Abstract
Homophilous behaviour plays a central role in the formation of human friendships. Individuals form social ties with others that show similar phenotypic traits, independently of relatedness. Evidence of such homophily can be found in bottlenose dolphins (Tursiops aduncus) in Shark Bay, Western Australia, where females that use marine sponges as foraging tools often associate with other females that use sponges. ‘Sponging’ is a socially learned, time-consuming behaviour, transmitted from mother to calf. Previous research illustrated a strong female bias in adopting this technique. The lower propensity for males to engage in sponging may be due to its incompatibility with adult male-specific behaviours, particularly the formation of multi-level alliances. However, the link between sponging and male behaviour has never been formally tested. Here, we show that male spongers associated significantly more often with other male spongers irrespective of their level of relatedness. Male spongers spent significantly more time foraging, and less time resting and travelling, than did male non-spongers. Interestingly, we found no difference in time spent socializing. Our study provides novel insights into the relationship between tool use and activity budgets of male dolphins, and indicates social homophily in the second-order alliance composition of tool-using bottlenose dolphins.
Keywords: bottlenose dolphins, tool use, alliance formation, activity budget, social networks, homophily
1. Introduction
Individuals acquire information and behavioural skills from conspecifics through social learning across a variety of taxa, including insects, fishes, reptiles, birds and mammals [1–4]. Despite the widespread prevalence of social learning, this strategy may not always be beneficial, as knowledge gained from conspecifics can be maladaptive with one's own behavioural patterns [5]. It is therefore important for individuals to learn selectively from others to maximize benefits [6]. Explanations for why, when and from whom individuals learn include adopting behaviour performed by the majority [7], behaviour performed by kin [8] or based on increased pay-offs [9], among others (reviewed in [4,10]). However, while social learning has received considerable attention in the literature, relatively little is known about what differences exist between the sexes and what consequences such differences might hold for adult life.
Sexual selection theory predicts that males should primarily engage in behaviours related to increasing mating opportunities, while females should invest more in behaviours related to increasing access to resources and offspring protection [11,12]. Differences in behavioural requirements or preferences are therefore expected to dictate sex biases in social learning. For example, both male and female chimpanzees (Pan troglodytes) learn socially to insert flexible tools made from vegetation into termite mounds in order to extract termites, yet females learn ‘termite fishing’ earlier, use it more frequently and do so more efficiently than males [13,14]. The differing priorities in learning to use a tool are reflective of the different strategies of male and female chimpanzees to maximize fitness. Chimpanzees use tools in foraging contexts; thus, the benefits of engaging in such a technique should be higher for females than males. Male chimpanzees form coalitions to compete for and maintain alpha male status, a social position that confers increased reproductive opportunity [15]. Consequently, males might be less inclined to invest in learning or improving complicated feeding techniques, but rather invest in social relationships with other males [16].
In the Indo-Pacific bottlenose dolphin (Tursiops aduncus) population of Shark Bay, Western Australia, sex bias is evident in a socially learned foraging technique involving the use of marine sponges as tools [17,18]. Sponge-carrying (sponging) is thought to protect the dolphin's rostrum while foraging for prey on the sea floor [17,19]. Sponging allows these dolphins (spongers) to exploit a novel ecological niche by providing access to prey not available to those dolphins unfamiliar with tool use [20]. Sponging is observed in both the eastern and western gulfs of Shark Bay, but only some members of particular matrilines use sponges (west: approx. 38% of all females [21]; east: approx. 13% of all females [22]). This is why sponging is thought to be an exclusively vertically transmitted behaviour [18,23]. Around 91% of female calves adopt sponging from their sponging mothers, while only 50% of males do so. The observed female bias in sponging is most likely to be reflective of a sex bias in social learning propensities at a young age [24–26].
Sponging females are distinctive with regard to their activity budget, spending more time foraging and less time resting than their non-sponging female counterparts [21,24]. When foraging, female spongers devote 95% of their time to sponging, compared with other foraging behaviours [24]. They are also seen alone more often than non-spongers [22,24]. However, when associating with other individuals, female spongers show a preference for other sponging females [22]. While there is a considerable amount of data on female spongers, much less is known about male spongers. For instance, why proportionally fewer males learn and specialize in this foraging technique, and if and how sponging influences adult male behaviour, remain unknown. The latter is of particular relevance as male dolphins in Shark Bay exhibit one of the most complex social structures outside humans (reviewed in [27]).
Bottlenose dolphins in Shark Bay live in an open fission–fusion society with changing group sizes and compositions [27,28]. Males form different levels of reproductive alliances with other males, driven by intense competition for access to receptive females [27]. Two to three males cooperate in ‘first-order’ alliances to consort single oestrus females [29]. These males also generally associate within larger ‘second-order’ alliances composed of 4–14 individuals, whose members cooperate to take females from rival alliances and to defend against such attacks [29]. First- and second-order allies are also frequently observed together in non-mating contexts [29]. Second-order alliances are considered the stable, core unit of male social organization in Shark Bay, while the stability of first-order alliances varies considerably [27]. These complex social relationships among males can last for decades and are critical to each male's reproductive success [27]. Alliances are considered costly, as each male must invest time in the formation and maintenance of these relationships [30].
Sponging is also a costly behaviour: it requires significant time investment and is associated with a decrease in overall sociability [22,24], as well as less time to rest and travel [21]. The investment of time and energy into male alliance behaviours may therefore preclude engaging in time-consuming, solitary foraging techniques, such as sponging. It has been proposed that sponging might put males at a disadvantage in forming and maintaining alliances compared with males that use foraging techniques that are both less time-consuming and less solitary [17,18,21,24]. However, these arguments assume that the time, social demands and energetic demands of sponging on males and females are similar, which has yet to be tested. Here, we assess the effect of sponging on male dolphin behaviour by comparing activity budgets, sociability and association patterns of male spongers to male non-spongers.
