Abstract
(1) Background: Rhubarb anthraquinones—a class of components with neuroprotective function—can be used to alleviate cerebral ischemia reperfusion injury. (2) Methods: The three pharmacodynamic indicators are neurological function score, brain water content, and cerebral infarction area; UPLC-MS/MS was used in pharmacokinetic studies to detect plasma concentrations at different time points, and DAS software was used to calculate pharmacokinetic parameters in a noncompartmental model. (3) Results: The results showed that the pharmacodynamics and pharmacokinetics of one of the five anthraquinone aglycones could be modified by the other four anthraquinones, and the degree of interaction between different anthraquinones was different. The chrysophanol group showed the greatest reduction in pharmacodynamic indicators comparing with other four groups where the rats were administered one of the five anthraquinones, and there was no significant difference between the nimodipine group. While the Aloe-emodin + Physcion group showed the most obvious anti-ischemic effect among the groups where the subjects were administered two of the five anthraquinones simultaneously. Emodin, rhein, chrysophanol, and physcion all increase plasma exposure levels of aloe-emodin, while aloe-emodin lower their plasma exposure levels. (4) Conclusions: This experiment provides a certain preclinical basis for the study of anthraquinone aglycones against cerebral ischemia and a theoretical basis for the study of the mechanism of interaction between anthraquinones.
Keywords: anthraquinones, pharmacodynamics, pharmacodynamics, pharmacokinetics, interaction
1. Introduction
Cerebral ischemic stroke is a major cause of severe acquired disability worldwide and the morbidity approximately account for 80% of global strokes [1,2]. However, the use of a thrombolysis agent is the only FDA-approved method for the treatment of acute ischemic stroke so far and there is a limited therapeutic window within 3 h, after which there is potential risk of hemorrhagic transformation [3,4,5,6]. Thus, alternative therapies are urgently acquired. It is a feasible direction to treat cerebral ischemia by using the active ingredients in Chinese medicine as a supplement and substitute therapy complementary and alternative treatment.
Modern Chinese medicine clinical research [7] shows that the basic pathogenesis of cerebral ischemia is deficiency, fire, wind, phlegm, qi, and blood, while qi deficiency and blood stasis are the main pathological mechanisms of ischemic stroke. Therefore, the treatment of tonifying qi and activating blood as a basic treatment can often receive good results. More and more studies have shown that cerebral ischemia and reperfusion are complex pathophysiological processes, and are inextricably linked to autophagy, apoptosis, oxidative stress, inflammation, and other reactions [8,9]. It has been preliminarily proven that anthraquinones have related pharmacological activities, such as reducing the activation of the NALP3 inflammatory response complex [10,11] and the upstream signaling pathway of nuclear factor kappa B (NF-κB), downregulation of inducible NO synthase [12,13] (i NOS), inhibits the massive production of NO, and reduces nerve damage. In addition, anthraquinones have a special effect on anticoagulant therapy in patients with primary brain tumors or secondary brain metastases [14]. In the current studies, rhubarb anthraquinone total aglycones have neuroprotective functions and can be used for the treatment of cerebral ischemic injury.
Chinese medicine places a high value on drug compatibility [15]. The compatibility of Chinese medicines is not the simple addition of the therapeutic effects of drugs; on the contrary, ingredients promote reciprocal absorption and entrance to the lesion site in the body, prolong residence time, increase blood drug concentration, change pharmacokinetic behavior, and synergistically enhance the drug therapeutic effect. Until now, most of the existing reports [16,17,18,19,20] concentrate on the overall pharmacodynamic study of rhubarb anthraquinone total aglycones, including aloe-emodin, emodin, rhein, chrysophanol, physcion, or the pharmacokinetic study of one of the five anthraquinone aglycones, so the pharmacokinetic interaction among the five anthraquinones should be deeply investigated. After all, these five aglycones, whose chemical structural formulas are shown in Figure S1, have the same parent group and may have the same target, resulting in similar effects. For example, published reports have showed that emodin cannot only significantly reduce the expression of tumor necrosis factor-α (TNF-α) protein and IL-1β protein in cerebral ischemic tissue and reduce the inflammatory response [21], but also enhance the activity of CAT in the brain of mice with cerebral ischemia-reperfusion, promote the decomposition of hydrogen peroxide, and reduce the generation of oxygen free radicals. Rhubarb aglycons have antioxidation, antibacterial, anti-inflammatory, and antiapoptosis effects, simultaneously [22,23,24]. These may cause an upgrade in the efficacy of the drug, and may also result in a weakness in treatment outcome due to antagonism between the drugs. Therefore, unambiguous interpretations and evidence about pharmacodynamic effect and pharmacokinetic interaction between the five anthraquinone aglycones were acquired for more effective compatibility.
Our investigation is the first to explore the systematic pharmacodynamics of the five anthraquinones (aloe-emodin, emodin, rhein, chrysophanol, and physcion) and pharmacokinetic interaction of two of the five anthraquinone aglycones in cerebral ischemia-reperfusion model rat. It was found that the experimental groups all showed anticerebral ischemic injury and the treatment intensity was consistent when different drug combinations were administered. On this basis, the pharmacokinetic study was carried out. We used UPLC-MS/MS to determine the plasma concentration of aloe-emodin, emodin, rhein, chrysophanol, and physcion at different time points, to calculate the pharmacokinetic parameters, and analyze the pharmacokinetic characteristics and pharmacokinetic interaction. Then, combining the pharmacodynamic and pharmacokinetic results, the functional mechanisms the anthraquinone glycosides on each other were speculated.
2. Results
2.1. Pharmacodynamic Results
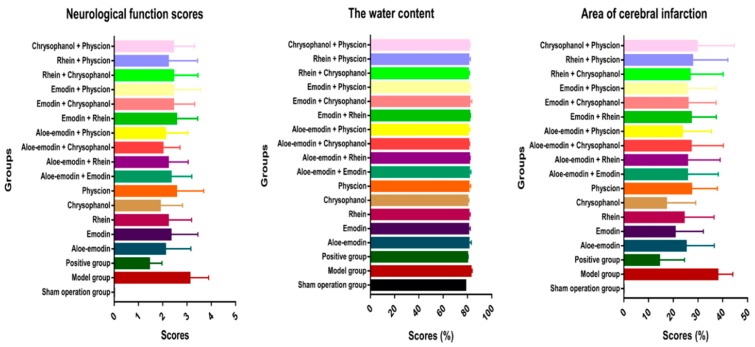
The neurological function scores, brain water content, and infarct size of the sham operation group, the model group, the positive group, and the 16 experimental groups are shown in Table 1 and Figure 1. Comparing the model group with the sham operation group, the neurological function score and brain tissue water content were significantly increased and the ischemic brain tissue staining showed a large area of white cerebral infarction. The results indicated that the cerebral ischemia model was established successfully and the pharmacodynamic characteristics could be reflected with the three selected pharmacodynamic indicators. Compared with the model groups, the three pharmacodynamic indicators were significantly decreased in the positive group and the Aloe-emodin, Rhein, Chrysophanol, Aloe-emodin + Rhein, and Aloe-emodin + Physcion groups. Besides, the Aloe-emodin + Physcion group, which showed no significant pharmacodynamic differences with the positive group, possessed the strongest efficacy of the 15 experimental groups.
