Abstract
Introduction
In the nationwide Dutch Acute Stroke Audit (DASA), consecutive patients with acute ischaemic stroke (AIS) and intracranial haemorrhage (ICH) are prospectively registered. Acute stroke care is a rapidly evolving field in which intravenous thrombolysis (IVT) and intra-arterial thrombectomy (IAT) play a crucial role in increasing odds of favourable outcome. The DASA can be used to assess the variation in care between hospitals and develop ‘best practice’ in acute stroke care. Patients and methods: We describe the initiation and design of the DASA as well as the results from 2015 and 2016.
Results
In 2015 and 2016, 55,854 patients with AIS and 7727 patients with ICH were registered in the DASA. Treatment with IVT was administered to 10,637 patients (with an increase of 1.3% in 2016) and 1740 patients underwent IAT (with an increase of 1% in 2016). Median door-to-needle time for IVT and median door-to-groin time for IAT have decreased from 27 to 25 min and 66 to 64 min, respectively. Mortality during admission was 4.9% in patients with AIS, whereas 26% of patients with ICH died. Modified Rankin Scale score at three months was registered in 49% of AIS patients and 45% of ICH patients.
Discussion
During the nationwide DASA, time to treatment is reduced for IVT as well as IAT. With the rapidly evolving treatment of acute stroke care, the DASA can be used to monitor the quality provided on patient- and hospital level.
Conclusion
Increasing completeness of registration of the outcome, in combination with adjustment for patient-related factors, is necessary to define and further improve the quality of the acute stroke care.
Keywords: Stroke, clinical audit, quality indicators
Introduction
Stroke is the second most common cause of death and one of the main causes of disability in the world.1 Therefore, a lot of research into treatment of strokes is carried out and as a result the field of acute stroke care is rapidly evolving. Intravenous thrombolysis (IVT), and the more recently introduced intra-arterial thrombectomy (IAT), both currently play an important part of the treatment of acute ischaemic stroke (AIS). In IVT as well as IAT, the treatment should be given in the first 4.5 or 6 h, respectively, and within these time frames as soon as possible (‘time is brain’).2 The faster these treatments are provided, the higher the odds for a favourable outcome.3,4 The treatment with IVT and IAT is logistically and technically complex and performed by a multidisciplinary and experienced team, consisting of neurologists, (intervention) radiologists, anaesthesiologists and nurses in often different hospitals. Therefore, a well-organised acute stroke care is a necessity.
By measuring everyday practice, more information can be obtained regarding quality of stroke care compared to the information provided by clinical trials alone. Clinical auditing is an instrument that seeks to assure the quality of patient care and aids to improve outcomes. It uses quality indicators to measure the performance of an individual hospital over time and compares the quality of care provided between hospitals. Therefore, clinical auditing can identify areas for improvement. The main clinical auditing tool for stroke in the Netherlands since 2014 is the Dutch Acute Stroke Audit (DASA). The DASA is a clinical audit concerning stroke care for patients with AIS and intracranial haemorrhage (ICH). The DASA can serve as an instrument to determine variation of care between hospitals to investigate the reason for this variation and to find ways to improve, for example by an in-depth study of best practices in acute stroke care.
Aims and/or hypothesis
The aim of this study was to present the structure of the DASA and evaluate the results of the registration of acute stroke care parameters in the DASA from 2015 up to and including 2016.
Materials and methods
Initiation of the DASA
In the Netherlands, the foundation ‘Kennisnetwerk CVA NL’ (KNCN) was set up in 2006 to secure and improve care by registering the multidisciplinary care of stroke patients on a regional level. In 2014, KNCN joined the Dutch Institute for Clinical Auditing (DICA) to initiate the Cerebrovascular Accident Benchmark (CVAB) to register stroke patients to evaluate stroke care in each hospital in the Netherlands. In 2016, the Netherlands Society of Neurology (NVN) took over the governance of the audit. Due to the high registration burden of the audit and the developments in acute stroke care treatment, the primary focus of the registry was shifted towards the acute treatment of AIS. In 2017, the CVAB registry was renamed to DASA.
