 n.s. | 0.65 |
| pTargetCrop | −1.61* | 0.77 | −3.75*** | 1.02 | −0.95 n.s. | 0.81 | −0.51 n.s. | 0.74 |
| iWood | −0.48 n.s. | 0.65 | 1.57* | 0.79 | 0.88 n.s. | 0.71 | 0.99 n.s. | 0.61 |
| iMeadow | 0.42 n.s. | 0.77 | −0.71 n.s. | 0.89 | 1.02 n.s. | 0.90 | 0.50 n.s. | 0.72 |
| iSNH | 0.02 n.s. | 0.80 | 0.32 n.s. | 0.96 | 1.47 n.s. | 0.93 | 0.63 n.s. | 0.76 |
| TFI*SHDI | −2.82 n.s. | 1.68 | −1.07 n.s. | 2.07 | −3.13 n.s. | 1.76 | −1.70 n.s. | 1.48 |
| TFI*pWood | 2.21 n.s. | 1.67 | −2.67 n.s. | 2.15 | −1.14 n.s. | 1.76 | −0.23 n.s. | 1.57 |
| TFI*pMeadow | −2.27 n.s. | 1.74 | −1.64 n.s. | 2.31 | −4.10* | 1.94 | −3.19* | 1.58 |
| TFI*pSNH | −1.24 n.s. | 1.88 | −2.67 n.s. | 2.45 | −3.88 n.s. | 2.01 | −2.87 n.s. | 1.67 |
| TFI*pTargetCrop | 1.57 n.s. | 1.87 | 6.95** | 2.48 | 0.43 n.s. | 1.96 | 1.07 n.s. | 1.81 |
| TFI*iWood | 0.49 n.s. | 1.59 | −2.60 n.s. | 1.97 | −1.55 n.s. | 1.71 | −3.82** | 1.48 |
| TFI*iMeadow | −2.18 n.s. | 1.69 | 0.84 n.s. | 1.97 | −3.20 n.s. | 1.91 | −0.63 n.s. | 1.54 |
| TFI*iSNH | −1.81 n.s. | 1.81 | −0.33 n.s. | 2.16 | −3.59 n.s. | 2.04 | −2.30 n.s. | 1.69 |
Figure 2.
Partial regression or interaction graphs for the significant effects in the averaged model. Shaded areas correspond to 95% confidence intervals. The boxplot at the top of each graph represents the distribution of the landscape variable; the one on the right represents the distribution of the predictions of the averaged model. For ground-level aphid cards, the significant effect of pSNH is not represented here but showed a similar pattern to the effect of SHDI; similarly, for crop-level aphids cards, the significant interaction between TFI and iWood is not represented here but showed a similar pattern to the interaction between TFI and pMeadow (see the electronic supplementary material, figure S7). Min., minimal TFI value; Max., maximal TFI value.
Figure 3.
Least-square means of the predation rate on sentinel prey and 95% confidence intervals for each crop type (best model). Different letters indicate significant differences and figures indicate the number of statistical individuals in each group.
(b). Ephestia predation
The predation of Ephestia eggs was positively influenced by the length of interfaces between crops and woods, irrespectively of the intensity of pesticide use (table 1 and figure 2b). There was also a significant interactive effect between pesticide use intensity and the proportion of target crop in the landscape (table 1). The proportion of target crop had a negative effect on the predation of Ephestia eggs in case of low than in high pesticide use intensity situations and, in a smaller extent, a positive effect in low pesticide use intensity situations. This also corresponded to a strong negative effect of the pesticide use intensity in landscape with a low proportion of target crop (figure 2c). Ephestia eggs were consumed less in oleaginous crops than in cereals, summer crops, apple orchards and meadows (figure 3). Their predation rate was enhanced when wind was low and humidity high (table 1, negative effect of PC1) and under low rain conditions (positive effect of PC3).