Table 1.
Pharmacodynamic index: the neurological function scores, the water content, and area of cerebral infarction. The results were expressed as means ± standard deviation. One-way ANOVA and bilateral inspection were used to analyze the data differences between the groups by some standards that Δ, * p < 0.05; ΔΔ, ** p < 0.01. Δ showed the comparison between the sham operation group, * showed the comparison between the model group.
| Group | Neurological Function Score | The Water Content (%) | Area of Cerebral Infarction (%) |
|---|---|---|---|
| Sham operation group | - | 78.40 ± 0.28 | - |
| Model group | 3.11 ± 0.78 ΔΔ | 82.84 ± 1.08 ΔΔ | 37.95 ± 6.13 ΔΔ |
| Positive group | 1.44 ± 0.53 ** | 79.91 ± 0.64 ** | 14.36 ± 10.20 ** |
| Aloe-emodin | 2.11 ± 1.05 * | 81.03 ± 2.15 ** | 25.09 ± 11.47 ** |
| Emodin | 2.33 ± 1.12 | 80.78 ± 1.52 ** | 20.65 ± 11.51 ** |
| Rhein | 2.22 ± 0.97 * | 81.08 ± 1.21 ** | 24.31 ± 12.19 ** |
| Chrysophanol | 1.89 ± 0.93 ** | 80.10 ± 1.21 ** | 17.16 ± 11.94 ** |
| Physcion | 2.56 ± 1.13 | 81.02 ± 1.64 * | 27.32 ± 10.57 * |
| Aloe-emodin + Emodin | 2.33 ± 0.87 | 81.42 ± 1.45 * | 25.62 ± 12.61 * |
| Aloe-emodin + Rhein | 2.22 ± 0.83 * | 81.52 ± 0.70 * | 25.72 ± 13.21 * |
| Aloe-emodin + Chrysophanol | 2.00 ± 0.71 ** | 80.91 ± 0.91 ** | 27.14 ± 13.14 |
| Aloe-emodin + Physcion | 2.11 ± 0.93 * | 80.52 ± 1.53 ** | 23.56 ± 11.92 * |
| Emodin + Rhein | 2.56 ± 0.88 | 81.65 ± 0.91 * | 27.16 ± 10.25 |
| Emodin + Chrysophanol | 2.44 ± 0.88 | 81.69 ± 1.98 * | 25.91 ± 11.41 * |
| Emodin + Physcion | 2.44 ± 1.13 | 81.34 ± 1.66 ** | 25.57 ± 11.63 * |
| Rhein + Chrysophanol | 2.44 ± 1.01 | 80.38 ± 1.27 ** | 26.66 ± 13.49 * |
| Rhein + Physcion | 2.22 ± 1.21 * | 80.89 ± 1.52 * | 27.70 ± 14.38 |
| Chrysophanol + Physcion | 2.44 ± 0.88 | 81.18 ± 1.11 * | 29.58 ± 15.04 |
Figure 1.
Pharmacodynamic index: the neurological function scores, the water content, and area of cerebral infarction. The results are expressed in terms of means ± standard deviation (n = 10).
2.2. Pharmacokinetics Interaction Studies
According to the guidelines for the bioanalytical validation method issued by the FDA (Center for Drug Evaluation and Research [25]), we conducted methodology validation of selectivity, linearity, LOQ (limit of quantitation), accuracy, precision, extraction recovery, matrix effect, and stability. The methodological verification results are shown in Tables S1–S4 and Figure S2. The chromatographic method has proven to be linear (r > 0.997), precise (relative standard deviation RSD < 8%), and accurate (relative error RE ± 6%) in the concentration range of the experiment. The minimum detection limits for aloe-emodin, emodin, rhein, chrysophanol, and physcion were 0.026, 0.098, 0.098, 0.538, and 0.317 ng, respectively. The experimental conditions we selected can detect the blood concentration of five rhubarb aglycones rapidly, stably, exclusively, and high sensitively.
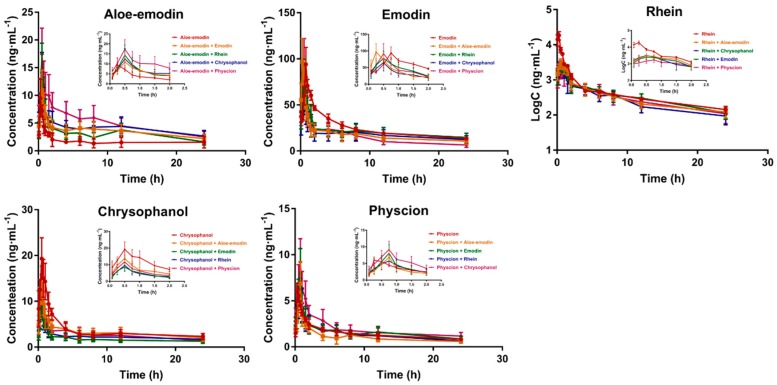
For each animal group the calculated mean plasma concentrations of the anthraquinone aglycones, plotted as a function of time, are shown in Figure 2. At all sampling times, the plasma level of aloe-emodin was increased by the co-administration of emodin, rhein, chrysophanol, or physcion; while plasma levels of emodin, rhein, and chrysophanol decreased when administered with aloe-emodin, emodin, rhein, chrysophanol, and physcion. Moreover, different pharmacokinetic parameters of the five anthraquinone aglycones were calculated using noncompartmental analysis, as presented in Table 2. Significant differences in AUC0–t, AUC0–∞, AUMC0–∞, and CL/F values were observed when comparing the groups in which subjects were administered one of the anthraquinone aglycones (aloe-emodin, emodin, rhein, chrysophanol, or physcion) with that groups where subjects were administered two of the anthraquinone aglycones.
Figure 2.
Mean plasma concentration–time curves.
Table 2.
Pharmacokinetic parameters of 5 substances in different groups (mean ± SD; n = 10). The pharmacokinetic parameters were calculated according to the blood concentration time of each subject, and then the mean values and SD values of 10 individual pharmacokinetic parameters in each group were calculated. One-way ANOVA and bilateral test were used to compare the pharmacokinetic differences between the compatibility groups and their corresponding groups which the subjects were administered one of the aglycones (* p < 0.05; ** p < 0.01).
