Abstract
Wei-LaPierre and Dirksen discuss new work investigating the molecular events underlying mitoflash biogenesis.
Mitoflashes are transient bursts of reactive oxygen species (ROS) that occur stochastically and concomitantly with matrix alkalinization and depolarization of the mitochondrial inner membrane potential (Ψm). They are observed in single mitochondria and local mitochondrial networks across a wide range of cell types (Wang et al., 2008; Pouvreau, 2010; Wei et al., 2011; Hou et al., 2012; Cao et al., 2013; Shen et al., 2014; Zhang et al., 2015; Urbanczyk et al., 2018). Mitoflashes were first described in cardiac myocytes using a mitochondrially targeted circularly permuted YFP (mt-cpYFP; Wang et al., 2008). Using other biosensors, events with similar characteristics were reported as “mitochondrial transients” in astrocytes (Azarias and Chatton, 2011) and “oxidative bursts” in neuronal cells (Breckwoldt et al., 2014). However, the precise molecular mechanisms responsible for the generation and termination of these multicomponent mitochondrial events have remained elusive. In this issue of JGP, Feng et al. use purified cardiac mitochondria from mt-cpYFP transgenic mice to carefully dissect the relative role of complexes I, II, and IV of the electron transport chain (ETC), the directionality of electron flow (e.g., forward vs. reverse electron transport) through each of these ETC complexes, and reverse-mode ATP synthase activity in the biogenesis of mitoflashes. The authors conclude that mitoflash activity is intimately linked to the buildup of mitochondrial electrochemical potential, rather than ETC activity per se, such that mitoflash discharges serve as a homeostatic autoregulator of the mitochondrial proton electrochemical potential (ΔμH+).
Mitoflash activity is a biomarker of mitochondrial metabolic state
Mitoflashes are observed in excitable and nonexcitable cells, as well as purified mitochondria, under both physiological and pathological conditions. Mitoflash frequency is a sensitive biomarker of mitochondrial metabolic activity (Wei et al., 2011; Gong et al., 2015). For example, mitoflash frequency is regulated by the activity of skeletal and cardiac muscle (Wei et al., 2011; Gong et al., 2014; Wei-LaPierre et al., 2019) in a manner that impacts ATP homeostasis (Wang et al., 2017). In neuronal cells, mitoflash activity has been associated with neuronal progenitor cell proliferation, early stage somatic cell reprograming, and induction of structural long-term potentiation (Hou et al., 2012; Ying et al., 2016; Fu et al., 2017). Mitoflash activity is also altered under pathological conditions including ischemic reperfusion, inflammation, ROS-induced apoptosis, insulin resistance, muscular dystrophy, malignant hyperthermia, muscle denervation, and Amyotrophic Lateral Sclerosis (Wang et al., 2008; Ma et al., 2011; Wei et al., 2011; Ding et al., 2015; Paolini et al., 2015; Zhang et al., 2015; Karam et al., 2017; Xiao et al., 2018). Given the intimate connection with cellular metabolic state, it is not surprising that mitoflash frequency is tightly linked to both ETC activity (Wang et al., 2008) and a robust mitochondrial proton motive force (Δp; Wang et al., 2008; Schwarzlander et al., 2011; Wei-LaPierre et al., 2013). Mitoflash activity is also altered by changes in matrix Ca2+ and ROS levels, proton uncaging, disturbance in mitochondrial fission/fusion, and shifts in energy demand (Hou et al., 2013; Li et al., 2016; Wang et al., 2016, 2017; Wei-LaPierre et al., 2019).
