Short abstract
Introduction
There is uncertainty regarding the optimal timing for initiation of oral anticoagulant treatment in patients with recent ischaemic stroke and atrial fibrillation. We surveyed the current UK practice and assessed clinician’s opinions of when to use oral anticoagulant in recent stroke patients with atrial fibrillation.
Patients and methods
An online survey was sent to stroke physicians within the United Kingdom via their national societies.
Results
One hundred and twenty-one clinicians responded to the survey. Ninety-five percent of responders agreed that there was uncertainty regarding timing of oral anticoagulant initiation after atrial fibrillation-related ischaemic stroke. Thirty-six percent of responders followed the ‘1–3–6–12’ European Society of Cardiology guidelines recommendation. Uncertainty was greater in cases of moderate stroke than in cases of transient ischaemic attack (TIA), mild or severe stroke. Eighty-eight percent of responders would be willing to participate in a clinical trial of early versus later initiation of oral anticoagulant after stroke. Direct-acting oral anticoagulants were the preferred oral anticoagulant of choice.
Discussion and Conclusion
There is a lack of consensus amongst stroke physicians for when to initiate oral anticoagulant to prevent recurrence in stroke patients with atrial fibrillation. There is little uncertainty regarding TIA. A clinical trial assessing the use of early versus later initiation of direct-acting oral anticoagulant in patients with recent ischaemic stroke and atrial fibrillation would be beneficial.
Keywords: Atrial fibrillation, oral anticoagulant, stroke, survey
Introduction
Atrial fibrillation (AF) is the most common heart rhythm disturbance in the United Kingdom1 and confers a near five-fold risk of stroke.2–4 The risk of stroke recurrence following AF-related stroke is also especially high during the first 14 days.5–7 Oral anticoagulants (OAC) reduce recurrence risk by two-thirds.8 Given the high early recurrence risk, use of OAC as early as possible seems desirable, but OAC use is associated with increased risk of intracerebral haemorrhage (ICH), including symptomatic ICH (sICH) after ischaemic stroke. Balancing these possible benefits and risks is challenging.
The European Society of Cardiology (ESC) guidelines recommend that OAC should be initiated between 1 and 12 days in patients with AF and recent acute ischaemic stroke depending on the stroke severity.9 This ‘1–3–6–12’ day rule states OAC would be started on day 1 following a transient ischaemic attack (TIA), day 3 following a mild stroke, day 6 following a moderate stroke and on day 12 following severe stroke.10 This pragmatic guidance is based on ‘expert opinion’ and such practice evolved during a time where only vitamin K antagonists (VKA) and heparins were available. Direct-acting oral anticoagulants (DOACs) are at least as effective as VKAs in preventing recurrent stroke in patients with ischaemic stroke and AF. DOACs have a rapid onset of action (0.5–4 h) along with lower rates of ICH.9 It may, therefore, be safer to use these drugs very early after stroke compared to VKA, but exactly when, and in whom, the balance of risk and benefit is in favour is unclear.
Whilst there is considerable uncertainty on the optimal latency after an acute stroke at which OACs should commence, the magnitude of this uncertainty, and the minimum and maximum delays that are acceptable to stroke physicians are unclear. Clarity in this regard would help inform the need and design of clinical trials in this area. We conducted a survey aimed to establish specialist opinion and current practice on the timing of OAC initiation in patients with recent ischaemic stroke and AF within the United Kingdom.
Methods
The study was discussed with the West of Scotland Research Ethics Service, and formal review was not required under the Governance Arrangements for Research Ethics Committees. A survey was created online using www.onlinesurveys.ac.uk. An online format was used to maximise response return and ease of dissemination. Questions aimed to assess what clinicians do in practice, rather than assess knowledge of guidelines. The survey consisted of 22 questions (see online Supplemental Data). The first four explored demographic data of responders. Further questions explored whether clinicians followed the ‘1–3–6–12’ day rule, whether they were uncertain regarding when to initiate OAC, and whether clinicians would be willing to participate in related clinical trials. Finally, the survey included four clinical vignettes of people with TIA and different severities of stroke. In each scenario, the patient had AF, no contraindications for anticoagulation, and scenarios were consistent regarding age, sex and cardiovascular risk factors (only severity of stroke differed), (see online Supplemental Data for the clinical vignettes) Responders were asked to give their view on the optimal time to start OAC, and the earliest and latest times they would be willing to start OAC in a clinical trial for each scenario. A free text section at the end of the survey was included.