2. Methods
(a). Study site and data collection
Data for this study were collected in the western gulf of Shark Bay, Western Australia, in an area that includes various habitat types, such as seagrass-rich shallow waters (less than 10 m) and deep-water channels with sandy substrates (greater than 10 m) [31]. We collected behavioural and genetic data during the austral winters from 2007 to 2015, identifying individual dolphins by photographs of their dorsal fins [32]. During boat-based surveys of dolphin groups, within the first 5 min, we recorded GPS position, environmental parameters (including sea state, water depth and temperature), group size and composition, as well as predominant group activity (rest, travel, forage, socialize or unknown; cf. [33] and electronic supplementary material). We defined group membership according to the 10 m chain rule [33]. Male dolphins that had been observed carrying a sponge while foraging at least twice on different days were classified as spongers [24], while males that had never been observed sponging were classified as non-spongers. Individuals that had been observed sponging only once were classified as ‘unknowns’. We obtained biopsy samples from dolphins on an opportunistic basis using a purpose-designed system for sampling small cetaceans [34]. The samples were used to genetically sex individuals [35] and determine pairwise genetic relatedness [18]. Further details of sampling and laboratory methods are provided in the electronic supplementary material. Unless otherwise specified, all analyses were conducted in R v. 1.1.453 [36].
(b). Data restriction
We included only independent/weaned males and excluded dependent calves [37]. Only males observed more than nine times and identified as spongers or non-spongers were included in our analyses. Sex was identified either genetically (see electronic supplementary material) or behaviourally by several observations of alliance-typical behaviour (being observed regularly travelling side-by-side engaging in synchronous surfacing, consorting of females or inter-group aggression with other males; cf. [27,38]). Furthermore, in order to assess males with similar association opportunities, we restricted our analyses to comparisons of male spongers with non-sponging males that also met habitat use criteria based on depth and home range overlap derived from data on sponging males. Further details on the calculation of these criteria are provided in the electronic supplementary material. Restricting the data in this manner resulted in a dataset containing 37 male dolphins, including 13 spongers and 24 non-spongers.
(c). Effect of sponging on male activity budgets
To investigate differences in activity budgets (proportions of resting, travelling, foraging and socializing behaviour) between male spongers and non-spongers, we conducted a multivariate analysis of variance (MANOVA) with the sole predictor of whether an individual was classified as sponger or non-sponger (hereafter: foraging technique). As dependent variables, we calculated activity budgets by dividing the number of individual sightings per activity by the total number of individual sightings. We used Pillai's trace (V) as a test statistic due to the unequal sample sizes in our dataset [39]. To investigate which activity proportions, in particular, differed between male spongers and non-spongers, we performed sequential Bonferroni-corrected, post hoc, independent t-tests (Welch's t-test [40]). While investigating the data structure of the multivariate activity budgets, we identified five outliers from the combined normal distribution. Thus, we conducted the MANOVA with outliers removed, retaining 32 males (spongers: n = 12, non-spongers: n = 20) in the dataset (see electronic supplementary material for analysis with the full dataset).
(d). Degree of sociability of male spongers and non-spongers
To investigate whether male spongers were more or less solitary than male non-spongers, we compared their levels of sociability. We constructed an index of sociability by dividing the number of solitary sightings by the total number of sightings per individual. We compared individual sociability indices of male spongers and male non-spongers in a two-sample permutation test (10 000 permutations) implemented in the ‘perm’ package [41].
To investigate the association pattern of male spongers and male non-spongers, we adhered to the following procedure. First, to maximize our ability to draw comparisons with other studies on cetaceans, we calculated half weight indices (HWIs) as a measure of the proportion of time two males spent together [42]. Based on the dyadic HWIs, we created a social network to analyse the association patterns between male spongers and male non-spongers. Second, we assessed whether associations in the social network followed a random pattern or whether two individuals were seen more or less often together than expected by chance [43,44]. For this analysis, we specified a daily sampling period. Third, to test whether the association indices between pairs consisting of males with similar foraging techniques (sponger–sponger; non-sponger–non-sponger) were higher than between pairs with different foraging techniques (sponger–non-sponger), we carried out a Mantel test on a similarity matrix and the matrix of dyadic associations with 10 000 permutations. The similarity matrix is a 1/0 matrix providing information on whether two individuals belong to the same group (either both spongers or both non-spongers = 1) or to different groups (sponger and non-sponger = 0). These analyses were conducted in SOCPROG 2.6 [45].
In a further step, we ran a double decker semi-partialling multiple regression quadratic assignment procedure (MRQAP-DSP; see below and [46]) to investigate whether the documented pattern of dyadic associations (between male pairs of spongers, pairs of non-spongers, and pairs of one sponger and one non-sponger) could be predicted by similarity in foraging technique, even when controlling for pairwise relatedness (based on 27 microsatellite loci; see electronic supplementary material for more detailed information). Similarity in foraging technique was presented in two matrices: in the first, we coded similarity in sponging as 1; and vice versa in the second, where similarity in non-sponging was coded as 1. Unequal dyads were assigned a value of 0 in both matrices. Separate similarity matrices allowed us to disentangle the contribution of similarity in sponging and non-sponging, respectively, to the association pattern.