Deconstructing mitoflash ETC dependence
Prior studies in isolated mitochondria and permeabilized cells have been limited to relatively straightforward comparisons of the effects of different mitochondrial substrates on mitoflash activity (Gong et al., 2014; Wang et al., 2017; Wei-LaPierre et al., 2019). By systematically dissecting the relative role and directionality of electron flux through each ETC complex and the F1-Fo ATP synthase, Feng et al. (2019) provide a significant advance in our understanding of the mechanism of mitoflash generation. The benefits of this approach are twofold: (1) careful dissection of each component of the ETC and its effect on mitoflash activity to allow the most critical components to be identified; (2) capitalization of extensive prior knowledge of mitochondrial bioenergetics in isolated mitochondria, which cannot easily be applied to intact cells. Using this approach, the authors make two important new observations: (1) identification of two types of mitoflashes based on the kinetics of ΔΨm depolarization, and (2) discovery of ETC-independent mitoflash events generated by reverse-mode ATP synthase activity. These results suggest that mitoflash activity regulates the mitochondrial proton electrochemical potential by producing discrete events of mitochondrial proton efflux when needed.
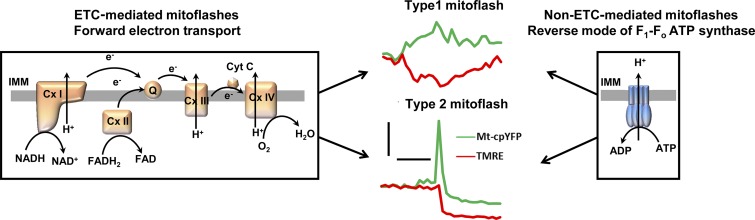
Type 1 and Type 2 mitoflashes
As mentioned above, a transient depolarization of the Ψm (a “dip” in tetramethylrhodamine, methyl ester [TMRM] fluorescence) occurs during each mitoflash. Feng et al. (2019) classified mitoflashes in isolated mitochondria into two groups according to the time course of Ψm depolarization: (1) Type 1 mitoflashes, in which TMRM dips precisely mirror the mitoflash time course (Fig. 1, middle panel, top) and (2) Type 2 mitoflashes, in which TMRM dips persist well beyond the duration of the mitoflash (Fig. 1, middle panel, bottom). Similar distinct populations of Ψm depolarization time courses were reported previously in intact cells (Wang et al., 2008; Fang et al., 2011; Li et al., 2012; Wei-LaPierre et al., 2013). Feng et al. (2019) showed a positive correlation between mitoflash and TMRM dip amplitudes for Type 1 mitoflashes, consistent with mitoflash and Ψm depolarization being tightly coupled during these events. A similar positive correlation between mitoflash and TMRM dip amplitudes has also been reported in intact cells (Wang et al., 2016; McBride et al., 2019). Feng et al. (2019) further found that the majority of Type 2 mitoflashes exhibited a prominent undershoot below baseline mt-cpYFP fluorescence following their termination when mitochondria were provided substrates for complex I (Cx I), complex II (Cx II), and ATP synthase operating in reverse (ATP hydrolysis) mode (Fig. 1, middle panel, bottom). Conversely, they rarely detected an undershoot for Type I mitoflashes while an undershoot was observed for ∼50% of all Type 2 mitoflash events in the presence of substrates for Complex IV (Cx IV).
Figure 1.
Two mechanisms for mitoflash generation. Forward-mode ECT-dependent (left) and reverse-mode ATP synthase–mediated (right) mechanisms for generation of Type 1 and Type 2 mitoflashes. Middle: representative traces for Type 1 and Type 2 mitoflashes from isolated cardiac mitochondria. Scale bars: horizontal, 10s; vertical, 1 ΔF/F0. IMM, inner mitochondrial membrane; Cx I-IV, complex I-IV; Cyt C, cytochrome C; Q, ubiquinone.
The authors attributed the Type 2 mitoflash undershoot to matrix acidification resulting from sustained activation of a large-conductance, proton-permeable pore (e.g., the mitochondrial permeability transition pore, mPTP). Nevertheless, the potential involvement of other mitochondrial inner membrane channels and/or transporters in the post-mitoflash undershoot cannot be excluded. Interestingly, compared with isolated mitochondria, mitoflashes with an undershoot occurred at a much lower frequency in intact skeletal and cardiac muscle cells (<30% mitoflashes with glucose as substrate). Thus, mitoflashes are likely subject to intracellular regulatory factors in intact cells that limit the incidence of high-conductance mPTP activity. This would minimize deleterious sustained Ψm depolarization, post-mitoflash acidification, and apoptosis.