DICA as a facilitator of clinical audits in the Netherlands
DICA is an independent organisation, founded by medical specialists, that facilitates national audits for various medical professions, including the DASA. The National Health Care Institute utilises DICA to fulfil the role given by the government of maintaining the quality and affordability of health care in the Netherlands as well as provide transparency in quality of care to the public. Funding for the audit is ensured by ‘Zorgverzekeraars Nederland’ (i.e. the umbrella organisation of nine health insurers in the Netherlands).
Dataset defined by experts in the field
The NVN formed a clinical audit board, consisting of mandated clinical experts in acute stroke care. Alongside neurologists, this board consists of a vascular surgeon (specialised in carotid interventions), an epidemiologist and additionally includes representation of the board of the NVN. The clinical audit board defines the indicators, containing performance indicators as well as outcome measures. These are based on Dutch evidence-based guidelines5 and emerge from conferences with neurologists active in the NVN and are updated with the latest developments in stroke care. They are used to assess the provided quality of care and identify variation between hospitals. Each variable in the dataset is evaluated by the clinical audit board for clinical relevancy on an annual basis.
Data collection
The data dictionary of the DASA is shown in Supplement 1. The data are collected with a waiver of patient consent as is common in clinical audits. Hospitals are free to decide who carries out the data registration (for instance (research) nurses, data managers or neurologists), but the final responsibility rests with the neurologist. Besides DICA and the NVN, a third party, Medical Research Data Management (MRDM), is involved to anonymise the data to comply with privacy legislature. Hospitals have three ways to provide the collected data to this data processor. First, an online survey through a secured web environment is available for hospitals to record the data. Second, hospitals can distribute the data in batches, i.e. data files in which large amounts of data can be transferred directly to the data processor. Third, to minimise registration burden, some hospitals took initiative to implement data linkage, i.e. extracting the data from their individual electronic patient health record to be automatically forwarded to MRDM. Thereafter, the data are reported in de-identified format to the clinical researcher of the DASA and the clinical audit board, who perform the nationwide data analysis.
Feedback of data
Results of the data are provided to participating hospitals through a secured website, MyDASA. Results are weekly updated. Annually, indicators shown to be valid (i.e. indicator is a valid tool to measure the defined care) and complete are selected by the clinical audit board for public transparency. These indicators are discussed with other stakeholders, such as medical specialists, hospitals, organisations representing patients and insurers and are then included in next year’s transparent indicators. During the next year of inclusion, the results of the established set of public indicators can be monitored in MyDASA and at the end of the year hospital-specific indicator results are published online and therefore visible to all relevant parties through DICA’s web-based Transparency Portal. In this way, the public, as well as the health care insurers and the government, are informed with meaningful information about the quality of the care provided by individual hospitals.
Patient domain
The DASA is a voluntary prospective clinical audit. Consecutive patients over 18 years of age that are presented in the acute phase with AIS and ICH at the hospital are included. Patients are considered to be in the acute phase of the stroke when they are presented to the emergency room or when they suffer a stroke while admitted to hospital. Excluded are patients with a transient ischaemic attack (TIA), cerebral venous thrombosis, subarachnoid haemorrhage, subdural and epidural haematoma. With respect to the DASA, TIA was defined as a neurological event caused by temporary lack of blood flow with neurological deficits that were not apparent at presentation anymore. Imaging-proven infarct with time of deficit of less than 24 h was classified as AIS. The minimal data requirements to consider a patient eligible for analysis are date of birth, type of stroke and date of presentation with symptoms of stroke at the hospital. The date of presentation at the hospital is used to determine the year of inclusion.