An MRQAP-DSP test is similar to a partial linear multiple regression with the exception that dependent and predictor variables are presented as matrices. Thus, this method tests whether an entered predictor variable significantly contributes to the explanation of the dependent matrix, while controlling for the other predictors. To control for the dependencies between data points, we used the MRQAP-DSP test as implemented and described in the ‘asnipe’ package [47] using 10 000 permutations. We did not include mitochondrial haplotypes in the predictors due to a previously documented high correlation with foraging technique [48]. Only males for which we had genetic data available were included in this test (spongers: n = 9, non-spongers: n = 16). We also repeated the MRQAP-DSP test including all genotyped males within our study population while additionaly correcting for home range overlaps (see electronic supplementary material).
To investigate whether the association patterns found in the previous analysis were also reflected in second-order alliance compositions, we defined second-order alliances based on dyadic HWIs. We lacked sufficient consortship data to define alliances functionally (i.e. through observation of consortship behaviour) for this study, so we could use only association strength as a proxy [33]. We used an average linkage agglomerative cluster analysis assuming a hierarchical social network structure [49] performed in SOCPROG [45], and defined and applied a threshold value at which a dyad can be considered to be part of the same second-order alliance. To find an appropriate threshold, we conducted a change point analysis employing the pruned exact linear time (PELT) method specified in the ‘changepoint’ package [50] (cf. [51] and electronic supplementary material for more detailed information).
3. Results
Between 2007 and 2015, we observed 124 male dolphins at least nine times. After applying the restrictions outlined above, the resulting dataset contained 37 male dolphins, of which 13 were spongers and 24 were non-spongers (number of sightings: mean = 35; range = 17–68). We computed HWIs from a total of 549 survey records over the 9-year study period. All males associated with at least five other individuals in the dataset.
(a). Effect of sponging on male activity budgets
We detected significantly different activity budgets between male spongers and non-spongers (V = 0.74, F4,27 = 19.6, p < 0.001). Thus, foraging techniques significantly contributed to explaining an individual male's activity budget. Post hoc analyses showed that male spongers foraged more, and rested and travelled less than male non-spongers. There was no significant difference in time spent socializing between male spongers and non-spongers (table 1).
Table 1.
Post hoc, Bonferroni-corrected t-tests on activity proportions of male spongers (n = 12) and non-spongers (n = 20). Significant p-values are indicated in italics.
| spongers |
non-spongers |
||||||
|---|---|---|---|---|---|---|---|
| proportion | mean | s.d. | mean | s.d. | t (d.f.) | r | p-value |
| forage | 0.45 | 0.02 | 0.20 | 0.02 | −9.42 (26.31) | 0.89 | <0.001 |
| rest | 0.18 | 0.01 | 0.28 | 0.01 | 4.83 (27.80) | 0.68 | <0.001 |
| travel | 0.16 | 0.02 | 0.31 | 0.02 | 4.83 (27.36) | 0.68 | <0.001 |
| socialize | 0.16 | 0.01 | 0.13 | 0.01 | −1.62 (29.99) | 0.28 | 0.23 |
(b). Degree of sociability of male spongers and male non-spongers
Male spongers were encountered significantly more often alone (sociability index: mean = 0.22, s.e. = 0.03) than male non-spongers (sociability index: mean = 0.04, s.e. = 0.01; p = 0.002).
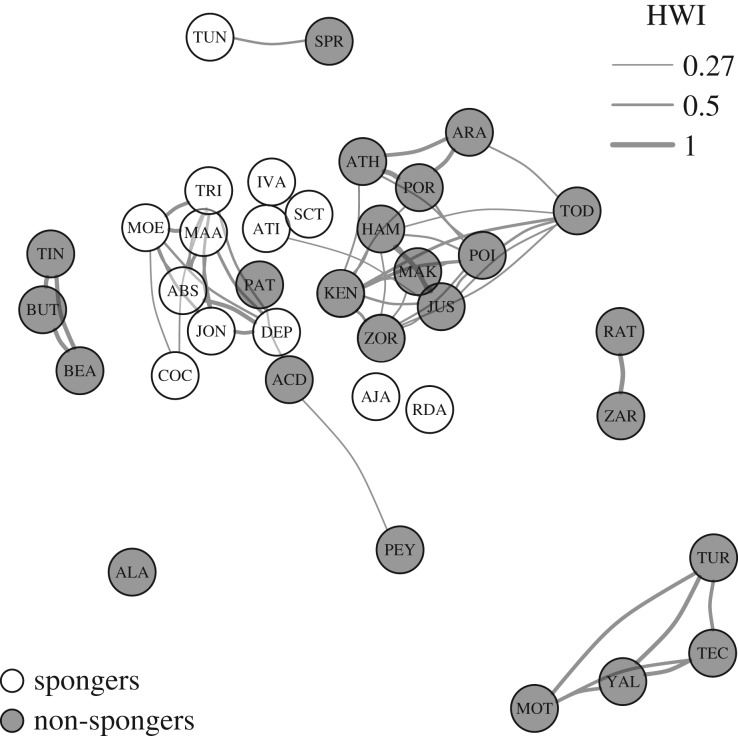
Among the 37 males, the overall mean HWI was 0.09 (1000 bootstraps: s.e. = 0.03), including the zeros of no associations. Considering only non-zero associations, the more conservative measure, the mean HWI was 0.17 (1000 bootstraps: s.e. = 0.05). The generated network based on the dyadic association indices (figure 1) represented a non-random social structure (10 000 permutations, 1000 switches; s.d.obs = 0.17, s.d.random = 0.14, p < 0.001). Thus, some males were observed more often in association than expected by chance alone, reflecting their well-documented alliance associations [27].
Figure 1.
Social network of the male dolphins in the restricted dataset (n = 37). The nodes represent individuals and are shaded according to foraging technique. Edges (lines) below 0.27 HWI are transparent and edge thickness corresponds to edge weight (see electronic supplementary material, figure S2 for the social network showing all edges). The graph was plotted with the force-directed Fruchterman–Reingold algorithm implemented in the ‘igraph’ package [52].