As considered previously (Wei and Dirksen, 2012; Wang et al., 2016), convincing experimental evidence for the mechanisms responsible for mitoflash termination is lacking. However, if mitoflash events are self terminating, one might expect a relationship between mitoflash amplitude and duration. Indeed, results from Feng et al. (2019) in isolated cardiac mitochondria suggest that larger amplitude mitoflash events terminate more quickly than lower amplitude events. For example, representative mitoflash event traces in Fig. 5 in Feng et al. (2019) reveal short duration events for several Type 2 mitoflashes with large amplitude, as well as numerous Type 1 mitoflashes with small amplitudes and long durations. In line with recent findings of an inverse correlation between mitoflash amplitude and duration in isolated mitochondria (Wei-LaPierre et al., 2019), these observations are consistent with an “amplitude threshold” mechanism for mitoflash termination.
Forward-mode ETC-dependent and reverse-mode ATP synthase–mediated mitoflashes
Previous studies have demonstrated that mitoflash activity is inhibited by blockers of each complex in the ETC (Wang et al., 2008; Pouvreau, 2010; Wei et al., 2011). Based on these and other findings (e.g., absence of mitoflash activity in ρ0 cells devoid of mitochondrial DNA), functional integrity of the ETC is believed to be essential for mitoflash genesis (Wang et al., 2008). Results from Feng et al. (2019) challenge this conventional wisdom. The authors investigated the role of each complex in the ETC in triggering mitoflashes by providing mitochondria with complex-specific substrates to initiate electron transport at different sites (Cx I, II, and IV). Complex-specific inhibitors were used under each experimental condition to examine the relative contribution of forward electron transport (FET) and reverse electron transport (RET) for each substrate entry site. Consistent with results obtained in intact cells, functional FET is required for mitoflash generation, regardless of entry site, when isolated mitochondria respire on substrates for Cx I, II, and IV in the absence of ADP (i.e., state II/IV respiration; Fig. 1, left panel). When Cx IV was used as the entry site, inhibition of RET at Cx III, II, and I dramatically increased mitoflash frequency, indicating that RET from Cx IV reduces mitoflash generation. This result is in contrast to that observed in intact cells, when flash activity was abolished in the presence of inhibitors for site IQ on Cx I (rotenone) and site IIIQo on Cx III (antimycin A), two major sites for RET. Thus, despite attempts to provide mitochondrial complex-specific substrates, entry through Cx I and FET dominate ETC activity in intact cells, at least under the experimental conditions used in these studies (Fang et al., 2011; Gong et al., 2014, 2015; Wei-LaPierre et al., 2019).
One of the most interesting and unexpected observations reported by Feng et al. (2019) is that mitoflash events can be generated in isolated mitochondria, in the absence of ETC activity, by driving reverse-mode F1-Fo ATP synthase activity (ATP hydrolysis mode) via the application of ATP in the absence of ETC substrates (Fig. 1, right panel). Under these conditions, the F1-Fo ATP synthase functions as an ATPase that hydrolyzes ATP to pump protons from the matrix into the intermembrane space, which increases the proton motive force to reenergize mitochondria. Mitoflashes recorded during reverse-mode ATP synthase activity were insensitive to Cx I-IV inhibitors, confirming that ETC activity was not involved in mitoflashes generated under these conditions. Similar to ETC-dependent mitoflashes, reverse-mode ATP synthase activity also resulted in Type 1 mitoflashes without a post-mitoflash undershoot and Type 2 mitoflashes with an undershoot (see Fig. 5 A, right panel in Feng et al. [2019]). However, reverse-mode ATP synthase–mediated mitoflashes exhibited distinct characteristics from those of forward-mode ETC-mediated mitoflashes. Both the frequency and amplitude of reverse-mode ATP synthase–mediated mitoflashes were significantly lower compared with those of ETC-mediated mitoflashes. In addition, mitoflashes observed under conditions of reverse-mode ATP synthase activity lacked the tight correlation between mitoflash and TMRM amplitudes that was observed for forward-mode ETC-dependent mitoflashes. ETC-dependent events consisted of a mixed signal that included an increase in superoxide/ROS production and a transient increase in mitochondrial matrix pH (Azarias and Chatton, 2011; Wei-LaPierre et al., 2013; Breckwoldt et al., 2014). Since ATP hydrolysis is not expected to generate superoxide, it will be important for future studies to assess the relative contribution of changes in superoxide/ROS and pH for mitoflashes generated during reverse-mode ATP synthase activity.