Indicators
The collected data of each patient can be divided into three categories: patient and/or disease characteristics, process indicators and outcome indicators. Age, gender and type of stroke are registered as relevant patient characteristics. Severity of stroke, i.e. National Institutes of Health Stroke Scale (NIHSS) score, was not registered in every day practice (i.e. outside of trial setting) for each stroke patient until 2017. Process indicators measure the activities concerning aspects of health care that were delivered by the health care providers. In the DASA, onset-to-door time was registered for both patients with AIS as well as patients with ICH. Onset time was defined as the time when symptoms of stroke started reported by patient or observer. In case the patient had a stroke during sleep, or the patient could not recall the time when symptoms began, the onset time was defined as unknown. Door time is defined as the time of arrival at emergency room. In case the patient has a stroke while admitted to the hospital, then the time when the neurologist sees the patient is the door time. Onset-to-door time is defined as the difference between onset time and door time. Other process indicators have been implemented, such as application of IVT and/or IAT including the derived door-to-needle time (DTNT) and door-to-groin time (DTGT), for only patients with AIS. DTNT is defined as the door time to the start of IVT. DTGT is the door time at the IAT centre to the groin puncture at the start of the IAT. To assess the functional outcome after three months, the modified Rankin Scale (mRS) score is used. Data for this indicator are collected by a research nurse over telephone. Additionally, during this time point, possible mortality will be recorded.
Methods
R Studio version 3.4.3 was used for statistical analysis. Patients with AIS and ICH registered in registration years 2014 up to and including 2016 were included. First, the volume of the DASA was described of the whole cohort. For further analysis, the year 2014 was excluded as this was a start-up year and was believed to be incomplete. Differences between patients and treatment characteristics are described using descriptive statistics. Categorical variables were compared using the chi-square trend test. Kruskal–Wallis test was used to assess age, onset-to-door time, DTNT and DTGT over the years of registration. Ordinal regression was used to determine the difference in mRS scores for each year. Logistic regression was used to determine the effect of IAT centre on dichotomised mRS (0–2 compared to 3–6). For this study, no informed consent or ethical approval was required under Dutch law.
Results
During the first three years of the audit, the DASA has included over 86,388 patients: 10,680 (12%) with ICH and 75,708 (88%) with AIS. In 2014, the first year of the audit, 75 hospitals participated. By 2016, 81 hospitals participated in the audit.
Patient characteristics