Association rates between pairs of males with similar foraging techniques (sponger–sponger; non-sponger–non-sponger; mean HWI = 0.14, s.d. = 0.09) were significantly higher (Mantel test, t = 5.75; p < 0.01; table 2) than associations between pairs with different foraging techniques (sponger–non-sponger: mean HWI = 0.05, s.d. = 0.04).
Table 2.
Mean association indices (HWI) by foraging technique of male spongers (n = 13) and non-spongers (n = 24), 666 dyadic relationships.
| pair composition | mean HWI (s.d.) |
|---|---|
| sponger–sponger | 0.21 (0.11) |
| non-sponger–non-sponger | 0.10 (0.05) |
| similar foraging technique | 0.14 (0.09) |
| different foraging technique | 0.05 (0.04) |
| overall | 0.09 (0.04) |
The MRQAP regression model showed that sponging was a significant predictor of male association patterns, even after controlling for relatedness (table 3). Related individuals did not associate above chance levels. These findings were also supported by the results of the MRQAP-DSP tests including all males within our study area (see electronic supplementary material for more information). Our analyses demonstrate that the association pattern of male dolphins inhabiting deep water and occupying similar home ranges can at least partly be explained by foraging technique.
Table 3.
MRQAP-DSP model including only genotyped males (n = 25; 300 dyadic relationships). Significant p-values are indicated in italics.
| variable | coefficient | p-value |
|---|---|---|
| sponger similarity | 0.19 | <0.001 |
| non-sponger similarity | 0.10 | <0.01 |
| relatedness | 0.21 | 0.24 |
| F3,297 = 34.5, adjusted R2 = 0.25, p-value <0.001 | ||
An average linkage agglomerative cluster analysis to define second-order alliances resulted in a tree diagram representing the underlying data well with a cophenetic correlation coefficient of 0.98 [45,53]. The PELT method resulted in a change point at HWI ≥ 0.27. This cut-off value is higher but well within the range of previous findings on the male dolphins of Shark Bay, in which an HWI of 0.20 has commonly been used in assigning males to second-order alliances [27,33]. Applying 0.27 as a threshold to define second-order alliances illustrated that the tendency of male spongers to associate with other male spongers was reflected in second-order alliance compositions. We identified nine second-order alliances, of which two consisted exclusively of spongers, one was of mixed composition (sponger and non-sponger) and the other six were composed exclusively of non-spongers (figure 2). Four individuals (three spongers, one non-sponger) could not be assigned to a second-order alliance. Five of the non-sponging alliances and both sponging alliances have also been observed engaging in functional alliance behaviour (e.g. consorting females). A similar pattern was found when we included all males in our study population (see electronic supplementary material for more detail).
Figure 2.
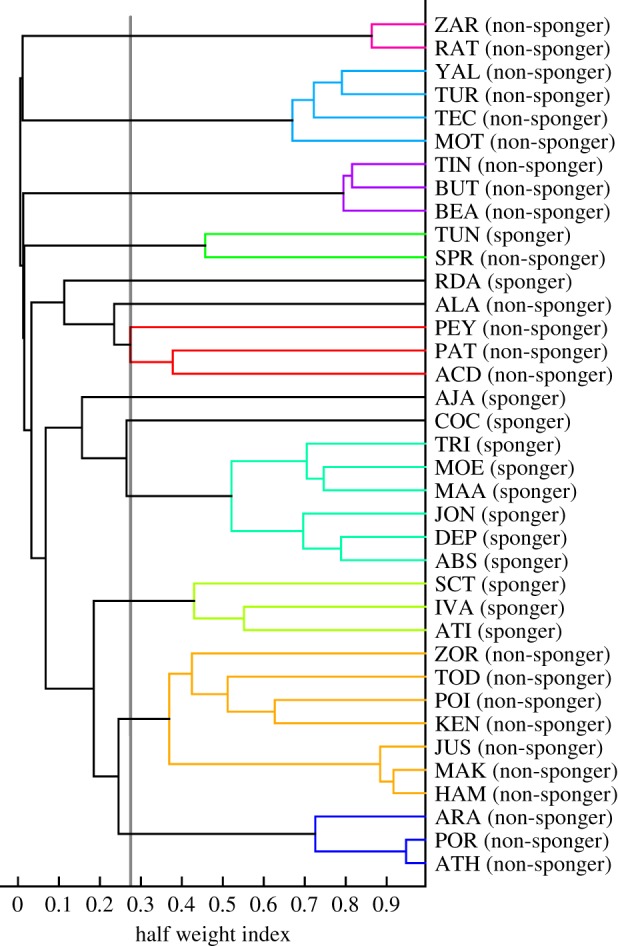
Hierarchical cluster diagram based on dyadic HWI measures. An HWI value of 0.27 was used as a cut-off value (grey line) to define communities (i.e. second-order alliances).
4. Discussion
It has been hypothesized that the investment of time and energy into the formation and maintenance of male alliances probably reduces the propensity for male dolphins to engage in time-consuming, solitary foraging techniques such as sponging, thereby resulting in the strong female bias previously documented [17,18,21,24]. This hypothesis was based on the assumptions that male spongers engage in different activity and social patterns than male non-spongers. Our results support these assumptions by revealing that, at least in the austral winters when data were collected, male spongers differed in their activity budgets, foraging more, and resting and travelling less, than male non-spongers. Interestingly, the time spent socializing was equal among male spongers and non-spongers despite the fact that male spongers spent more time alone than male non-spongers. When male spongers were observed with other males, they associated significantly more often with other male spongers.
