Abstract
In this study, we synthetized a series of 5-aryl-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine derivatives containing tetrazole and other heterocycle substituents, i.e., triazole, methyltriazole, and triazolone. The forced swim test (FST) and tail suspension test (TST) were used to evaluate the antidepressant activity of the target compounds. The compound 5-[4-(trifluoromethyl)phenyl]-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4i) showed the highest antidepressant activity, with a reduced immobility time of 55.33% when compared with the control group. Using an open-field test, compound 4i was shown to not affect spontaneous activity of mice. The determination of in vivo 5-hydroxytryptamine (5-HT) concentration showed that compound 4i may have an effect in the mouse brain. The biological activities of all synthetized compounds were verified by molecular docking studies. Compound 4i showed significant interactions with residues of the 5-HT1A receptor homology model.
Keywords: synthesis, antidepressant, FST, 5-HT, molecular docking studies
1. Introduction
The structure of the thiophene moiety has been gaining much attention owing to its significant influence on biological activity, showing anti-inflammatory [1], antibacterial [2], anti-anxiety [3], antitumor [4,5], and antidepressant effects [6]. It is therefore essential to further investigate derivatives with a thiophene structure.
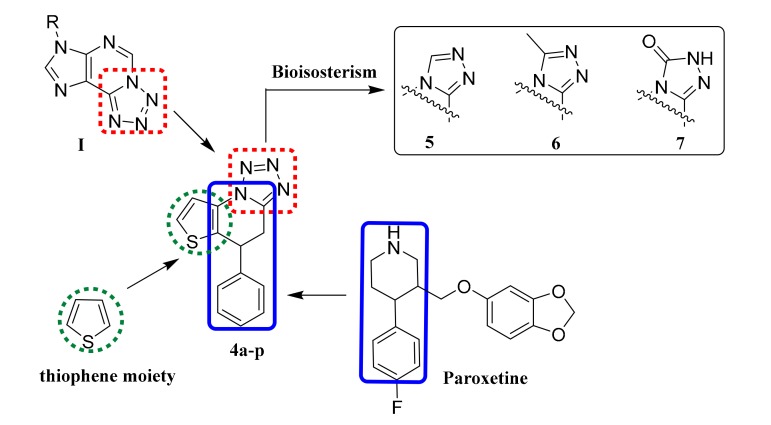
It is well-known that in compounds with a tetrazole fragment, this fragment has a great influence on the biological activity of the compound, showing, for instance, antifungal [7], antibacterial [8], antileishmanial [9], anticancer [10,11], anticonvulsant [12,13], and antidepressant effects [14]. Our group has studied a series of 7-alkyl-7H-tetrazolo [1,5-g]purine derivatives (I) (Figure 1), where most of the target compounds showed a significant antidepressant activity [15]. Paroxetine is primarily used for the treatment of depression; the 4-arylpiperidine fragment of this drug has a great influence on its antidepressant activity. In this study, we designed and synthesized a series of 5-aryl-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4a–p) derivatives by combining biologically active structural fragments such as tetrazole, thiophene ring, and 4-phenylpiperidine. To investigate the effect of the tetrazole fragment on antidepressant activity, the tetrazole ring was replaced, following the bioisosteric principle, by other heterocycles such as triazole, methyltriazole, and triazolone to obtain the target compounds 5, 6, and 7, respectively.
Figure 1.
Structures of compound I, Paroxetine, and target compounds 4a–p, 5, 6 and 7.
The forced swim test (FST) was used to evaluate the antidepressant activity of the target compounds. Moreover, the most active compound was further tested using a tail suspension test (TST). The concentration of 5-hydroxytyramine (5-HT) was determined in mouse brain to explore the possible mechanism of action of compound 4i. A homology model of the 5-HT1A receptor was built using Discovery Studio software and used to perform a molecular docking study. The synthesized target compounds were characterized using infrared spectroscopy, high resolution mass spectrometry, and 1H-nuclear/13C-nuclear magnetic resonance spectroscopy techniques.
2. Results and Discussion
2.1. Chemistry
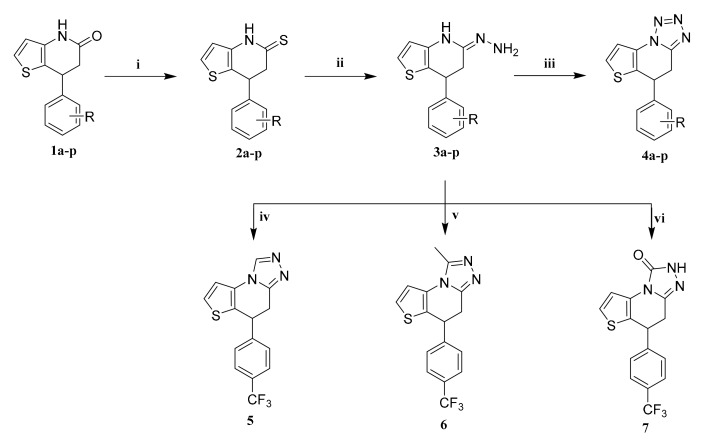
The synthesis of the target compounds is outlined in Scheme 1. Compound 1a–p [16] (differently substituted on the benzene ring using electron withdrawing such as Cl, F, CF3, Br, 2-Cl-6-F or electron donating such as OCH3, 3,4,5-OCH3, as well as unsubstituted on the benzene ring) was mixed with P2S5 in acetonitrile in the presence of triethylamine to obtain the intermediate 2a–p. Compound 3a–p can be easily obtained with a high yield under reflux conditions after the reaction of compound 2a–p and hydrazine hydrate with ethanol as a solvent, where ethanol can also be replaced by methanol or tetrahydrofuran [17]. The target compounds, 7-phenyl-4,5,6,7-tetrahydrothieno[3,2-b]pyridine derivatives containing tetrazole, triazole, methyltriazole, and triazolone (compounds 4a–p, 5, 6, and 7, respectively), were synthesized by mixing compound 3a–p with NaNO2 (i.e., 5% HCl solution cooled in ice water, withNaNO2 slowly added to prevent the increase of by-products caused by excessive drip acceleration), HC(OC2H5)3 (that acts as both a solvent and a reactant at 100 °C), CH3COOH (that acts as both a solvent and a reactant, and a high yield can be obtained by refluxing), and NH2CONH2 (the temperature must be controlled at around 170 °C), respectively [18]. The structures of the target compounds were characterized using spectral methods (see Supplementary Materials).