From January 2015 to December 2016, 55,854 patients with AIS and 7727 patients with ICH were registered in the DASA. The median age of patients with AIS was 74.0 years (IQR 64–82) and 52% was male. Of the patients with ICH, the median age was 76.0 years (IQR 66–83) and 52% was male. The median age and sex distribution did not significantly change over the years for both AIS and ICH, as shown in Table 1.
Table 1.
Patient and disease characteristics, process indicators and outcome indicators registered in the DASA.
|
Acute ischaemic stroke (n = 55,854) |
Intracranial haemorrhage (n = 7727) |
||||||
|---|---|---|---|---|---|---|---|
| 2015 | 2016 | p-value | 2015 | 2016 | p-value | ||
| Number of patients | 28,820 | 27,034 | 4145 | 3582 | |||
| Number of hospitals | 78 | 80 | 79 | 75 | |||
| Patient and disease characteristics | |||||||
| Age in years (median, IQR) | 74 (64–82) | 74 (64–82) | 0.92 | 76 (65–83) | 76 (66–84) | 0.17 | |
| Male sex (n,%) | 14,555 (52.4) | 13,839 (52.6) | 0.62 | 1997 (51.8) | 1802 (52.5) | 0.61 | |
| Onset-to-door time in minutes (median, IQR) | 171 (74–544) | 175 (74–570) | 0.42 | 138 (61–430) | 130 (61–408) | 0.45 | |
| Process indicators | |||||||
| Intravenous thrombolysis (n, %) | 5338 (20.4) | 5299 (21.7) | <0.001 | – | – | – | |
| Door-to-needle time in minutes (median, IQR) | 27 (20–37) | 25 (19–35) | <0.001 | – | – | – | |
| Intra-arterial thrombectomy (n, %) | 755 (3.1) | 985 (4.1) | <0.001 | – | – | – | |
| Door-to-groin time in minutes (median, IQR) | 66 (41–99) | 64 (35–95) | 0.02 | – | – | – | |
| Outcome indicators | |||||||
| In-hospital mortality (n, %) | 1310 (5.0) | 1161 (4.8) | 0.19 | 995 (25.4) | 863 (26.4) | 0.35 | |
| Modified Rankin Scale (mRS) scorea | |||||||
| No symptoms, mRS 0 (n, %) | 2144 (17.8) | 2294 (19.8) | <0.001 | 89 (7.1) | 87 (7.5) | <0.001 | |
| Mild symptoms, mRS 1–2 (n, %) | 6178 (51.2) | 5893 (51.0) | 478 (37.9) | 459 (39.6) | |||
| Moderate to severe symptoms, mRS 3-5 (n, %) | 2783 (23.1) | 2608 (22.5) | 478 (37.9) | 414 (35.8) | |||
| Death, mRS 6 (n, %) | 953 (7.9) | 769 (6.6) | 215 (17.1) | 198 (17.1) | |||
IQR: interquartile range. P-values < 0.05 are printed bold.
amRS was not obtained of patients that died during admission.
Process indicators
Onset-to-door time. Median onset-to-door time was 173 min (IQR 74–544) in patients with AIS and 134 min (IQR 61–421) in patients with ICH. Onset-to-door time did not change over time in both AIS and ICH.
IVT. Seventy-five hospitals administrating IVT are registered in the DASA. In 2015, 20.4% (n = 5338) of the patients with AIS received IVT. This percentage increased to 21.7% (n = 5299) in 2016 (p < 0.001).
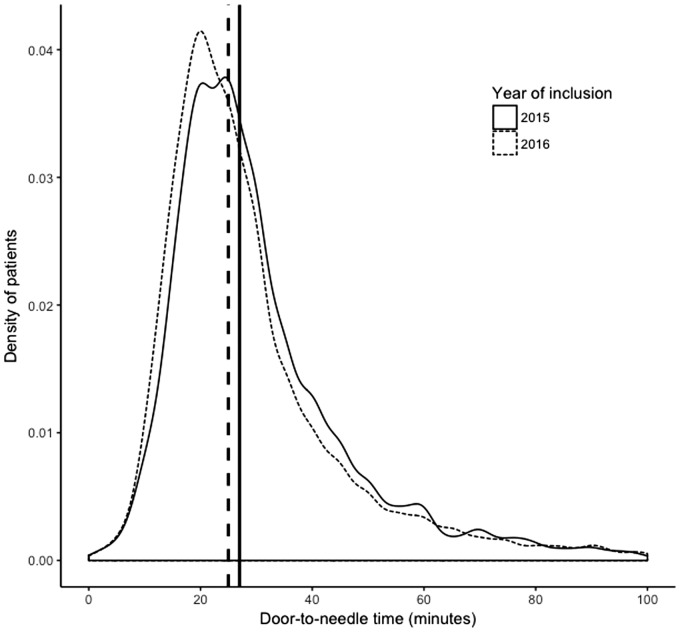
During the registry, the median DTNT reduced from 27 min (IQR 20–37) to 25 min (IQR 19–35) (p < 0.001). The range of distribution of IQR decreased over the years, implying that variation in DTNT reduced, as shown in the density plot in Figure 1. Of all patients receiving IVT during the study period, 92% was given within 60 min.
Figure 1.
Distribution of DTNT in patients with AIS treated with IVT for each year. The vertical lines represent the annual median.
