Abstract
This paper presents the synthesis of -halo--lactones, -iodo--lactones and -hydroxy--lactones from readily available organic substrates such as trans-crotonaldehyde and aryl bromides. Crystal structure analysis was carried out for lactones that were obtained in crystalline form. All halo--lactones and -hydroxy--lactones were highly cytotoxic against gastric cancer AGS cells with values in the range of 0.0006–0.0044 mM. Some lactones showed high bactericidal activity against E. coli ATCC 8739 and S. aureus ATCC 65389, which reduced the number of CFU/mL by 70–83% and 87% respectively.
Keywords: halolactones, translactonization, hydroxylactones, anti-cancer properties, bactericidal properties
1. Introduction
Lactones are compounds with unusual biological activities. The natural source of lactones are plants [1,2] but they are also found in microorganisms [3] and animals [4]. They exhibit, among others, anti-cancer activity [5,6], cytotoxic [7,8,9], bacterio- [10,11,12] and fungistatic [13,14], antiviral [15,16] and deterrent properties [17,18]. This biological potential is widely used in medicine and agriculture. Lactones also have sensory properties [19] used in the cosmetics and food industries but they can also be toxic, such as mycotoxins produced by mold fungi [20]. The properties and application possibilities of lactones are they reason they are still intensively studied by many groups. New natural derivatives are still isolated, their properties are examined and new synthetic analogs with desirable properties are sought. This interesting group of compounds also attracted our attention. Thus far, we have developed a synthesis of -ayl--iodo--lactones [21], aryl--hydroxy--lactones [22], and trans--halo--lactones [23], and tested their cytotoxic and bactericidal activities. Here, we present stereoselective substrate-controlled synthesis of new -halo--lactones. Some of the obtained iodo--lactones can easily undergo translactonization reactions leading to -hydroxy--lactones with high yields. Their biological activities were also evaluated: antimicrobial activity against Escherichia coli strain ATCC 8739 and Staphylococcus aureus strain ATCC 65389, and cytotoxicity against L929 cell lines (mouse fibroblasts) and human gastric cancer cell line AGS.
2. Results and Discussion
2.1. Synthesis
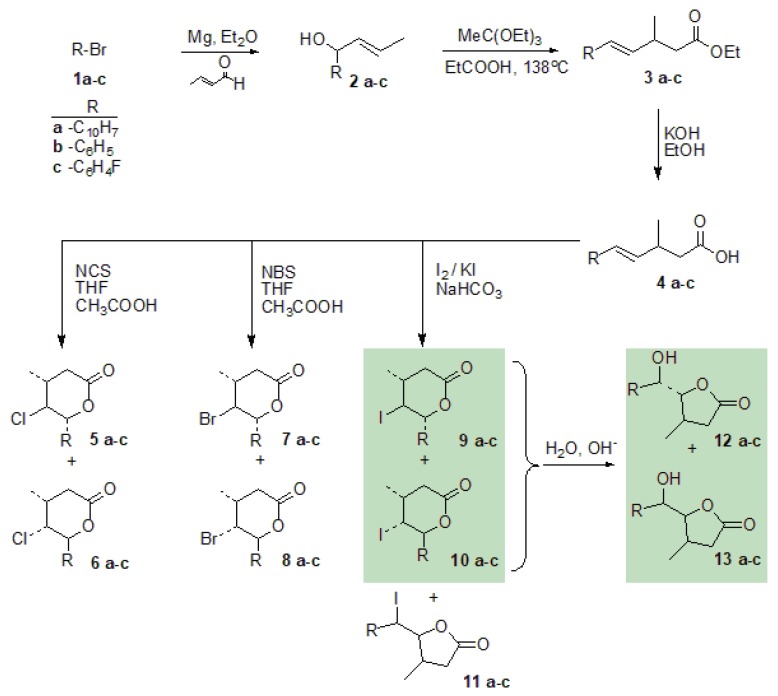
Halolactones were obtained as a result of the four-step reaction [23] shown in Figure 1. The first step of the synthesis was a Grignard reaction between selected aryl bromides 1a–c and trans-crotonaldehyde, which gave us unsaturated allyl alcohols 2a–c with yields 48–62% [24]. The Johnson–Claisen rearrangement [25] of these alcohols with triethyl orthoacetate and catalytic amount of propionic acid obtained ,-unsaturated ethyl esters 3a–c with yields 49–63%. In the third step, the esters were subjected to alkaline hydrolysis in ethanol to obtain ,-unsaturated carboxylic acids 4a–c. Thus obtained substrates, with suitably substituted double bonds, enforced selectivity of halolactonization in the last step of the synthesis. The yields and products distribution are shown in Table 1. Only two of these compounds have been described thus far in the literature [26].
Figure 1.
A four-step synthesis of -halo--lactones and -hydroxy--lactones.
Table 1.
The yields and products distribution from halolactonization of unsaturated carboxylic acids 4a–c (according to GC).