Scheme 1.
Synthesis of the target compounds. Reagents and conditions: (i) P2S5, CH3CN, 4–7 h; (ii) NH2NH2.H2O, CH3OH, reflux, 1 h; (iii) NaNO2, 5% HCl, 0–5 °C, 5 h; (iv) HC(OC2H5)3, 100 °C, 6 h; (v) CH3COOH, reflux, 2 h; (vi) NH2CONH2, 170 °C, 4 h.
2.2. Pharmacology
2.2.1. Forced Swim Test (FST)
The FST is the most direct and effective method for screening antidepressants. A variety of antidepressant drugs have been developed using this method to evaluate their efficacy. In this study, we used FST as a preliminary antidepressant activity test for all target compounds. Fluoxetine, a widely used selective 5-HT reuptake inhibitor (SSRI), selectively inhibits 5-HT transporters and blocks pre-synaptic membrane reuptake of 5-HT. This extends and increases the effects of 5-HT, resulting in antidepressant effects. In these experiments, we used fluoxetine as a positive control, as already reported in many studies [19]. Results obtained for FST and antidepressant activity are shown in Table 1.
Table 1.
Antidepressant activities of compounds 4a–p, 5, 6, and 7 in forced swim test (FST).
| Compound | R | Antidepressant Activities a | |
|---|---|---|---|
| Duration of Immobility(s) | Change from Control | ||
| (mean ± S.E.M.) b | (%) | ||
| 4a | o-Cl | 87.21 ± 16.95 * | 24.25 |
| 4b | m-Cl | 99.02 ± 13.12 | 13.99 |
| 4c | p-Cl | 65.15 ± 12.73 ** | 43.41 |
| 4d | o-F | 85.22 ± 14.20 * | 25.98 |
| 4e | m-F | 79.72 ± 19.41 * | 30.76 |
| 4f | p-F | 53.61 ± 15.50 ** | 53.44 |
| 4g | o-CF3 | 68.43 ± 11.87 ** | 40.56 |
| 4h | m-CF3 | 73.63 ± 17.58 ** | 36.05 |
| 4i | p-CF3 | 51.43 ± 13.76 ** | 55.33 |
| 4j | o-OCH3 | 95.26 ± 18.83 | 17.26 |
| 4k | m-OCH3 | 86.54 ± 16.72 * | 24.83 |
| 4l | p-OCH3 | 66.32 ± 15.61 ** | 42.40 |
| 4m | p-Br | 83.53 ± 14.23 * | 27.45 |
| 4n | -H | 78.13 ± 20.91 * | 32.14 |
| 4o | 2-Cl-6-F | 98.28 ± 16.32 | 14.64 |
| 4p | 3,4,5-OCH3 | 102.41 ± 19.18 | 11.05 |
| 5 | p-CF3 | 89.38 ± 12.39 * | 22.37 |
| 6 | p-CF3 | 93.63 ± 16.81 | 18.70 |
| 7 | p-CF3 | 82.58 ± 15.15 * | 28.27 |
| Fluoxetine | - | 54.21 ± 11.97 ** | 52.91 |
| Control | - | 115.13 ± 18.67 | - |
a Target compounds and fluoxetine were administered at 40 mg/kg. b Values represent the mean ± S.E.M. (n = 8). * significantly different from control (0.01 < p < 0.05). ** very significantly different from control (p < 0.01).
As illustrated in Table 1, most of the compounds in the 4a–p series showed a better antidepressant activity (40 mg/kg, intraperitoneal injection) when compared with the control group. Among those, compounds 4a, 4d–e, 4k, and 4m–n showed a significant difference when compared with the control (0.01 < p < 0.05). Furthermore, the six compounds 4c, 4f–i, and 4l showed a substantial significant difference when compared with the control group (p < 0.01). Overall, compound 4i demonstrated the best antidepressant activity and compound 4i led to a mice immobility time of 51.43 s, which was similar to that of fluoxetine, i.e., 54.21 s. In a second step, the most active compound 4i was structurally modified by replacing the biologically active tetrazole fragment with other azole moieties (i.e., triazole, methyltriazole, and triazolone), to potentially synthetize a more active compound. The antidepressant activity of compounds 5, 6 and 7 was found to be lower than that of compound 4i. The structure–activity relationship was obtained based on the pharmacological results shown in Table 1. Compounds containing electron-withdrawing groups (i.e., Cl, F, and CF3) showed an antidepressant activity order of p-Cl > m-Cl > o-Cl, p-F > m-F > o-F, p-CF3 > o-CF3 > m-CF3. This indicates that a compound having an electron-withdrawing group substituted at its p-position shows a better activity than a substitution in the o and m-positions. On the contrary, in the presence of the electron-donating group OCH3, the activity order was p-OCH3 > m-OCH3 > o-OCH3, while substitution in the p-position also led to the highest compound activity. The most active compound 4i was found to be more active than compounds 5, 6, and 7, with an activity order of 4i > 7 > 5 > 6.
Compound 4i and fluoxetine were further tested to evaluate their antidepressant activity at different doses, i.e., 10, 20, and 40 mg/kg. Table 2 shows that compound 4i and fluoxetine have different antidepressant activities at different doses, with the best antidepressant activity observed with a dose of 40 mg/kg. Therefore, the antidepressant activity of compound 4i appeared to be dose-dependent, with a gradually increased antidepressant activity observed when increasing the dose.
Table 2.
Antidepressant activities of compound 4i and fluoxetine in FST at different doses.
| Compound | Dose (mg/kg) | Antidepressant Activities a | |
|---|---|---|---|
| Duration of Immobility(s) | Change from Control | ||
| (mean ± S.E.M.) b | (%) | ||
| 10 | 81.27 ± 16.13 * | 27.69 | |
| 4i | 20 | 67.41 ± 14.91 ** | 40.02 |
| 40 | 53.39 ± 12.85 ** | 52.53 | |
| 10 | 82.41 ± 15.72 ** | 27.56 | |
| Fluoxetine | 20 | 69.18 ± 14.56 ** | 38.44 |
| 40 | 55.02 ± 10.17 ** | 51.04 | |
| Control | - | 112.39 ± 12.14 | - |
a Compound 4i and fluoxetine were administered at 40, 20, 10 mg/kg, respectively. b Values represent the mean ± S.E.M. (n = 8). * significantly different from control (0.01 < p < 0.05). ** very significantly different from control (p < 0.01).