Previous studies on female activity budgets in Shark Bay also found that spongers spent a greater proportion of their time foraging and less time resting and travelling than their non-sponging counterparts [21,24], suggesting that time investment could be a proximate cost of sponging in comparison with other foraging techniques for both sexes. A comparison between the sexes warrants further investigation. Interestingly, socializing proportions for males seem not to be affected by these time investments, suggesting that a comparatively smaller amount of time spent resting might be the proximate cost of sponging. However, these potential costs might be offset by having fewer competitors for food, as sponging may decrease competition for resources by providing access to a novel ecological niche [19,20]. Indeed, the role of intraspecific competition on niche expansion has been reported across several taxa [54,55].
Our finding that male spongers and male non-spongers spent equal amounts of time socializing contradicts the hypothesis that sponging conflicts with cooperative male alliance behaviour. However, when comparing sociability, we found that male spongers had higher proportions of solitary sightings compared with male non-spongers. Our findings thereby corroborate previous studies indicating that sponging is a largely solitary activity [21,24]. The increased solitariness of male spongers might still affect cooperative male alliance behaviour negatively to some degree, even though there is no difference in socializing time.
Our examination of male social structure in deep-water habitat revealed that male spongers tended to associate with other male spongers rather than male non-spongers, as demonstrated by their clustering in the social network. Sponging was a significant predictor of the observed association patterns of males sharing similar home ranges even after controlling for pairwise relatedness and similarity in non-sponging. Likewise, when we repeated our analysis and included all genotyped males, similarity in sponging remained a significant predictor for social structuring (see electronic supplementary material for more information). These results contradict a previous study on male dolphins in eastern Shark Bay [22], which did not detect a significant effect of similarity in foraging technique on social structuring. This was most likely to be a result of low sample size as there are far fewer spongers, and particularly male spongers, in the eastern gulf of Shark Bay compared with the western gulf [22,31]. Remarkably, in our study, while similarity in foraging technique was significant in terms of impact on social structuring, pairwise relatedness was not (table 3). The absence of an effect of relatedness on the social structuring of male dolphins seems plausible; previous studies on male associations and relatedness of second-order alliances reported ambiguous patterns, with only a minority of alliances showing higher relatedness than the population average [56].
The high social affinity among male spongers could either indicate social learning of tool use from alliance partners or be explained by homophilous behaviour (i.e. increased associations due to similar behaviour). The established pattern of strict vertical transition of sponging [18,23] and the reported homophily related to sponging in female dolphins of Shark Bay [22] make homophily among male spongers the more parsimonious explanation. Whether the observed homophily among male spongers is driven by the males themselves or emerges as a by-product of the high social affinity of female spongers (i.e. mothers) remains unknown. Research in eastern Shark Bay has shown that juvenile males preferentially stayed in proximity to their natal associates [57], and the number of associates stays constant from infancy through the juvenile period [58]. If the natal associates of spongers were also male spongers, this could explain the high social bonds between pairs or trios of sponging males. As sponging females—and hence, mothers of sponging males—are shown to cluster together [22], such a scenario seems plausible.
The ultimate benefit of such homophilous behaviour in male spongers could be their ability to maintain the use of such a foraging technique while simultaneously remaining in close proximity to males ‘of a similar ilk’ (i.e. with whom they can also engage in alliance behaviours). This argument is further strengthened when considering the composition of second-order alliances. There was only one mixed second-order alliance, while the other eight alliances in our dataset consisted of either only male spongers or male non-spongers. The threshold resulting from our PELT analysis to identify second-order alliances was higher than previously documented in Shark Bay [29], resulting in the delineation of a greater number of alliances, with some having fewer members than typically reported for second-order alliances [27,29]. The higher threshold of 0.27 may have split some second-order alliances that associated at levels of greater than 0.20 but less than 0.27. Thus, the smaller second-order alliances identified in our study comprising only two to three males are probably first-order allies. Yet, irrespective of the threshold used to define alliances, when considering the hierarchical structure of the social network (i.e. dyadic associations assorted in a dendrogram, figure 2), social homophily is apparent. Given the need to synchronize activities when living in groups (i.e. in alliances) [59], males in alliances containing sponging and non-sponging individuals might be at a disadvantage relative to non-mixed alliances. Future research needs to examine whether there are differences in the structure and complexity of second- and first-order alliances between male spongers and non-spongers. Here, we suggest that the benefits of social homophily may, to a certain extent, mitigate the costs of sponging for male alliance behaviour.
Apart from social homophily, behavioural plasticity might manifest itself by allied male spongers reducing the amount of time invested in sponging during the peak mating season, thus further mitigating the costs of being a male sponger to some degree. Nevertheless, the mating season in Shark Bay is only moderately seasonal, with consortships occurring during all months of the year, and a diffuse peak between September and December [60].
In summary, we show that while previous assumptions that sponging affects male activity budgets and social pattern hold true, this might not necessarily stand in conflict with male alliance behaviour. The apparent cost-mitigating behaviours together with the observed absence of differences in socializing proportions between male spongers and non-spongers weaken the hypothesis that sponging stands in conflict with male alliance behaviour, thereby leading to a female bias in sponging. In fact, preliminary data suggest rates of female monopolization do not differ between male spongers and male non-spongers (M.R.B. 2016, unpublished data). Future research might explore the costs of sponging and how it might be mitigated in more detail, leaving room for other plausible explanations regarding female bias in social learning of sponging. For instance, time constraints on a male dolphin during its early life may play an important role. Males are weaned earlier than females [61], and therefore have less time to learn sponging from their mothers; instead, they may need to invest time in developing social bonds with other males. Indeed, juvenile male dolphins invest more time in developing social skills than juvenile females, who instead increase their foraging rates [58]. In addition, a recent study showed that an extensive training period (decades) is crucial to achieve peak performance in sponging [26].