| Group | Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t1/2 (h) | Tmax (h) | Cmax (ng/mL) | AUC0-t | AUC0-∞ | AUMC0-∞ | MRT0-∞ (h) | V/F (L) | Cl/F (L/h) | ||
| (ng·h/mL) | (ng·h/mL) | (ng·h2/mL) | ||||||||
| Aloe-emodin | Aloe-emodin | 14.98 ± 6.48 | 0.32 ± 0.12 | 9.88 ± 2.9 | 42.77 ± 10.09 | 63.18 ± 24.20 | 1524 ± 566.3 | 21.73 ± 8.35 | 4287 ± 1948 | 244.2 ± 118.0 |
| Aloe-emodin + Emodin | 33.26 ± 6.4 ** | 0.45 ± 0.11 | 10.85 ± 1.45 | 88.02 ± 8.32 ** | 214.4 ± 14.32 ** | 9303 ± 703.5 ** | 45.3 ± 6.39 ** | 2857 ± 171.6 * | 59.6 ± 7.76 ** | |
| Aloe-emodin + Rhein | 21.51 ± 6.36 | 0.55 ± 0.11 ** | 14.1 ± 3.04 * | 77.58 ± 7.3 ** | 126.6 ± 15.53 ** | 3323 ± 240.7 ** | 26.61 ± 5.61 | 3181 ± 271.5 | 104.0 ± 12.18 ** | |
| Aloe-emodin+ Chrysophanol | 28.34 ± 4.72 ** | 0.45 ± 0.11 | 12.41 ± 2.53 | 99.62 ± 11.7 ** | 210.4 ± 18.37 ** | 7955 ± 508.8 ** | 38.15 ± 5.3 ** | 2488 ± 330.0 ** | 62.78 ± 5.30 ** | |
| Aloe-emodin + Physcion | 15.49 ± 3.64 | 0.55 ± 0.11 ** | 17.8 ± 4.18 ** | 121.5 ± 19.59 ** | 177.7 ± 16.70 ** | 3641 ± 261.1 ** | 21.2 ± 6.57 | 1556 ± 262.4 ** | 75.65 ± 15.29 ** | |
| Emodin | Emodin | 23.13 ± 3.56 | 0.75 ± 0.00 | 91.65 ± 16.82 | 624.7 ± 73.35 | 1108 ± 191.1 | 33673 ± 10503 | 29.90 ± 4.71 | 1052 ± 119.8 | 31.99 ± 5.15 |
| Emodin + Aloe-emodin | 26.13 ± 4.44 | 0.3 ± 0.11 ** | 95.15 ± 5.47 | 419.5 ± 21.62 ** | 798.8 ± 40.85 ** | 27693 ± 2767 | 32.5 ± 5.44 | 1550 ± 113.0 ** | 44.82 ± 5.12 ** | |
| Emodin + Rhein | 28.97 ± 6.17 * | 0.45 ± 0.11 ** | 77.83 ± 6.2 * | 511.8 ± 40.91 ** | 1194 ± 226.7 | 45531 ± 5933 ** | 41.11 ± 5.04 ** | 1282 ± 165.4 ** | 30.98 ± 4.08 | |
| Emodin + Chrysophanol | 26.73 ± 3.18 | 0.45 ± 0.11 ** | 65.19 ± 4.96 ** | 433.4 ± 33.69 ** | 906.6 ± 29.35 * | 33509 ± 3389 | 38.18 ± 4.87 ** | 1475 ± 151.6 ** | 37.92 ± 4.57 | |
| Emodin + Physcion | 5.49 ± 0.78 ** | 0.5 ± 0 ** | 91.21 ± 8.89 | 373.3 ± 54.31 ** | 397.4 ± 82.77 ** | 3464 ± 655.3 ** | 8.64 ± 0.83 ** | 689.7 ± 49.64 ** | 87.65 ± 7.74 ** | |
| Rhein | Rhein | 10.20 ± 2.50 | 0.23 ± 0.06 | 19290 ± 5420 | 18866 ± 1739 | 20893 ± 2262 | 157175 ± 52352 | 7.44 ± 1.87 | 12.08 ± 2.07 | 0.83 ± 0.09 |
| Rhein + Aloe-emodin | 5.21 ± 0.89 ** | 0.6 ± 0.29 ** | 3136 ± 412.6 ** | 10272 ± 1478 ** | 10909 ± 1119 ** | 71400 ± 5075 | 7.17 ± 0.78 | 13.51 ± 1.46 | 1.56 ± 0.53 ** | |
| Rhein + Emodin | 9.26 ± 0.87 | 0.5 ± 0 ** | 2554 ± 511.0 ** | 10861 ± 1055 ** | 11515 ± 1366 ** | 116864 ± 16106 ** | 10.76 ± 1.10 ** | 18.33 ± 1.19 ** | 1.47 ± 0.55 * | |
| Rhein + Chrysophanol | 8.98 ± 0.74 | 0.5 ± 0.18 ** | 3076 ± 299.8 ** | 8914 ± 535.7 ** | 10548 ± 1333 ** | 92427 ± 5960 * | 10.07 ± 1.54 ** | 21.9 ± 2.25 ** | 1.98 ± 0.28 ** | |
| Rhein + Physcion | 10.8 ± 1.12 | 0.75 ± 0.18 ** | 1746 ± 102.8 ** | 8857 ± 845.1 ** | 10500 ± 1367 ** | 124300 ± 17430 ** | 11.42 ± 1.61 ** | 24.18 ± 2.45 ** | 1.72 ± 0.54 ** | |
| Chrysophanol | Chrysophanol | 37.47 ± 14.54 | 0.54 ± 0.10 | 19.01 ± 3.93 | 88.97 ± 10.58 | 185.2 ± 56.36 | 8755 ± 3200 | 42.24 ± 23.55 | 10372 ± 2880 | 236.8 ± 97.59 |
| Chrysophanol + Aloe-emodin | 34.61 ± 4.86 | 0.65 ± 0.11 ** | 15.22 ± 4.55 | 75.17 ± 8.9 ** | 173.6 ± 11.3 | 7441 ± 368 | 44.73 ± 5.03 | 10815 ± 1527 | 248.6 ± 40.56 | |
| Chrysophanol + Emodin | 47.34 ± 11.03 | 0.62 ± 0.15 ** | 8.74 ± 2.09 ** | 45.87 ± 7.42 ** | 121.8 ± 15.29 ** | 7125 ± 450 | 54.53 ± 9.19 | 20629 ± 2753 ** | 313.0 ± 42.9 * | |
| Chrysophanol + Rhein | 38.18 ± 5.76 | 0.72 ± 0.12 ** | 9.17 ± 1.49 ** | 52.47 ± 3.07 ** | 160.2 ± 4.80 | 8390 ± 418 | 57.27 ± 6.35 | 14625 ± 1072 ** | 241.8 ± 19.13 | |
| Chrysophanol + Physcion | 15.75 ± 2.18 ** | 0.67 ± 0.13 ** | 11.8 ± 2.36 ** | 59.38 ± 4.14 ** | 96.01 ± 11.77 ** | 2478 ± 345.7 ** | 19.06 ± 2.38 ** | 9866 ± 1354 | 416.4 ± 48.03 ** | |
| Physcion | Physcion | 30.47 ± 14.4 | 0.38 ± 0.14 | 6.24 ± 0.59 | 36.38 ± 5.58 | 72.50 ± 41.70 | 4492 ± 2151 | 41.50 ± 17.65 | 13170 ± 6030 | 433.4 ± 161.6 |
| Physcion + Aloe-emodin | 26.38 ± 4.99 | 0.75 ± 0.16 ** | 6.78 ± 0.81 | 26.71 ± 4.72 ** | 48.29 ± 6.73 * | 1556 ± 220 | 32.09 ± 5.62 | 20255 ± 4540 ** | 545.1 ± 52.62 * | |
| Physcion + Emodin | 16.65 ± 3.17 | 0.7 ± 0.11 ** | 8.05 ± 0.56 ** | 39.51 ± 2.23 | 60.89 ± 3.57 | 1359 ± 156.28 | 22.82 ± 6.99 | 10336 ± 1131 | 436.5 ± 38.8 | |
| Physcion + Rhein | 12.88 ± 4.28 | 0.75 ± 0 ** | 5.82 ± 0.7 | 34.51 ± 3.92 | 45.52 ± 4.41 * | 759.2 ± 34.69 | 16.52 ± 2.82 | 10773 ± 1823 | 580.0 ± 46.03 ** | |
| Physcion + Chrysophanol | 27.49 ± 2.62 | 0.75±0 ** | 9.14 ± 0.37 ** | 48.45 ± 3.47 ** | 94.39 ± 5.18 | 3346 ± 260 | 35.89 ± 4.75 | 10747 ± 1803 | 276.7 ± 16.66 ** | |
3. Discussion
In this experiment, the matrix effect and extraction recovery rate of five rhubarb aglycones and an internal standard were investigated under the conditions that methanol, acetonitrile, and methanol: acetonitrile (1:1) were used as precipitation solvent, respectively. The results showed that when pure methanol was used as a protein removal agent the impurity interference was minimal and the matrix effect were in the range of 75 to 115%; recovery rates were higher than 90%. Next, we compared four mobile phase systems—methanol–water (A), acetonitrile–water (B), methanol–0.1% formic acid water (C), and methanol-3 mmol/mL ammonium acetate (D). It was found that the organic phase acetonitrile did not possess peak shape following to inaccurate integral value; meanwhile methanol could avoid this phenomenon. Formic acid water can improve the detection of the quasi-ion peak [M + H]+ of rhubarb aglycone. However, according to the fact that the five aglycones contain a plurality of phenolic hydroxyl groups [17,26], the five anthraquinones are more likely to exhibit a correspondingly strong signal quasi-ion peak [M − H]− in an alkaline environment. Therefore, methanol—3 mmol/mL ammonium acetate (D) was finally selected as the mobile phase system. Referring to published literature [27], the Cmax, t1/2, and AUC0–t in thrombotic focal cerebral ischemia (TFCI)-induced rats were almost twice that in normal rats, and the CL value was significantly lower than in normal rats (p < 0.05), showing that the pharmacokinetic characteristics of the five anthraquinones were modified under pathological conditions. Thus, in order to match the treatment background, cerebral ischemia reperfusion model rats were selected as the research objects in this experiment. In order to provide some theoretical basis for the drug administration methods of the five drugs (single or combined), our research studied the pharmacokinetic characteristics and pharmacodynamics of the five anthraquinone glycosides in the cerebral ischemia reperfusion model rats and the pharmacokinetic interactions and pharmacodynamics of the five anthraquinone glycosides in the pairwise administration.
