Abstract
McManus, Werley, and Dempsey highlight new work showing that nicotine rapidly equilibrates in the ER after extracellular application.
Scientific discoveries that impact human health motivate many of us and also provide a rationale for public support. Nicotine use disorder (NUD) remains a significant public health burden affecting over 20% of adults in the United States and over 30% of those with other mental health disorders (Chou et al., 2016). Tobacco use is the primary component of NUD and is the leading cause of preventable morbidity and mortality in the United States, being responsible for approximately one in five of such deaths (more than 480,000 deaths annually; Camenga and Klein, 2016; Centers for Disease Control and Prevention, 2019). In spite of intensive government public information campaigns that have highlighted the health risks associated with nicotine use over the past five decades, a fraction of the population continues to suffer NUD. Moreover, the emergence of alternative nicotine delivery products, for example electronic nicotine delivery systems like vaporizers or electronic cigarettes, provide fresh challenges to reducing NUD (U.S. Department of Health and Human Services, 2014).
Why does NUD persist in the face of widespread distribution of overwhelming evidence of its dangers? Clearly, the addictive properties of nicotine are a primary obstacle to reducing its use (Davis et al., 1988). An understanding of the molecular mechanism of nicotine dependence could form the basis for developing new and improved NUD therapeutics. The immediate effects of nicotine administration that users seek are mediated by a variety of nicotinic acetylcholine receptor (nAchR) subunit combinations in the plasma membrane of neurons involved in reward circuity (Koob and Volkow, 2016). In addition, nicotine can act on nicotinic receptors positioned at intracellular locations, which affect cell function by an “inside-out” mechanism. The Lester laboratory at Caltech and their collaborators at Janelia Farm have worked to unravel the components of inside-out nicotinic receptor biology and its role in disease mechanisms including Parkinson’s disease and NUD. In this issue of the Journal of General Physiology, Shivange et al. describe the development and characterization of a novel optical nicotine sensor iNicSnFR, which also detects varenicline (Chantix)—a drug that acts on nicotinic receptors and is used as a smoking cessation aid. iNicSnFR affords a means to answer key questions concerning the levels and timing of nicotine and drug exposure at intracellular and extracellular sites, as well as providing a basis for mechanistic investigations of NUD and a possible path to improved therapeutics.
nAchRs and inside-out pharmacology
nAchRs are among the most intensively studied ion channel receptors due to their important roles in neuromuscular transmission and signaling in both the central and peripheral nervous system. nAChR signaling has been most thoroughly studied at the neuromuscular junction (Sine, 2012), which has in turn led to much of the broader mechanistic understanding of synaptic transmission (e.g., Katz, 1999). The earliest concept of a receptor molecule originated, in part, from studies of effects of nicotine and curare on this synapse (Langley, 1914). The following century or more of work has led to an impressive accumulation of mechanistic understanding, which has largely been driven by preceding technical advances including intracellular recordings (Fatt and Katz, 1951), electron microscopy (De Robertis and Bennett, 1954; Palade and Palay, 1954), voltage clamp recording (Magleby and Stevens, 1972), fluctuation analysis (Katz and Miledi, 1972; Anderson and Stevens, 1973), single channel recording (Neher and Sakmann, 1976) and cryo-EM (Walsh et al., 2018).