An open question, not fully addressed by the authors, is under what conditions might reverse-mode ATP synthase-mediated mitoflash activity be important in intact cells? Under physiological conditions, with a normal mitochondrial membrane potential, the F1-Fo ATP synthase operates in forward mode to drive ATP synthesis. Reversal of the ATP synthase in order to hydrolyze ATP to restore the proton electrochemical potential occurs under conditions of significant depolarization of Ψm, for example, during hypoxia/ischemia. As ETC activity stops when the ATP synthase reverses to hydrolyze ATP, further investigation will be required to determine whether reverse-mode ATP synthase–mediated, and forward-mode ETC-dependent, mitoflash generation can occur at the same time in intact cells. Because the reversal potential of the adenosine nucleotide translocase (ANT) is more positive than that of the F1-Fo ATP synthase (Chinopoulos, 2011), the ANT reverses at even more depolarized Ψm than the ATP synthase. In the reverse-mode ATP synthase experiments by Feng et al. (2019), mitochondria were almost completely depolarized before the addition of substrate (ATP). Under these conditions, the ANT operates in a reverse mode to pump ATP into the mitochondrial matrix (in exchange for ADP) to be used for reverse-mode ATP synthase–mediated mitoflash generation. However, as a complete reversal of both the ATP synthase and ANT requires a larger Ψm depolarization, it is less likely to occur than reversal of only the F1-Fo ATP synthase (Chinopoulos et al., 2010; Chinopoulos, 2011). Future studies are needed to determine whether reverse-mode ATP synthase–mediated mitoflash activity occurs in the absence of reversal of the ANT and to what degree reverse-mode ATP synthase–mediated mitoflash generation occurs in intact cells under pathological conditions.
Proton motive force is the ultimate mitoflash determinant
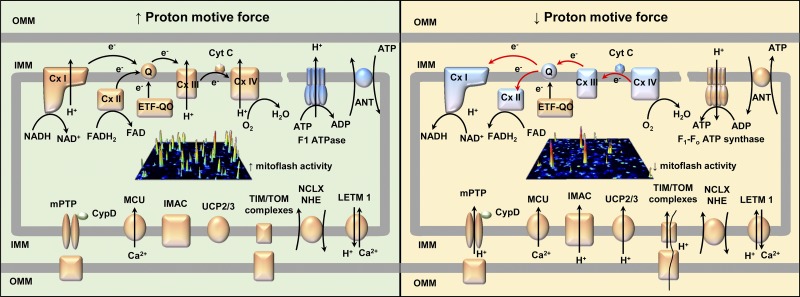
In intact skeletal and cardiac muscle cells, the proton motive force (Δp) regulates mitoflash generation in a biphasic manner. While a modest reduction of Δp using a low concentration of nigericin (0.001–0.3 µM) stimulates mitoflash activity (Wei-LaPierre et al., 2013; Wang et al., 2016), collapse of Δp with higher concentrations of nigericin (5 µM) abolishes mitoflash activity (Wei-LaPierre et al., 2013). These observations are explained by a modest reduction in Δp being sufficient to stimulate respiratory chain activity and enhance proton pumping across the inner membrane in order to restore the pH gradient. In contrast, a collapse in Δp could uncouple mitochondria and explain abolition of mitoflash activity. These results are consistent with mitoflashes responding to modest increases in H+ in the mitochondrial matrix, while at the same time requiring a robust Δp. The discovery by Feng et al. (2019) of ETC-independent, reverse-mode ATP synthase–mediated mitoflash activity further supports the critical role of Δp in mitoflash generation. In their studies in isolated mitochondria, mitoflash activity increased in all experimental conditions that promoted Δp (both ETC-dependent FET and reverse-mode ATP synthase activity; Fig. 2, left) and decreased in all conditions that reduced Δp (both ETC-dependent RET and State III respiration; Fig. 2, right).