| -Chloro--lactones | -Bromo--lactones | |||||
|---|---|---|---|---|---|---|
| yield [%] | trans,trans | cis,trans | yield [%] | trans,trans | cis,trans | |
| 51 | 5a 82% | 6a 18% | 72 | 7a 82% | 8a 18% | |
| 82 | 5b 84% | 6b 16% | 72 | 7b 78% | 8b 22% | |
| 62 | 5c 81% | 6c 19% | 78 | 7c 76% | 8c 24% | |
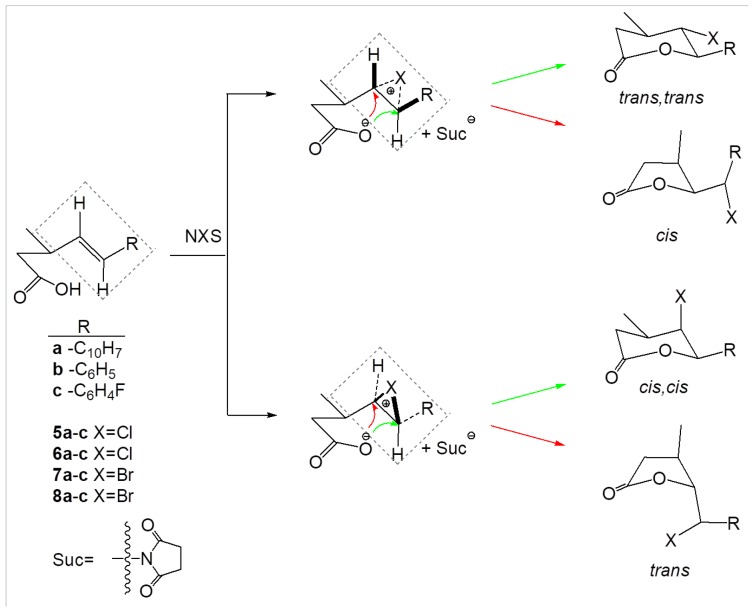
According to the literature [27,28], halolactonization of ,-unsaturated carboxylic acids, carried out with N-chlorosuccinimide (NCS) or N-bromosuccinimide (NBS), leads to a mixture of - and -lactones, with an excess of 6-endo cyclization products. The regioselectivity of the reaction is influenced by steric hindrance at carbon atoms adjacent to the double bond. In the case of acids with substituents at the C-3, nucleophilic attack at the -position is hindered and the amount of products formed by 5-exo cyclization decreases. This is also confirmed by our results. The chlorolactonization of unsaturated carboxylic acids 4a–c with N-chlorosuccinimide (NCS) in THF, only proceeded through a 6-endo cyclization mechanism and gave new -halo--lactones 5a–c and 6a–c. The analysis of post-reaction mixtures showed that in each case only isomers trans,trans and cis,trans were formed (Figure 2). The thermodynamically more stable isomers trans,trans were always present in large excess, up to 80% (Table 1 and Table 2). An analogous synthesis using ,-unsaturated carboxylic acids with a methyl group in the -position led to a mixture of products. Lactonization of unsaturated carboxylic acids with two methyl groups in the -position have only one product, trans--chloro--lactone [23].
Figure 2.
Mechanisms of chloro- and bromolactonization of ,-unsaturated carboxylic acids with NCS and NBS.
Table 2.
The yields and products distribution from iodolactonization of unsaturated carboxylic acids 4a–c (according to GC).
| cis--Iodo--lactones | -Iodo--lactones | -Hydroxy--lactones | |||||
|---|---|---|---|---|---|---|---|
| yield [%] | trans | cis | trans,trans | cis,trans | trans | cis | |
| - | - | 12a 10% | 13a 90% | ||||
| 75 | - | 11b 10% | 9b 42% | 10b 33% | 12b 4% | 13b 11% | |
| 69 | - | 11c 8% | 9c 37% | 10c 30% | 12c 7% | 13c 18% | |
Bromolactones 7a–c and 8a–c were obtained by reacting 4a–c with N-bromosuccinimide (NBS) in THF with a yield of 72–78%. Mechanisms for lactonization of ,-unsaturated carboxylic acids with NCS and NBS are similar [27] (Figure 2) and lead to the same products of 6-endo cyclization. The analysis of post-reaction mixtures showed that in bromolactonization of unsaturated carboxylic acids 4a–c in each case only isomers trans,trans and cis,trans were formed. The yield of trans,trans--bromo--lactones was 76–82% and yield of cis,trans--bromo--lactones was only 18–24%. The previously described reactions of ,-unsaturated carboxylic acids with in saturated solution, carried out under kinetic control, resulted in mixtures of - and -iodolactones, in which the products formed by 5-exo cyclization predominated. The regioselectivity-determining step for the reaction is the attack of the negatively charged oxygen atom of the carboxyl group on the iodine-double bond complex. The presence of substituents in the -position of unsaturated carboxylic acids hindered nucleophilic attack at the C-4 position. This limited the formation of five-membered lactones. The effect of steric hindrance at the C-6 negatively influenced the formation of lactones and favored -lactones [27].
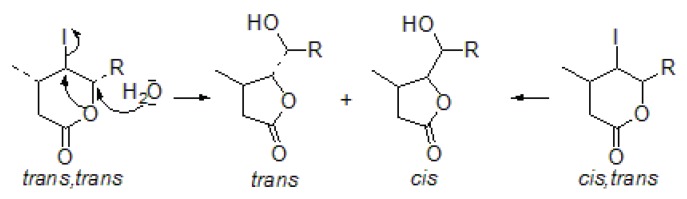
Iodolactonization of 4a–c acids was carried out with and in a water/diethyl ether mixture [29]. Analysis of the reaction mixtures unexpectedly showed the formation of two new isomers of -hydroxy--lactone 12a and 13a. The yield of reactions was in the range of 69–85%. The reaction conditions prevented the direct synthesis of these compounds because the reaction was carried out in an alkaline solution, whereas hydroxylactonization requires an acidic environment [22]. Cis-isomers are more thermodynamically stable than trans-isomers and predominantly formed in this reaction. The excess of trans-isomer is usually observed in the synthesis of -lactones. However, the reaction initially gave -iodo--lactones, which, through a translactonization reaction, were transformed into the final products–hydroxylactones 12a and 13a. This was confirmed by observations of purified isomers trans,trans and cis,trans -iodo--lactones 9b and 10b, and 9c and 10c. These isomers underwent rapid decomposition with release of iodine. The analysis of residues showed that pure isomers trans,trans or cis,trans transformed into an equimolar mixture of isomers trans and cis hydroxylactones (Figure 3). These results indicate that the translactonization of -iodo--lactones involved elimination of the iodine, which was a good leaving group and rearrangement of the six-membered lactone ring into the five-membered one, according to the SN1 mechanism. The carbocation was stabilized by the electron lone pairs of the oxygen atom present in the water molecule. The preparation of hydroxylactones from iodolactones has been described thus far only for biotransformation experiments. The synthesis described here allows obtaining these compounds without microorganisms.
Figure 3.
The translactonization mechanism of -iodo--lactones.
Iodolactonization of ,-unsaturated carboxylic acids 4b and 4c also led to results different from previous studies (Table 1). In both reactions, five products with the lactone ring were identified. The main products were isomers trans,trans--iodo--lactones 9b (yield 42%) and 9c (yield 37%). Isomers cis,trans--iodo--lactones 10b,c were formed with yields of 30% and 33% and isomers cis--iodo--lactones 11b,c only with yields of 10% and 8%. This result was new and unexpected, because iodolactonization of ,-unsaturated carboxylic acid usually gives a mixture of -lactones as the main product and only a trace of -lactones [27]. This reaction also produced -hydroxy--lactones. Isomers trans 12b,c were formed with yields of 5% and 7% and isomers cis 13b,c with yields of 14% and 18%.