(c). Ground-level aphid predation
Predation of aphids on the soil surface was positively related to the landscape diversity and the proportion of semi-natural habitats, irrespectively of the intensity of pesticide use (table 1, figure 2d and electronic supplementary material, figure S7-a). There was a significant interaction between pesticide use intensity and the proportion of meadows. The proportion of meadows had a positive effect on aphid predation when pesticide use intensity was low but a negative effect when pesticide use intensity was high, although the regression slope was low in both cases (figure 2e). This also corresponded to a negative effect of pesticide use intensity stronger in landscapes covered by a high proportion of meadows than in landscapes with few meadows. The predation rate of aphids at ground level was significantly lower in oleaginous crops and grass–legume mixtures than in cereals and apple orchards (figure 3). Meteorological conditions did not affect predation of aphids at ground level.
(d). Crop-level aphid predation
Aphid predation on the crop was significantly affected by the interactions between pesticide use intensity and two landscape variables: the proportion of meadows and the length of the interface between crops and woods (table 1). The length of the crop–woodland interface and the proportion of meadows had a positive effect on aphid predation when pesticide use intensity was low, and a neutral effect when it was high. It also reflected a higher negative effect of pesticide use intensity in landscapes with a high proportion of meadows or a high length of interfaces between crops and woods (figure 2f and electronic supplementary material, figure S7-b). Aphid predation rate at the crop-level was also significantly lower in oleaginous crops compared with the cereals, summer crops, apple orchards and meadows (figure 3) and was unaffected by meteorological conditions.
4. Discussion
A major finding from this study is that, regardless of the crop type and once the effects of meteorological conditions on pest biocontrol were accounted for, landscape effects on biocontrol were sometimes modified by the intensity of local pesticide use. However, some landscape characteristics had an effect on biocontrol irrespective of local pesticide use intensity. The landscape proportional cover of suitable habitat for pests of the target crop negatively affected natural biocontrol for weed seeds, whereas the length of crop-wood land interfaces and landscape diversity positively affected natural biocontrol of Ephestia eggs and ground-level aphids. In all cases, this corresponded to a negative effect of different components of the landscape simplification. Here, the proportion of target crop in the landscape was never positively correlated to local pesticide use in our dataset (electronic supplementary material, figure S3 and table S7), i.e. fields located in landscapes dominated by the same crop do not appear to be managed more intensively than those located in more diversified landscape contexts. Yet, we cannot exclude that the proportion of target crop could in some way represent the landscape-scale pesticide pressure, which would explain its negative impact on biocontrol. An alternative explanation is that the abundance of natural enemies and thereby the predator–prey ratio decreases as landscape simplification increases, because of the loss of a diversity of habitats and the niches and trophic resources they supply [28–31].
Pesticide use intensity at the local field-level had a negative effect on the predation rates of all types of prey. This negative effect was higher in complex landscape contexts, particularly for Ephestia eggs (low proportion of target crop) and crop-level aphids (high proportion of meadows and high length of interfaces between crops and woods). This suggests that, in these landscape contexts, the predation activity of a wide range of natural enemies (feeding on plant and animal pests both on the soil surface and on crop plants) was reduced when pesticide pressure was high, most probably because of a decrease in their diversity and/or abundance. Such a negative effect of pesticides on the biodiversity of agroecosystems has been reported at the pan-European scale [32], and there is some evidence that increasing pesticide use can reduce the intensity of pest control, for example, weed seed predation [15]. Pesticides can directly increase mortality and so reduce the abundance of natural enemies [33], they can also have sub-lethal effects that induce modifications in the biocontrol pattern and behaviour of natural enemies [34] such as carabid beetles [35] and spiders [36]. In addition, herbicide use decreases the diversity of weeds in arable fields [37,38] with a subsequent reduction in habitat and trophic resources for natural enemies [39]. Sub-lethal pesticide effects and poor resource availability can together reduce the fitness of natural enemies in arable fields [40].