2.2.2. Tail Suspension Test (TST)
The most active compound 4i was selected for TST (Table 3) to further confirm the antidepressant activity measured in FST. Fluoxetine was also used as positive control. TST experiments were carried out with a dose of 40 mg/kg for compound 4i and fluoxetine. As illustrated in Table 3, compound 4i also demonstrated a significant antidepressant activity during TST. The immobility time of mice was 77.18 s, a substantially significant difference relative to the control group (p < 0.01).
Table 3.
Antidepressant activities of compound 4i and fluoxetine in the TST test.
| Compound | Dose (mg/kg) | Antidepressant Activities a | |
|---|---|---|---|
| Duration of Immobility (s) | Change from Control | ||
| (mean ± S.E.M.) b | (%) | ||
| 4i | 40 | 72.33 ± 14.61 ** | 43.73 |
| Fluoxetine | 40 | 77.18 ± 16.52 ** | 39.95 |
| Control | - | 128.54 ± 25.37 | - |
a Compound 4i and fluoxetine were administered at 40 mg/kg. b Values represent the mean±S.E.M. (n = 8). ** very significantly different from control (p < 0.01).
2.2.3. Open-Field Test
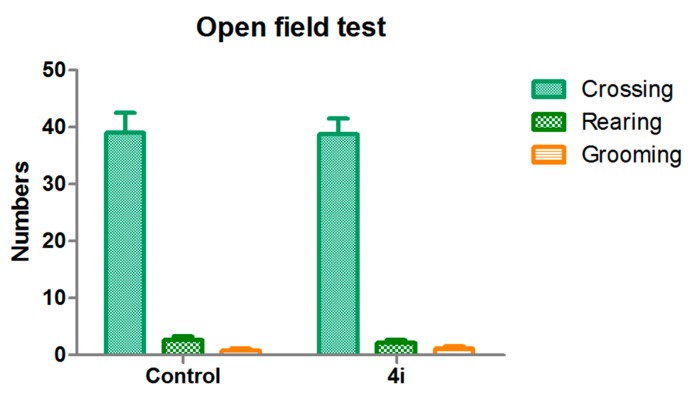
The open-field test was used to determine whether compound 4i affects the spontaneous locomotor activity of mice (Figure 2). This test is a classical animal experimental model to assess the autonomic effects of drugs and the general animal activities. Since the reduced immobility time in behavioral despair and depression animal models may be caused by excitation of sympathetic nerves by the drug, the open-field experiment was used to evaluate the central excitability of 4i [20,21]. Compared with the control group, no significant difference was observed for the compound 4i (p > 0.05, motor activity: crossing, rearing, and grooming). These findings thus exclude any false positive results attributed to central activity excitability.
Figure 2.
Exploratory activity (counts) in the open-field test. The behavioral parameters were recorded for 3 min. Locomotion: number of line crossings; rearing: number of times seen standing on hind legs; grooming: number of modifications; 4i (40 mg/kg) was administered 60 min before the test. The values represent the mean ±SEM (n = 8).
2.2.4. Determination of 5-HT Concentration
Nowadays, monoamine neurotransmitters such as 5-HT are recognized to play a role in the neurobiochemical mechanisms of depression. The brain is scattered with monoamine neurotransmitter pathways that primarily control physiological activities. Changes in neurotransmitters levels affect the monoamine-based transmitter pathways, resulting in a variety of clinical depressive symptoms. The results of a pathological autopsy of depression showed a decrease in 5-HT levels in the brainstem and frontal lobe, as well as a decrease in the total amount of 5-HT receptors in the hippocampus [22]. In the present study, the effect of compound 4i on the concentration of 5-HT in mouse brain tissues was determined using enzyme-linked immunosorbent assay (ELISA). The results showed that the concentration of 5-HT in brain tissue in the group treated with compound 4i and the fluoxetine group (40 mg/kg) was significantly higher than that of the control group (Table 4).
Table 4.
Effect of compound 4i and fluoxetine on brain 5-HT level in mice.
| Compound | Dose (mg/kg) | 5-HT (ng/mg) a |
|---|---|---|
| 4i | 40 | 1.276 ± 0.094 *,b |
| Fluoxetine | 40 | 1.328 ± 0.105 * |
| Control | - | 1.015 ± 0.098 |
a Compound 4i and fluoxetine were analyzed (the concentration of 5-HT in the mice brain) 1 h after oral administration. b Represent the mean ± SEM. (n = 8). * significantly different from control (0.01 < p < 0.05).
2.2.5. Docking Study
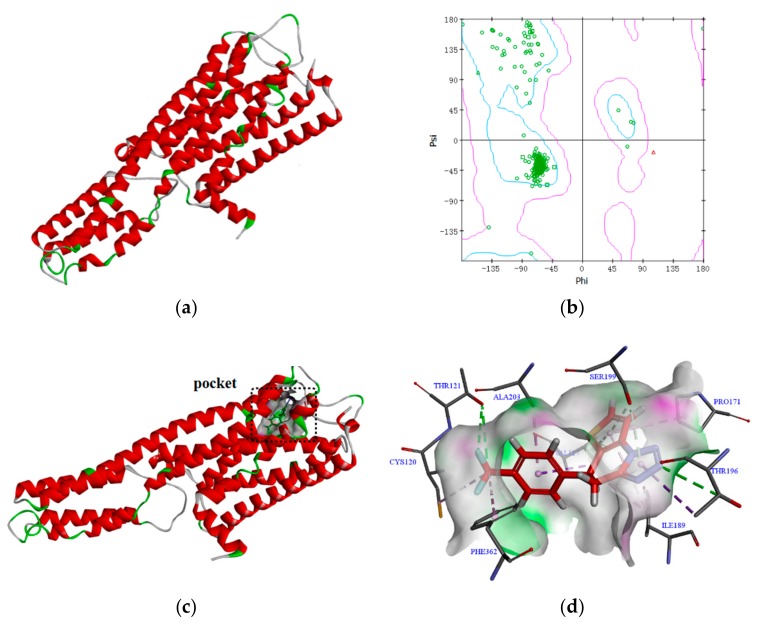
Molecular docking is an important means to explore the possible mechanisms of biologically active compounds. The 5-HT1A receptor plays a role in the pathogenesis of various mental and neurological diseases. Activation of postsynaptic 5-HT1A receptors is important for an adequate response to antidepressants [23,24]. Here, we used molecular docking to investigate the interaction between compound 4i and different residues of the 5-HT1A receptor homology model. The docking results are shown in Figure 3.
Figure 3.
