Abstract
Mundiña-Weilenmann and Mattiazzi examine new work revealing the mechanism by which nitroxide modifies uptake of Ca2+ into the SR.
Cardiovascular disease is the leading cause of morbidity and mortality worldwide. Calcium (Ca2+) mishandling is one of the most striking abnormalities in this wide spectrum of pathologies, among which heart failure (HF) remains the leading cause of death in developed countries (Benjamin et al., 2018). A hallmark of HF in both human and animal models is impaired Ca2+ sequestration into the SR, which contributes to the decreased contractile performance in this disease (Gwathmey et al., 1987; Meyer et al., 1995; del Monte et al., 2002). Not surprisingly, this defective mechanism has been targeted with novel therapeutic strategies that are now undergoing experimental and clinical testing in animals and patients (Pfeffer et al., 2015; Hulot et al., 2016, 2017; Motloch et al., 2018). In this issue of JGP, Keceli et al. provide novel insights into the molecular mechanism from which nitroxyl (HNO), nitric oxide (NO)’s one-electron-reduced and protonated sibling, recently emerged as a promising candidate for HF treatment.
Overview of excitation–contraction coupling
In normal hearts, Ca2+ enters the cell through voltage-dependent Ca2+ channels (L-type Ca2+ channels) and subsequently binds to and activates the SR Ca2+ release channels or ryanodine receptor 2 (RYR2) to trigger further Ca2+ release. (Fig. 1) This process, known as CICR (Fabiato and Fabiato, 1977), essentially amplifies the Ca2+ signal, increasing cytosolic Ca2+ to produce contraction at the myofilament level. Relaxation occurs when cytosolic Ca2+ returns to basal diastolic values. The majority of this task is accomplished by SERCA2a, which mediates Ca2+ uptake into the SR, and to a lesser extent by the Na+/Ca2+ exchanger (NCX), which normally transfers Ca2+ to the extracellular space in exchange for entry of Na+ (Bers, 2001).
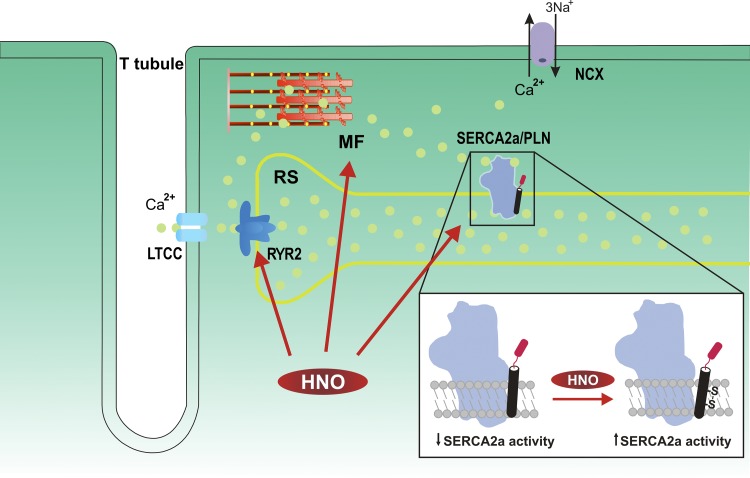
Figure 1.
Schematic depicting excitation−contraction coupling and the mechanism of HNO action. The main HNO targets in cardiac myocytes are shown as well as the proposed mechanism by which HNO modifies SERCA2a/PLN interaction. Upon membrane depolarization, Ca2+ (yellow spheres) enters the cell through L-type Ca2+ channels (LTCC) and activates the RYR2 of the SR to produce further Ca2+ release. Ca2+ binds to the myofilaments (MF) to produce contraction. Most of the Ca2+ is then reuptaken by SERCA2a, and a small fraction is extruded from the cell through NCX. HNO activates RYR2 and SR Ca2+ release, SR Ca2+ uptake, and MF Ca2+ sensitivity, without significantly affecting LTCC and NCX. The inset of the figure shows the mechanism proposed by Keceli et al. (2019) by which HNO modifies thiol groups in PLN. It is suggested that when the HNO/thiol stoichiometry approaches a 1:1 ratio, similar to that anticipated in vivo under normal physiological conditions, an intramolecular disulfide bond between two cysteines of the transmembrane domain of PLN is formed, relieving the preexisting SERCA2a inhibition.