The local pesticide use intensity and the landscape proportion of target crop had interactive effects on the biocontrol of Ephestia eggs. Biocontrol was high in two very different contexts, either when both the intensity of local pesticide use and the proportional area of target crop were at low levels, or when they were both at high levels. In the less intensive context, the predation of eggs could result from the biocontrol activity of a diversified natural enemy community that require a diversity of crop types in the landscape and respond negatively to local pesticide pressure. Conversely, in very intensive situations, the predation of eggs could result from the activity of one or a few predators that thrive in a specific crop type and are less sensitive to the direct and indirect effects of pesticides. In another study, the percentage of annual crops in the landscape was shown to positively influence the predation rate of squash bug eggs [41]. A possible explanation could be that in simple landscapes with a high percentage of crops, the background food quantity is reduced, which in turn increases food patch exploitation and foraging time, increasing predation rates [42,43]. In different studies, natural enemies were found to be positively impacted by the proportion of cultivated land [44,45] or negatively by the proportion of semi-natural habitat [31]. Our findings therefore suggest that in these two contrasting contexts, different communities of predators consuming Ephestia eggs could be at play leading to the same intensity of pest control but with different underlying ecological processes. In a recent meta-analysis approach, Muneret et al. [46] showed that organic cropping systems had more weeds, less pathogens and an equivalent level of animal pests, which overall translated to a higher total level of pest infestation compared to conventional cropping systems. A possibility is therefore that a low intensive situation locally provides more resources for some natural enemies, but less for others.
Similarly, the influence of the proportion of meadows and of the length of crop–woodland interfaces in the landscape on aphid predation varied according to local pesticide pressure. These landscape metrics affected biocontrol positively only when pesticide pressure was low, and, in a lower extent, negatively when pesticide pressure was high. This provides additional evidence that non-crop habitats do not universally increase natural biocontrol [8] and reflects the great variability in the way natural enemies respond to semi-natural habitats [47]. Previous studies have shown how local management factors may interact with landscape to explain variation in weed biocontrol. Fischer et al. [43] showed that weed seed predation decreased with increasing percentage of arable land in organic fields (negative effect of landscape simplification), but increased with increasing percentage of arable land in conventional ones (positive effect of landscape simplification).
Our results suggest that in a low local pesticide pressure situation, natural enemies require non-crop habitats, with spillover processes taking place from semi-natural habitats to crops. In other studies, the amount of hedges and grassland in the landscape was shown to increase the abundance of natural enemies of winter wheat aphids [48], whereas a meta-analysis revealed a negative impact of landscape simplification on aphid predation [7]. Conversely, high local pesticide pressure situations probably harm less specialized communities of natural enemies favoured by crops and whose abundance decreases as semi-natural habitat area or connectivity increase in the landscape. For some generalist natural enemy species, crops may provide more resources than semi-natural habitats [49] in such a way to counteract the expected positive effect of semi-natural habitats in the landscape. There is evidence that some predators such as hoverflies are quite independent from semi-natural habitats, overwintering inside crops and finding complementary resources on the weed flora [50]. Moreover, the spillover processes that occur under low pesticide pressure may be counteracted by agricultural practices in high pesticide use situations [49,51]. Another alternative explanation of the negative effect of semi-natural habitats could be that in high pesticide use context, food resources are very low in crops and natural enemies are more attracted by semi-natural habitats that provide more resources.
5. Conclusion
Accounting for field-scale agricultural practices, and specifically pesticide use intensity, appears essential to fully understand the role of landscape characteristics on natural biocontrol, and to provide accurate management guidelines to identify landscape features or landscape configurations reinforcing biocontrol of insect pests or weeds. The present study shows that expected positive effects of semi-natural habitats can be hampered by high pesticide use. Conversely, our results suggest that some intensively managed agricultural landscapes could achieve an effective predation of crop pests, even though they may badly perform in terms of biodiversity conservation and preservation of environmental resources [52]. Reducing pesticide use could therefore disadvantage specific communities of natural enemies selected by landscape-scale intensive agricultural situations. Pesticide use reduction, required by environmental and health concerns [53], should thus be accompanied with semi-natural habitat enhancement to ensure a habitat relay and an effective change in the communities of natural enemies.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Monitored fields are parts of the long-term research and observation system for environmental research ‘SOERE ECOSCOPE’. We are very grateful to farmers who accepted to participate. We warmly thank Cyrille Auguste, Romane Blaya, Laurent Burnel, Nicolas Chague, Erwan Chavonnet, Jérémy Cortello, Eloïse Couthouis, Chantal Ducourtieux, Thomas Defferier, Bruno Dumora, Diane Esquerré, Emilie François, Ninon Frémaux, Kristel Jegou, Guillomette Laurens, Wilfried Heintz, Ruoying Long, Augustine Perrin, Magali Philippe, Antoine Picot, Marie Pillods, Elodie Proux, Laurent Raison, Jean-Luc Roger, Claude Sarasin, Jean-François Toubon, and Jérôme Willm for their essential contribution to GIS analyses, fieldwork and farm surveys. We also thank Fabrice Dessaint and Guillaume Adeux for their advice on statistical analyses and Adam Vanbergen for his helpful comments on the manuscript.