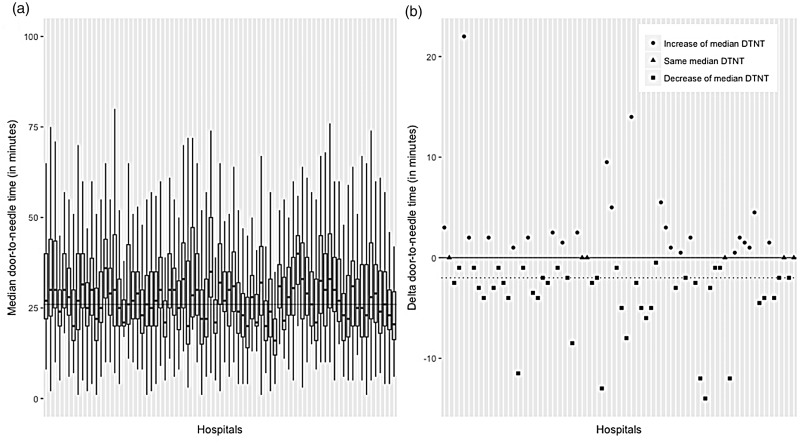
On a hospital level, the median DTNT ranged from 15.5 to 48 min over the course of 2015 and 2016, as shown in Figure 2(a). Figure 2(b) shows the delta for each hospital, i.e. the difference of median DTNT between 2015 and 2016. Forty-three hospitals lowered their median DTNT when comparing 2015 and 2016, six hospitals have similar median DTNTs and 23 hospitals show an increase of median DTNT.
Figure 2.
(a) Boxplots of DTNT with range of distribution from fifth to 95th percentile for 2015 and 2016 combined for each hospital registering in the DASA and (b) difference in median DTNT in minutes (i.e. delta) between 2015 and 2016 for each hospital registering in the DASA. The dotted line reflects the nationwide trend of reduction of median DTNT. DTNT: door-to-needle time.
IAT. The Multicenter Randomized Clinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands (MR CLEAN) proved irrevocably as first international randomised controlled trial that IAT is an effective and safe therapy, when administered within 6 h after AIS caused by proximal intracranial occlusion of the anterior circulation.6 All 17 IAT hospitals, which also participated in the MR CLEAN registry, registered their IAT patients in the DASA. In 2015, 3.1% of patients (n = 755) with IAT treatment were registered; by 2016, this increased to 4.1% (n = 986; p < 0.001).
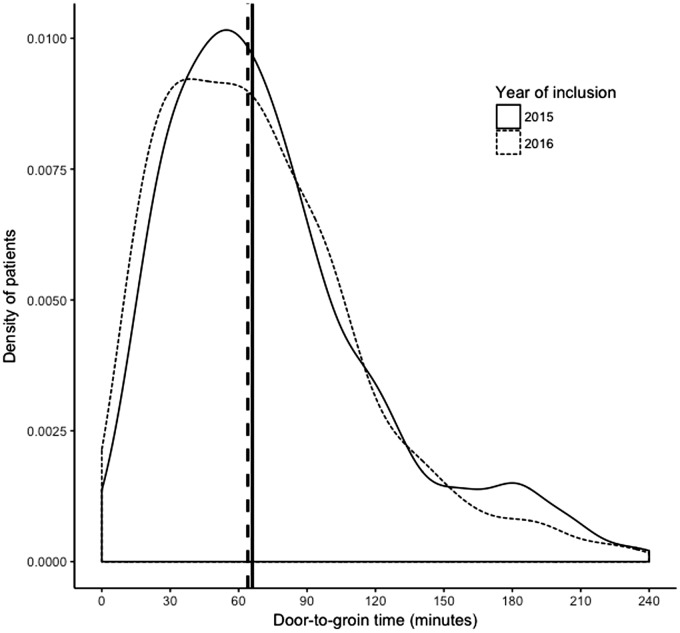
During the study period, the median DTGT reduced from 66 min (IQR 41–99) to 64 min (IQR 35–95). Similar to the DTNT in treatment with IVT, the variation in DTGT reduced, as shown in Figure 3.
Figure 3.
Annual distribution of DTGT in patients with AIS treated with IAT. The vertical lines represent the annual median.