Regulation of SR Ca2+ uptake by phospholamban
The activity of SERCA2a is reversibly regulated by phospholamban (PLN), a 52–amino acid protein (Tada et al., 1975) localized to the longitudinal SR (Jorgensen and Jones, 1987). Sequence analysis of PLN has shown that the protein is organized into three domains: two cytosolic domains (Ia and Ib) and a transmembrane domain (II; Simmerman and Jones, 1998). Domain Ia contains Ser16 and Thr17, the sites of phosphorylation by PKA and CaMKII, respectively (Wegener et al., 1989). It is well known that dephosphorylated PLN binds to the transmembrane domains of SERCA2a (Kimura et al., 1996) and allosterically inhibits SR Ca2+ reuptake (MacLennan and Kranias, 2003). Conversely, phosphorylation of PLN relieves SERCA2a inhibition, increasing the affinity of the enzyme for Ca2+ and enhancing the rates of SR Ca2+ uptake and myocardial relaxation. This in turn leads to an increased SR Ca2+ load, SR Ca2+ release, and contractility (Lindemann et al., 1983; Lindemann and Watanabe, 1985; Mundiña-Weilenmann et al., 1996).
Experimental evidence suggests two possible mechanistic models by which PLN phosphorylation releases its inhibitory action. In one, phosphorylation results in the physical dissociation of PLN from SERCA2a (Chen et al., 2007). In the other, phosphorylation shifts the cytoplasmic domain of PLN toward a noninhibitory conformational state, leaving the transmembrane domain anchored to the Ca2+ pump (Gustavsson et al., 2013). Another point to consider is the dynamic equilibrium that exists between monomeric and pentameric forms of PLN in SR membranes, which is also influenced by the phosphorylation status of the protein (Simmerman and Jones, 1998). Although evidence supports the assertion that the monomer is the active species of PLN (MacLennan and Kranias, 2003), x-ray crystallography studies indicate that SERCA2a also interacts with pentameric PLN, pointing to a potential role of this oligomeric form in regulating SERCA2a activity (Stokes et al., 2006; Glaves et al., 2011).
New insights on SERCA2a regulation: PLN partners and redox modifications. The emergence of HNO
The classical view of SERCA2a regulation by PLN phosphorylation was modified and enriched by further investigations. These revealed that PLN does not act alone but in association with a set of proteins that regulate SR Ca2+ uptake: the PLN/SERCA2a interactome (for reviews, see Haghighi et al., 2014; Mattiazzi and Kranias, 2014). Moreover, it became clear that PLN is also regulated by posttranslational modifications that are different from phosphorylation and evoked by reactive oxygen and nitrogen species (ROS and RNS, respectively; Bigelow and Squier, 2005; Froehlich et al., 2008; Lancel et al., 2009; Ha et al., 2011; Sivakumaran et al., 2013; Irie et al., 2015). Among the RNS is HNO, the one-electron reduction product of NO. HNO avidly reacts with nucleophiles, especially thiols, to yield either disulfides or sulfinamides; a redox change exclusive to HNO. Furthermore, the hydrophobic nature of HNO makes it more likely to react with thiols in hydrophobic regions. Thus, HNO reactivity toward thiol-containing proteins is different from its redox sibling NO and other recognized oxidizing agents. Intriguingly, the putative physiological relevance of HNO, as well as the possibility of an endogenous source in biological systems, has been difficult to establish, owing to its short-lived characteristics. However, the emergence of HNO donors as ideal pharmacological agents in the treatment of HF (Paolocci et al., 2001, 2003; also see below), has recently gained considerable interest.
Cardiac effects of HNO
HNO was initially identified as a vasorelaxant agent (for review, see Bullen et al., 2011). Later on, its importance as a positive inotropic and lusitropic agent was recognized in seminal works by Paolocci et al. (2001, 2003), performed in conscious instrumented dogs with normal or failing hearts. The effects were independent of the cardiac loading conditions (e.g., alterations in pre- and afterload) as well as of β-adrenergic signaling (Paolocci et al., 2003). These novel findings signaled the onset of a new era of scientific research that raised the significance of HNO from an almost overlooked agent to a potential candidate for HF treatment (Hasenfuss and Teerlink, 2011). Further work demonstrated that the effects resulted from a direct action of HNO on SERCA2a and RYR2, leading to increased SR Ca2+ uptake and release. These HNO cardiac effects were fully reversible and independent of both cAMP/PKA and cGMP/PKG signaling.