The survey was piloted, reviewed and then circulated to members of the British Association of Stroke Physicians (BASP), Association of British Neurologists (ABN), Scottish Association of Neurological Science (SANS), British Cardiovascular Society (BCS), Scottish Cardiac Society (SCS) and Society of Acute Medicine (SAM) along with a cover letter detailing the background of the project. It was also disseminated via the UK Clinical Research Networks. The survey was distributed via email with a hyperlink embedded allowing access to the survey. Responders were provided 6 weeks to complete the survey, with reminders sent 2 weeks prior to the closing date. Descriptive statistics were produced to describe responses.
Results
Demographics
A total of 121 clinicians completed the survey. Responders were distributed throughout the United Kingdom with 54% from England, 31% from Scotland, 11% from Wales and 4% from Northern Ireland. The commonest speciality of responders was geriatric medicine (n = 88, 73%) followed by neurology (n = 18, 15%) and acute internal medicine (n = 5, 4%). Three responders selected ‘other’ but stated a special interest in stroke medicine. In the United Kingdom, acute stroke management is undertaken by either neurologists, geriatricians or internists with specialist interest in stroke medicine. Free text comments regarding timing of initiation of OAC are shown in online Supplemental Table I.
Use of OAC after stroke
One hundred and fifteen (95%) responders answered that there was uncertainty regarding when to administer OAC after AF-related ischaemic stroke. One hundred and four (86%) of the responders replied that they would be willing to participate in a clinical trial of early versus later initiation of OAC. Forty-three (36%) responders used the 1–3–6–12 guidelines outlined y the ESC. Sixty (50%) responders reported using DOACs early after ischaemic stroke in patients with AF, 60 (50%) reported that their drug choice differed between patients but none reported a preference for VKA.
TIA scenario
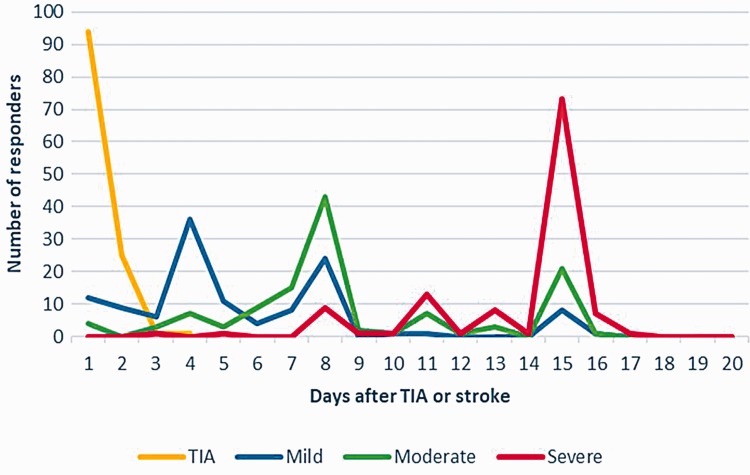
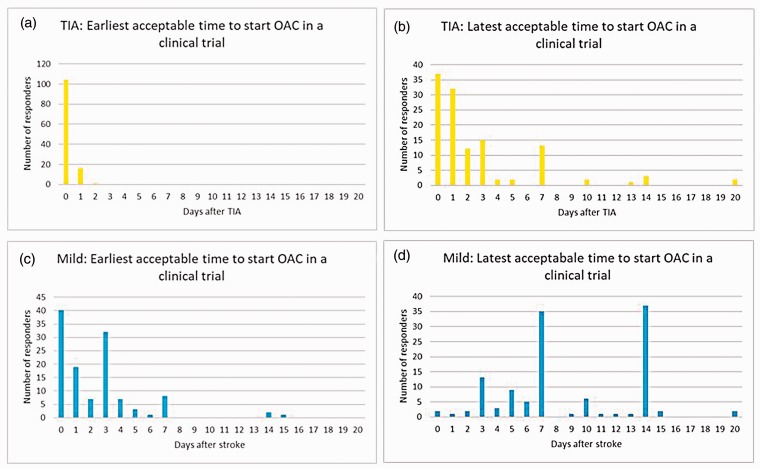
All responders reported that they currently initiate OAC within 3 days of a TIA (78% on the same day, median day 0, IQR day 0 to day 0) (Figure 1). The earliest and the latest acceptable times to start OAC in a clinical trial for patients with TIA are shown in Figure 2(a) and (b).