2.2. The Structural Analysis of Compounds
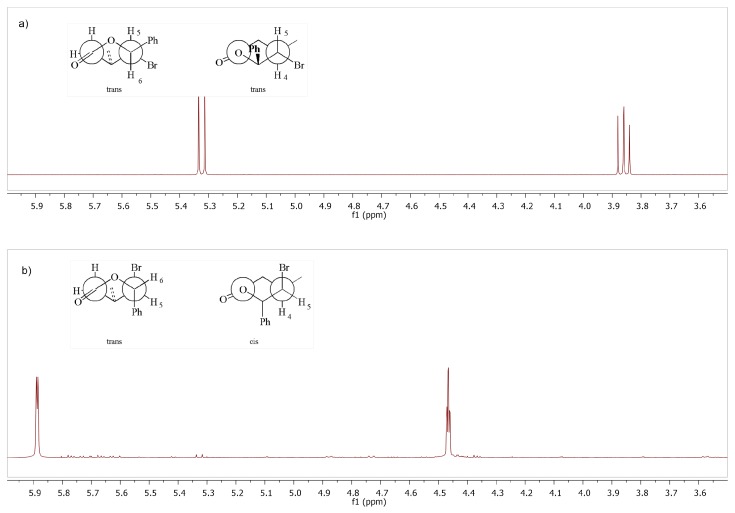
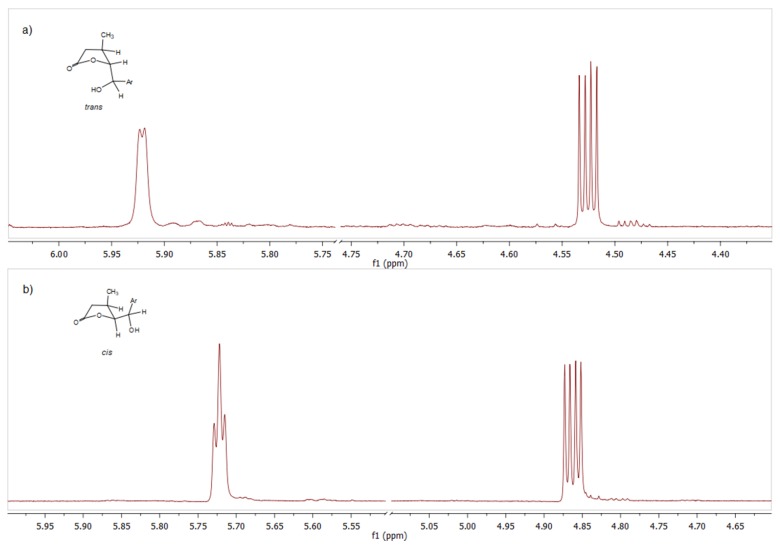
Spectroscopic and spectrometric analyses were performed for all obtained compounds. The X-ray structural analysis was carried out for the solid products of lactonization. The NMR spectra of analogous isomers of -halo--lactones are similar to each other in most respects. In cis,trans--bromo--lactone 8b, the H-6 proton was observed at 5.89 ppm as a doublet with coupling constants 2.5 Hz (Figure 4). The signal of proton H-5 appears at 4.46 ppm as a triplet due to coupling with protons H-4 and H-6 and the coupling constant 2.5 Hz. According to Karplus, the coupling constant of vicinal protons depends on the dihedral angle between them. The equatorial-equatorial and axial-equatorial coupling constants are in the range 1–5 Hz. The axial-axial coupling constant is normally large but these magnitudes are decreased (9–10 Hz) by the antiperiplanar arrangement of the hydrogens and oxygen atom of the carbonyl group [30,31].
Figure 4.
The comparison of the NMR signals for H-6 and H-5 protons of: (a) trans,trans--bromo--lactone 7b and (b) cis,trans--bromo--lactone 8b.
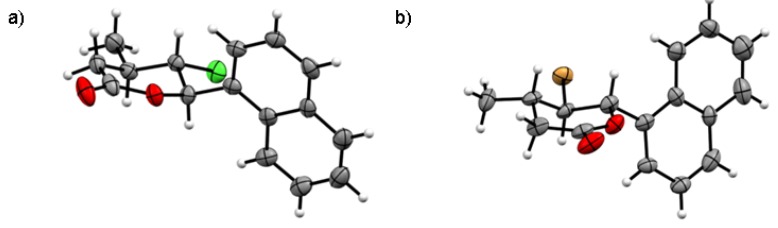
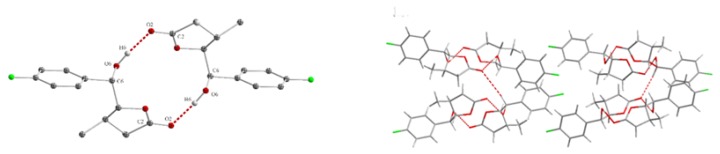
The magnitudes of these coupling constants indicate that the protons H-5 and H-6 are in aquatorial positions and proton H-5 is in axial position. This causes that in cis,trans--chloro--lactones 8b Br and Ph substituents are on opposite sides of the lactone ring, in the axial position at C-5 and C-6 and CH substituent is in equatorial position at C-4 carbon atom. In the case of trans,trans isomer 7b, coupling constant between protons H-4, H-5 and H-6 is much larger. The signal of protons H-6 observed at 5.32 ppm as a doublet with coupling constant J = 10.3 Hz. The triplet observed at 3.86 ppm for H-5 proton have a coupling constant 10.2 Hz. This means that all the substituents are in the equatorial position, alternately above and below the lactone ring plane. The pyranose chair conformations with substituents in the equitorial position are more stable than in the axial position due to torsional strain and 1,3-diaxial interactions. This is reflected in the composition of the reaction mixture where trans isomers are dominant. The structure of the trans,trans--halo--lactones also have been confirmed by single crystal X-ray diffraction analysis. Figure 5a shows the crystalline structure of trans,trans--chloro--lactone 5a [32]. The dihedral angles C1-C6-C5-Cl and C7-C4-C5-Cl being and respectively. Thus, the aryl substituent at C-6, chlorine atom at C-5 and methyl group at C4 are in the equatorial positions in gauche,gauche arrangement. Hydrogen atoms H-4, H-5 and H-6 occupy the axial position with the dihedral angles between H-4 and H-5 and between H-5 and H-6 equal to and , respectively. X-ray analysis of trans,trans--chloro- and trans,trans--bromo--lactones showed that the structure of the lactone rings of the same isomers is analogous. Figure 5b shows the crystalline structure of trans,trans--bromo--lactone 7a [33].