Additional results indicated that PLN plays a central role in the HNO enhancement of SERCA2a activity. When the three cysteines (Cys) of PLN transmembrane domain were replaced by alanine, the stimulatory action of HNO was lost (Froehlich et al., 2008). Similar to one of the suggested mechanisms for how PLN phosphorylation reduces SERCA2a inhibition (Gustavsson et al., 2013, and see above), it was proposed that HNO functionally uncouples PLN from SERCA without altering the physical association between both proteins (Sivakumaran et al., 2013).
New mechanistic insights into the effects of HNO
Keceli et al. (2019) extend these previous results by adding significant insight into the mechanism by which HNO affects SR Ca2+ uptake. By using 15N-edited NMR spectroscopy (a technique that allows detection of sulfinamides, redox changes on thiols that are unique to HNO) in concert with measurements of SERCA2a activity and immunoblots, the authors were able to address two important remaining questions regarding the mechanisms by which HNO modifies PLN function and SR Ca2+ sequestration. Specifically, they discovered that Cys41 and Cys46 are the main targets of HNO that functionally uncouple PLN from SERCA2a and activate SR Ca2+ pump. In addition, they found that when the HNO:thiol ratio approaches values similar to those expected in vivo in cardiac cells, intramolecular disulfide bond formation between Cys41 and Cys46 in PLN monomer predominates over irreversible sulfinamide links (Fig. 1). This is important because, unlike sulfinamide formation, reduction of disulfide bonds can be completed within a few minutes at physiological pH and temperature. These results match the fast reversibility of HNO-induced effects previously observed in vitro and in vivo (Paolocci et al., 2001, 2003; Froehlich et al., 2008; Sivakumaran et al., 2013), strongly suggesting that the physiological effects of HNO donors on the cardiac system are likely due to the formation of reversible disulfide linkages rather than sulfinamides. These latter results constitute an important clue for the potential role of HNO as a signaling molecule in the cardiovascular system. In addition, the results reinforce previous findings, which indicate that the HNO signaling and contractile effects are different from the more traditional β1/β2 agonists.
Perspectives
Collectively, the results of Keceli et al. (2019) deepen understanding of the mechanism by which HNO increases SR Ca2+ uptake at the molecular level and provide new insights into the reactive redox switches of PLN. Several questions still remain, however. For instance, as the authors recognize, new accurate detection methods are needed to know where and how much HNO is produced in vivo. Moreover, it is not clear whether HNO-induced enhancement of SR Ca2+ uptake also involves direct effects on SERCA2a. Keceli et al. (2019) present evidence supporting the idea that PLN is the main HNO target. Indeed, they accurately show that mutation of Cys41 and Cys46 of PLN to alanine reduces the HNO-induced enhancement of SERCA2a activity. In contrast, prior results from Lancel et al. (2009) indicated that HNO-induced increases in SR Ca2+ uptake are due to direct SERCA2a activation, independently of PLN, via S-glutathiolation at Cys674. An alternative explanation to these somewhat controversial results is that the action of HNO on PLN and SERCA might represent complementary or even redundant mechanisms (assuming that HNO is a physiological signaling molecule), which contribute similarly at the intact myocyte level.
The novel mechanistic insights presented by Keceli et al. (2019) also provide new support for the use of HNO donors in HF treatment. In this scenario, the diversity of actions evoked by HNO on excitation–contraction coupling proteins different from PLN and SERCA2a deserves comment (Fig. 1). It has been shown that HNO does not significantly affect L-type Ca2+ current (Kohr et al., 2010), but increases myofilament Ca2+ sensitivity, which would add to the potency of HNO as an inotropic agent and favor its beneficial effect on HF (Gao et al., 2012). It has also been shown that HNO enhances RYR2 activity (Tocchetti et al., 2007). This effect occurs in association with increased SR Ca2+ uptake, increased fractional Ca2+ release, and no change in SR Ca2+ load.