Figure 1.
Response analysis of current initiation of oral anticoagulant after TIA and stroke.
Figure 2.
The earliest and latest acceptable oral anticoagulant initiation timings after TIA (a and b, respectively) and mild stroke (c and d, respectively) in patients with atrial fibrillation for a potential clinical trial.
Mild stroke scenario
All responders reported that they currently initiate OAC within 15 days of a mild stroke (10% on the same day and 71% within 7 days) (Figure 1). The earliest and the latest acceptable times to start OAC in a clinical trial for patients with mild stroke are shown in Figure 2(c) and (d). The median response regarding the earliest acceptable time to start OAC in a clinical trial was day 2 (IQR: day 0–3). The median response regarding the latest time was day 7 (IQR: day 6–14).
Moderate stroke scenario
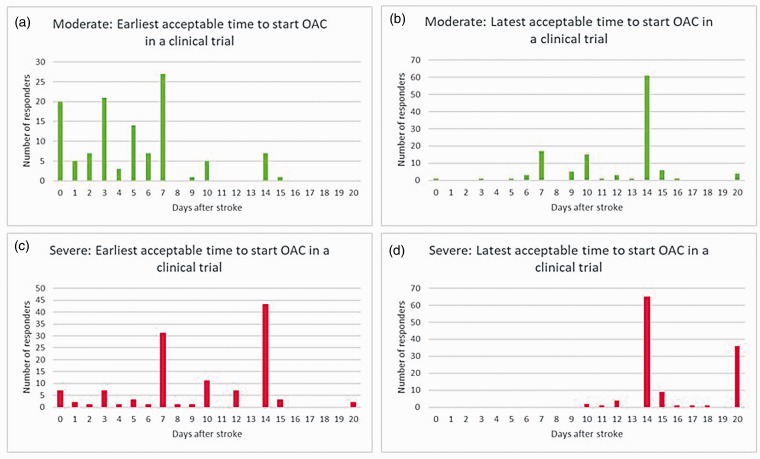
All responders reported that they currently initiate OAC within 15 days of a moderate stroke (3% on the same day and 34% within 7 days) (Figure 1). The earliest and the latest acceptable times to start OAC in a clinical trial for patients with moderate stroke are shown in Figure 3(a) and (b). The median for the earliest acceptable time to start OAC in a clinical trial setting was day 5 (IQR: days 2–7). Meanwhile, the median for the latest time of such was day 14 (IQR: days 10–14).
Figure 3.
The earliest and latest acceptable oral anticoagulant initiation timings after a moderate stroke (a and b, respectively) and severe stroke (c and d, respectively) in patients with atrial fibrillation for a potential clinical trial.
Severe stroke scenario
All responders reported that they currently initiate OAC within 20 days of a severe stroke (0% on the same day and 2% within 7 days) (Figure 1). The earliest and the latest acceptable times to start OAC in a clinical trial for patients with moderate stroke are shown in Figure 3(c) and (d). The median for the earliest acceptable time to start OAC in a clinical trial was day 10 (IQR: days 7–14). Meanwhile, the median for the latest time of such was day 14 (IQR: days 14–20).