Figure 2.
Proton motive force is required to drive mitoflash generation. Conditions that increase proton motive force increase mitoflash activity (left) while conditions that severely compromise proton motive force decrease mitoflash activity (right). OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; Cyt C, cytochrome C; Q, ubiquinone; ANT, adenosine nucleotide translocase; ETF-QO, electron transferring flavoprotein dehydrogenase; CypD, cyclophilin D; MCU, mitochondrial Ca2+ uniporter; UCP, uncoupling protein; TIM/TOM complexes, translocase of the inner/outer membrane; NCLX, Ca2+/Na+ exchanger, NHE, Na+/H+ exchanger; LETM1, Ca2+(K+)/H+ antiporter.
Given the critical role of Δp in mitoflash activity, further investigation is warranted to assess the impact of transporters/channels within the mitochondrial inner membrane that could alter Δp (by altering pH, Ψm, or both). Potential transporters/channels include, but are not limited to, mPTP, mitochondrial uncoupling proteins (UCP1-3), inner membrane anion channel (IMAC), and translocator of inner/outer membrane complexes. Interestingly, while some of these players are not required for mitoflash activity under basal conditions, they impact regulation of mitoflash activity under specific metabolic, stimulated, and/or pathological conditions. For example, although inhibition of UCP2 activity does not alter mitoflash frequency in the presence of glucose in quiescent cardiac myocytes, it enhances mitoflash activity in the presence of pyruvate (Wang et al., 2017). In addition, UCP2 overexpression inhibits mitoflash generation in rat neonatal cardiomyocytes and protects the myocardium from lipopolysaccharide-induced injury (Chen et al., 2018). In skeletal muscle fibers, CypD-dependent mPTP activity modulates mitoflash activity during muscle activity and under pathological conditions, but does not impact mitoflash activity under basal conditions (Pouvreau, 2010; Wei et al., 2011; Karam et al., 2017; Xiao et al., 2018; Wei-LaPierre et al., 2019). Furthermore, while basal mitoflash activity in skeletal muscle is unaltered by either UCP3 ablation or IMAC inhibition (Pouvreau, 2010; McBride et al., 2019), a potential regulatory role for UCP3 and IMAC under other conditions remains to be determined. Finally, other channels and exchangers that mediate ion flux across the mitochondrial inner membrane (e.g., mitochondrial Ca2+ uniporter, K+ channels, Ca2+/Na+ exchanger, Na+/H+ exchanger, and Ca2+(K+)/H+ antiporter) may also affect the proton motive force, and thus, modulate mitoflash activity (Dzbek and Korzeniewski, 2008; Zotova et al., 2010; Boyman et al., 2013; Quan et al., 2015).
What’s next?
The study by Feng et al. (2019) provides new insights into the molecular mechanisms underlying mitoflash generation. The identification of an ETC-independent, reverse-mode ATP synthase–mediated mechanism for mitoflash production counters the conventional view that these events are obligatorily linked to ETC activity and further emphasizes the essential role of the mitochondrial proton motive force in mitoflash production. However, whether the biphasic regulation of mitoflash activity by Δp in intact cells is also relevant for both forward-mode ETC-dependent and reverse-mode ATP synthase-mediated mitoflash activity in isolated mitochondria remains to be determined. In addition, it will be important for future work to determine the mechanisms and degree to which changes in mitoflash activity initiate downstream signaling mechanisms that impact mitochondrial substrate utilization and energy production. Translating the current work in experimentally defined conditions in isolated mitochondria to intact cells will also require additional attention. For example, deciphering the relative physiological contributions of mitoflash activity driven by different substrates and entry points within the ETC will be a major challenge moving forward. Similarly, the potential role of reverse-mode ATP synthase–mediated mitoflash generation under different pathological states, cell types, and metabolic conditions remains to be determined. As the reverse-mode ATP synthase mechanism for mitoflash generation in intact cells is likely to be operative primarily under pathological conditions in which mitochondrial Ψm is markedly depolarized, approaches designed to specifically quantify these events could be used as a biomarker for certain diseases. Confirming the expected differences in signal composition of forward-mode ETC-dependent (e.g., superoxide and pH) mitoflashes and reverse-mode ATP synthase-mediated (presumably only pH) mitoflashes could provide one avenue to differentiate between these two mechanisms in intact cells. Such an advance could lead to improved diagnosis and treatment of a wide range of oxidative stress-related disorders including cancer, neurodegeneration, stroke, arteriosclerosis, and ischemia/reperfusion injury.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (AR059646 to R.T. Dirksen).