In conclusion, our study explored the impacts of sponging on male dolphin behaviour. We suggest that potential costs associated with sponging for male dolphins might be mitigated by social homophily. Revealing social homophily in bottlenose dolphins is interesting, as in humans, homophilous behaviour is a key factor in the emergence and maintenance of subcultures [62], and the establishment of attachment and close friendships [63]. Our study thereby provides another example of convergence in social complexity, innovation and cultural behaviour between cetaceans and great apes [20,22,64,65].
Supplementary Material
Supplementary Material
Acknowledgements
We thank Shark Bay Resources and the Useless Loop community for their generous, long-term, in-kind and logistical support. We also thank all field assistants for their help during this study.
Data accessibility
All used datasets are available as electronic supplementary material to this study.
Authors' contributions
Conceived and designed study: M.K., S.J.A. and M.R.B. Carried out field and laboratory work: S.J.A., L.G., S.Wil., S.L.K., M.K., S.Wit., W.R.F., R.C.C. and M.R.B. Performed statistical analyses: M.R.B. Wrote the manuscript: M.R.B., S.J.A. and M.K. Edited the manuscript: S.L.K., L.G., S.Wil., R.C.C., W.R.F. and S.Wit.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by a Swiss National Science Foundation grant (31003A_149956) to M.K. Further financial assistance was provided by grants from the National Geographic Society, W. V. Scott Foundation, SeaWorld Research and Rescue Foundation Inc., A. H. Schultz Stiftung, and the University of Zurich. S.L.K. was supported by The Branco Weiss Fellowship—Society in Science. W.R.F. was supported by a Graduate Fellowship in Anthropogeny from the University of California, San Diego.
References
- 1.Laland K, Janik V. 2006. The animal cultures debate. Trends Ecol. Evol. 21, 542–547. ( 10.1016/j.tree.2006.06.005) [DOI] [PubMed] [Google Scholar]
- 2.Leadbeater E, Chittka L. 2007. Social learning in insects—from miniature brains to consensus building. Curr. Biol. 17, R703–R713. ( 10.1016/j.cub.2007.06.012) [DOI] [PubMed] [Google Scholar]
- 3.Hoppitt W, Laland KN. 2008. Social processes influencing learning in animals: a review of the evidence. Adv. Stud. Behav. 38, 105–165. ( 10.1016/S0065-3454(08)00003-X) [DOI] [Google Scholar]
- 4.Fogarty L, Laland KN, Morgan TJH, Webster MM, Hoppitt WJE, Rendell L. 2011. Cognitive culture: theoretical and empirical insights into social learning strategies. Trends Cogn. Sci. 15, 68–76. ( 10.1016/j.tics.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 5.Giraldeau L, Valone TJ, Templeton JJ. 2002. Potential disadvantages of using socially acquired information. Phil. Trans. R Soc. Lond. B 357, 1559–1566. ( 10.1098/rstb.2002.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laland KN. 2011. Social learning strategies. Anim. Learn. Behav. 32, 4–14. ( 10.3758/bf03196002) [DOI] [PubMed] [Google Scholar]
- 7.Pike TW, Laland KN. 2010. Conformist learning in nine-spined sticklebacks' foraging decisions. Biol. Lett. 6, 466–468. ( 10.1098/rsbl.2009.1014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrich J, Henrich N. 2010. The evolution of cultural adaptations: Fijian food taboos protect against dangerous marine toxins. Proc. R. Soc. B 277, 3715–3724. ( 10.1098/rspb.2010.1191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendal JR, Rendell L, Pike TW, Laland KN. 2009. Nine-spined sticklebacks deploy a hill-climbing social learning strategy. Behav. Ecol. 20, 238–244. ( 10.1093/beheco/arp016) [DOI] [Google Scholar]
- 10.Hoppitt W, Laland KN. 2013. Social learning: an introduction to mechanisms, methods, and models. Princeton, NJ: Princeton University Press. [Google Scholar]
- 11.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man 1871–1971 (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine. [Google Scholar]
- 12.Bateman AJ. 1984. Intra-sexual selection in Drosophila. Heredity (Edinb) 2, 349–368. [DOI] [PubMed] [Google Scholar]
- 13.Lonsdorf EV. 2005. Sex differences in the development of termite-fishing skills in the wild chimpanzees, Pan troglodytes schweinfurthii, of Gombe National Park, Tanzania. Anim. Behav. 70, 673–683. ( 10.1016/j.anbehav.2004.12.014) [DOI] [Google Scholar]
- 14.Lonsdorf EV, Anderson KE, Stanton MA, Shender M, Heintz MR, Goodall J et al. 2014. Boys will be boys: sex differences in wild infant chimpanzee social interactions. Anim. Behav. 88, 79–83. ( 10.1016/j.anbehav.2013.11.015 ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873–885. ( 10.1016/j.anbehav.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE.. 2013. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373–381. ( 10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smolker R, Richards A, Connor R, Mann J, Berggren P. 1997. Sponge carrying by dolphins (Delphinidae, Tursiops sp.): a foraging specialization involving tool use? Ethology 103, 454–465. ( 10.1111/j.1439-0310.1997.tb00160.x) [DOI] [Google Scholar]