Figure 5.
The crystalline structure of: (a) trans,trans--chloro--lactone 5a; and (b) trans,trans--bromo--lactone 7a.
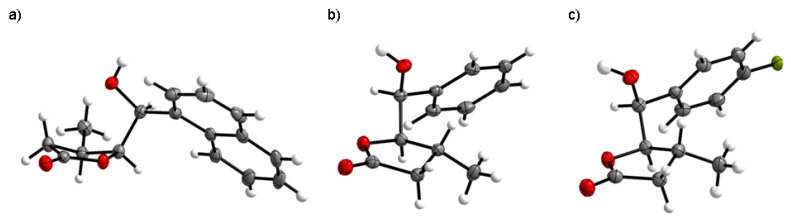
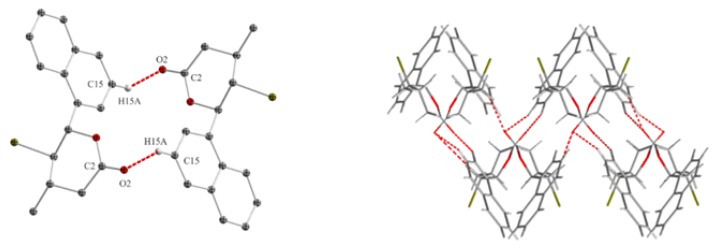
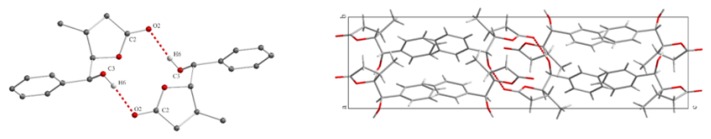
The crystalline structure of -hydroxylactone 13a [34], obtained as a result of iodolactonization of acid 4a in 90% yield, is shown in Figure 6a. The dihedral angles C7-C4-C5-C6 and H4-C4-C5-H5 are and , respectively. Thus, the substituents at C-4 and C-5 are in syn-periplanar orientation, on the same side of the lactone ring, forming the cis isomer.
Figure 6.
The crystalline structure of: (a) cis--hydroxy--lactone 13a; (b) trans--hydroxy--lactone 12b; and (c) trans--hydroxy--lactone 12c.
The structure of -hydroxylactones was also confirmed by NMR spectroscopic analysis. In the NMR spectra of cis--hydroxylactone 13a, the signal of proton H-5 appears at = 4.86 ppm as a double doublet, due to coupling with protons H-4 and H-6 (J = 7.2 Hz, J = 3.4 Hz). The analogous coupling constants for trans--hydroxylactone 12a are smaller and equal to 5.5 Hz and 2.8 Hz, respectively (Figure 7). This means that the substituents are in the equatorial position in trans-arrangement.
Figure 7.
The comparison of the NMR signals for H-5 and H-6 protons of: (a) trans--hydroxy--lactone 12b; and (b) cis--hydroxy--lactone 13a.
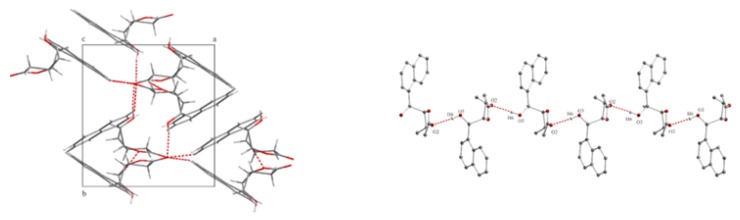
The structure of the lactone rings was also confirmed by NMR and IR spectroscopy. The carbon signals at : 68.97 (13a) and 72.13 ppm (12a) are characteristic of carbon atoms bonded to the hydroxyl group. The typical chemical shift values for the ester carbon atoms in -lactones also have signals at 177.11 ppm (13a) and 175.98 ppm (12a). In the IR spectrum, the stretching bands of the C = O group appeared at 1747 cm (13a) and 1774 cm (12a). Spectroscopic analysis showed a similar structure of lactones 13b,c and 12b,c. The crystalline structures of -hydroxylactones 12b [35] and 12c [36] are shown in Figure 6b,c. The dihedral angles C7-C4-C5-C6 and H4-C4-C5-H5, being and (12b), and (12c), respectively, are characteristic of antiperiplanar conformers and trans configuration of the lactone rings.
The iodolactonization of ,-unsaturated carboxylic acids 4b,c also gave -iodo--lactones 11b and 11c in low yields (7% and 8%, respectively). Their structure was determined by spectroscopic methods. In the IR spectra, the absorption bands of the C = O group were observed at 1765 cm (11b) and 1760 cm (11c). This is the typical range for -lactones. The analysis of NMR spectra showed the signal of the carbonyl carbon at 176.00 ppm (11b) and 176.11 ppm (11c). The signals observed at : 28.38 ppm (11b) and 28.45 ppm (11c) were assigned to carbons connected to the iodine. The signal of proton H-5 appears as a double doublet, due to coupling with protons H-4 and H-6 (J = 11.2 Hz, J = 4.5 Hz 11b,c). This indicates the syn-periplanar orientation of the protons H-4 and H-5, and cis configuration of -lactones.
2.3. Bactericidal Properties
The results of CFU counting method are shown in Table 3. The lactones 6c, 9c and 10c showed the highest bactericidal activity against E. coli ATCC 8739 (70–78% less CFU/mL). The other lactones exhibited moderate bactericidal properties. The highest bactericidal activity against S. aureus ATCC 65389 was found for lactone 5a which reduced the number of CFU/mL by 87%. The bactericidal properties against tested bacterial strain also showed lactones 6b, 8c and 7a (78–83% less CFU/mL). Lactones 9a and 12a did not show bactericidal activity against S. aureus ATCC 65389.