Outcome indicators
Of patients with AIS registered from 2014 to and including 2016, the mortality during admission was 4.9% with no significant difference between the years of inclusion (p = 0.19). Of the patients that were discharged, 7.3% had died at three-month follow-up. After three months, 70% of patients were functionally independent (mRS score of 0–2) of which 19% had no symptoms at all (mRS of 0). When comparing IAT centres to non-IAT centres, the IAT centres have significantly more patients with severe outcome (mRS score of 3–6) than non-IAT centres (OR 1.09; 95% CI 1.04–1.15; p = 0.001).
Of patients with ICH from 2014 to and including 2016, the mortality during admission was 26%. At the three-month follow-up, of the patients that were discharged from hospital another 17% had died. After three months, 47% of patients was functionally independent (mRS score of 0–2) of which 7% had no symptoms at all (mRS score of 0). Moderate or severe disability (mRS of 3–5) was assessed in 37% of the follow-up patients. When comparing both years, no significant differences in independent functioning at three months were found (p = 0.35).
After discharge, the follow-up was registered in 49% of the patients with AIS and 45% of the patients with ICH. For both AIS and ICH, the percentage of completeness of registration for each hospital ranges from 0 to 99.7% and 0 to 100%, respectively.
Discussion
This is the first report of the DASA. Since the start of the DASA in 2014, over 86,000 stroke patients have been registered. During the DASA, the median time to treatment for both IVT and IAT decreased and showed a narrower range of distribution of treatment time, indicating less variability.
Clinical auditing, in this case by using DASA, can be used to define a standard by using process indicators and outcome indicators to assess the quality of the treatment provided by hospitals.7–9 Due to the ongoing continuous character of the auditing cycle, an up-to-date benchmark can be provided. Several studies have shown that a reduction of the DTNT in stroke care can be reached, using quality registration as a tool for improvement.10–12 As a result, the requirement of a median DTNT under 45 min for each hospital has now been formulated in the Dutch national quality standard of acute stroke to stimulate local interventions aiming at a reduction of time to treatment. Local quality improvement processes in the Netherlands for DTNT have shown to be effective. For instance, Zinkstok et al.13 and Van Schaik et al.14 used information obtained from an auditing cycle to significantly reduce the median DTNT in IVT. With the addition of IAT as a second acute treatment option for patients with AIS, the DASA can be utilised to evaluate the DTGT. IAT is only performed in selected centres, therefore referral from a centre where the patient primarily presented is often necessary. On short term and based on the data in the DASA, it will be possible to calculate door-to-door-to-groin times to evaluate the regional organisation of stroke care.
There are several limitations to the DASA. First, severity of stroke was not registered in the DASA during the first three years of the registry. However, such influencing prognostic patient-related factors are necessary to register to understand the measured outcome. From January 2014 until September 2016, five hospitals in the DASA registered a more extensive set of variables to identify possible influential factors. Three were determined: age, type of stroke (either AIS or ICH) and the severity of the stroke (defined by the NIHSS score). Earlier research verified these as important prognostic factors for which 30-day outcome measures must be adjusted.15 Since 2017, the NIHSS score was added to the dataset and these case-mix factors are used in order to provide reliable benchmark information.
A second possible limitation is that to date the mRS score after three months has been registered in 49% of the patients with AIS and in 45% of patients with ICH. This percentage of completion needs to improve to better assess quality improvement. When correcting for patient-related factors, the outcome measure could be able to rank hospitals, as mentioned earlier. It is challenging for hospitals in the Netherlands to complete these data, as they do not get financial support to aid this process. Other national audits seem to experience the same difficulty with completeness range from 5 to 100%.16 The NVN is actively appealing to the neurologists to implement NIHSS score registration in the acute phase of AIS as well as to increase the completeness concerning three-month follow-up.
A final limitation is that data completeness is difficult to determine as only patients presented to the emergency room in the acute phase of the stroke are registered in the DASA. Patients seen in the outpatient clinic are not included, but are assigned the same diagnosis–treatment–combination hospital reimbursement code. To assess data accuracy, we will perform data verification on a random sample in the near future.