Although these results emphasize the value of HNO donors as innovative pharmacological tools to enhance myocardial Ca2+ cycling, an important concern not yet clarified is their potential arrhythmogenicity. An increase in SR Ca2+ uptake that would potentially increase (or maintain) SR Ca2+ load and an enhancement in RYR2 activity (Tocchetti et al., 2007; Keceli et al., 2019) are two conditions that favor arrhythmogenic SR Ca2+ leak (Zima et al., 2014). Importantly, an increase in SR Ca2+ sparks has been observed in “healthy” myocytes in the presence of the HNO donor (Tocchetti et al., 2007). An excess of SR Ca2+ sparks may produce Ca2+ waves that propagate through cardiac cells. Spontaneous Ca2+ waves are arrhythmogenic because, by activating inward membrane currents (mainly the electrogenic NCX working in the forward mode), they depolarize the cell membrane and eventually produce an ectopic beat (Pogwizd and Bers, 2004; Laurita a nd Rosenbaum, 2008). Considering that HF myocytes often have a leaky RYR2 and are prone to pro-arrhythmic events (Belevych et al., 2011; Zima et al., 2014), it is still uncertain whether the beneficial effects on contractility produced by HNO donors could be compromised in nonhealthy myocytes by a possible arrhythmogenic effect. The work of Keceli et al. (2019) and previous work from the same group (Froehlich et al., 2008; Tocchetti, 2007; Sivakumaran et al., 2013), provides valuable mechanistic insight into the role of HNO on SR Ca2+ uptake at the molecular level. Similar mechanistic studies on the effect of HNO on SR Ca2+ release will be most welcome in the near future to provide a better understanding of the effects of HNO donors on HF.
Acknowledgments
This work was supported by Consejo de Investigaciones Científicas y Técnicas (PIP #0507 to C. Mundiña-Weilenmann and PIP #0305 to A. Mattiazzi).
The authors declare no competing financial interests.
Henk L. Granzier served as editor.
References
- Belevych A.E., Terentyev D., Terentyeva R., Nishijima Y., Sridhar A., Hamlin R.L., Carnes C.A., and Györke S.. 2011. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc. Res. 90:493–502. 10.1093/cvr/cvr025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . 2018. Heart disease and stroke Statistics-2018 update: a report from the American Heart Association. Circulation. 137:e67–e492. 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- Bers D.M. 2001. Excitation-contraction coupling and cardiac contractile force. Kluwer Academic Publishers, Dordrecht, The Netherlands: 10.1007/978-94-010-0658-3 [DOI] [Google Scholar]
- Bigelow D.J., and Squier T.C.. 2005. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim. Biophys. Acta. 1703:121–134. 10.1016/j.bbapap.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Bullen M.L., Miller A.A., Andrews K.L., Irvine J.C., Ritchie R.H., Sobey C.G., and Kemp-Harper B.K.. 2011. Nitroxyl (HNO) as a vasoprotective signaling molecule. Antioxid. Redox Signal. 14:1675–1686. 10.1089/ars.2010.3327 [DOI] [PubMed] [Google Scholar]
- Chen Z., Akin B.L., and Jones L.R.. 2007. Mechanism of reversal of phospholamban inhibition of the cardiac Ca2+-ATPase by protein kinase A and by anti-phospholamban monoclonal antibody 2D12. J. Biol. Chem. 282:20968–20976. 10.1074/jbc.M703516200 [DOI] [PubMed] [Google Scholar]
- del Monte F., Harding S.E., Dec G.W., Gwathmey J.K., and Hajjar R.J.. 2002. Targeting phospholamban by gene transfer in human heart failure. Circulation. 105:904–907. 10.1161/hc0802.105564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., and Fabiato F.. 1977. Calcium release from the sarcoplasmic reticulum. Circ. Res. 40:119–129. 10.1161/01.RES.40.2.119 [DOI] [PubMed] [Google Scholar]
- Froehlich J.P., Mahaney J.E., Keceli G., Pavlos C.M., Goldstein R., Redwood A.J., Sumbilla C., Lee D.I., Tocchetti C.G., Kass D.A., et al. . 2008. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 47:13150–13152. 10.1021/bi801925p [DOI] [PubMed] [Google Scholar]