(a) 3D model; (b) Ramachandran plot; (c) Binding pocket of compound 4i to 5-HT1A receptor; (d) Compound 4i showing interactions with residues of the 5-HT1A receptor.
According to existing literature [25], the main amino acid residues present at the active site of the 5-HT1A receptor homology model are Ala93, Ala263, Ala365, Ala383, Asn386, Asp116, Cys120, Thr379, Gln97, Gly382, Ile113, Ile124, Ile167, Phe112, Phe361, Phe362, Ser199, Thr196, Thr121, Thr200, and Val117. Molecular docking showed that compound 4i interacts with amino acid residues present at the 5-HT1A receptor active site, for instance via hydrogen bond interaction between the N atom in the triazole fragment and the Thr196 residue. Furthermore, at the site of the active pocket of the 5-HT1A receptor, compound 4i can form a hydrophobic and hydrogen bond interaction with the amino acid residues surrounding this active pocket. Therefore, the mode of action of compound 4i in exerting antidepressant activity may be closely related to its interaction with the 5-HT1A receptor.
3. Experimental Section
3.1. Chemistry
An X-4 binocular microscope melting point apparatus (Ningbo Biocotek scientific equipment Co., Ltd, Ningbo, China) was used to determine the melting points (Mp). All NMR (1H and 13C NMR) experiments were performed using an AV-300 spectrometer (Bruker Bioscience, Billerica, MA, USA), with CDCl3 or DMSO-d6 as solvent, as well as tetramethylsilane as internal standard. Infrared (IR) spectra of the target compounds were obtained with an IR Prestige-21 instrument (in KBr, Shimadzu, Tokyo, Japan). High resolution mass spectra (HRMS) were obtained with mass spectrometry (Bruker Daltonik GmbH, Bremen, Germany). Most chemicals used for the synthesis were commercial products without further purification. The synthesis of starting material 1a–p was based on the synthesis method of Zhang et al. [16].
3.1.1. Synthesis of 7-substitutedphenyl-6,7-dihydrothieno[3,2-b]pyridine-5(4H)-thiones (2a–p)
A hundred milliliters of a solvent mixture composed of acetonitrile/triethylamine (3:1) was added to a 250 mL round bottom flask, and P2S5 (7.5 mmol) was added in small portions in an ice bath. The intermediate compound 1a–p (5.0 mmol) was then added to the reaction solution followed by reflux for 4–7 h. After completion of the reaction, the solvent was evaporated under reduced pressure. Dichloromethane (50 mL) was then added to the solid residue and washed three times with water. The dichloromethane layer was subsequently dried over anhydrous magnesium sulfate, filtered and evaporated, producing the white crude compound 2a–p.
3.1.2. Synthesis of 5-hydrazono-7-substitutedphenyl-4,5,6,7-tetrahydrothieno[3,2-b]pyridines (3a–p)
Compound 2a–p (3.0 mmol) and hydrazine hydrate (6.0 mmol) were added to a round bottom flask containing 20 mL of methanol. The reaction took place at 70 °C for 1 h. After the reaction was completed, most of the methanol was removed under reduced pressure and cooled. Next, a small amount of cold methanol was used for filtration and rinsing, leading to a pale yellow or white solid.
3.1.3. Synthesis of 5-aryl-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4a–p)
Selected intermediates from compound 3a–p (3.0 mmol) were added to a round bottom flask containing 10 mL of 5% HCl, and the mixture was cooled in ice water. An aqueous solution of NaNO2 was then slowly added. The reaction was monitored by thin liquid chromatography (TLC). After completion of the reaction, the crude product was obtained by suction filtration under reduced pressure. Purification was performed using column chromatography (methanol/dichloromethane = 1/80) to give a white solid.
5-(2-Chlorophenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4a): Yield 68%. Mp: 159–160 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.59–3.82 (m, 2H, CH2), 5.18 (t, 1H, J = 7.4 Hz, CH), 6.89–7.28 (m, 4H, Ar-H), 7.45 (d, 1H, J = 5.3 Hz, S-C=C-H), 7.81 (d, 1H, J = 5.3 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 27.5, 35.9, 117.5, 127.2, 127.7, 128.2, 128.6, 129.5, 130.3, 131.3, 133.0, 137.6, 149.5. IR (KBr, cm−1): 1479 (C=N). ESI-HRMS calcd for C13H10ClN4S+ ([M + H]+): 289.0309; found: 289.0302.
5-(3-Chlorophenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4b): Yield 77%. Mp: 169–170 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.47–3.82 (m, 2H, CH2), 4.61 (t, 1H, J = 8.4 Hz, CH), 7.10–7.32 (m, 4H, Ar-H), 7.43 (d, 1H, J = 5.3 Hz, S-C=C-H), 7.58 (d, 1H, J = 5.3 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.0, 39.4, 117.6, 125.6, 127.2, 127.6, 128.7, 129.5, 130.6, 130.7, 135.1, 142.3, 149.6. IR (KBr, cm−1): 1479 (C=N). ESI-HRMS calcd for C13H10ClN4S+ ([M + H]+): 289.0309; found:289.0305.
5-(4-Chlorophenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4c): Yield 72%. Mp: 108–110 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.44–3.80 (m, 2H, CH2), 4.61 (t, 1H, J = 8.5 Hz, CH), 7.16 (d, 2H, J = 4.1 Hz, Ar-H), 7.35 (d, 2H, J = 4.2 Hz, Ar-H), 7.41 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.56 (d, 1H, J = 5.4 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.1, 39.2, 117.6, 127.1, 128.8, 128.8, 129.5, 129.5, 129.9, 130.7, 134.3, 138.8, 149.7. IR (KBr, cm−1): 1479 (C=N). ESI-HRMS calcd for C13H10ClN4S+ ([M + H]+): 289.0309; found: 289.0303.
5-(2-Flulorophenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4d): Yield 62%. Mp: 122–123 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.60–3.81 (m, 2H, CH2), 4.97 (t, 1H, J = 7.7 Hz, CH), 6.92–7.36 (m, 4H, Ar-H), 7.43 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.59 (d, 1H, J = 5.4 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 27.6, 32.8, 116.3, 117.6, 124.9, 126.9, 127.3, 128.4, 128.7, 130.2, 130.9, 149.7, 158.4, 161.7. IR (KBr, cm−1): 1485 (C=N). ESI-HRMS calcd for C13H10FN4S+ ([M + H]+): 273.0605; found: 273.0609.