- 18.Krützen M, Mann J, Heithaus MR, Connor RC, Bejder L, Sherwin WB. 2005. Cultural transmission of tool use in bottlenose dolphins. Proc. Natl Acad. Sci. USA 102, 8939–8943. ( 10.1073/pnas.0500232102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson EM, Mann J. 2011. The ecological conditions that favor tool use and innovation in wild bottlenose dolphins (Tursiops sp.). PLoS ONE 6, e22243 ( 10.1371/journal.pone.0022243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krützen M, Kreicker S, MacLeod CD, Learmonth J, Kopps AM, Walsham P, Allen SJ. 2014. Cultural transmission of tool use by Indo-Pacific bottlenose dolphins (Tursiops sp.) provides access to a novel foraging niche. Proc. R. Soc. B 281 20140374 ( 10.1098/rspb.2014.0374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopps AM, Krützen M, Allen SJ, Bacher K, Sherwin WB. 2014. Characterizing the socially transmitted foraging tactic ‘sponging’ by bottlenose dolphins (Tursiops sp.) in the western gulf of Shark Bay, Western Australia. Mar. Mammal Sci. 30, 847–863. ( 10.1111/mms.12089) [DOI] [Google Scholar]
- 22.Mann J, Stanton MA, Patterson EM, Bienenstock EJ, Singh LO. 2012. Social networks reveal cultural behaviour in tool-using using dolphins. Nat. Commun. 3, 980 ( 10.1038/ncomms1983) [DOI] [PubMed] [Google Scholar]
- 23.Bacher K, Allen S, Lindholm AK, Bejder L, Krützen M. 2010. Genes or culture: are mitochondrial genes associated with tool use in bottlenose dolphins (Tursiops sp.)? Behav. Genet. 40, 706–714. ( 10.1007/s10519-010-9375-8) [DOI] [PubMed] [Google Scholar]
- 24.Mann J, Sargeant BL, Watson-Capps JJ, Gibson QA, Heithaus MR, Connor RC, Patterson E. 2008. Why do dolphins carry sponges? PLoS ONE 3, e3868 ( 10.1371/journal.pone.0003868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann J, Patterson EM. 2013. Tool use by aquatic animals. Phil. Trans. R. Soc. B 368, 20120424 ( 10.1098/rstb.2012.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson EM, Krzyszczyk E, Mann J. 2016. Age-specific foraging performance and reproduction in tool-using wild bottlenose dolphins. Behav. Ecol. 27, 401–410. ( 10.1093/beheco/arv164) [DOI] [Google Scholar]
- 27.Connor RC, Krützen M. 2015. Male dolphin alliances in Shark Bay: changing perspectives in a 30-year study. Anim. Behav. 103, 223–235. ( 10.1016/j.anbehav.2015.02.019) [DOI] [Google Scholar]
- 28.Connor RC, Wells RJ, Mann J, Read A. 2000. The bottlenose dolphin: social relationships in a fission–fusion society. In Cetacean societies: field studies of dolphins and whales (eds Mann J, Connor R, Tyack P, Whitehead H), pp. 91–126. Chicago, IL: University of Chicago Press. [Google Scholar]
- 29.Connor RC, Smolker RA, Richards AF. 1992. Two levels of alliance formation among male bottlenose dolphins (Tursiops sp). Proc. Natl Acad. Sci. USA 89, 987–990. ( 10.1073/pnas.89.3.987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerber L, et al. In review Multi-level cooperation in wild male bottlenose dolphins is predicted by long-term friendships. Behav. Ecol.
- 31.Tyne JA, Loneragan NR, Kopps AM, Allen SJ, Krützen M, Bejder L. 2012. Ecological characteristics contribute to sponge distribution and tool use in bottlenose dolphins Tursiops sp. Mar. Ecol. Prog. Ser. 444, 143–153. ( 10.3354/meps09410) [DOI] [Google Scholar]
- 32.Würsig B, Würsig M. 1977. The photographic determination of group size, composition, and stability of coastal porpoises (Tursiops truncatus). Science 198, 755–756. [Google Scholar]
- 33.Smolker RA, Richards AF, Connor RC, Pepper JW. 1992. Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour 123, 38–69. ( 10.1163/156853992X00101) [DOI] [Google Scholar]
- 34.Krützen M, Barre L, Möller L, Heithaus M, Simms C, Sherwin W. 2002. A biopsy system for small cetaceans: darting success and wound healing in Tursiops spp. Mar. Mammal Sci. 18, 863–878. ( 10.1111/j.1748-7692.2002.tb01078.x) [DOI] [Google Scholar]
- 35.Gilson A, Syvanen M, Levine K, Banks J. 1998. Deer gender determination by polymerase chain reaction: validation study and application to tissues, bloodstains, and hair forensic samples from California. Calif. Fish Game 84, 159–169. [Google Scholar]
- 36.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.r-project.org [Google Scholar]
- 37.Mann J, Connor RC, Barré LM, Heithaus MR. 2000. Female reproductive success in bottlenose dolphins (Tursiops sp.): life history, habitat, provisioning, and group-size effects. Behav. Ecol. 11, 210–219. ( 10.1093/beheco/11.2.210) [DOI] [Google Scholar]
- 38.Connor RC, Smolker R, Bejder L. 2006. Synchrony, social behaviour and alliance affiliation in Indian Ocean bottlenose dolphins, Tursiops aduncus. Anim. Behav. 72, 1371–1378. ( 10.1016/j.anbehav.2006.03.014) [DOI] [Google Scholar]
- 39.Tabachnick BG, Fidell LS, Ullman JB. 2007. Using multivariate statistics. Boston, MA: Pearson. [Google Scholar]
- 40.Welch WJ. 1990. Construction of permutation tests. J. Am. Stat. Assoc. 85, 693–698. ( 10.1080/01621459.1990.10474929) [DOI] [Google Scholar]