Data accessibility
Data are provided in the electronic supplementary material file ‘Ricci_ProceedingsB_Data.txt’ and the description of variables are given in the electronic supplementary material file ‘Ricci_ProceedingsB_DataLabels.txt’.
Authors' contributions
B.R. participated in the design of the study, carried out the statistical analyses and drafted the manuscript; C.L. participated in the design of the study, participated in data analysis and helped draft the manuscript; A.A., S.A., P.F., M.P. and A.V. participated in the design of the study, helped analyse the data and helped draft the manuscript; J.-C.B. participated in the design of the study; F.M. participated in data analysis; A.J. and J.-P.C. critically revised the manuscript; L.B.-D. collected field data, carried out GIS analyses and performed farm surveys; S.L. carried out GIS analyses; G.S. and C.T. collected field data; S.P. conceived and coordinated the study, participated in data analysis and helped draft the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was carried out with financial support from the Ecophyto Plan of the French Ministry of Agriculture and Food and the French Foundation for Research on Biodiversity (FRB), project ‘SEBIOPAG-PHYTO’.
References
- 1.Landis DA, Gardiner MM, van der Werf W, Swinton SM. 2008. Increasing corn for biofuel production reduces biocontrol services in agricultural landscapes. Proc. Natl Acad. Sci. USA 105, 20 552–20 557. ( 10.1073/pnas.0804951106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tschumi M, Albrecht M, Entling MH, Jacot K.. 2015. High effectiveness of tailored flower strips in reducing pests and crop plant damage. Proc. R. Soc. B 282, 20151369 ( 10.1098/rspb.2015.1369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bommarco R, Kleijn D, Potts SG.. 2013. Ecological intensification: harnessing ecosystem services for food security. Trends Ecol. Evol. 28, 230–238. ( 10.1016/j.tree.2012.10.012) [DOI] [PubMed] [Google Scholar]
- 4.Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C.. 2005. Landscape perspectives on agricultural intensification and biodiversity: ecosystem service management. Ecol. Lett. 8, 857–874. ( 10.1111/j.1461-0248.2005.00782.x) [DOI] [Google Scholar]
- 5.Rusch A, Valantin-Morison M, Sarthou JP, Roger-Estrade J.. 2010. Biological control of insect pests in agroecosystems. Effects of crop management, farming systems, and seminatural habitats at the landscape scale: a review. Adv. Agron. 109, 219–259. ( 10.1016/B978-0-12-385040-9.00006-2) [DOI] [Google Scholar]
- 6.Veres A, Petit S, Conord C, Lavigne C.. 2013. Does landscape composition affect pest abundance and their control by natural enemies? A review. Agric. Ecosyst. Environ. 166, 110–117. ( 10.1016/j.agee.2011.05.027) [DOI] [Google Scholar]
- 7.Rusch A, et al. 2016. Agricultural landscape simplification reduces natural pest control: a quantitative synthesis. Agric. Ecosyst. Environ. 221, 198–204. ( 10.1016/j.agee.2016.01.039) [DOI] [Google Scholar]
- 8.Karp DS, et al. 2018. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl Acad. Sci. USA 115, E7863–E7870. ( 10.1073/pnas.1800042115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begg GS, et al. 2017. A functional overview of conservation biological control. Crop Prot. 97, 145–158. ( 10.1016/j.cropro.2016.11.008) [DOI] [Google Scholar]
- 10.Chaplin-Kramer R, Kremen C.. 2012. Pest control experiments show benefits of complexity at landscape and local scales. Ecol. Appl. 22, 1936–1948. ( 10.1890/11-1844.1) [DOI] [PubMed] [Google Scholar]