Considering the drug concentration–time curve, aloe-emodin exhibits different degrees of absorption double-peaks. This may be because, when administered orally, aloe-emodin was easily combined with glucose in the form of glycoside [16], and when the concentration of aloe-emodin in the blood reduced, a small amount of glycoside was hydrolyzed, so that aloe-emodin has a smaller peak. After all, aloe-emodin still exists in the form of a metabolite [28]. This phenomenon was consistent with previous studies; aloe-emodin met the two-compartment model [16]. The four aglycones (emodin, rhein, chrysophanol, and physcion) all prolonged aloe-emodin peak times, Tmax; increased peak concentrations, Cmax; reduced elimination rates; and extended t1/2. The increasing AUC0–t and AUC0–∞ showed increasing aloe-emodin plasma exposure level. Therefore, the absorption was increased, the elimination was slowed, and action time was prolonged. In addition, the decrease in apparent volume of distribution may cause the drug to concentrate on the pathological site, reducing the side effects of aloe-emodin. Comparing the effects of the four anthraquinones on the pharmacokinetics of aloe-emodin, physcion possessed the greatest influence on it, while rhein showed the least effect. Aloe-emodin, rhein, chrysophanol, and physcion all reduced the AUC0–t value of Emodin, and the AUC0–t value decreased to 67.1%, 72.1%, 59.7%, and 35.9%, respectively. However, they have different ways of changing the pharmacokinetics of emodin. Aloe-emodin increases the exposure level of emodin by shortening and increasing the apparent volume of distribution, V, while physcion shortens the t1/2 and improves the clearance rate. The significantly shortened Tmax indicated that the absorption of emodin was faster and the effect was rapider with the co-administered with aloe-emodin, rhein, chrysophanol, or physcion. Aloe-emodin, emodin, chrysophanol, and physcion all significantly reduced the AUC0–t, AUC0–∞, and Cmax of Rhein and significantly increased Tmax, V, and CL, which suggested that the drug–time curves become gentle and the absorption is slowed down. This result may create the reductive possibility of drug accumulation in a certain part to cause the side effects. After all, the drug′s therapeutic effect or toxic side effects often depend on whether the drug dose or concentration exceeds a certain value. In general, aloe-emodin can decrease the AUC of the other four anthraquinones and increase the clearance rate and apparent distribution volume, indicating that aloe-emodin has an inhibitory effect on their utilization; meanwhile, emodin, rhein, and chrysophanol increase and reduce the absorption of aloe-emodin. A large number of experiments [29,30,31,32] have shown that both emodin and chrysophanol can inhibit the expression of TNF-α, IL-1β, and vascular adhesion molecule (VCAM-1), enhance the activity of antioxidant enzymes, inhibit the excessive production of NO, scavenge free radicals, and inhibit apoptosis after cerebral ischemic injury. Thus, many identical neuroprotective functions have demonstrated that these two components may have the same targets in the treatment of cerebral ischemia and exhibit competitive inhibition, which may also be the cause of chrysophanol can cause emodin performed reduced absorption, accelerated elimination, and extensive distribution. The effects of emodin, rhein, and chrysophanol on their mutual pharmacokinetic characteristics are similar.
Three pharmacodynamic indicators were considered as a whole. The anticerebral ischemia effect of chrysophanol was the most obvious when subjects were administered one of the five anthraquinones, while the effect of physcion was not obvious. According to the literature [33,34], chrysophanol can attenuate nitrosative/oxidative stress injury and inhibit the endoplasmic reticulum stress response induced by ischemia/reperfusion damage in the brain. In our study, chrysophanol significantly reduced brain water content and cerebral infarct size with no significant difference from positive pharmacodynamics. There was no significant difference in the neurological function scores between the physcion and the model group. However, the pharmacodynamic index, water content, and cerebral infarct size values were higher than other anthraquinone groups. The three pharmacodynamic indicators of aloe-emodin + rhein and aloe-emodin + physcion were significantly different from the model group and were statistically significant. Comprehensive evaluation of the values of the three indicators, the neurological function score, brain water content and cerebral infarct size values in the aloe-emodin + physcion group were smaller than other compatibility groups, indicating that the group showed the best effect among the compatibility groups. The three indicators of the aloe-emodin + physcion group were lower than those in the aloe-emodin group and physcion group, but, comparing with the model group, the deviation was not equal to the sum of reduction values in the corresponding groups in which the subjects were administered only one of the two anthraquinones. Aloe-emodin and physcion increased the reciprocal anti-ischemic effect, and their interaction was not a simple additive effect but a synergistic effect. Comparing the group with emodin and the compatibility groups with the combination of emodin and the other four anthraquinones, physcion reduced the two pharmacodynamic indicators values of cerebral water content and cerebral infarction area significantly. Therefore, when emodin was combined with other rhubarb aglycones to treat cerebral ischemic injury, physcion may be the best choose. The three indexes in the chrysophanol group were lower than the chrysophanol compatibility groups, and the cerebral infarction area was significantly different from the chrysophanol compatibility groups, indicating that the anticerebral ischemia effect of chrysophanol was better than the chrysophanol compatibility group.