Even with the significant reduction of DTNT and DTGT, the DASA can continue to play an important role in monitoring and improving stroke care in the future. The DAWN trial17 and the DEFUSE-3 trial18 have recently proven that IAT is effective in a selection of patients with large vessel occlusion with an onset of stroke symptoms for more than 6 h and less than 24 h. This indicates that these patients with long-standing symptoms must be assessed in the acute setting as well, resulting in extra strain on the acute stroke services. This warrants an adjustment in regional logistic protocol. The DASA can be used to monitor the implementation of this adjusted protocol and indicate domains for improvement.
Conclusion
From 2014 to and including 2016 the DASA, a national audit in the Netherlands, has registered over 86,000 patients with AIS and ICH. Over the course of time, the number of patients who received IVT and/or IAT and have been enrolled into the registry has significantly risen and the median time from the door of the hospital to treatment has been reduced. Increasing completeness of registration of the outcome, in combination with adjustment for patient-related factors, is necessary to define and further improve the quality of the acute stroke care provided by each hospital. Due to the rapidly evolving field of acute stroke care and the broadening of the selection of patients that are eligible for treatment, there is a continuing need for monitoring and assessing stroke care as done by the DASA.
Supplemental Material
Supplemental material for The Dutch Acute Stroke Audit: Benchmarking acute stroke care in the Netherlands by Laurien S Kuhrij, Michel WJM Wouters, Renske M van den Berg-Vos, Frank-Erik de Leeuw and Paul J Nederkoorn in European Stroke Journal
Acknowledgements
We thank the Clinical Audit board of the DASA for its contribution: Lingsma HF, de Borst GJ, van Norden AGW and Eysink Smeets MM.
We also want to thank the DASA consortium: Aerden LAM (Reinier de Graaf Ziekenhuis), Alblas CL (Franciscus Vlietland Ziekenhuis), de Beer F (Spaarne Gasthuis), Bienfait PH (Gelre Ziekenhuis), Boon AE (St Anna Ziekenhuis), Bor S (Rode Kruis Ziekenhuis), Boreas AMHP (Antonius Ziekenhuis Sneek), Bronner I (Flevoziekenhuis), Brouns R (St ZorgSaam Terneuzen), Brouwers PJAM (Medisch Spectrum Twente), Brugman F (Ziekenhuis Rivierenland), Dane ML (Rivas Beatrix Ziekenhuis), Fransen PSS (Isala Klinieken), van Gemert HMA (Meander Medisch Centrum), van Golde AEL (Ziekenhuisgroep Twente), de Graaf MT (Slotervaart ziekenhuis), Hani L (Noordwest Ziekenhuis Den Helder), Hilkens PH (St Antonius Ziekenhuis), ten Holter JBM (Deventer ziekenhuis), de Jong SW (St Jansdal Harderwijk), Kapelle LJ (University Medical Center Utrecht), Keizer K (Catharina ziekenhuis), Keunen R (HagaZiekenhuis), Kloppenborg RP (St Franciscus Gasthuis), Kok AJM (Elkerkliek Ziekenhuis), Koops L (Isala Diaconessenhuis Meppel), Kruyt ND (Leiden University Medical Center), de Leeuw FE (Radboud University Medical Center), Lövenich H (St Jans Gasthuis), Luijckx GJ (University Medical Center Groningen), Maasland E (Bethesda Ziekenhuis), van der Meijden AMHG (VieCuri Medisch Centrum), Miedema I (Ziekenhuis Gelderse Vallei), Nederkoorn PJ (Academic Medical Center), van Norden AGW (Amphia Ziekenhuis), Persoon S (Wilhelmina Ziekenhuis), Peters EW (Admiraal de Ruyter Ziekenhuis), van der Ree TC (Westfries Gasthuis), Rozeman AD (Albert Schweitzer Ziekenhuis), Saxena R (Maasstad Ziekenhuis), van Schaik S (Ons Lieve Vrouwe Gasthuis), de Schryver ELLM (Alrijne Ziekenhuis), Schuiling WJ (Medisch Centrum Leeuwarden), Schut ES (Martiniziekenhuis), Staals JEA (Maastricht University Medical Center), Stalpers X (Maxima medisch centrum), Tjeerdsma H (Bravis Ziekenhuis), van Tuijl JH (St Elisabeth Ziekenhuis), Vermeer SE (Rijnstate ziekenhuis), Visser MC (Vrije Universiteit Medical Center), van den Wijngaard I (Haaglanden Medical Center), van Zagten MSG (Jeroen Bosch Ziekenhuis) and Zylicz SA (Langeland Zoetermeer).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent
Not applicable.