- Gao W.D., Murray C.I., Tian Y., Zhong X., DuMond J.F., Shen X., Stanley B.A., Foster D.B., Wink D.A., King S.B., et al. . 2012. Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ. Res. 111:1002–1011. 10.1161/CIRCRESAHA.112.270827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaves J.P., Trieber C.A., Ceholski D.K., Stokes D.L., and Young H.S.. 2011. Phosphorylation and mutation of phospholamban alter physical interactions with the sarcoplasmic reticulum calcium pump. J. Mol. Biol. 405:707–723. 10.1016/j.jmb.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson M., Verardi R., Mullen D.G., Mote K.R., Traaseth N.J., Gopinath T., and Veglia G.. 2013. Allosteric regulation of SERCA by phosphorylation-mediated conformational shift of phospholamban. Proc. Natl. Acad. Sci. USA. 110:17338–17343. 10.1073/pnas.1303006110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey J.K., Copelas L., MacKinnon R., Schoen F.J.S., Feldman M.D., Grossman W., and Morgan J.P.. 1987. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ. Res. 61:70–76. 10.1161/01.RES.61.1.70 [DOI] [PubMed] [Google Scholar]
- Ha K.N., Masterson L.R., Hou Z., Verardi R., Walsh N., Veglia G., and Robia S.L.. 2011. Lethal Arg9Cys phospholamban mutation hinders Ca2+-ATPase regulation and phosphorylation by protein kinase A. Proc. Natl. Acad. Sci. USA. 108:2735–2740. 10.1073/pnas.1013987108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K., Bidwell P., and Kranias E.G.. 2014. Phospholamban interactome in cardiac contractility and survival: A new vision of an old friend. J. Mol. Cell. Cardiol. 77:160–167. 10.1016/j.yjmcc.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfuss G., and Teerlink J.R.. 2011. Cardiac inotropes: current agents and future directions. Eur. Heart J. 32:1838–1845. 10.1093/eurheartj/ehr026 [DOI] [PubMed] [Google Scholar]
- Hulot J.S., Ishikawa K., and Hajjar R.J.. 2016. Gene therapy for the treatment of heart failure: promise postponed. Eur. Heart J. 37:1651–1658. 10.1093/eurheartj/ehw019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulot J.S., Salem J.E., Redheuil A., Collet J.P., Varnous S., Jourdain P., Logeart D., Gandjbakhch E., Bernard C., Hatem S.N., et al. AGENT-HF Investigators . 2017. Effect of intracoronary administration of AAV1/SERCA2a on ventricular remodelling in patients with advanced systolic heart failure: results from the AGENT-HF randomized phase 2 trial. Eur. J. Heart Fail. 19:1534–1541. 10.1002/ejhf.826 [DOI] [PubMed] [Google Scholar]
- Irie T., Sips P.Y., Kai S., Kida K., Ikeda K., Hirai S., Moazzami K., Jiramongkolchai P., Bloch D.B., Doulias P.T., et al. . 2015. S-Nitrosylation of Calcium-Handling Proteins in Cardiac Adrenergic Signaling and Hypertrophy. Circ. Res. 117:793–803. 10.1161/CIRCRESAHA.115.307157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A.O., and Jones L.R.. 1987. Immunoelectron microscopical localization of phospholamban in adult canine ventricular muscle. J. Cell Biol. 104:1343–1352. 10.1083/jcb.104.5.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keceli G., Majumdar A., Thorpe C.N., Jun S., Tocchetti C.G., Lee D.I., Mahaney J.E., Paolocci N., and Toscano J.P.. 2019. Nitroxyl (HNO) targets phospholamban cysteines 41 and 46 to enhance cardiac function. J. Gen. Physiol. 10.1085/jgp.201812208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Kurzydlowski K., Tada M., and MacLennan D.H.. 1996. Phospholamban regulates the Ca2+-ATPase through intramembrane interactions. J. Biol. Chem. 271:21726–21731. 10.1074/jbc.271.36.21726 [DOI] [PubMed] [Google Scholar]
- Kohr M.J., Kaludercic N., Tocchetti C.G., Dong Gao W., Kass D.A., Janssen P.M., Paolocci N., and Ziolo M.T.. 2010. Nitroxyl enhances myocyte Ca2+ transients by exclusively targeting SR Ca2+-cycling. Front. Biosci. (Elite Ed.). 2:614–626 (Elite edition). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancel S., Zhang J., Evangelista A., Trucillo M.P., Tong X., Siwik D.A., Cohen R.A., and Colucci W.S.. 2009. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ. Res. 104:720–723. 10.1161/CIRCRESAHA.108.188441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurita K.R., and Rosenbaum D.S.. 2008. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J. Mol. Cell. Cardiol. 44:31–43. 10.1016/j.yjmcc.2007.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann J.P., and Watanabe A.M.. 1985. Phosphorylation of phospholamban in intact myocardium. Role of Ca2+-calmodulin-dependent mechanisms. J. Biol. Chem. 260:4516–4525. [PubMed] [Google Scholar]