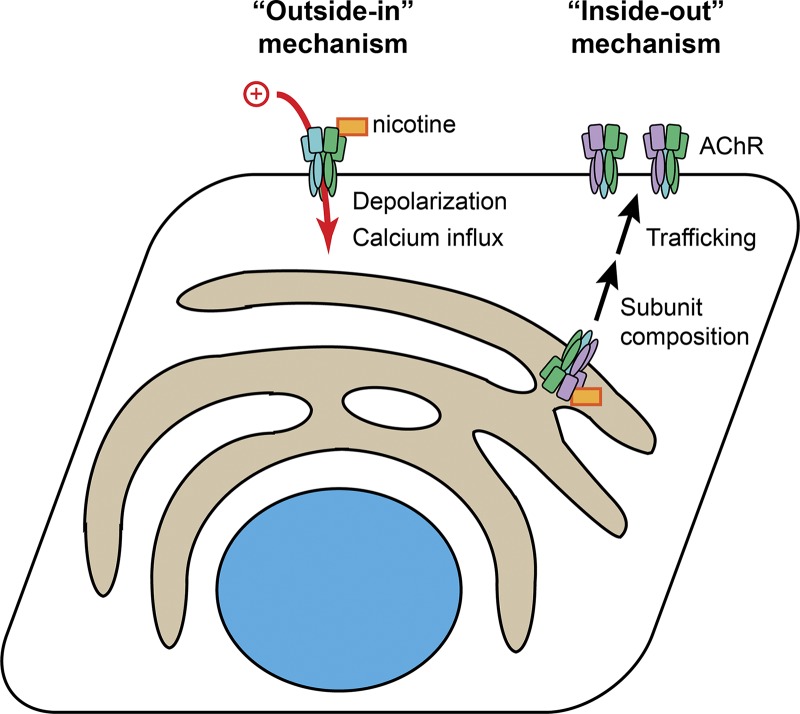
Nicotine interacts with nAChRs via two different mechanisms (Fig. 1). In the canonical “outside-in” pathway, nicotine binds to cell surface nAChRs and rapidly elicits channel opening, membrane depolarization, and intracellular calcium signaling. Nicotine can also lead to nAChR desensitization, as, unlike acetylcholine, it is not degraded in milliseconds by extracellular acetylcholinesterase enzymes. This outside-in pathway mediates the acute effects of nicotine in the central nervous system and also suppression of nicotine withdrawal symptoms (Miwa et al., 2011). In contrast, the inside-out pathway of nicotine signaling results from the membrane permeability of nicotine, which, unlike acetylcholine, can pass through cell membranes in its neutral form and bind to intracellular nAChRs. Although the consequences of inside-out signaling by nicotine are not fully elucidated, nicotine binding to nAChRs in the endoplasmic reticulum (ER) is known to stabilize the interface between α4 and β2 nAChR subunits, thereby chaperoning channels to the plasma membrane to increase surface expression and modify subunit composition (Lester et al., 2009). This likely leads to sustained enhancement of outside-in signaling for both acetylcholine and nicotine. The paper by Shivange et al. (2019) provides the first direct measurements of the time course and concentration of nicotine in the ER following its transient extracellular application.
Figure 1.
Outside-in and inside-out mechanisms of nicotine signaling. In the outside-in mechanism, nicotine binds directly to nAChR channels in the plasma membrane, causing membrane depolarization, calcium influx, and eventual channel densitization. In the inside-out mechanism, nicotine travels inside the cell and stabilizes particular nAChR subunit combinations in the ER. This leads to increased channel trafficking to the plasma membrane and a modified distribution of subunits, which modifies endogenous acetylcholine signaling.
Intensity-based nicotine-sensing fluorescent reporters (iNicSnFRs): A fluorescent nicotine sensor
Determining the relative importance of outside-in and inside-out nicotine signaling was previously impossible because the nicotine concentration inside the ER was unknown. To address this knowledge gap, Shivange et al. (2019) developed a novel set of engineered protein-based fluorescent sensors called iNicSnFRs. Because the protein-based sensors can be targeted to different subcellular locations, they can provide detailed information about nicotine concentration and dynamics in different organelles.