Combined with pharmacodynamic results and pharmacokinetic results, it was found that the pharmacodynamic intensity of the drugs often related to the plasma concentration of the drug in plasma. In the pharmacokinetic study: the AUC0–t of chrysophanol was significantly decreased under the influence of the other four anthraquinone aglycones. Aloe-emodin, emodin, rhein, and physcion reduced the AUC0–t of chrysophanol by 15.5%, 48.4%, 4.1%, and 33.3%, respectively, and had similar trends with brain water content in pharmacodynamic studies. Brain water content: Emodin + Chrysophanol > Physcion + Chrysophanol > Aloe-emodin + Chrysophanol > Rhein + Chrysophanol > Chrysophanol.
4. Materials and Methods
4.1. Material
4.1.1. Chemicals and Materials
The reference standards of aloe-emodin, emodin, rhein, chrysophanol, physcion, and 1,8-dihydroxyanthraquinone (internal standard (IS)) were purchased from the National Institutes for Food and Drug Control (Beijing, China). The chemical purity of these reference substances was higher than 98% as determined by HPLC analysis. The raw materials had a purity of ≥ 90%; aloe-emodin, emodin, rhein, chrysophanol, and physcion were obtained from Xi′an Xin Rui Bio Technology Co., Ltd. 2,3,5-triphenyltetrazolium chloride (TTC) was obtained from Beijing Solarbio Science & Technology Co. (Beijing, China), Ltd. (Lot. No. 20160310), and chloral hydrate was provided by the Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Nimodipine was purchased from Shandong Xinhua Pharmaceutical Co., Ltd. (Shangdong, China), penicillin sodium for injection was purchased from North China Pharmaceutical Co., Ltd. (Hebei, China), and sodium carboxymethyl cellulose (CMC-Na) was purchased from Tianjin Hengxing Chemical Reagent Manufacturing Co., Ltd. (Tianjin, China). Methanol, provided by Tedia Company (Fairfield, OH, USA), and ammonium acetate, purchased from Fisher Company (Pittsburgh, PA, USA), were of mass spectrum grade. The ultrapure water was produced by the Millipore Milli-Q system (Bedford, MA, USA). When aloe-emodin, emodin, rhein, chrysophanol, and physcion were used, the corresponding concentration suspension was prepared with 0.5% sodium carboxymethyl cellulose.
4.1.2. Animals and Cerebral Ischemia Reperfusion Model in Rats
Male Sprague-Dawley (SD) Rats weighing 260–300g were purchased from the Hebei Province Experimental Animal Center (Hebei, China, SCXK2012-1-003). The study protocol is as described by the Experimental Animal Care and Ethics Committee of the First Affiliated Hospital, Henan University of Chinese Medicine (Henan, China, SYXK (Yu) 2015-0005). Before operation, all rats were exposed to 12 h light/12 h dark, temperature 22 ± 2 °C, relative humidity 50 ± 2% conditions for 7 days to adapt to the environment. All breeding and research on experimental animals strictly abide by the regulations on the administration of experimental animals in Henan province. In this study, we used a method previously known as middle cerebral artery occlusion [35,36,37,38] (MCAO) to model cerebral ischemia reperfusion in rats.
Briefly, after anesthesia using 10% chloral hydrate, we separated the left common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA). A six-0 nylon suture monofilament with a rounded tip (Beijing, China, Beijing salon Biotechnology Co., Ltd.) was inserted through a small incision on the left common carotid artery and moved forward into the internal carotid artery until small resistance was felt, which signifies blocking the entrance of middle cerebral artery. Then ligate the proximal end of the internal carotid artery, suture the wound, and place the rats in a rat cage with a clean pad. At this point, the brain deficit model has been established. After 2 h, the filament was slowly withdrawn until there is resistance to allow reperfusion. The sham group exposed only the left side of the vessel without the insertion.
4.2. Pharmacodynamic Study of Anticerebral Ischemia-Reperfusion Injury
4.2.1. Grouping and Administration
After 7 days of acclimatization, 180 rats were randomly assigned to one of the following 18 groups (n = 10 each); Sham operation group, MCAO model group, nimodipine positive control group, and 15 experimental groups MCAO + (Aloe-emodin; Emodin; Rhein; Chrysophanol; Physcion; Aloe-emodin + Emodin; Aloe-emodin+ Rhein; Aloe-emodin+ Chrysophanol; Aloe-emodin+ Physcion; Emodin + Rhein; Emodin + Chrysophanol; Emodin + Physcion; Rhein + Chrysophanol; Rhein + Physcion; Chrysophanol + Physcion). Drug concentrations were as follows; Aloe-emodin 13.05 mg/kg, Emodin 34.65 mg/kg, Rhein 17.25 mg/kg, Chrysophanol 39.45 mg/kg, Physcion 26.4 mg/kg, and the positive drug nimodipine 10.8 mg/kg. All of the above drugs were given orally with the corresponding concentration of suspension prepared with 0.5%CMC-Na before use. The dosage was calculated at one percent of the rat′s body weight. The sham operation group and the model group were given 0.5%CMC-Na of the corresponding volume. Rats in the experimental groups and the positive group were intragastrically administered drugs once a day for seven consecutive days. The rats were fasted but water-free at 12 h before the last dose, and then a cerebral apoplexy reperfusion model was established 2 h after the last administration.
4.2.2. Measurement and Evaluation
(1) The neurological function of rats was evaluated by Longa method [39,40,41]. Scoring criteria: 0 points: no symptoms of neurological deficit, normal activity; 1 point: cannot extend the opposite front claw completely; 2 points: turn to hemiplegia side when crawling; 3 points: when walking, the body falls to the side of hemiplegia; 4 points: unable to walk spontaneously, loss of consciousness; 5 points: death. One-to-three points were selected as successful samples of cerebral ischemia. (2) The scope and area of infarction after cerebral ischemia reperfusion were determined by TTC staining, and the infarction area of each group was calculated by Image analysis software. After staining, the nonischemic area was rose-red and the infarction area was white. (3) The dry and wet method was used to the measurement of brain water content on cerebral ischemia side. Cerebral ischemia reperfusion can induce significant increase of brain water content and lead to cerebral edema in rats. Moisture content of brain tissue = (wet brain weight − dry brain weight)/wet brain weight × 100%.