- 41.Fay M, Shaw PA. 2010. Exact and asymptotic weighted logrank tests for interval censored data: the interval R Package. J. Stat. Softw. 36, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cairns SJ, Schwager SJ. 1987. A comparison of association indices. Anim. Behav. 35, 1454–1469. ( 10.1016/S0003-3472(87)80018-0) [DOI] [Google Scholar]
- 43.Bejder L, Fletcher D, Bräger S. 1998. A method for testing association patterns of social animals. Anim. Behav. 56, 719–725. ( 10.1006/anbe.1998.0802) [DOI] [PubMed] [Google Scholar]
- 44.Whitehead H, Bejder L, Ottensmeyer CA. 2005. Testing association patterns: issues arising and extensions. Anim. Behav. 69, e1–e6. ( 10.1016/j.anbehav.2004.11.004) [DOI] [Google Scholar]
- 45.Whitehead H. 2009. SOCPROG: program for analyzing social structure. Behav. Ecol. Sociobiol. 63, 765–778. ( 10.1007/s00265-008-0697-y ) [DOI] [Google Scholar]
- 46.Dekker D, Krackhardt D, Snijders TAB. 2007. Sensitivity of MRQAP tests to collinearity and autocorrelation conditions. Psychometrika 72, 563–581. ( 10.1007/s11336-007-9016-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farine DR. 2013. Animal social network inference and permutations for ecologists in R using asnipe. Methods Ecol. Evol. 4, 1187–1194. ( 10.1111/2041-210X.12121) [DOI] [Google Scholar]
- 48.Kopps AM, Ackermann CY, Sherwin WB, Allen SJ, Bejder L, Krützen M. 2014. Cultural transmission of tool use combined with habitat specializations leads to fine-scale genetic structure in bottlenose dolphins. Proc. R. Soc. B 281, 20133245 ( 10.1098/rspb.2013.3245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitehead H. 2008. Analyzing animal societies: quantitative methods for vertebrate social analysis, pp. 161–168. Chicago, IL: University of Chicago Press; See http://public.eblib.com/choice/publicfullrecord.aspx?p=408184 [Google Scholar]
- 50.Killick R, Haynes K, Eckley I, Fearnhead P, Lee J. 2016. ‘changepoint’: methods for changepoint detection. See https://cran.r-project.org/web/packages/changepoint/changepoint.pdf
- 51.King SL, et al. 2018. Bottlenose dolphins retain individual vocal labels in multi-level alliances. Curr. Biol. 28, 1993–1999. ( 10.1016/j.cub.2018.05.013) [DOI] [PubMed] [Google Scholar]
- 52.Csárdi G, Nepusz T. 2014. The igraph software package for complex network research. J. Comput. Appl. Complex Sy:9. See https://igraph.org/r.
- 53.Bridge PD, Fry J. 1993. Classification. In Biological data analysis (ed. J Fry), pp. 219–242. Oxford, UK: Oxford University Press. [Google Scholar]
- 54.Smith TB, Skúlason S. 1996. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Syst. 27, 111–133. ( 10.1146/annurev.ecolsys.27.1.111) [DOI] [Google Scholar]
- 55.Bolnick DI. 2001. Intraspecific competition favours niche width expansion in Drosophila melanogaster. Nature 410, 463–466. ( 10.1038/35068555) [DOI] [PubMed] [Google Scholar]
- 56.Krützen M, Sherwin WB, Connor RC, Barré LM, Van De Casteele T, Mann J, Brooks R. 2003. Contrasting relatedness patterns in bottlenose dolphins (Tursiops sp.) with different alliance strategies. Proc. R. Soc. B 497–502. ( 10.1098/rspb.2002.2229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai YJJ, Mann J. 2012. Dispersal, philopatry, and the role of fission–fusion dynamics in bottlenose dolphins. Mar. Mammal Sci. 29, 261–279. ( 10.1111/j.1748-7692.2011.00559.x) [DOI] [Google Scholar]
- 58.Krzyszczyk E, Patterson EM, Stanton MA, Mann J. 2017. The transition to independence: sex differences in social and behavioural development of wild bottlenose dolphins. Anim. Behav. 129, 43–59. ( 10.1016/j.anbehav.2017.04.011) [DOI] [Google Scholar]
- 59.Tosi CH, Ferreira RG. 2010. Differences between solitary and group time budgets in Guiana dolphin (Sotalia guianensis) at northeastern Brazil. In Whales and dolphins behavior, biology and distribution (ed. CA Murray), pp. 139–150. New York, NY: Nova Science Publishers. [Google Scholar]
- 60.Smolker RA, Connor RC. 1996. ‘Pop’ goes the dolphin: a vocalization male bottlenose dolphins produce during consortships. Behaviour 133, 643–662. ( 10.1163/156853996X00404) [DOI] [Google Scholar]
- 61.Mann J, Sargeant BL. 2003. Like mother like calf: the ontogeny of foraging traditions in wild Indian Ocean bottlenose dolphins (Tursiops sp.). In The biology of traditions (eds Fragaszy DM, Perry S), pp. 236–266. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 62.McPherson M, Smith-Lovin L, Cook JM. 2001. Birds of a feather: homophily in social networks. Annu. Rev. Sociol. 27, 415–444. ( 10.1146/annurev.soc.27.1.415) [DOI] [Google Scholar]
- 63.Rivera MT, Soderstrom SB, Uzzi B. 2010. Dynamics of dyads in social networks: assortative, relational, and proximity mechanisms. Annu. Rev. Sociol. 36, 91–115. ( 10.1146/annurev.soc.34.040507.134743) [DOI] [Google Scholar]
- 64.Allen SJ, King SL, Krützen M, Brown AM. 2017. Multi-modal sexual displays in Australian humpback dolphins. Sci. Rep. 7, 13644 ( 10.1038/s41598-017-13898-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lonsdorf EV, Eberly LE, Pusey AE. 2004. Sex differences in learning in chimpanzees. Nature 428, 715–716. ( 10.1038/428715a) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All used datasets are available as electronic supplementary material to this study.