In the feedback section, whilst a clinical trial measuring use of early versus later initiation of DOACs in ischaemic stroke in patients with AF would be welcomed, opinions were mixed regarding the structure and intricacies of such a trial. The free text comments are shown in the online Supplemental Table II.
Discussion
The results of this UK-wide online survey amongst stroke physicians indicated that there was a lack of consensus regarding when to administer OAC after a stroke in patients with AF. It was clear from the survey that physicians felt that a clinical trial to answer this clinical conundrum would be beneficial.
Current clinical practice was shaped during an era where warfarin was the only available oral drug. Warfarin, compared to acetylsalicylic acid, increases the risk of bleeding by two-thirds (2.2 vs. 1.3 events per 100 patient years).11 DOACs are at least as effective as warfarin for stroke prevention in AF and cause lower rates of intracranial haemorrhage. In the ARISTOTLE trial, the rate of intracranial haemorrhage was half with apixaban versus warfarin (hazard ratio (HR) = 0.51, 95% confidence interval (CI) = 0.35–0.70), and in the AVERROES trial, it was similar to aspirin.12,13 Similar data in comparison to warfarin have been shown for other DOACs.14–16 DOACs may therefore offer a better risk-benefit ratio early after stroke, but this cannot be concluded at present. Most trials of DOACs excluded patients who had suffered a recent stroke, defined as within the last 7 days after stroke onset, and in some trials much later.
Interestingly, the survey found that only 36% of clinicians used the ‘1–3-6–12’ day outlined in ESC guidelines. However, from the responses, it is clear that most followed the basic principle of delaying OAC initiation in proportion to stroke severity. For those who did not use the ‘1–3–6–12’ rule, our free text comments suggest their decisions were based in a pragmatic way using their clinical judgement to assess for factors such as; swallow status, haemorrhagic transformation, likelihood of falls and size of infarct. The survey found little uncertainty regarding timing of OAC initiation after TIA. There was little disagreement in severe stroke but more uncertainty in cases of moderate stroke. Clinicians were also asked their opinions regarding the earliest/latest time for OAC intervention in the context of a clinical trial. Answers regarding the earliest and latest acceptable time points were more varied for severe and mild stroke, respectively. The nature of the uncertainty, therefore, differs with severity of stroke but exists nonetheless. It is clear that there is support from clinicians for a clinical trial in this area.
We used an online survey and case vignettes. Each scenario was carefully selected, and all external risk factors were kept constant. This was to ensure the survey was easy to complete, and that we could identify clear patterns of practice that could inform trial design. There were no contraindications for OAC in each case although this oversimplification would not apply to real life clinical practice where many contraindications make the decision-making process more complex. To differentiate between the stroke severity in each of the cases provided, the definitions from the ELAN clinical protocol11 were used along with a National Institute of Health Stroke Scale (NIHSS) score. We felt, given the patient in each vignette had suffered TIA or stroke and had AF, that they were already of high risk of recurrence and had a CHA2DS2VASc score of at least 2 and that ABCD2 score may not alter decision to anticoagulate. Our data do not allow us to explore how hyperacute thrombectomy therapy would influence decision making.
Limitations
The design of this survey had several limitations. There is a risk of selection bias, where clinicians with a more active interest in this subject, or perhaps with more uncertainty, were more likely to answer. Social desirability bias was another potential limitation, where responders would answer in a way they thought they should answer rather than what they would actually do. The survey was anonymised to try to reduce bias. This was a UK survey so results may not be generalisable to other European and non-European countries. This may be particularly relevant given that most responders were trained in geriatric medicine. The multiple-choice options were kept short, and the list was not exhaustive which may have caused some responders to answer too quickly or in an over-simplistic manner. The survey was distributed via email with a hyperlink embedded allowing access to the survey. Thus, we were also unable to provide the proportion of active clinicians from each organisation who completed the survey.