4.3. Pharmacokinetic Interaction among Anthraquinone Aglycones in Model Rats
4.3.1. Apparatus and Operation Conditions
For the LC separation, an Ultimate3000 HPLC (Thermo Scientific, San Jose, CA, USA) equipped with a quaternary pump, a cooling autosampler, a column temperature compartment, and UV detector were utilized. An XBridgeTMC18 (2.1 mm × 150 mm, 5 μm) was employed at 35 °C. The mobile phase consisted of methanol (A) and 3 mmol/L ammonium acetate (B), which was delivered at a flow rate of 0.3 mL/min. The applied gradient elution is as follows; 30–60% A from 0 to 2 min, 60–95% A from 2 to 10 min, 95–60% A from 10 to 11 min, 60–30% A from 11 to 12 min, and 30% A from 12 to 13 min. The injection volume was 5 mL.
For MS, a Q-Orbitrap mass spectrometer (Thermo Scientific, San Jose, CA, USA) equipped with a heat electrospray ionization (HESI) operated in the full-scan mode. The sheath gas, auxiliary gas, and sweep gas pressure was 35 bar, 10 bar, and 10 bar, respectively. Positive and negative ions were simultaneous scanned and the spray voltage was set at +3.5 kV or −2.8 kV under positive or negative mode. The capillary temperature and auxiliary gas heater temperature were maintained at 350 °C and 200 °C. The full scan ranges were from 150 to 1500 m/z with resolution of 70,000. The ions to be measured and labeled for quantitative analysis are as follows; aloe-emodin m/z 269.0455 [M − H]−; emodin m/z 269.0455 [M − H]−; rhein m/z 283.0248 [M − H]−; chrysophanol m/z 253.03.506 [M − H]−; and physcion m/z 283.0612 [M − H]−.
4.3.2. Plasma Sample Preparation
Rat plasma samples were directly subjected to protein precipitation [42,43] with methanol. Plasma samples (100 mL) were spiked with 100 mL of IS (0.916 mg/mL) and 200 mL of methanol, vortexed for 15 min, and centrifuged at 15,000 r/min for 20 min, and then the supernatant was transferred to a clean test tube, evaporated to dryness at 40 °C under a flow of nitrogen gas, the residue was reconstituted in 100 mL of methanol–water (30:70, v/v) and subsequently, a 5 mL aliquot was injected for HPLC-MS/MS measurement. Because the concentration of rhein is too high, it is not within the range of our standard curve linear range. In the actual operation of measuring the blood concentration of rhein, we diluted the plasma samples after the above treatment with methanol at ratio 1:50 (v:v).
4.3.3. Standard Solution Preparation and Sample Quality Control
Five reference products (aloe-emodin, emodin, rhein, chrysophanol, and physcion) of rhubarb were accurately weighed, and methanol was used as solvent to prepare stock solutions with appropriate concentration. The five stock solutions were moderately mixed to afford a final mixed standard solution. A series of working solutions of these five analytes were freshly prepared by diluting the mixed standard solution with methanol gradiently. 1,8-Dihydroxyanthraquinone, an IS, was prepared at a concentration of 0.916 µg/mL by methanol. Calibration samples were prepared by spiking 100 µL working solutions of the corresponding concentrations; 100 µL methanol and 100 µL of IS solution to 100 µL of blank rat plasma. Then, calibration samples were pretreated according to the “4.3.2 Plasma sample preparation”. Low-, medium-, and high-quality control (QC) samples were also prepared in the same way; the medium concentrations were of aloe-emodin 93.2 ng/mL; emodin 77.6 ng/mL; rhein 82.8 ng/mL; chrysophanol 85.6 ng/mL; and physcion 86.4 ng/mL, and were used to measure the precision and accuracy of our methods. All stock solutions were refrigerated at 4 °C.
4.3.4. Administration and Sample Collection
After seven days of acclimatization, 150 rats were randomly divided into groups of 10 each. And those subjects were fasted but free to drink water for 12 h before building the cerebral ischemia model. The rats were administered when the cerebral ischemia model established for 12 h. The administered composition and dose of the 15-group pharmacokinetic study were the same as those of the 15 experimental groups in the pharmacodynamic study (4.2.1). Blood was collected from the tail vein before and after administration at 0.083, 0.25, 0.75, 1, 2, 4, 6, 8, 12, 24 h, respectively, and collected in the heparin centrifuge tube. The blood was then centrifuged in a refrigerated centrifuge for 20 min (6000 r/min) to obtain plasma. Plasma was stored in a −80 °C refrigerator for cryopreservation.
4.4. Statistical Methods
SPSS19.0 analysis software was adopted for data analysis. The results were expressed in terms of means ± standard deviation and one-way ANOVA was used to analyze the data differences between the groups by some standards; p < 0.05 indicates statistical significance, p < 0.01 indicates significant difference.
5. Conclusions
In each group rats were administered with one of the five anthraquinone aglycones; the anticerebral ischemia effect of chrysophanol was the most obvious. In the compatibility groups, the anticerebral ischemia effect of aloe-emodin + physcion group was the most obvious. After co-administration with Aloe-emodin, the anticerebral ischemia effect of emodin, rhein, and chrysophanol was weakened. Therefore, when treating cerebral ischemic injury, it was not recommended to administer aloe-emodin with emodin, rhein, and chrysophanol. The pharmacokinetic interaction between the five anthraquinones aglycones was intricate. Emodin, rhein, chrysophanol, and physcion increased the absorption and slowed the elimination of aloe-emodin, while aloe-emodin reduced the AUC0–t values of the above four anthraquinones. Emodin, chrysophanol, and rhein have a similar pharmacokinetic effect on each other. It was speculated that the three anthraquinones had the same targets in anticerebral ischemia. In this study, the pharmacodynamics and pharmacokinetics of five rhubarb aglycones were systematically studied, and their pharmacodynamics and pharmacokinetic interaction were explored, which provided a theoretical basis for the study of the mechanism of action.
Acknowledgments
The authors thank all the volunteers involved in the study and all the people in the Henan Key Laboratory of Chinese Medicine for Respiratory Disease for their help. The authors greatly appreciate the Zhengzhou Key Laboratory of Chinese Medicine Quality Control and Evaluation and Collaborative Innovation Center for Respiratory Disease Diagnosis and Treatment & Chinese Medicine Development of Henan Province for their financial support.
Supplementary Materials
The following are available online, Figure S1. Chemical formula for five anthraquinones (Rhein, Emodin, Chrysophanol, Aloe-emodin, Physcion) and IS. Figure S2. Six-channel chromatogram of five anthraquinones (Aloe-emodin, Aloe, Rhein, Chrysophanol, Pphyscion). Table S1. Calibration curves for the 5 anthraquinones in rat plasma. Table S2. Intra-day and inter-day precision and accuracy for determination of the 5 anthraquinones in rat plasma. Table S3. Matrix effects and extraction recovery of the 5 anthraquinones in rat plasma. Table S4. Stability of the 5 anthraquinones in rat plasma.