- 11.Petit S, Trichard A, Biju-Duval L, McLaughlin B, Bohan DA.. 2017. Interactions between conservation agricultural practice and landscape composition promote weed seed predation by invertebrates. Agric. Ecosyst. Environ. 240, 45–53. ( 10.1016/j.agee.2017.02.014) [DOI] [Google Scholar]
- 12.Winqvist C, et al. 2011. Mixed effects of organic farming and landscape complexity on farmland biodiversity and biological control potential across Europe. J. Appl. Ecol. 48, 570–579. ( 10.1111/j.1365-2664.2010.01950.x) [DOI] [Google Scholar]
- 13.Puech C, Poggi S, Baudry J, Aviron S.. 2014. Do farming practices affect natural enemies at the landscape scale? Landsc. Ecol. 30, 125–140. ( 10.1007/s10980-014-0103-2) [DOI] [Google Scholar]
- 14.Lechenet M, Dessaint F, Py G, Makowski D, Munier-jolain N.. 2017. Reducing pesticide use while preserving crop productivity and profitability on arable farms. Nat. Plants 3, 17008 ( 10.1038/nplants.2017.8) [DOI] [PubMed] [Google Scholar]
- 15.Trichard A, Alignier A, Biju-Duval L, Petit S.. 2013. The relative effects of local management and landscape context on weed seed predation and carabid functional groups. Basic Appl. Ecol. 14, 235–245. ( 10.1016/j.baae.2013.02.002) [DOI] [Google Scholar]
- 16.Albert L, Franck P, Gilles Y, Plantegenest M.. 2017. Impact of agroecological infrastructures on the dynamics of Dysaphis plantaginea (hemiptera: Aphididae) and its natural enemies in apple orchards in northwestern France. Environ. Entomol. 46, 528–537. ( 10.1093/ee/nvx054) [DOI] [PubMed] [Google Scholar]
- 17.Emmerson M, et al. 2016. Chapter two - how agricultural intensification affects biodiversity and ecosystem services. Adv. Ecol. Res. 55, 43–97. [Google Scholar]
- 18.Roubos CR, Rodriguez-Saona C, Isaacs R.. 2014. Mitigating the effects of insecticides on arthropod biological control at field and landscape scales. Biol. Control 75, 28–38. ( 10.1016/j.biocontrol.2014.01.006) [DOI] [Google Scholar]
- 19.Lefebvre M, Franck P, Toubon JF, Bouvier JC, Lavigne C.. 2016. The impact of landscape composition on the occurrence of a canopy dwelling spider depends on orchard management. Agric. Ecosyst. Environ. 215, 20–29. ( 10.1016/j.agee.2015.09.003) [DOI] [Google Scholar]
- 20.Westerman PR, Wes JS, Kropff MJ, Van Der Werf W.. 2003. Annual losses of weed seeds due to predation in organic cereal fields. J. Appl. Ecol. 40, 824–836. ( 10.1046/j.1365-2664.2003.00850.x) [DOI] [Google Scholar]
- 21.Sigsgaard L, Jacobsen SK.. 2017. Functional agrobiodiversity: a novel approach to optimize pest control in fruit production. Landsc. Manag. Funct. Biodivers. IOBC-WPRS Bull. 122, 26–28. [Google Scholar]
- 22.Östman Ö. 2004. The relative effects of natural enemy abundance and alternative prey abundance on aphid predation rates. Biol. Control 30, 281–287. ( 10.1016/j.biocontrol.2004.01.015) [DOI] [Google Scholar]
- 23.OECD . 2001. Environmental indicators for agriculture, volume 3: methods and results Paris, France: OECD. [Google Scholar]
- 24.French Ministry of Agriculture and Agribusiness. 2016. Ephy website. Le catalogue des produits phytopharmaceutiques et de leurs usages des matières fertilisantes et des Supports de culture homologués en France. See https://ephy.anses.fr/.
- 25.Boussard H, Baudry J. 2017. Chloe4.0: a software for landscape pattern analysis. See https://www6.rennes.inra.fr/bagap/PRODUCTIONS/Logiciels.