Table 3.
The bactericidal activity of the investigated lactones against Escherichia coli ATCC 8739 and Staphylococcus aureus ATCC 65389.
| Compound | E. coli ATCC 8739 | S. aureus ATCC 65389 |
|---|---|---|
| Number of CFU/mL (× ) | ||
| 5a | 6.41 | 2.74 |
| 5b | 4.06 | 7.63 |
| 5c | 9.46 | 14.83 |
| 6a | 7.58 | 5.20 |
| 6b | 6.32 | 3.58 |
| 6c | 2.06 | 7.39 |
| 7a | 5.21 | 4.63 |
| 7b | 8.63 | 10.96 |
| 7c | 6.52 | 6.42 |
| 8a | 7.14 | 9.29 |
| 8b | 5.83 | 9.04 |
| 8c | 8.87 | 3.60 |
| 9b | 9.02 | 21.25 |
| 9c | 2.43 | 9.76 |
| 10b | 7.65 | 5.92 |
| 10c | 2.87 | 6.30 |
| 12a | 9.43 | 21.15 |
| 13a | 6.02 | 9.12 |
| Control | 9.47 | 21.36 |
| Control with DMSO | 9.30 | 20.85 |
The 3-(4,5-Dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium Bromide ( Mtt) Reduction Effectiveness
All lactones inhibited metabolic activity of the cell lines in the concentration range of 0.1–50 g/mL. The ability of cells to reduce MTT to formazan decreased by 37–93% (Table 4 and Table 5). The cytotoxicity of tested lactones against normal eukaryotic cells may exclude their use as antimicrobial agents in medicine but does not exclude their use in anti-cancer drugs. Our results showed that lactones 10b, 10c and 6b exhibit the lowest values against L929 cells. All halo--lactones and -hydroxy--lactones were highly cytotoxic against gastric cancer AGS cells with values in the range of 0.0006–0.0044 M. These values are much lower than = 0.025 M of doxorubicin, the reference anticancer drug [37]. The differences between values for AGS and L929 cell line were evaluated by non-parametric Mann–Whitney U test. Statistical significance (p ≤ 0.05) was observed for all tested lactones except 6b (p = 0.1).
Table 4.
The cytotoxic activity of tested lactones against AGS cell lines.
| Lactone | Concentration [g/mL] | 5% DMSO | [M] | |||||
|---|---|---|---|---|---|---|---|---|
| 50 | 20 | 10 | 5 | 0.5 | 0.1 | |||
| 5a | 15 ± 0.01 | 18 ± 0.08 | 27 ± 0.10 | 43 ± 0.09 | 48 ± 0.05 | 54 ± 0.07 | 85 ± 0.1 | 0.0019 |
| 5b | 8 ± 0.09 | 11 ± 0.07 | 14 ± 0.12 | 16 ± 0.06 | 20 ± 0.09 | 35 ± 0.06 | 0.0023 | |
| 5c | 10 ± 0.12 | 21 ± 0.08 | 34 ± 0.09 | 44 ± 0.10 | 47 ± 0.12 | 54 ± 0.12 | 0.0006 | |
| 6a | 8 ± 0.05 | 8 ± 0.09 | 19 ± 0.01 | 23 ± 0.09 | 28 ± 0.07 | 35 ± 0.06 | 0.0019 | |
| 6b | 8 ± 0.06 | 14 ± 0.07 | 21 ± 0.02 | 28 ± 0.03 | 35 ± 0.02 | 42 ± 0.02 | 0.0044 | |
| 6c | 6 ± 0.08 | 14 ± 0.07 | 16 ± 0.10 | 28 ± 0.06 | 37 ± 0.05 | 49 ± 0.10 | 0.0029 | |
| 7a | 8 ± 0.03 | 20 ± 0.06 | 21 ± 0.03 | 25 ± 0.10 | 26 ± 0.05 | 39 ± 0.02 | 0.0021 | |
| 7b | 10 ± 0.06 | 14 ± 0.03 | 20 ± 0.06 | 23 ± 0.02 | 25 ± 0.10 | 40 ± 0.10 | 0.0036 | |
| 7c | 8 ± 0.07 | 18 ± 0.10 | 25 ± 0.12 | 42 ± 0.02 | 49 ± 0.06 | 63 ± 0.03 | 0.0035 | |
| 8a | 9 ± 0.09 | 17 ± 0.07 | 25 ± 0.05 | 39 ± 0.09 | 47 ± 0.06 | 61 ± 0.10 | 0.0016 | |
| 8b | 11 ± 0.05 | 17 ± 0.10 | 28 ± 0.09 | 31 ± 0.02 | 35 ± 0.10 | 44 ± 0.15 | 0.0019 | |
| 8c | 10 ± 0.09 | 11 ± 0.03 | 18 ± 0.07 | 43 ± 0.10 | 58 ± 0.10 | 69 ± 0.15 | 0.0025 | |
| 9b | 8 ± 0.09 | 15 ± 0.06 | 26 ± 0.03 | 43 ± 0.12 | 52 ± 0.15 | 54 ± 0.15 | 0.0014 | |
| 9c | 15 ± 0.03 | 18 ± 0.06 | 23 ± 0.02 | 35 ± 0.10 | 43 ± 0.15 | 51 ± 0.10 | 0.0014 | |
| 10b | 10 ± 0.06 | 14 ± 0.08 | 16 ± 0.09 | 37 ± 0.03 | 43 ± 0.02 | 58 ± 0.07 | 0.0018 | |
| 10c | 8 ± 0.03 | 10 ± 0.07 | 13 ± 0.03 | 20 ± 0.02 | 23 ± 0.15 | 38 ± 0.06 | 0.0017 | |
| 12a | 9 ± 0.10 | 13 ± 0.08 | 18 ± 0.05 | 22 ± 0.09 | 26 ± 0.05 | 39 ± 0.09 | 0.0043 | |
| 13a | 8 ± 0.08 | 10 ± 0.10 | 18 ± 0.12 | 29 ± 0.05 | 39 ± 0.09 | 44 ± 0.12 | 0.0037 | |
The cytotoxicity was assessed by (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) reduction assay. The cell viability was calculated for four experiments including three repeats for each compound. Complete RPMI-1640 medium was used as a positive control (Control +) of cell viability (100% viable cells) and 0.03% HO as a negative control (Control −) of cell viability (100% dead inactive cells). All values were expressed as the mean percentage of viable cells ± SD. The differences between positive control and treated with tested compounds were evaluated by non-parametric Mann–Whitney U test. Statistical significance: p < 0.05. Dimethyl sulfoxide (DMSO) was used as a solvent; , half maximal inhibitory concentration.