Author Contributions
Conceptualization, S.-X.F. and J.-S.L.; Data Curation, R.-R.L., S.-X.F., and L.-B.Q.; Formal Analysis, R.-R.L. and S.-X.F.; Funding Acquisition, S.-X.F. and J.-S.L.; Investigation, X.-F.L. and N.Z.; Methodology, R.-R.L. and X.-F.L.; Resources, S.-X.F. and J.-S.L.; Software, R.-R.L.; Supervision, X.-F.L.; Validation, S.-N.S. and P.-Y.W.; Visualization, L.-B.Q.; Writing—Original Draft, R.-R.L.; Writing—Review & Editing, S.-X.F.
Funding
This research was supported financially by Collaborative Innovation Center for Respiratory Disease Diagnosis and Treatment & Chinese Medicine Development of Henan Province and Zhengzhou Key Laboratory of Chinese Medicine Quality Control and Evaluation (project number: 81274139; 30873265). Both of these laboratories contributed to the study design, research, and interpretation of data, and the writing, reviewing, and approving of the publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Russo T., Felzani G., Marini C. Stroke in the very old: A systematic review of studies on incidence, outcome, and resource use. J. Aging Res. 2011;2011:108785. doi: 10.4061/2011/108785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed N., Lees K.R., Ringleb P.A., Bladin C., Collas D., Toni D., Ford G.A. Outcome after stroke thrombolysis in patients >80 years treated within 3 hours vs. > 3–4.5 hours. Neurology. 2017;89:1561–1568. doi: 10.1212/WNL.0000000000004499. [DOI] [PubMed] [Google Scholar]
- 3.Liang J., Qi Z.F., Liu W.L., Wang P., Shi W.J., Dong W., Ji X.M., Luo Y.M., Liu K.J. Normobaric hyperoxia slows blood-brain barrier damage and expands the therapeutic time window for tissue-type plasminogen activator treatment in cerebral ischemia. Stroke. 2015;46:1344–1351. doi: 10.1161/STROKEAHA.114.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnan G.A., Davis S.M., Parsons M.W., Ma H., Dewey H.M., Howells D.W. How to make better use of thrombolytic therapy in acute ischemic stroke. Nat. Rev. Neurol. 2011;7:400–409. doi: 10.1038/nrneurol.2011.89. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed N., Kellert L., Lees K.R., Mikulik R., Tatlisumak T., Toni D. Results of intravenous thrombolysis within 4.5 to 6 hours and updated results within 3 to 4.5 hours of onset of acute ischemic stroke recorded in the Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register (SITS-ISTR): An observational study. JAMA Neurol. 2013;70:837–844. doi: 10.1001/jamaneurol.2013.406. [DOI] [PubMed] [Google Scholar]
- 6.Puyal J., Ginet V., Clarke P.G. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: A challenge for neuroprotection. Prog. Neurobiol. 2013;105:24–48. doi: 10.1016/j.pneurobio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Miao X.L., Huang Y., Pei J., Yang Y.S., Wang X.Z., Tan J.L., Li J., Gu W., Cao X.L., Dong M.J., et al. The Regression Analysis on Syndromes Grade for Yin-yang Syndrome Differentiation of in Acute Phase of Ischemic Stroke. Chin. J. Integr. Trad. West. Med. 2007;5:1166–1167. [Google Scholar]
- 8.Wang Y., Ren Q.Y., Zhang X., Lu H.L., Chen J. Neuroprotective Mechanisms of Calycosin Against Focal Cerebral Ischemia and Reperfusion Injury in Rats. Cell Physiol. Biochem. 2018;45:537–546. doi: 10.1159/000487031. [DOI] [PubMed] [Google Scholar]
- 9.Liu P., Zhao H.P., Wang R.L., Wang P., Tao Z., Gao L., Yan F., Liu X.G., Yu S., Ji X.M., et al. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke. 2015;46:513–519. doi: 10.1161/STROKEAHA.114.007482. [DOI] [PubMed] [Google Scholar]
- 10.Zhang N., Zhang X.J., Liu X.X., Wang H., Xue J., Yu J.Y., Kang N., Wang X.L. Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. Mediat. Inflamm. 2014;2014:370530. doi: 10.1155/2014/370530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Amara M., Yang S.Y., Quaglia A., Rowley P., Fuller B., Seifalian A., Davidson B. Role of endothelial nitric oxide synthase in remote ischemic preconditioning of the mouse liver. Liver Transpl. 2011;17:610–619. doi: 10.1002/lt.22272. [DOI] [PubMed] [Google Scholar]
- 12.Chen S.D., Yang D.I., Lin T.K., Shaw F.Z., Liou C.W., Chuang Y.C. Roles of oxidative stress, apoptosis, PGC-1 alpha and mitochondrial biogenesis in cerebral ischemia. Int. J. Mol. Sci. 2011;12:7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadiiska M.B., Peddada S., Herbert R.A., Basu S., Hensley K., Jones D.P., Hatch G.E., Mason R.P. Biomarkers of oxidative stress study VI. Endogenous plasma antioxidants fail as useful biomarkers of endotoxin-induced oxidative stress. Free Radic. Biol. Med. 2015;81:100–106. doi: 10.1016/j.freeradbiomed.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pejin B., Jovanović K.K., Mojović M., Savić A.G. New and highly potent antitumor natural products from marine-derived fungi: Covering the period from 2003 to 2012. Curr. Top. Med. Chem. 2013;13:2745–2766. doi: 10.2174/15680266113136660197. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Gou L.L., Yang G., Wang Y.Y. Methodology and prospects of study on theory of compatibility of prescriptions in traditional Chinese medicine. World Sci. Tech./Mod. Trad. Chin. Med. Mater. Med. 2006;8:1–5. [Google Scholar]
- 16.Yu C.P., Shia C.S., Lin H.J., Hsieh Y.W., Lin S.P., Hou Y.C. Analysis of the pharmacokinetics and metabolism of aloe-emodin following intravenous and oral administrations in rats. Biomed. Chromatogr. 2016;30:1641–1647. doi: 10.1002/bmc.3735. [DOI] [PubMed] [Google Scholar]
- 17.Ullah H.M.A., Kim J., Rehman N.U., Kim H.J., Ahn M.J., Chuang H.J. A Simple and Sensitive Liquid Chromatography with Tandem Mass Spectrometric Method for the Simultaneous Determination of Anthraquinone Aglycones and Their Aglycones in Rat Plasma: Application to a Pharmacokinetic Study of Rumex acetosa Extract. Pharmaceutics. 2018;10:100. doi: 10.3390/pharmaceutics10030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun H., Luo G.W., Xiang Z., Cai X.J., Chen D.H. Pharmacokinetics and pharmacodynamics study of rhein treating renal fibrosis based on metabonomics approach. Phyto. Med. 2016;23:1661–1670. doi: 10.1016/j.phymed.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Sun H., Yin Q.W., Zhang A.H., Wang X.J. UPLC-MS/MS performing pharmacokinetic and biodistribution studies of rhein. J. Sep. Sci. 2012;35:2063–2068. doi: 10.1002/jssc.201200378. [DOI] [PubMed] [Google Scholar]