5-(3-Flulorophenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4e): Yield 61%. Mp: 124–125 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.49–3.83 (m, 2H, CH2), 4.63 (t, 1H, J = 8.3 Hz, CH), 6.91–7.39 (m, 4H, Ar-H), 7.43 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.59 (d, 1H, J = 5.4 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.0, 39.4, 114.6, 115.6, 117.6, 123.1, 127.1, 129.6, 130.7, 131.0, 142.7, 149.7, 161.4, 164.7. IR (KBr, cm−1): 1485 (C=N). ESI-HRMS calcd for C13H10FN4S+ ([M + H]+): 273.0605; found: 273.0611.
5-(4-Flulorophenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4f): Yield 55%. Mp: 123–124 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.44–3.80 (m, 2H, CH2), 4.62 (t, 1H, J = 8.5 Hz, CH), 7.03–7.27 (m, 4H, Ar-H), 7.41 (d, 1H, J = 5.3 Hz, S-C=C-H), 7.56 (d, 1H, J = 5.3 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.3, 39.1, 116.1, 116.4, 117.6, 126.9, 129.0, 129.1, 130.4, 130.6, 136.0, 149.8, 160.8, 164.1. IR (KBr, cm−1): 1485 (C=N). ESI-HRMS calcd for C13H10FN4S+ ([M + H]+): 273.0605; found: 273.0601.
5-[2-(Trifluoromethyl)phenyl]-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4g): Yield 51%. Mp: 201–202 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.44–3.90 (m, 2H, CH2), 5.08 (t, 1H, J = 8.4 Hz, CH), 7.24–7.78 (m, 4H, Ar-H), 7.44 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.61 (d, 1H, J = 5.4 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.3, 35.3, 117.5, 125.9, 126.4, 127.4, 128.0, 128.4, 129.2, 129.7, 131.2, 132.9, 139.6, 149.4. IR (KBr, cm−1): 1485 (C=N). ESI-HRMS calcd for C14H10F3N4S+ ([M + H]+): 323.0573; found: 323.0577.
5-[3-(Trifluoromethyl)phenyl]-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4h): Yield 53%. Mp: 141–142 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.49–3.87 (m, 2H, CH2), 4.72 (t, 1H, J = 8.5 Hz, CH), 7.40–7.64 (m, 4H, Ar-H), 7.44 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.59 (d, 1H, J = 5.4 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.1, 39.6, 117.7, 121.9, 124.4, 125.4, 127.3, 129.3, 129.9, 130.8, 130.9, 131.9, 141.3, 149.6. IR (KBr, cm−1): 1485 (C=N). ESI-HRMS calcd for C14H10F3N4S+ ([M + H]+): 323.0573; found: 323.0579.
5-[4-(Trifluoromethyl)phenyl]-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4i): Yield 48%. Mp: 155–156 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.50–3.86 (m, 2H, CH2), 4.72 (t, 1H, J = 8.3 Hz, CH), 7.36 (d, 2H, J = 7.9 Hz, Ar-H), 7.45 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.60 (d, 1H, J = 5.4 Hz, S-C-H), 7.66 (d, 2H, J = 8.0 Hz, Ar-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.0, 39.5, 117.7, 126.4, 127.3, 127.3, 127.8, 127.8, 127.8, 127.8, 129.2, 130.9, 144.3, 149.5. IR (KBr, cm−1): 1485 (C=N). ESI-HRMS calcd for C14H10F3N4S+ ([M + H]+): 323.0573; found: 323.0577.
5-(2-Methoxyphenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4j): Yield 42%. Mp: 139–141 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.67 (d, 2H, J = 7.2 Hz, CH2), 3.82 (s, 3H, OCH3), 5.02 (t, 1H, J = 7.2 Hz, CH), 6.85–7.30 (m, 4H, Ar-H), 7.38 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.56 (d, 1H, J = 5.4 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 26.9, 33.5, 55.4, 110.9, 117.3, 120.9, 126.3, 127.5, 128.5, 129.3, 129.9, 130.7, 150.3, 156.4. IR (KBr, cm−1): 1510 (C=N). ESI-HRMS calcd for C14H13N4OS+ ([M + H]+): 285.0805; found: 285.0810.
5-(3-Methoxyphenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4k): Yield 66%. Mp: 104–105 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.49–3.82 (m, 2H, CH2), 3.80 (s, 3H, OCH3), 4.58 (t, 1H, J = 8.5 Hz, CH), 6.78–7.33 (m, 4H, Ar-H), 7.40 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.58 (d, 1H, J = 5.4 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.1, 39.8, 55.3, 113.3, 113.5, 115.1, 117.5, 119.5, 126.8, 130.4, 130.5, 141.8, 149.9, 160.2. IR (KBr, cm−1): 1510 (C=N). ESI-HRMS calcd for C14H13N4OS+ ([M + H]+): 285.0805; found: 285.0809.
5-(4-Methoxyphenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4l): Yield 60%. Mp: 156–157 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.43–3.79 (m, 2H, CH2), 3.81 (s, 3H, OCH3), 4.56 (t, 1H, J = 8.7 Hz, CH), 6.89 (d, 2H, J = 8.4 Hz, Ar-H), 7.18 (d, 2H, J = 8.3 Hz, Ar-H), 7.38 (d, 1H, J = 5.3 Hz, S-C=C-H), 7.56 (d, 1H, J = 5.2 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.3, 39.1, 55.3, 114.6, 114.6, 117.6, 126.7, 128.5, 128.5, 130.4, 131.4, 132.2, 150.1, 159.5. IR (KBr, cm−1): 1510 (C=N). ESI-HRMS calcd for C14H13N4OS+ ([M + H]+): 285.0805; found: 285.0811.
5-(4-Bromophenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4m): Yield 56%. Mp: 119–121 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.43-3.80 (m, 2H, CH2), 4.59 (t, 1H, J = 8.4 Hz, CH), 7.10 (d, 2H, J = 8.3 Hz, Ar-H), 7.41 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.50 (d, 2H, J = 8.2 Hz, Ar-H), 7.56 (d, 1H, J = 5.40 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.0, 39.3, 50.8, 117.6, 122.4, 127.1, 129.1, 129.1, 129.9, 130.7, 132.5, 132.5, 139.3, 149.7. IR (KBr, cm−1): 1500 (C=N). ESI-HRMS calcd for C13H10BrN4S+ ([M + H]+): 322.9804; found: 322.9809.