- 26.Rand TA, Waters DK, Blodgett SL, Knodel JJ, Harris MO. 2014. Increased area of a highly suitable host crop increases herbivore pressure in intensified agricultural landscapes. Agric. Ecosyst. Environ. 186, 135–143. ( 10.1016/j.agee.2014.01.022) [DOI] [Google Scholar]
- 27.Fenlon JS, Faddy MJ. 2006. Modelling predation in functional response. Ecol. Modell. 198, 154–162. ( 10.1016/j.ecolmodel.2006.04.002) [DOI] [Google Scholar]
- 28.Thies C, et al. 2011. The relationship between agricultural intensification and biological control: experimental tests across Europe. Ecol. Appl. 21, 2187–2196. ( 10.1890/10-0929.1) [DOI] [PubMed] [Google Scholar]
- 29.Werling BP, Meehan TD, Robertson BA, Gratton C, Landis DA. 2011. Biocontrol potential varies with changes in biofuel-crop plant communities and landscape perenniality. GCB Bioenergy 3, 347–359. ( 10.1111/j.1757-1707.2011.01092.x) [DOI] [Google Scholar]
- 30.Blaauw BR, Isaacs R. 2015. Wildflower plantings enhance the abundance of natural enemies and their services in adjacent blueberry fields. Biol. Control 91, 94–103. ( 10.1016/j.biocontrol.2015.08.003) [DOI] [Google Scholar]
- 31.Rusch A, Binet D, Delbac L, Thiéry D. 2016. Local and landscape effects of agricultural intensification on carabid community structure and weed seed predation in a perennial cropping system. Landsc. Ecol. 31, 2163–2174. ( 10.1007/s10980-016-0390-x) [DOI] [Google Scholar]
- 32.Geiger F, et al. 2010. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 11, 97–105. [Google Scholar]
- 33.Kunkel BA, Held DW, Potter DA. 2001. Lethal and sublethal effects of bendiocarb, halofenozide, and imidacloprid on Harpalus pennsylvanicus (Coleoptera: Carabidae) following different modes of exposure in turfgrass. J. Econ. Entomol. 94, 60–67. ( 10.1603/0022-0493-94.1.60) [DOI] [PubMed] [Google Scholar]
- 34.Desneux N, Decourtye A, Delpuech J-M. 2007. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106. ( 10.1146/annurev.ento.52.110405.091440) [DOI] [PubMed] [Google Scholar]
- 35.Mauchline AL, Osborne JL, Powell W. 2004. Feeding responses of carabid beetles to dimethoate-contaminated prey. Agric. For. Entomol. 6, 99–104. ( 10.1111/j.1461-9563.2004.00208.x) [DOI] [Google Scholar]
- 36.Baatrup E, Bayley M. 1993. Effects of the pyrethroid insecticide cypermethrin on the locomotor activity of the wolf spider Pardosa amentata: quantitative analysis employing computer-automated video tracking. Ecotoxicol. Environ. Saf. 26, 138–152. ( 10.1006/eesa.1993.1046) [DOI] [PubMed] [Google Scholar]
- 37.Roschewitz I, Gabriel D, Tscharntke T, Thies C. 2005. The effects of landscape complexity on arable weed species diversity in organic and conventional farming. J. Appl. Ecol. 42, 873–882. ( 10.1111/j.1365-2664.2005.01072.x) [DOI] [Google Scholar]
- 38.Petit S, Gaba S, Grison AL, Meiss H, Simmoneau B, Munier-Jolain N, Bretagnolle V. 2016. Landscape scale management affects weed richness but not weed abundance in winter wheat fields. Agric. Ecosyst. Environ. 223, 41–47. ( 10.1016/j.agee.2016.02.031) [DOI] [Google Scholar]
- 39.Letourneau DK, et al. 2011. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 21, 9–21. [DOI] [PubMed] [Google Scholar]
- 40.Labruyere S, Ricci B, Lubac A, Petit S. 2016. Crop type, crop management and grass margins affect the abundance and the nutritional state of seed-eating carabid species in arable landscapes. Agric. Ecosyst. Environ. 231, 183–192. ( 10.1016/j.agee.2016.06.037) [DOI] [Google Scholar]