- 20.Shia C.S., Hou Y.C., Tsai S.Y., Huieh P.H., Leu Y.L., Chao P.W.L. Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats. J. Pharm. Sci. 2010;99:2185–2195. doi: 10.1002/jps.21978. [DOI] [PubMed] [Google Scholar]
- 21.Li J.S., Liu J.X., Zhang W.Y., Liang S.W., Wang D., Fang J. Preventive effects of emodin on cerebral ischemia injury and expression of the inflammtory factors in rats with cerebral ischemia. China J. Chin. Mater. Med. 2005;30:1939–1943. [PubMed] [Google Scholar]
- 22.Liu T., Hu H.T., Sun Q.R. Neuroprotective effects of emodin on primary rat cortical neurons apoptosis induced by hydrogen peroxide. Chin. Med. Met. 2010;33:1116–1119. [PubMed] [Google Scholar]
- 23.Ma B.L., Ma Y.M., Yan D.M., Zhou H., Shi R., Wang T.M., Yang Y., Wang C.H., Zhang N. Effective constituents in xiexin decoction for anti-inflammation. J. Ethnopharmacol. 2009;125:151–156. doi: 10.1016/j.jep.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 24.Lu K., Zhang C., Wu W.J., Zhou M., Tang Y.M., Peng Y. Rhubarb extract has a protective role against radiation-induced brain injury and neuronal cell apoptosis. Mol. Med. Rep. 2015;12:2689–2694. doi: 10.3892/mmr.2015.3693. [DOI] [PubMed] [Google Scholar]
- 25.Guidance for Industy, Bioanalytical Method Validation. US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CV); Washington, DC, USA: 2001. pp. 4–10. [Google Scholar]
- 26.Xue J.T., Shi Y.L., Ye L.M., Yang Q.M., Li C.Y., Chen X.Y., Jing Y. Near-infrared spectroscopy for rapid and simultaneous determination of five main active components in rhubarb of different geographical origins and processing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018;205:419–427. doi: 10.1016/j.saa.2018.07.055. [DOI] [PubMed] [Google Scholar]
- 27.Feng S.X., Li J.S., Qu L.B., Shi Y.M., Zhao D. Comparative pharmacokinetics of five rhubarb anthraquinones in normal and thrombotic focal cerebral ischemia-induced rats. Phytother. Res. 2013;27:1489–1494. doi: 10.1002/ptr.4890. [DOI] [PubMed] [Google Scholar]
- 28.Song R., Lin H., Zhang Z., Li Z., Xu L., Dong H., Tian Y. Profiling the metabolic differences of anthraquinone derivatives using liquid chromatography/tandem mass spectrometry with data-dependent acquisition. Rapid Commun. Mass Spectrom. 2009;23:537–547. doi: 10.1002/rcm.3907. [DOI] [PubMed] [Google Scholar]
- 29.Zhao W., Wang S., Li F.J. Effects of chrysophanol on antioxidative stress andAQP4 in brain tissue of mice induced by cerebral ischemia-reperfusion injury. Chin. Pharmacol. Bull. 2015;31:1477–1478. [Google Scholar]
- 30.Wang S.H., Liang C.L., Zhang H.H., Wang S. Effects of chrysophanol on NO of brain tissue and anti-anoxia in mice with cerebral ischemia-reperfusion injury. Tianjin Med. J. 2017;45:593–595. [Google Scholar]
- 31.Wang S., Zhang H.H., Xue G.P. Effects of Emodin on Exploratory and Cognitive Function of Mice Experiencing Cerebral Ischemia Reperfusion. Acta Neuropharmacol. 2013;3:8–13. [Google Scholar]
- 32.Dong X., Fu J., Yin X.B., Cao S., Li X.C., Lin L.F., Huyiligeqi, Ni J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016;30:1207–1218. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y., Fang Y., Zhao H., Li J., Duan Y., Shi W., Huang Y., Gao L., Luo Y. Chrysophanol inhibits endoplasmic reticulum stress in cerebral ischemia and reperfusion mice. Eur. J. Pharmacol. 2018;818:1–9. doi: 10.1016/j.ejphar.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y., Huang Y., Fang Y., Zhao H., Shi W., Li J., Duan Y., Sun Y., Gao L., Luo Y. Chrysophanol attenuates nitrosative/oxidative stress injury in a mouse model of focal cerebral ischemia/reperfusion. J. Pharmacol. Sci. 2018;138:16–22. doi: 10.1016/j.jphs.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Bian H.T., Hu Q., Liang X.P., Chen D., Li B., Tang J.P., Zhang J.H. Hyperbaric oxygen preconditioning attenuates hemorrhagic transformation through increasing PPARγ in hyperglycemic MCAO rats. Exp. Neurol. 2015;265:22–29. doi: 10.1016/j.expneurol.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matel N., Camara J., McBride D., Camara R., Xu N., Tang J.P., Zhang J.H. Intranasal wnt3a Attenuates Neuronal Apoptosis through Frz1/PIWIL1a/FOXM1 Pathway in MCAO Rats. J. Neurosci. 2018;38:6787–6801. doi: 10.1523/JNEUROSCI.2352-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McBride D.W., Zhang J.H. Precision Stroke Animal Models: The permanent MCAO Model Should Be the Primary Model, Not Transient MCAO. Transl. Stroke Res. 2017;8:397–404. doi: 10.1007/s12975-017-0554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J., Yan H., Li S., Zhang M. Berberine Ameliorates MCAO Induced Cerebral Ischemia/Reperfusion Injury via Activation of the BDNF-TrkB-PI3K/Akt Signaling Pathway. Neurochem. Res. 2018;43:702–710. doi: 10.1007/s11064-018-2472-4. [DOI] [PubMed] [Google Scholar]
- 39.Morris G.P., Wright A.L., Tan R.P., Gladbach A., Ittner M., Vissel B. A Comparative Study of Variables Influencing Ischemic Injury in the Longa and Koizumi Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Mice. PLoS ONE. 2016;11:e0148503. doi: 10.1371/journal.pone.0148503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachour S.P., Hevesi M., Bachour O., Sweis B.M., Mahmoudi J., Brekke J.A., Divani A.A. Comparisons between Garcia, Modo, and Longa rodent stroke scales: Optimizing resource allocation in rat models of focal middle cerebral artery occlusion. J. Neurol. Sci. 2016;364:136–140. doi: 10.1016/j.jns.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Sun T., Zeng G.R., Jiang D.J. Neurological behavior in pharmacology and toxicology evaluation. Cent. South Pharm. 2014;12:732–734. [Google Scholar]
- 42.Gong Z.G., Hu J., Wu X., Xu Y.J. The Recent Developments in Sample Preparation for Mass Spectrometry-Based Metabolomics. Crit. Rev. Anal. Chem. 2017;47:325–331. doi: 10.1080/10408347.2017.1289836. [DOI] [PubMed] [Google Scholar]
- 43.Jin Q.Z., Wang N., Liu Y.F. Study on the Pretreatment of Biological Samples of Traditional Chinese Medicine. Asia-Pac. Tradit. Med. 2014;10:67–69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.