5-Phenyl-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4n): Yield 68%. Mp: 101–102 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.48–3.81 (m, 2H, CH2), 4.61 (t, 1H, J = 8.5 Hz, CH), 7.23–7.38 (m, 5H, Ar-H), 7.39 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.57 (d, 1H, J = 5.3 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.1, 39.8, 117.5, 126.8, 127.4, 127.4, 128.4, 129.3, 129.3, 130.5, 130.7, 140.3, 149.9. IR (KBr, cm−1): 1481 (C=N). ESI-HRMS calcd for C13H11N4S+ ([M + H]+): 255.0699; found: 255.0693.
5-(2-Chloro-6-fluorophenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4o): Yield 54%. Mp: 179–180 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.76–3.84 (m, 2H, CH2), 5.36 (t, 1H, J = 9.3 Hz, CH), 7.04–7.32 (m, 3H, Ar-H), 7.35 (d, 1H, J = 5.5 Hz, S-C=C-H), 7.54 (d, 1H, J = 5.3 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 25.4, 50.8, 115.4, 117.5, 117.5, 126.2, 126.2, 128.5, 129.9, 130.4, 130.5, 134.6, 149.9. IR (KBr, cm−1): 1485 (C=N). ESI-HRMS calcd for C13H9ClFN4S+ ([M + H]+): 307.0215; found: 307.0209.
5-(3,4,5-Trimethoxyphenyl)-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4p): Yield 62%. Mp: 200–201 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.47–3.85 (m, 2H, CH2), 3.77–3.85 (m, 9H, (OCH3)3), 4.53 (t, 1H, J = 8.6 Hz, CH), 6.45 (s, 2H, Ar-H), 7.41 (d, 1H, J = 5.4 Hz, S-C=C-H), 7.57 (d, 1H, J = 5.4 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.3, 40.2, 50.8, 56.2, 60.9, 104.4, 104.4, 117.5, 126.9, 130.6, 130.7, 135.7, 137.9, 150.0, 153.8, 153.8. IR (KBr, cm−1): 1510 (C=N). ESI-HRMS calcd for C16H17N4O3S+ ([M + H]+): 345.1016; found: 345.1021.
3.1.4. Synthesis of 5-[4-(trifluoromethyl)phenyl]-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyridine (5)
The intermediate 3i (3.0 mmol) and 10 mL of triethyl orthoformate were mixed into a 50 mL round bottom flask, and then reacted at 100 °C for 6 h. After completion of the reaction, the triethyl orthoformate was removed under reduced pressure using a vacuum pump. Thirty milliliters of water were added to obtain a solid residue, which was then filtered and dried to give a crude product. Purification was performed using column chromatography (methanol/dichloromethane = 1/100) to give a white solid.
5-[4-(Trifluoromethyl)phenyl]-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyridine (5): Yield 61%. Mp: 158–160 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.40–3.71 (m, 2H, CH2), 4.59 (t, 1H, J = 7.8 Hz, CH), 7.22–7.23 (d, 1H, S-C=C-H), 7.35–7.64 (m, 4H, Ar-H), 7.38–7.40 (d, 1H, S-C-H), 8.52 (s, 1H, Triazole-H). 13C-NMR (CDCl3, 75 MHz) δ: 29.7, 39.6, 116.8, 126.1, 126.2, 126.7, 127.9, 128.1, 128.1, 130.2, 130.2, 130.6, 137.6, 144.9, 148.9. IR (KBr, cm−1): 1502 (C=N). ESI-HRMS calcd for C15H11F3N3S+ ([M + H]+): 322.0620; found: 322.0626.
3.1.5. Synthesis of 1-methyl-5-[4-(trifluoromethyl)phenyl]-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyridine (6)
The selected intermediates from compound 3i synthesis (3.0 mmol) were added to a round bottom flask containing 15 mL of acetic acid and reacted at 140 °C for 2 h. After the reaction was completed, acetic acid was removed under reduced pressure. Thirty milliliters of water were added, filtered, and dried to give a crude product. Purification was performed using column chromatography (methanol/dichloromethane = 1/100) to give a white solid.
1-Methyl-5-[4-(trifluoromethyl)phenyl]-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyridine (6): Yield 48%. Mp: 117–119 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 2.76 (s, 3H, CH3), 3.41–3.60 (m, 2H, CH2), 4.54 (s, 1H, CH), 7.32–7.63 (m, 4H, Ar-H), 7.38 (s, 1H, S-C=C-H), 7.62 (d, 1H, J = 5.5 Hz, S-C-H). 13C-NMR (CDCl3, 75 MHz) δ: 12.5, 29.9, 39.7, 117.0, 122.1, 126.0, 126.1, 126.1, 127.9, 127.9, 127.9, 129.4, 130.2, 131.0, 144.7, 175.2. IR (KBr, cm−1): 1666 (C=N). ESI-HRMS calcd for C16H13F3N3S+ ([M + H]+): 336.0777; found: 336.0785.
3.1.6. Synthesis of 5-[4-(trifluoromethyl)phenyl]-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyridin-1(2H)-one (7)
Selected intermediates from 3i (5.0 mmol) and urea (10.0 mmol) were added into a 50 mL round bottom flask, and the mixture was heated to 170 °C for reaction for 4 h. After the reaction was completed, 30 mL of water was added, suction filtered, and dried to give a crude product. Purification was performed using column chromatography (methanol/dichloromethane = 1/60) to give a white solid. The yield, melting point and nuclear magnetic data of the target compounds are shown below.
5-[4-(Trifluoromethyl)phenyl]-4,5-dihydrothieno[2,3-e][1,2,4]triazolo[4,3-a]pyridin-1(2H)-one (7): Yield 62%. Mp: 229–231 °C. 1H-NMR (CDCl3, 300 MHz, ppm) δ: 3.19–3.35 (m, 2H, CH2), 4.55 (t, 1H, J = 7.6 Hz, CH), 7.29–7.64 (m, 4H, Ar-H), 7.29–7.81 (m, 2H, S-C=C-H and S-C-H), 9.38 (s, 1H, CONH). 13C-NMR (CDCl3, 75 MHz) δ: 29.9, 38.1, 117.4, 124.6, 125.5, 126.3, 126.5, 128.7, 128.7, 128.7, 128.7, 130.8, 142.5, 147.2, 151.5. IR (KBr, cm−1): 1707 (C=O). ESI-HRMS calcd for C15H11F3N3OS+ ([M + H]+): 338.0569; found: 338.0573.