The authors declare no competing financial interests.
Eduardo Ríos served as editor.
References
- Azarias G., and Chatton J.Y.. 2011. Selective ion changes during spontaneous mitochondrial transients in intact astrocytes. PLoS One. 6:e28505 10.1371/journal.pone.0028505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman L., Williams G.S.B., Khananshvili D., Sekler I., and Lederer W.J.. 2013. NCLX: the mitochondrial sodium calcium exchanger. J. Mol. Cell. Cardiol. 59:205–213. 10.1016/j.yjmcc.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckwoldt M.O., Pfister F.M., Bradley P.M., Marinković P., Williams P.R., Brill M.S., Plomer B., Schmalz A., St Clair D.K., Naumann R., et al. 2014. Multiparametric optical analysis of mitochondrial redox signals during neuronal physiology and pathology in vivo. Nat. Med. 20:555–560. 10.1038/nm.3520 [DOI] [PubMed] [Google Scholar]
- Cao Y., Zhang X., Shang W., Xu J., Wang X., Hu X., Ao Y., and Cheng H.. 2013. Proinflammatory Cytokines Stimulate Mitochondrial Superoxide Flashes in Articular Chondrocytes In Vitro and In Situ. PLoS One. 8:e66444 10.1371/journal.pone.0066444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Luo S., Xie P., Hou T., Yu T., and Fu X.. 2018. Overexpressed UCP2 regulates mitochondrial flashes and reverses lipopolysaccharide-induced cardiomyocytes injury. Am. J. Transl. Res. 10:1347–1356. [PMC free article] [PubMed] [Google Scholar]
- Chinopoulos C. 2011. Mitochondrial consumption of cytosolic ATP: not so fast. FEBS Lett. 585:1255–1259. 10.1016/j.febslet.2011.04.004 [DOI] [PubMed] [Google Scholar]
- Chinopoulos C., Gerencser A.A., Mandi M., Mathe K., Töröcsik B., Doczi J., Turiak L., Kiss G., Konràd C., Vajda S., et al. 2010. Forward operation of adenine nucleotide translocase during F0F1-ATPase reversal: critical role of matrix substrate-level phosphorylation. FASEB J. 24:2405–2416. 10.1096/fj.09-149898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Fang H., Shang W., Xiao Y., Sun T., Hou N., Pan L., Sun X., Ma Q., Zhou J., et al. 2015. Mitoflash altered by metabolic stress in insulin-resistant skeletal muscle. J. Mol. Med. (Berl.). 93:1119–1130. 10.1007/s00109-015-1278-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzbek J., and Korzeniewski B.. 2008. Control over the contribution of the mitochondrial membrane potential (DeltaPsi) and proton gradient (DeltapH) to the protonmotive force (Deltap). In silico studies. J. Biol. Chem. 283:33232–33239. 10.1074/jbc.M802404200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., Chen M., Ding Y., Shang W., Xu J., Zhang X., Zhang W., Li K., Xiao Y., Gao F., et al. 2011. Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals. Cell Res. 21:1295–1304. 10.1038/cr.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G., Liu B., Li J., Cheng T., Huang Z., Wang X., and Cheng H.P.. 2019. Mitoflash biogenesis and its role in the autoregulation of mitochondrial proton electrochemical potential. J. Gen. Physiol.:jgp.201812176 10.1085/jgp.201812176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z.X., Tan X., Fang H., Lau P.M., Wang X., Cheng H., and Bi G.Q.. 2017. Dendritic mitoflash as a putative signal for stabilizing long-term synaptic plasticity. Nat. Commun. 8:31 10.1038/s41467-017-00043-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G., Liu X., and Wang W.. 2014. Regulation of metabolism in individual mitochondria during excitation-contraction coupling. J. Mol. Cell. Cardiol. 76:235–246. 10.1016/j.yjmcc.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G., Liu X., Zhang H., Sheu S.