Table 5.
The cytotoxic activity of tested lactones against L929 cell lines.
| Lactone | Concentration [g/mL] | 5% DMSO | [M] | |||||
|---|---|---|---|---|---|---|---|---|
| 50 | 20 | 10 | 5 | 0.5 | 0.1 | |||
| 5a | 1 ± 0.01 | 5 ± 0.06 | 8 ± 0.07 | 10 ± 0.03 | 15 ± 0.02 | 17 ± 0.09 | 79 ± 0.07 | 0.0058 |
| 5b | 1 ± 0.03 | 4 ± 0.02 | 7 ± 0.06 | 12 ± 0.05 | 18 ± 0.15 | 20 ± 0.08 | 0.0071 | |
| 5c | 2 ± 0.12 | 4 ± 0.06 | 10 ± 0.03 | 14 ± 0.09 | 21 ± 0.15 | 26 ± 0.03 | 0.0078 | |
| 6a | 2 ± 0.09 | 6 ± 0.03 | 7 ± 0.07 | 9 ± 0.15 | 11 ± 0.08 | 15 ± 0.03 | 0.0083 | |
| 6b | 2 ± 0.05 | 4 ± 0.15 | 16 ± 0.03 | 25 ± 0.02 | 27 ± 0.12 | 32 ± 0.07 | 0.0041 | |
| 6c | 2 ± 0.02 | 4 ± 0.08 | 9 ± 0.05 | 18 ± 0.12 | 22 ± 0.05 | 24 ± 0.03 | 0.0047 | |
| 7a | 1 ± 0.12 | 2 ± 0.03 | 6 ± 0.07 | 12 ± 0.02 | 18 ± 0.12 | 25 ± 0.02 | 0.0043 | |
| 7b | 2 ± 0.09 | 5 ± 0.15 | 10 ± 0.05 | 13 ± 0.12 | 18 ± 0.08 | 25 ± 0.03 | 0.0048 | |
| 7c | 1 ± 0.03 | 5 ± 0.05 | 11 ± 0.08 | 17 ± 0.02 | 21 ± 0.08 | 27 ± 0.08 | 0.0066 | |
| 8a | 1 ± 0.15 | 4 ± 0.02 | 7 ± 0.06 | 14 ± 0.03 | 19 ± 0.03 | 23 ± 0.03 | 0.0044 | |
| 8b | 1 ± 0.02 | 4 ± 0.08 | 8 ± 0.05 | 15 ± 0.03 | 17 ± 0.08 | 24 ± 0.03 | 0.0055 | |
| 8c | 1 ± 0.05 | 5 ± 0.02 | 11 ± 0.03 | 15 ± 0.03 | 20 ± 0.08 | 25 ± 0.03 | 0.0044 | |
| 9b | 1 ± 0.12 | 4 ± 0.15 | 11 ± 0.06 | 13 ± 0.07 | 18 ± 0.03 | 23 ± 0.03 | 0.0044 | |
| 9c | 1 ± 0.05 | 3 ± 0.09 | 6 ± 0.03 | 9 ± 0.06 | 12 ± 0.08 | 15 ± 0.03 | 0.0068 | |
| 10b | 1 ± 0.02 | 5 ± 0.06 | 7 ± 0.15 | 15 ± 0.08 | 20 ± 0.03 | 25 ± 0.08 | 0.0040 | |
| 10c | 2 ± 0.05 | 2 ± 0.15 | 6 ± 0.09 | 12 ± 0.07 | 18 ± 0.03 | 22 ± 0.05 | 0.0041 | |
| 12a | 2 ± 0.06 | 3 ± 0.03 | 8 ± 0.06 | 10 ± 0.02 | 15 ± 0.05 | 19 ± 0.15 | 0.0062 | |
| 13a | 1 ± 0.07 | 5 ± 0.12 | 6 ± 0.06 | 9 ± 0.03 | 12 ± 0.02 | 16 ± 0.09 | 0.0081 | |
The cytotoxicity was assessed by (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) reduction assay. The cell viability was calculated for four experiments including three repeats for each compound. Complete RPMI-1640 medium was used as a positive control (Control +) of cell viability (100% viable cells) and 0.03% HO as a negative control (Control −) of cell viability (100% dead inactive cells). All values were expressed as the mean percentage of viable cells ± SD. The differences between positive control and treated with tested compounds were evaluated by non-parametric Mann–Whitney U test. Statistical significance: p < 0.05. Dimethyl sulfoxide (DMSO) was used as a solvent; , half maximal inhibitory concentration.
Such cytostatic activity of lactones was reported by various research groups. Bai et al. showed the cytotoxic effects of sesquiterpene lactones from Inula britannica against four human cancer cell lines: COLO 205, HT 29, HL-60 and AGS [38]. The high cytotoxicity of halo lactones with -phenyl--lactone or -phenyl--lactone framework against cancer lines Jurkat (human leukaemia) and D17 (canine osteosarcoma) was reported by Mazur et al. [28,39,40].
3. Materials and Methods
All used chemicals were purchased from Sigma-Aldrich and Fluka and used without further purification. The NMR spectra were obtained in CDCl on a Bruker Avance DRX 500 MHz. FTIR spectra were recorded using an attenuated total reflection technique on a Perkin Elmer Spectrum 400 spectrometer. High-resolution electrospray ionization mass spectra (HR-ESI-MS) were acquired on a Bruker micrOTOF-Q II. Gas chromatography was performed using a Thermo Scientific-Trace 1310 chromatograph equipped with a TG-5HT column (30 m × 0.25 mm). Melting points were determined with a Boetius micro melting point apparatus and are uncorrected. The refractive index was determined with an Abbe refractometer (Atago RX-7000 CX). TLC analysis was performed on silica gel F254 plates (Merck). Column chromatography was performed on silica gel 60 (230–400 mesh, Merck) using mixtures of hexane, ethyl acetate and acetone as eluents. The crystal structure was determined by single-crystal X-ray diffraction (Xcalibur, Sapphire 2 CCD detector).