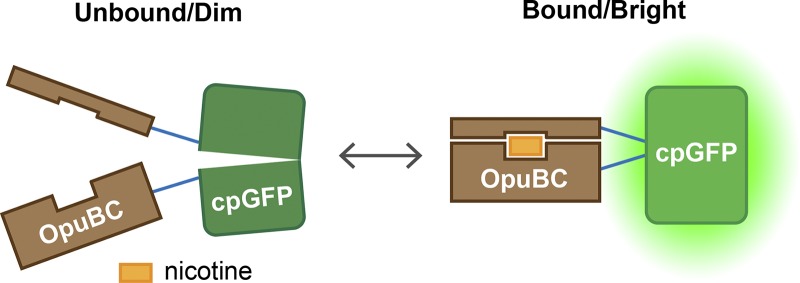
The fluorescent sensor design followed that of the glutamate sensor iGluSnFR (Marvin et al., 2013). Pieces of a nicotine-binding protein are fused with short peptide linkers to a circularly permuted GFP (cpGFP; Fig. 2). The N and C terminals of the cpGFP are fused together, and the protein is instead cut in the middle of the β-barrel that protects the GFP fluorophore. If these new N- and C-ends are pulled apart (no nicotine bound), the fluorophore is perturbed and goes dark. When nicotine binds, however, the native GFP conformation is restored, and the GFP becomes bright. The nicotine binding protein was derived from OpuBC, a choline/betaine periplasmic binding protein from T. spX513. Nicotine binding and iNicSnFR sensitivity was engineered into the sensor by directed evolution: ∼12,000 mutants were tested over multiple iterations, with a focus on the linkers and residues in the binding pocket. The final version, iNicSnFR3a, was equipped with an epitope tag for purification and immunostaining. The resulting half maximal effective concentration (EC50) for nicotine was 19 µM with subsecond on and off rates at physiologically relevant nicotine concentrations (Shivange et al., 2019). iNicSnFR3a can therefore detect 1 µM nicotine, which is a typical value found in the blood and cerebrospinal fluid during smoking (Benowitz et al., 1991).
Figure 2.
iNicSnFR fluorescent nicotine sensor mechanism. The engineered nicotine binding protein OpuBC is fused to a circularly permuted GFP protein. In the absence of nicotine, the GFP chromophore is perturbed and sensor fluorescence is dim. Upon nicotine binding, the GFP conformation is restored and the senor becomes bright.
The authors targeted expression of the sensor to two different cellular compartments, the ER and plasma membrane, in order to enable direct measurement of nicotine in each. Imaging and colabeling of the ER with DsRed2-ER revealed that the sensors (iNicSnFR3a_ER and iNicSnFR3a_PM) show the expected localization patterns. Functional validation was performed using nicotine pulse-chase experiments. Robust fluorescence changes were detected at concentrations as low as 250 nM nicotine in both cellular regions. Further validation of the compartmentalization of the sensors was provided using membrane impermeant ligands: iNicSnFR3a_ER did not detect membrane impermeable molecules N’MeNic and ACh but did detect nicotine, whereas iNicSnFR3a_PM detected all ligands. Observations for HEK293, SH-SY5Y, and human iPS cell–derived dopaminergic neurons tracked observations in HeLa cells, showing the generality of the approach. One caveat of the sensors is their strong pH sensitivity, which constrains their utility in acidic organelles.
Findings in the paper
The primary finding of the publication is that the nicotine concentration in the ER rapidly (<10 s) equilibrates with the extracellular nicotine concentration (within twofold). Thus, the outside-in and inside-out mechanisms experience approximately equal nicotine exposure throughout the day. Because the inside-out mechanism (EC50 ∼30 nM) is activated at much lower concentrations than the outside-in mechanism (EC50 0.3–4 µM), it is plausible that the inside-out mechanism dominates in some conditions. The authors used simulations of nicotine pharmacokinetics/pharmacodynamics to show that the concentration of nicotine in the blood/cerebrospinal fluid, and thus also the ER, is greater than the EC50 for chaperoning during the 1-h period between cigarettes for a typical smoker. In other words, the residual nicotine that remains in the body between cigarettes, the rapid entry of nicotine to the ER, and the achieved nicotine concentrations at the ER lend themselves to the inside-out mechanism of up-regulation of nAChRs as a potential culprit for addictive behavior. Table 1 summarizes the outside-in and inside-out mechanisms in light of these new data.
Table 1. Summary of inside-out and outside-in signaling during smoking.
| Outside-in: Activated nAChRs in the plasma membrane | Inside-out: Chaperoned nAChRs in the ER | |
|---|---|---|
| Nominal cellular effects | Na+ and Ca2+ influx, increased neuronal excitability, calcium signaling, nAChR desensitization | Stabilization of α4β2 subunit combinations, increased nAChR in the plasma membrane, biased subunit composition |
| EC50 for effect | 0.3 – 4 µMa (Buisson et al., 1996; Gopalakrishnan et al., 1997; Pacheco et al., 2001; Kuryatov et al., 2005) | 30 nM (Kuryatov et al., 2005) |
| Fraction average activation for a typical smoking habitb | 0.1 | 0.7 |
| Hypothesized organismal effects | Sense of well-being, cognitive boost, appetite suppression, increased tolerance of stressful stimuli, suppression of withdrawal (Miwa et al., 2011; Naudé et al., 2015; Nees, 2015; Picciotto et al., 2015) | Early events of nicotine dependence (Govind et al., 2009; Henderson and Lester, 2015) |
| Timescale of organismal effects | Minutes | Days to weeks |
Dependent on subunit composition and assay.