Ethical approval
For this study, no informed consent or ethical approval was required under Dutch law.
Guarantor
PJN.
Contributorship
LSK and PJN wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.GBD 2016 Cause of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017; 390: 1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 3.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016; 316: 1279–1289. [DOI] [PubMed] [Google Scholar]
- 5.Nederlandse Vereniging voor Neurologie. Richtlijn herseninfarct en hersenbloeding, https://www.zorginzicht.nl/bibliotheek/acute-beroertezorg/KwaliteitsstandaardenDocumenten/Richtlijn%20herseninfarct%20en%20hersenbloeding.pdf (2017, accessed 3 May 2018).
- 6.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 7.Leersum van NJ, Snijders HS, Henneman D, et al. The Dutch Surgical Colorectal Audit. EJSO 2013; 39: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 8.Busweiler LAD, Wijnhoven BPL, Berge Henegouwen van MI, et al. Early outcomes from the Dutch Upper Gastrointestinal Cancer Audit. Br J Surg 2016; 103: 1855–1863. [DOI] [PubMed] [Google Scholar]
- 9.Bommel van ACM, Spronk PER, Vrancken Peeters Mjtfd, et al. Clinical auditing as an instrument for quality improvement in breast cancer in the Netherlands: the national NABON Breast Cancer Audit. J Surg Oncol 2017; 115: 243–249. [DOI] [PubMed] [Google Scholar]
- 10.Meretoja A, Strbian D, Mustanoja S, et al. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology 2012; 79: 306–313. [DOI] [PubMed] [Google Scholar]
- 11.Dickson R, Nedelcut A, Nedelcut MM. Stop stroke: a brief report on door-to-needle times and performance after implementing an acute care coordination medical application and implications to emergency medical services. Prehosp Disaster Med 2017; 32: 343–347. [DOI] [PubMed] [Google Scholar]
- 12.Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014; 311: 1632–1640. [DOI] [PubMed] [Google Scholar]
- 13.Zinkstok SM, Beenen LF, Luitse JS, et al. Thrombolysis in stroke within 30 minutes: results of the acute brain care intervention study. PLoS One 2016; 11: e0166668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Schaik SM, Van der Veen B, Van den Berg-Vos RM, et al. Achieving a door-to-needle time of 25-minutes in thrombolysis for acute ischemic stroke: a quality improvement project. J Stroke Cerebrovasc Dis 2014; 23: 2900–2906. [DOI] [PubMed] [Google Scholar]
- 15.Lees KR, Bluhmki E, Kummer R, von, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 16.Meretoja A, Roine RO, Kaste M, et al. Stroke monitoring on a national level: PERFECT stroke, a comprehensive, registry-linkage stroke database in Finland. Stroke 2010; 41: 2239–2246. [DOI] [PubMed] [Google Scholar]
- 17.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 478: 11–21. [DOI] [PubMed] [Google Scholar]
- 18.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Eng J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for The Dutch Acute Stroke Audit: Benchmarking acute stroke care in the Netherlands by Laurien S Kuhrij, Michel WJM Wouters, Renske M van den Berg-Vos, Frank-Erik de Leeuw and Paul J Nederkoorn in European Stroke Journal