3.1. X-ray Study
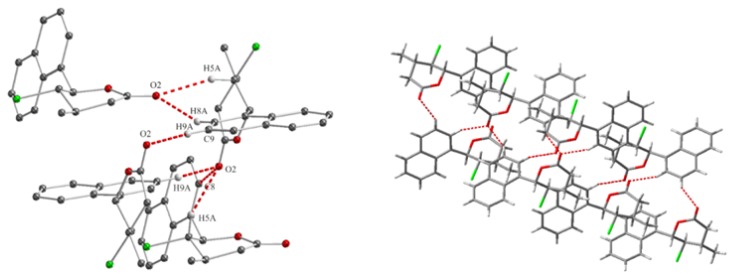
The single crystal X-ray diffraction for lactones was carried out on a Rigaku Oxford Diffraction SuperNova diffractometer equipped with a micro focus Cu X-ray source. The crystals were maintained at 100 K by using an Oxford Cryosystems nitrogen gas-flow device. Data collection strategies were optimized using CrysAlisPro software package [41]. The crystal structures of lactones were solved using the charge-flipping method implemented in SUPERFLIP and refined with the JANA package [42]. The crystal data, data collection, and refinement parameters for lactones are given in Table 6. Crystallographic data, as CIF files, have been deposited with the Cambridge Crystallographic Data Centre. The solid-state structure of lactone was determined by single-crystal X-ray diffraction. The lactones 5a (Figure 8), 7a (Figure 9), and 13a (Figure 10) crystallized in the centrosymmetric space group while lactones 12b (Figure 11) and 12c (Figure 12) in the orthorhombic space group Pbca. Only one molecule is present in the asymmetric unit. Experimental details of the crystallographic analysis are given in Table 5.
Table 6.
Experimental details of the crystallographic analysis for lactones 5a, 7a, 13a, 12b and 12c.
| 5a | 7a | 13a | 12b | 12c | |
|---|---|---|---|---|---|
| Empirical formula | CHOCl | CHOBr | CHO | CHO | CHFO |
| Formula weight | 274.73 | 319.19 | 256.29 | 206.24 | 224.22 |
| Temperature (K) | 293.15 | 293.15 | 100 | 100 | 293.15 |
| Wavelength (Å) | 0.71073 | 0.71073 | 1.54184 | 1.54184 | 0.71073 |
| Crystal system, space group | monoclinic, | monoclinic, | monoclinic, | orthorhombic P b c a () | orthorhombic P b c a |
| a (Å) | 13.670(3) | 13.9026(9) | 11.4137(2) | 10.1332(2) | 10.042(3) |
| b(Å) | 8.9220(2) | 8.9784(4) | 11.46100(10) | 7.62820(10) | 7.591(2) |
| c(Å) | 11.890(3) | 11.8318(9) | 10.7848(2) | 27.2405(2) | 28.174(3) |
| ( ) | 111.73(3) | 111.418(8) | 117.359(2) | 90 | 90 |
| Volume (Å) | 1347.1(5) | 1374.89(17) | 1252.982 (4) | 2105.64(5) | 2147.8(9) |
| Z, Calculated density (Mg/m) | 4, 1.355 | 4, 1.542 | 4, 0.154 | 8, 0.1831 | 8, 1.387 |
| Absorption coefficient (mm) | 0.278 | 2.984 | 0.126 | 0.150 | 0.110 |
| F(000) | 576 | 648 | 544 | 880 | 944 |
| Theta range for data collection () | 2.935 −28.804 | 2.927 −26.999 | 3.86 −76.29 | 3.24 −75.38 | 3.441 −28.867 |
| Index ranges | −17 ≤ h ≤ 18, −11 ≤ k ≤ 12, −16 ≤ l ≤ 9 | −17≤ h≤ 17, −11 ≤ k ≤ 11, −12 ≤ l ≤ 15 | −14 ≤ h ≤ 14, −14 ≤ k ≤ 14, −12 ≤ l ≤ 13 | −142 ≤ h≤ 10, −9 ≤ k ≤ 9, −32 ≤ l ≤ 33 | −13 ≤ h≤ 13, −8≤ k ≤ 10, −36 ≤ l ≤ 37 |
| Reflections Collected unique observed [I > 2sigma(I)] | 9623 | 9471 | 17416 | 13106 | 14345 |
| 3259 | 2957 | 2623 | 2170 | 2677 | |
| 1811 | 1923 | 2483 | 2017 | 1951 | |
| 0.0713 | 0.0959 | 0.0281 | 0.0401 | 0.0501 | |
| Completeness to (%) | 25.242, 99.1 | 25.242, 99.2 | 76.29, 99.0 | 75.38, 99.0 | 25.242, 99.8 |
| Refinement method | Full-matrix least-squares on | Full-matrix least-squares on | Full-matrix least-squares on | Full-matrix least-squares on | Full-matrix least-squares on |
| Data/restraints/parameters | 3259/0/172 | 2957/0/172 | 2623/0/259 | 2170/0/140 | 2677/0/149 |
| Goodness-of-fit on | 0.883 | 1.436 | 4.030 | 3.16 | 1.052 |
| Final R indices [I > 2sigma(I)] | = 0.0569 | = 0.0703 | = 0.0408 | = 0.0408 | = 0.0485 |
| = 0.1289 | = 0.1686 | = 0.1184 | = 0.0534 | = 0.1315 | |
| R indices (all data) | = 0.1079 | = 0.1149 | = 0.0422 | = 0.0429 | = 0.0669 |
| = 0.1433 | = 0.2216 | = 0.1192 | = 0.0540 | = 0.1384 |
Figure 8.
The arrangement of 5a in the unit cell box.
Figure 9.
The arrangement of 7a in the unit cell box.
Figure 10.
The arrangement of 13a in the unit cell box.
Figure 11.
The arrangement of 12b in the unit cell box.
Figure 12.
The arrangement of 12c in the unit cell box.