-S., and Wang W.. 2015. Mitochondrial flash as a novel biomarker of mitochondrial respiration in the heart. Am. J. Physiol. Heart Circ. Physiol. 309:H1166–H1177. 10.1152/ajpheart.00462.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T., Zhang X., Xu J., Jian C., Huang Z., Ye T., Hu K., Zheng M., Gao F., Wang X., and Cheng H.. 2013. Synergistic triggering of superoxide flashes by mitochondrial Ca2+ uniport and basal reactive oxygen species elevation. J. Biol. Chem. 288:4602–4612. 10.1074/jbc.M112.398297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Ouyang X., Wan R., Cheng H., Mattson M.P., and Cheng A.. 2012. Mitochondrial superoxide production negatively regulates neural progenitor proliferation and cerebral cortical development. Stem Cells. 30:2535–2547. 10.1002/stem.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam C., Yi J., Xiao Y., Dhakal K., Zhang L., Li X., Manno C., Xu J., Li K., Cheng H., et al. 2017. Absence of physiological Ca2+ transients is an initial trigger for mitochondrial dysfunction in skeletal muscle following denervation. Skelet. Muscle. 7:6 10.1186/s13395-017-0123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Zhang W., Fang H., Xie W., Liu J., Zheng M., Wang X., Wang W., Tan W., and Cheng H.. 2012. Superoxide flashes reveal novel properties of mitochondrial reactive oxygen species excitability in cardiomyocytes. Biophys. J. 102:1011–1021. 10.1016/j.bpj.2012.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Sun T., Liu B., Wu D., Qi W., Wang X., Ma Q., and Cheng H.. 2016. Regulation of Mitoflash Biogenesis and Signaling by Mitochondrial Dynamics. Sci. Rep. 6:32933 10.1038/srep32933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Fang H., Shang W., Liu L., Xu Z., Ye T., Wang X., Zheng M., Chen Q., and Cheng H.. 2011. Superoxide flashes: early mitochondrial signals for oxidative stress-induced apoptosis. J. Biol. Chem. 286:27573–27581. 10.1074/jbc.M111.241794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride S., Wei-LaPierre L., McMurray F., MacFarlane M., Qiu X., Patten D.A., Dirksen R.T., and Harper M.E.. 2019. Skeletal muscle mitoflashes, pH, and the role of uncoupling protein-3. Arch. Biochem. Biophys. 663:239–248. 10.1016/j.abb.2019.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini C., Quarta M., Wei-LaPierre L., Michelucci A., Nori A., Reggiani C., Dirksen R.T., and Protasi F.. 2015. Oxidative stress, mitochondrial damage, and cores in muscle from calsequestrin-1 knockout mice. Skelet. Muscle. 5:10 10.1186/s13395-015-0035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouvreau S. 2010. Superoxide flashes in mouse skeletal muscle are produced by discrete arrays of active mitochondria operating coherently. PLoS One. 5:e13035 10.1371/journal.pone.0013035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan X., Nguyen T.T., Choi S.-K., Xu S., Das R., Cha S.-K., Kim N., Han J., Wiederkehr A., Wollheim C.B., and Park K.-S.. 2015. Essential role of mitochondrial Ca2+ uniporter in the generation of mitochondrial pH gradient and metabolism-secretion coupling in insulin-releasing cells. J. Biol. Chem. 290:4086–4096. 10.1074/jbc.M114.632547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzlander M., Logan D.C., Fricker M.D., and Sweetlove L.. 2011. The circularly permuted yellow fluorescent protein cpYFP that has been used as a superoxide probe is highly responsive to pH but not superoxide in mitochondria: implications for the existence of superoxide ‘flashes’. Biochem. J. 437:381–387. 10.1042/BJ20110883 [DOI] [PubMed] [Google Scholar]