- Lindemann J.P., Jones L.R., Hathaway D.R., Henry B.G., and Watanabe A.M.. 1983. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J. Biol. Chem. 258:464–471. [PubMed] [Google Scholar]
- MacLennan D.H., and Kranias E.G.. 2003. Phospholamban: a crucial regulator of cardiac contractility. Nat. Rev. Mol. Cell Biol. 4:566–577. 10.1038/nrm1151 [DOI] [PubMed] [Google Scholar]
- Mattiazzi A., and Kranias E.G.. 2014. The role of CaMKII regulation of phospholamban activity in heart disease. Front. Pharmacol. 5:5 10.3389/fphar.2014.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Schillinger W., Pieske B., Holubarsch C., Heilmann C., Posival H., Kuwajima G., Mikoshiba K., Just H., Hasenfuss G., et al. . 1995. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 92:778–784. 10.1161/01.CIR.92.4.778 [DOI] [PubMed] [Google Scholar]
- Motloch L.J., Cacheux M., Ishikawa K., Xie C., Hu J., Aguero J., Fish K.M., Hajjar R.J., and Akar F.G.. 2018. Primary Effect of SERCA 2a Gene Transfer on Conduction Reserve in Chronic Myocardial Infarction. J. Am. Heart Assoc. 7:e009598 10.1161/JAHA.118.009598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundiña-Weilenmann C., Vittone L., Ortale M., de Cingolani G.C., and Mattiazzi A.. 1996. Immunodetection of phosphorylation sites gives new insights into the mechanisms underlying phospholamban phosphorylation in the intact heart. J. Biol. Chem. 271:33561–33567. 10.1074/jbc.271.52.33561 [DOI] [PubMed] [Google Scholar]
- Paolocci N., Saavedra W.F., Miranda K.M., Martignani C., Isoda T., Hare J.M., Espey M.G., Fukuto J.M., Feelisch M., Wink D.A., and Kass D.A.. 2001. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc. Natl. Acad. Sci. USA. 98:10463–10468. 10.1073/pnas.181191198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolocci N., Katori T., Champion H.C., St John M.E., Miranda K.M., Fukuto J.M., Wink D.A., and Kass D.A.. 2003. Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: independence from beta-adrenergic signaling. Proc. Natl. Acad. Sci. USA. 100:5537–5542. 10.1073/pnas.0937302100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer M.A., Claggett B., Assmann S.F., Boineau R., Anand I.S., Clausell N., Desai A.S., Diaz R., Fleg J.L., Gordeev I., et al. . 2015. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation. 131:34–42. 10.1161/CIRCULATIONAHA.114.013255 [DOI] [PubMed] [Google Scholar]
- Pogwizd S.M., and Bers D.M.. 2004. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc. Med. 14:61–66. 10.1016/j.tcm.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Simmerman H.K., and Jones L.R.. 1998. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol. Rev. 78:921–947. 10.1152/physrev.1998.78.4.921 [DOI] [PubMed] [Google Scholar]
- Sivakumaran V., Stanley B.A., Tocchetti C.G., Ballin J.D., Caceres V., Zhou L., Keceli G., Rainer P.P., Lee D.I., Huke S., et al. . 2013. HNO enhances SERCA2a activity and cardiomyocyte function by promoting redox-dependent phospholamban oligomerization. Antioxid. Redox Signal. 19:1185–1197. 10.1089/ars.2012.5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D.L., Pomfret A.J., Rice W.J., Glaves J.P., and Young H.S.. 2006. Interactions between Ca2+-ATPase and the pentameric form of phospholamban in two-dimensional co-crystals. Biophys. J. 90:4213–4223. 10.1529/biophysj.105.079640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Kirchberger M.A., and Katz A.M.. 1975. Phosphorylation of a 22,000-dalton component of the cardiac sarcoplasmic reticulum by adenosine 3′:5′-monophosphate-dependent protein kinase. J. Biol. Chem. 250:2640–2647. [PubMed] [Google Scholar]
- Tocchetti C.G., Wang W., Froehlich J.P., Huke S., Aon M.A., Wilson G.M., Di Benedetto G., O’Rourke B., Gao W.D., Wink D.A., et al. . 2007. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ. Res. 100:96–104. 10.1161/01.RES.0000253904.53601.c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener A.D., Simmerman H.K., Lindemann J.P., and Jones L.R.. 1989. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J. Biol. Chem. 264:11468–11474. [PubMed] [Google Scholar]
- Zima A.V., Bovo E., Mazurek S.R., Rochira J.A., Li W., and Terentyev D.. 2014. Ca handling during excitation-contraction coupling in heart failure. Pflugers Arch. 466:1129–1137. 10.1007/s00424-014-1469-3 [DOI] [PMC free article] [PubMed] [Google Scholar]