1 cigarette/hour for 12 h leads to ∼100 nM sustained nicotine concentration.
The authors offer some preliminary insights into the mechanism of action of varenicline (Chantix), the prescription medication used as a smoking cessation agent. iNicSnFR3a is, fortuitously, also sensitive to varenicline concentration. Much like nicotine, varenicline also rapidly equilibrates in the ER and could therefore activate the inside-out molecular chaperoning, which could be responsible for the efficacy and/or side effects of the drug.
Conclusions
The authors have built a powerful new tool for studying nicotine dynamics in cells, which has enabled them to strengthen the case for the inside-out mechanism as a critical factor in nicotine addiction. In addition, the authors demonstrate how to develop a fluorescent sensor for a drug through protein engineering, which could help elucidate key mechanisms underlying addiction or therapeutic function.
Acknowledgments
Merritt C. Maduke served as editor.
References
- Anderson C.R., and Stevens C.F.. 1973. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J. Physiol. 235:655–691. 10.1113/jphysiol.1973.sp010410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N.L., Jacob P. III, Denaro C., and Jenkins R.. 1991. Stable isotope studies of nicotine kinetics and bioavailability. Clin. Pharmacol. Ther. 49:270–277. 10.1038/clpt.1991.28 [DOI] [PubMed] [Google Scholar]
- Buisson B., Gopalakrishnan M., Arneric S.P., Sullivan J.P., and Bertrand D.. 1996. Human α4β2 neuronal nicotinic acetylcholine receptor in HEK 293 cells: A patch-clamp study. J. Neurosci. 16:7880–7891. 10.1523/JNEUROSCI.16-24-07880.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenga D.R., and Klein J.D.. 2016. Tobacco use disorders. Child Adolesc. Psychiatr. Clin. N. Am. 25:445–460. 10.1016/j.chc.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2019. Fast Facts: Smoking & Tobacco Use. Available at: https://www.cdc.gov/tobacco/about/osh/index.htm (accessed March 18, 2019).
- Chou S.P., Goldstein R.B., Smith S.M., Huang B., Ruan W.J., Zhang H., Jung J., Saha R.P., Pickering R.P., and Grant B.F.. 2016. The Epidemiology of DSM-5 Nicotine Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III J. Clin. Psychiatry. 77:1404–1412. 10.4088/JCP.15m10114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.M., Novotny T.E., Lynn W.R., Benowitz N.L., Grunberg N.E., Henningfield J.E., and Lando H.A.. 1988. Health Consequences of Smoking: Nicotine Addiction. Available at: https://profiles.nlm.nih.gov/ps/access/nnbbzd.pdf (accessed March 18, 2019).