Future directions
It is clear that randomised, controlled trials are needed to address this uncertainty. Four randomised, controlled clinical trials investigating early versus late initiation of DOACs in patients with AF-related ischemic stroke have been initiated: Early versus Late initiation of direct oral Anticoagulants in post-ischaemic stroke patients with AF (ELAN, NCT03148457, Switzerland); OPtimal TIMing of Anticoagulation after AF-associated acute ischaemic Stroke (OPTIMAS, British Heart Foundation, UK); Timing of Oral Anticoagulant Therapy in Acute Ischaemic Stroke with Atrial Fibrillation (TIMING, NCT02961348, Sweden) and Optimal Delay Time to Initiate Anticoagulation After Ischemic Stroke in Atrial Fibrillation (START, NCT03021928, USA). Results are expected in 2021.
Conclusion
This online survey of UK stroke physicians showed a lack of consensus for when to initiate OAC for patients with an acute stroke and AF, and clinical trials are needed.
Supplemental Material
Supplemental material, Supplementary Data and Tables for A survey of opinion: When to start oral anticoagulants in patients with acute ischaemic stroke and atrial fibrillation? by David Munn, Azmil H Abdul-Rahim, Urs Fischer, David J Werring, Thompson G Robinson and Jesse Dawson in European Stroke Journal
Acknowledgements
We thank the ABN, SANS, BCS, SCS, SAM and NIHR (Stroke) for the distribution of the survey. TGR is an NIHR Senior Investigator.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JD and TGR have received speaker and advisory board fees from manufacturers of DOACs.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Guarantor
Not applicable.
Contributorship
JD and AHAR jointly supervised the project. DM, AHAR and JD designed the survey. DM collected and analysed the responses. DM drafted the initial article. DM, AHAR and JD were involved in reviewing and reporting of the work. All authors provided a critical revision of the article for important intellectual content and approved the final version.
References
- 1.NHS choices. Causes of atrial fibrillation, www.nhs.uk/Conditions/Atrialfibrillation/Pages/Causes.aspx (2017, accessed 19 April 2017).
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22: 983–988. [DOI] [PubMed] [Google Scholar]
- 3.Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population based study. Stroke 2005; 36: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 4.Albertsen I, Rasmussen L, Overvad T, et al. Risk of stroke or systemic embolism in atrial fibrillation patients treated with warfarin: a systematic review and meta-analysis. Stroke 2013; 44: 1329–1336. [DOI] [PubMed] [Google Scholar]
- 5.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 6.NICE guidelines: management of atrial fibrillation, www.nice.org.uk/guidance/cg180/chapter/1recommendations (2014, accessed 20 April 2017).
- 7.Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. Lancet 2000; 355: 1205–1210. [DOI] [PubMed] [Google Scholar]
- 8.Connolly S. Anticoagulation for patients with atrial fibrillation and risk factors for stroke. BMJ 2000; 320: 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruff C, Giugliano R, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2018; 283: 955–962. [DOI] [PubMed] [Google Scholar]
- 10.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962. [DOI] [PubMed] [Google Scholar]
- 11.Fischer U. Early versus late initiation of direct oral anticoagulants in postischaemic stroke patients with atrial fibrillation (ELAN): an international, multicentre, randomised-controlled, two-arm, assessor-blinded trial. Clinical Trial Protocol. University of Bern. [DOI] [PMC free article] [PubMed]
- 12.Lopes RD, Alexander JH, Al-Khatib SM, et al. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J 2010; 159: 331–339. [DOI] [PubMed] [Google Scholar]
- 13.Granger CB, Alexander JH, McMurray JV, et al. Apixaban versus warfarin in patients with atrial fibrillation (AVERROES) trial. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 14.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 15.Patel MR, Kenneth MW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 16.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary Data and Tables for A survey of opinion: When to start oral anticoagulants in patients with acute ischaemic stroke and atrial fibrillation? by David Munn, Azmil H Abdul-Rahim, Urs Fischer, David J Werring, Thompson G Robinson and Jesse Dawson in European Stroke Journal