3.2. Pharmacology
All target compounds were evaluated in vivo in Kunming mice (18–22 g). Mice had free access to food and water before the experiments. Target compounds were dissolved in dimethyl sulfoxide or 0.5% methylcellulose. Fluoxetine was used as positive control. Two experimental models, FST and TST, were used to estimate the antidepressant activity of target compounds. The 5-HT enzyme-linked immunosorbent assay (ELISA) kits were used to determine the concentrations of 5-HT in mouse tissues.
3.2.1. Evaluation of Antidepressant Activity
As reported in the literature, antidepressant activities of the target compounds were determined using FST [26,27]. Based on the preliminary FST screening results, the potent active compound 4i was selected to further evaluate its antidepressant activity using TST [28,29]. Next, the highly active compound 4i was assessed for its activity on animals using an open-field test [30]. 5-HT concentration was measured using the ELISA method to determine whether compound 4i showed an effect in the mouse brain.
3.2.2. Molecular Docking Studies
The sequence of the 5-HT1A receptor was retrieved from Research Collaboratory for Structural Bioinformatics (RCSB) in Protein Data Bank (PDB) (web site: https://www.rcsb.org/). Structure crystals with high homology (PDB: 4IAR, 4IAQ, 5V54, and 6G79) were used to construct a homology model of the 5-HT1A receptor using Discovery Studio software. The structures of the ligands were sketched using ChemBioDraw Ultra 14.0. LibDock protocol was followed and the top LibDock Score was used for further analysis. The docking results were analyzed with Discovery Studio.
3.3. Statistical Analysis
Data were expressed as mean ± standard deviation. One-Way ANOVA analysis of variance was performed using GraphPad Prism 5.0 statistical software. 0.01 < p < 0.05 indicated significant difference. p < 0.01 indicates that the difference is very significant.
4. Conclusions
In the present study, a series of 5-aryl-4,5-dyhidrotetrazolo[1,5-a]thieno[2,3-e]pyridine derivatives containing tetrazole, triazole, methyltriazole, and triazolone moieties were synthesized. Two experimental methods, FST and TST, were used to evaluate the antidepressant activity of the target compounds. The pharmacological results showed that most of the target compounds exhibited a significant antidepressant activity in FST experiments. Among these compounds, 5-[4-(trifluoromethyl)phenyl]-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine (4i) was found to be the most potent. In the open-field test experiments, compound 4i did not affect spontaneous activity. The determination of in vivo 5-HT concentrations revealed that compound 4i may have an effect in the mouse brain. Molecular docking experiments showed that compound 4i significantly interacted with residues of the 5-HT1A receptor homology model. Therefore, the mode of action of compound 4i supporting its antidepressant activity may be closely related to the 5-HT1A receptor.
Supplementary Materials
The 1H-NMR, and 13C-NMR spectrums of compounds 4a–p, 5, 6, and 7 are available online at https://www.mdpi.com/1420-3049/24/10/1857/s1.
Author Contributions
S.W. and Z.Q. conceived and wrote the paper; S.W., H.L., X.W., K.L. and G.L. performed the experiments and analyzed the data.
Funding
The work was supported by Natural Science Foundation of Shandong (No. ZR2017BH037), Doctoral Foundation of Liaocheng University (No. 318051517), National Natural Science Foundation of China (No. 31702817 and 81803360) and Taishan scholar research Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Badr S.M.I. Synthesis and anti-inflammatory activity of novel 2,5-disubstituted thiophene derivatives. Turk. J. Chem. 2010;35:131–143. [Google Scholar]
- 2.Foroumadi A., Mansouri S., Kiani Z., Rahmani A. Synthesis and in vitro antibacterial evaluation of N-[5-(5-nitro-2-thienyl)-1,3,4-thiadiazole-2-yl]piperazinyl quinolones. Eur. J. Med. Chem. 2003;38:851–854. doi: 10.1016/S0223-5234(03)00148-X. [DOI] [PubMed] [Google Scholar]
- 3.Amer A.E., Sherif M.H., Assy M.G., Al-Omar M.A., Ragab I. Antiarrhythmic, serotonin antagonist and antianxiety activities of novel substituted thiophene derivatives synthesized from 2-amino-4,5,6,7-tetrahydro-N-phenylbenzo[b]thiophene-3-carboxamide. Eur. J. Med. Chem. 2010;45:5935–5942. doi: 10.1016/j.ejmech.2010.09.059. [DOI] [PubMed] [Google Scholar]
- 4.Fadda A.A., Abdel-Latif E., El-Mekawy R.E. Synthesis of some new arylazothiophene and arylazopyrazole derivatives as antitumor agents. J. Pharm. Pharmacol. 2012;3:148–157. doi: 10.4236/pp.2012.32022. [DOI] [Google Scholar]
- 5.Ali K.A., Abdalghfar H.S., Mahmoud K., Ragab E.A. Synthesis and antitumor activity of new Polysubstituted Thiophenes and 1,3,4-thiadiazoles incorporating 2,6-pyridine moieties. J. Heterocycl. Chem. 2013;50:1157–1164. doi: 10.1002/jhet.1613. [DOI] [Google Scholar]
- 6.Mathew B., Jerad S., Anbazhagan S. Synthesis, in silico preclinical evaluation, antidepressant potential of 5-substituted phenyl-3-(thiophen-2-yl)-4,5-dihydro-1hpyrazole-1-carboxamides. Biomed. Aging Pathol. 2014;4:327–333. doi: 10.1016/j.biomag.2014.08.002. [DOI] [Google Scholar]
- 7.Łukowska-Chojnacka E., Kowalkowska A., Gizińska M., Koronkiewicz M., Staniszewska M. Synthesis of tetrazole derivatives bearing pyrrolidine scaffold and evaluation of their antifungal activity against Candida albicans. Eur. J. Med. Chem. 2019;164:106–120. doi: 10.1016/j.ejmech.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 8.Sribalan R., Banuppriya G., Kirubavathi M., Padmini V. Synthesis, biological evaluation and in silico studies of tetrazole heterocycle hybrids. J. Mol. Struct. 2019;1175:577–586. doi: 10.1016/j.molstruc.2018.07.114. [DOI] [Google Scholar]