- De Robertis E.D.P., and Bennett H.S.. 1954. Submicroscopic vesicular component in the synapse. Fed. Proc. 13:35. [DOI] [PubMed] [Google Scholar]
- Fatt P., and Katz B.. 1951. An analysis of the end-plate potential recorded with an intracellular electrode. J. Physiol. 115:320–370. 10.1113/jphysiol.1951.sp004675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan M., Molinari E.J., and Sullivan J.P.. 1997. Regulation of human α4β2 neuronal nicotinic acetylcholine receptors by cholinergic channel ligands and second messenger pathways. Mol. Pharmacol. 52:524–534. 10.1124/mol.52.3.524 [DOI] [PubMed] [Google Scholar]
- Govind A.P., Vezina P., and Green W.N.. 2009. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem. Pharmacol. 78:756–765. 10.1016/j.bcp.2009.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B.J., and Lester H.A.. 2015. Inside-out neuropharmacology of nicotinic drugs. Neuropharmacology. 96(Pt B):178–193. 10.1016/j.neuropharm.2015.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. 1999. On the Quantal Mechanism of Neural Transmitter Release. In Nobel Lectures, Physiology or Medicine 1963-1970. World Scientific Publishing, River Edge, NJ: 485–492. [Google Scholar]
- Katz B., and Miledi R.. 1972. The statistical nature of the acetycholine potential and its molecular components. J. Physiol. 224:665–699. 10.1113/jphysiol.1972.sp009918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., and Volkow N.D.. 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 3:760–773. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A., Luo J., Cooper J., and Lindstrom J.. 2005. Nicotine acts as a pharmacological chaperone to up-regulate human α4β2 acetylcholine receptors. Mol. Pharmacol. 68:1839–1851. 10.1124/mol.105.012419 [DOI] [PubMed] [Google Scholar]
- Langley J.N. 1914. The antagonism of curari and nicotine in skeletal muscle. J. Physiol. 48:73–108. 10.1113/jphysiol.1914.sp001649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H.A., Xiao C., Srinivasan R., Son C.D., Miwa J., Pantoja R., Banghart M.R., Dougherty D.A., Goate A.M., and Wang J.C.. 2009. Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J. 11:167–177. 10.1208/s12248-009-9090-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K.L., and Stevens C.F.. 1972. A quantitative description of end-plate currents. J. Physiol. 223:173–197. 10.1113/jphysiol.1972.sp009840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin J.S., Borghuis B.G., Tian L., Cichon J., Harnett M.T., Akerboom J., Gordus A., Renninger S.L., Chen T.-W., Bargmann C.I., et al. 2013. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods. 10:162–170. 10.1038/nmeth.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa J.M., Freedman R., and Lester H.A.. 2011. Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron. 70:20–33. 10.1016/j.neuron.2011.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudé J., Dongelmans M., and Faure P.. 2015. Nicotinic alteration of decision-making. Neuropharmacology. 96(Pt B):244–254. 10.1016/j.neuropharm.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Nees F. 2015. The nicotinic cholinergic system function in the human brain. Neuropharmacology. 96(Pt B):289–301. 10.1016/j.neuropharm.2014.10.021 [DOI] [PubMed] [Google Scholar]
- Neher E., and Sakmann B.. 1976. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 260:799–802. 10.1038/260799a0 [DOI] [PubMed] [Google Scholar]
- Pacheco M.A., Pastoor T.E., Lukas R.J., and Wecker L.. 2001. Characterization of human α4β2 neuronal nicotinic receptors stably expressed in SH-EP1 cells. Neurochem. Res. 26:683–693. 10.1023/A:1010995521851 [DOI] [PubMed] [Google Scholar]
- Palade G.E., and Palay S.L.. 1954. Electron microscope observations of intraneuronal and neuromuscular synapses. Anat. Rec. 118:35.13124786 [Google Scholar]
- Picciotto M.R., Lewis A.S., van Schalkwyk G.I., and Mineur Y.S.. 2015. Mood and anxiety regulation by nicotinic acetylcholine receptors: A potential pathway to modulate aggression and related behavioral states. Neuropharmacology. 96(Pt B):235–243. 10.1016/j.neuropharm.2014.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivange A.V., Borden P.M., Muthusamy A.K., Nichols A.L., Bera K., Bao H., Bishara I., Jeon J., Mulcahy M.J., Cohen B., et al. 2019. Determining the pharmacokinetics of nicotinic drugs in the endoplasmic reticulum using biosensors. J. Gen. Physiol. 10.1085/jgp.201812201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S.M. 2012. End-plate acetylcholine receptor: structure, mechanism, pharmacology, and disease. Physiol. Rev. 92:1189–1234. 10.1152/physrev.00015.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services 2014. The Health Consequences of Smoking—50 Years of Progress. Available at: https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf (accessed March 18, 2019).
- Walsh R.M.J. Jr., Roh S.-H., Gharpure A., Morales-Perez C.L., Teng J., and Hibbs R.E.. 2018. Structural principles of distinct assemblies of the human α4β2 nicotinic receptor. Nature. 557:261–265. 10.1038/s41586-018-0081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]