- Shen E.Z., Song C.Q., Lin Y., Zhang W.H., Su P.F., Liu W.Y., Zhang P., Xu J., Lin N., Zhan C., et al. 2014. Mitoflash frequency in early adulthood predicts lifespan in Caenorhabditis elegans. Nature. 508:128–132. 10.1038/nature13012 [DOI] [PubMed] [Google Scholar]
- Urbanczyk S., Stein M., Schuh W., Jäck H.M., Mougiakakos D., and Mielenz D.. 2018. Regulation of Energy Metabolism during Early B Lymphocyte Development. Int. J. Mol. Sci. 19:E2192 10.3390/ijms19082192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J., Wang X., Li K., Han P., Zheng M., et al. 2008. Superoxide flashes in single mitochondria. Cell. 134:279–290. 10.1016/j.cell.2008.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang X., Huang Z., Wu D., Liu B., Zhang R., Yin R., Hou T., Jian C., Xu J., et al. 2016. Protons Trigger Mitochondrial Flashes. Biophys. J. 111:386–394. 10.1016/j.bpj.2016.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang X., Wu D., Huang Z., Hou T., Jian C., Yu P., Lu F., Zhang R., Sun T., et al. 2017. Mitochondrial flashes regulate ATP homeostasis in the heart. eLife. 6:e23908 10.7554/eLife.23908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., and Dirksen R.T.. 2012. Mitochondrial superoxide flashes: from discovery to new controversies. J. Gen. Physiol. 139:425–434. 10.1085/jgp.201210790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Salahura G., Boncompagni S., Kasischke K.A., Protasi F., Sheu S.S., and Dirksen R.T.. 2011. Mitochondrial superoxide flashes: metabolic biomarkers of skeletal muscle activity and disease. FASEB J. 25:3068–3078. 10.1096/fj.11-187252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei-LaPierre L., Gong G., Gerstner B.J., Ducreux S., Yule D.I., Pouvreau S., Wang X., Sheu S.S., Cheng H., Dirksen R.T., and Wang W.. 2013. Respective contribution of mitochondrial superoxide and pH to mitochondria-targeted circularly permuted yellow fluorescent protein (mt-cpYFP) flash activity. J. Biol. Chem. 288:10567–10577. 10.1074/jbc.M113.455709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei-LaPierre L., Ainbinder A., Tylock K.M., and Dirksen R.T.. 2019. Substrate-dependent and cyclophilin D-independent regulation of mitochondrial flashes in skeletal and cardiac muscle. Arch. Biochem. Biophys. 665:122–131. 10.1016/j.abb.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Karam C., Yi J., Zhang L., Li X., Yoon D., Wang H., Dhakal K., Ramlow P., Yu T., et al. 2018. ROS-related mitochondrial dysfunction in skeletal muscle of an ALS mouse model during the disease progression. Pharmacol. Res. 138:25–36. 10.1016/j.phrs.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z., Chen K., Zheng L., Wu Y., Li L., Wang R., Long Q., Yang L., Guo J., Yao D., et al. 2016. Transient Activation of Mitoflashes Modulates Nanog at the Early Phase of Somatic Cell Reprogramming. Cell Metab. 23:220–226. 10.1016/j.cmet.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Zhang M., Sun T., Jian C., Lei L., Han P., Lv Q., Yang R., Zhou X., Xu J., Hu Y., et al. 2015. Remodeling of Mitochondrial Flashes in Muscular Development and Dystrophy in Zebrafish. PLoS One. 10:e0132567 10.1371/journal.pone.0132567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotova L., Aleschko M., Sponder G., Baumgartner R., Reipert S., Prinz M., Schweyen R.J., and Nowikovsky K.. 2010. Novel components of an active mitochondrial K(+)/H(+) exchange. J. Biol. Chem. 285:14399–14414. 10.1074/jbc.M109.059956 [DOI] [PMC free article] [PubMed] [Google Scholar]