- 9.Aziz H., Saeed A., Jabeen F., Din N.U., Flörke U. Synthesis, single crystal analysis, biological and docking evaluation of tetrazole derivatives. Heliyon. 2018;4:e00792. doi: 10.1016/j.heliyon.2018.e00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Z., Chong S., Lin H., Yang Q., Wang X., Zhang W., Zhang X., Zeng Z., Su Y. Design, synthesis and biological evaluation of tetrazole-containing RXRα ligands as anticancer agents. Eur. J. Med. Chem. 2019;164:562–575. doi: 10.1016/j.ejmech.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 11.Bommagani S., Penthala N.R., Balasubramaniam M., Kuravi S., Caldas-Lopes E., Guzman M.L., Balusu R., Crooks P.A. A novel tetrazole analogue of resveratrol is a potent anticancer agent. Bioog. Med. Chem. Lett. 2019;29:172–178. doi: 10.1016/j.bmcl.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng X.Q., Wei C.X., Song M.X., Chai K.Y., Sun Z.G., Quan Z.S. Synthesis and studies on anticonvulsant and antidepressant activities of 5-alkoxy-tetrazolo[1,5-a]quinolines. Bull. Korean. Chem. Soc. 2010;31:447–452. doi: 10.5012/bkcs.2010.31.02.447. [DOI] [Google Scholar]
- 13.Wang S.B., Piao G.C., Zhang H.J., Quan Z.S. Synthesis of 5-Alkoxythieno[2,3-e][1,2,4]triazolo[4,3-c]pyrimidine Derivatives and Evaluation of Their Anticonvulsant Activities. Molecules. 2015;20:6827–6843. doi: 10.3390/molecules20046827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X.Y., Wei C.X., Deng X.Q., Sun Z.G., Quan Z.S. Synthesis and primary anticonvulsant activity evaluation of 6-alkyoxyl-tetrazolo[5,1-a]phthalazine derivatives. Arzneimittelforschung. 2010;60:289–292. doi: 10.1055/s-0031-1296289. [DOI] [PubMed] [Google Scholar]
- 15.Wang S.B., Deng X.Q., Liu D.C., Zhang H.J., Quan Z.S. Synthesis and evaluation of anticonvulsant and antidepressant activities of 7-alkyl-7H-tetrazolo[1,5-g]purine derivatives. Med. Chem. Res. 2014;23:4619–4626. doi: 10.1007/s00044-014-1030-0. [DOI] [Google Scholar]
- 16.Zhang M.M., Wang X.S., Li Q., Yao C.S., Tu S.J. Three-Component One-Pot Synthesis of 1-Aryl-4-benzo[f]quinoline Derivatives in Aqueous Media. Chin. J. Org. Chem. 2008;28:881–884. [Google Scholar]
- 17.Sun X.Y., Zhang L., Wei C.X., Piao H.R., Quan Z.S. Design, synthesis of 8-alkoxy-5,6-dihydro-[1,2,4]triazino[4,3-a]quinolin-1-ones with anticonvulsant activity. Eur. J. Med. Chem. 2009;44:1265–1270. doi: 10.1016/j.ejmech.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Sun X.Y., Wei C.X., Chai K.Y., Piao H.R., Quan Z.S. Synthesis and anti-inflammatory activity evaluation of novel 7-alkoxy-1-amino-4,5-dihydro[1,2,4]triazole[4,3-a]quinolines. Arch. Pharm. 2008;341:288–293. doi: 10.1002/ardp.200700182. [DOI] [PubMed] [Google Scholar]
- 19.Choi B.D., Lim H.J., Lee S.Y., Lee M.H., Kil K.S., Lim D.S., Jeong S.J., Jeong M.J. Synthesis of Novel Benzazole Derivatives and Evaluation of Their Antidepressant-Like Activities with Possible Underlying Mechanisms. Molecules. 2018;23:2881. doi: 10.3390/molecules23112881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galdino P.M., Nascimento M.V., Sampaio B.L., Ferreira R.N., Paula J.R., Costa E.A. Antidepressant-like effect of Lafoensia pacari A. St.-Hil. ethanolic extract and fractions in mice. J. Ethnopharmacol. 2009;124:581–585. doi: 10.1016/j.jep.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Sakakibara H., Ishida K., Grundmann O., Nakajima J., Seo S., Butterweck V., Minami Y., Saito S., Kawai Y., Nakaya Y., et al. Antidepressant effect of extracts from Ginkgo biloba leaves in behavioral models. Biol. Pharm. Bull. 2006;29:1767–1770. doi: 10.1248/bpb.29.1767. [DOI] [PubMed] [Google Scholar]
- 22.Meyer J.H., McMain S., Kennedy S.H., Korman L., Brown G.M., DaSilva J.N., Wilson A.A., Blak T., Eynan-Harvey R., Goulding V.S., et al. Dysfunctional attitudes and 5-HT2A receptors during depression self-harm. Am. J. Psychiatry. 2003;160:90–99. doi: 10.1176/appi.ajp.160.1.90. [DOI] [PubMed] [Google Scholar]
- 23.Ostrowska K., Grzeszczuk D., Głuch-Lutwin M., Gryboś A., Siwek A., Leśniak A., Sacharczuk M., Trzaskowski B. 5-HT1A and 5-HT2A receptors affinity, docking studies and pharmacological evaluation of a series of 8-acetyl-7-hydroxy-4-methylcoumarin derivatives. Bioorg. Med. Chem. 2018;26:527–535. doi: 10.1016/j.bmc.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Blier P., Ward N.M. Is there a role for 5-HT1A agonists in the treatment of depression? Biol. Psychiatry. 2003;53:93–103. doi: 10.1016/S0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- 25.Sherin D.R., Geethu C.K., Prabhakaran J., Mann J.J., Dileep J.S., Manojkumar T.K. Molecular docking, dynamics simulations and 3D-QSAR modeling of arylpiperazine derivatives of 3,5-dioxo-(2H,4H)-1,2,4-triazine as 5-HT1AR agonists. Comput. Biol. Chem. 2019;78:108–115. doi: 10.1016/j.compbiolchem.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Porsolt R.D. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 27.Zomkowski A.D., Santos A.R., Rodrigues A.L. Evidence for the involvement of the opioid system in the agmatine antidepressant-like effect in the forced swimming test. Neurosci. Lett. 2005;381:279–283. doi: 10.1016/j.neulet.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Streu L., Chermat R., Thierry B., Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacol. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 29.Sairanen M., Lucas G., Ernfors P., Castrén M., Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronalturnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott P.J., Chan J., Parker Y.M., Nemeroff C.B. Behavioral effects of neurotensin in the open field: Structure-activity studies. Brain. Res. 1986;381:259–265. doi: 10.1016/0006-8993(86)90075-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.