- 41.Phillips BW, Gardiner MM. 2016. Does local habitat management or large-scale landscape composition alter the biocontrol services provided to pumpkin agroecosystems? Biol. Control 92, 181–194. ( 10.1016/j.biocontrol.2015.10.001) [DOI] [Google Scholar]
- 42.Westerman PR, Borza JK, Andjelkovic J, Liebman M, Danielson B. 2008. Density-dependent predation of weed seeds in maize fields. J. Appl. Ecol. 45, 1612–1620. ( 10.1111/j.1365-2664.2008.01481.x) [DOI] [Google Scholar]
- 43.Fischer C, Thies C, Tscharntke T. 2011. Mixed effects of landscape complexity and farming practice on weed seed removal. Perspect. Plant Ecol. Evol. Syst. 13, 297–303. ( 10.1016/j.ppees.2011.08.001) [DOI] [Google Scholar]
- 44.Gardiner MM, Landis DA, Gratton C, Schmidt N, O'Neal M, Mueller E, Chacon J, Heimpel GE, Difonzo CD. 2009. Landscape composition influences patterns of native and exotic lady beetle abundance. Divers. Distrib. 15, 554–564. ( 10.1111/j.1472-4642.2009.00563.x) [DOI] [Google Scholar]
- 45.Caballero-López B, Bommarco R, Blanco-Moreno JM, Sans FX, Pujade-Villar J, Rundlöf M, Smith HG. 2012. Aphids and their natural enemies are differently affected by habitat features at local and landscape scales. Biol. Control 63, 222–229. ( 10.1016/j.biocontrol.2012.03.012) [DOI] [Google Scholar]
- 46.Muneret L, Mitchell M, Seufert V, Aviron S, Djoudi EA, Pétillon J, Plantegenest M, Thiéry D, Rusch A. 2018. Evidence that organic farming promotes pest control. Nat. Sustain. 1, 361–368. ( 10.1038/s41893-018-0102-4) [DOI] [Google Scholar]
- 47.Holland JM, Bianchi FJ, Entling MH, Moonen A-C, Smith BM, Jeanneret P. 2016. Structure, function and management of semi-natural habitats for conservation biological control: a review of European studies. Pest Manag. Sci. 72, 1638–1651. ( 10.1002/ps.4318) [DOI] [PubMed] [Google Scholar]
- 48.Alignier A, Raymond L, Deconchat M, Menozzi P, Monteil C, Sarthou JP, Vialatte A, Ouin A. 2014. The effect of semi-natural habitats on aphids and their natural enemies across spatial and temporal scales. Biol. Control 77, 76–82. ( 10.1016/j.biocontrol.2014.06.006) [DOI] [Google Scholar]
- 49.Tscharntke T, et al. 2016. When natural habitat fails to enhance biological pest control: five hypotheses. Biol. Conserv. 204, 449–458. ( 10.1016/j.biocon.2016.10.001) [DOI] [Google Scholar]
- 50.Vialatte A, Tsafack N, Hassan DAl, Duflot R, Plantegenest M, Ouin A, Villenave-Chasset J, Ernoult A. 2017. Landscape potential for pollen provisioning for beneficial insects favours biological control in crop fields. Landsc. Ecol. 32, 465–480. ( 10.1007/s10980-016-0481-8) [DOI] [Google Scholar]
- 51.Lefebvre M, Papaïx J, Mollot G, Deschodt P, Lavigne C, Ricard JM, Mandrin JF, Franck P. 2017. Bayesian inferences of arthropod movements between hedgerows and orchards. Basic Appl. Ecol. 21, 76–84. ( 10.1016/j.baae.2017.05.002) [DOI] [Google Scholar]
- 52.MacFadyen S, Cunningham SA, Costamagna AC, Schellhorn NA. 2012. Managing ecosystem services and biodiversity conservation in agricultural landscapes: are the solutions the same? J. Appl. Ecol. 49, 690–694. ( 10.1111/j.1365-2664.2012.02132.x) [DOI] [Google Scholar]
- 53.Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L. 2016. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front. Public Heal. 4, 1–8. ( 10.3389/fpubh.2016.00148) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided in the electronic supplementary material file ‘Ricci_ProceedingsB_Data.txt’ and the description of variables are given in the electronic supplementary material file ‘Ricci_ProceedingsB_DataLabels.txt’.