3.2. Claisen Rearrangement
The ,-unsaturated ethyl esters 3a–c were obtained by Claisen rearrangement [25]. A mixture of triethyl orthoacetate (0.15 mol), ethanol (0.02 mol) and a catalytic amount (one drop) of propionic acid was heated at 138 C for 5 h while distilling off alcohol. When the reaction was completed (TLC), the excess of triethyl orthoacetate was removed by evaporation. The crude product was purified by column chromatography on silica gel using a mixture of ethyl acetate and hexane (1:80). Spectral data are given below.
Ethyl 3-methyl-5-(naphthalen-1-yl)pent-4-enoate (3a): The product was obtained as a yellow oil with a yield of 62.75%; = 1.5641; = 0.38 (EtOAc:hexane 1:20); H NMR (CDCl, 600 MHz), [ppm]: 1.28 (d, 3H, J = 6.8 Hz, )), 1.29 (t, 3H, J = 7.1 Hz, OCHCH), 2.48 (dd, 1H, = 14.7 Hz, = 7.1 Hz, CHaHb), 2.55 (dd, 1H, = 14.7 Hz, = 7.6 Hz, CHaHb), 2.99–3.10 (m, 1H, )), 4.12 (dd, 2H, = 7.1 Hz, = 1.7 Hz, ), 6.19 (dd, 1H, = 15.6 Hz, = 7.6 Hz, CH = CH), 7.19 (d, 1H, J = 15.6 Hz, CH = CH), 7.45–7.60 (m, 4H, HAr), 7.79 (d, 1H, J = 8.2 Hz, HAr), 7.87 (d, 1H, J = 7.7 Hz, HAr), 8.13 (d, 1H, J = 8.2 Hz, HAr); C NMR (151 MHz, CDCl), [ppm]: 14.34 (OCHCH), 20.39 ()), 34.57 (CH(CH), 41.93 (CH), 60.38 (OCHCH), 125.90 (CH = CH), 133.62 (CH = CH), 172.50 (C = O), CAr: 123.74, 123.92, 125.65, 125.71, 125.90, 126.23, 127.55, 128.50, 131.21, 135.33, 137.66; IR (cm): 1727, 1589, 1507, 1455, 1392, 1365, 1345, 1268, 1239, 1164, 1094, 1028, 968, 861, 791, 775, 732; HR-MS (ESI-TOF) calculated for CHO, m/z [M + Na]: 291.136092; experimental value: 291.137301.
Eethyl 3-methyl-5-phenylpent-4-enoate (3b): The product was obtained as a yellow oil with a yield of 59.26%; = 1.5201; = 0.37 (EtOAc:hexane 1:20); H NMR (CDCl, 500 MHz), [ppm]: 1.15 (d, 3H, J = 6.8 Hz, CH(CH)), 1.23 (t, 3H, J = 7.1 Hz, OCHCH), 2.35 (dd, 1H, = 14.8 Hz, = 7.3 Hz, CHaHb), 2.42 (dd, 1H, = 14.8 Hz, = 7.3 Hz, CHaHb), 2.80–2.92 (m, 1H, )), 4.12 (q, 2H, J = 7.1 Hz, OCHCH), 6.14 (dd, 1H, = 15.9 Hz, = 7.6 Hz, CH = CH), 6.40 (d, 1H, J = 15.9 Hz, CH = CH), 7.17–7.39 (m, 5H, HAr); C NMR (125 MHz, CDCl), [ppm]: 13.82 (OCHCH), 19.74 ()), 33.62 (), 41.30 (CH), 59.79 (OCHCH), 125.63 (CH = CH), 133.79 (CH = CH), 171.91 (C = O), CAr: 126.08, 126.63, 128.01, 136.95; IR (cm): 1731, 1597, 1494, 1450, 1368, 1348, 1298, 1285, 1239, 1162, 1100, 1079, 1071, 1030, 964, 929, 909, 842, 747, 690; HR-MS (ESI-TOF) calculated for CHO, m/z [M + Na]: 241.120443; experimental value: 241.121304.
Ethyl 5-(4-fluorophenyl)-3-methylpent-4-enoate (3c): The product was obtained as a yellow oil with a yield of 48.50%; = 1.5201, = 0.38 (EtOAc:hexane 1:20); H NMR (CDCl, 500 MHz), [ppm]: 1.15 (d, 3H, J = 6.8 Hz, CH(CH)), 1.23 (t, 3H, J = 7.1 Hz, OCHCH), 2.35 (dd, 1H, = 14.8 Hz, = 7.2 Hz, CHaHb), 2.41 (dd, 1H, = 14.8 Hz, = 7.4 Hz, CHaHb), 2.78–32.92 (m, 1H, CH(CH)), 4.13 (q, 2H, J = 7.1 Hz, OCHCH)), 6.05 (dd, 1H, = 15.9 Hz, = 7.6 Hz, CH = CH), 6.36 (d, 1H, J = 15.9 Hz, CH = CH), 6.98 (t, 2H, J = 8.7 Hz, HAr), 7.24–37.32 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 14.27 (OCHCH), 20.19 (CH(CH)), 34.04 (CH(CH)), 41.74 (CH), 60.26 (OCHCH), 127.68 (CH = CH), 133.99 (CH = CH), 172.32 (C = O), CAr: 115.32, 127.53, 133.55, 162.03; IR (cm): 1732, 1603, 1506, 1459, 1415, 1370, 1348, 1298, 1283, 1225, 1207, 1174, 1156, 1095, 1079, 1028, 970, 935, 851, 814, 772; HR-MS (ESI-TOF) calculated for CHFO, m/z [M + Na]: 259.111021; experimental value: 259.111099.
3.3. Basic Hydrolysis of Esters
The ,-unsaturated carboxylic acids were obtained by alkaline hydrolysis of esters 3a–c. The reaction occurred in ethanolic KOH under reflux conditions (2 h). The excess of ethanol was evaporated and the residue was acidified with 0.1 M hydrochloric acid. The product was extracted with diethyl ether and dried over anhydrous magnesium sulfate. Spectral data are given below.
3-Methyl-5-(naphthalen-1-yl)pent-4-enoic acid (4a): The product was obtained as a colorless oil with a yield of 98%; = 1.5940; = 0.16 (EtOAc:hexane 1:5); H NMR (CDCl, 500 MHz), [ppm]: 1.23 (d, 3H, J = 6.8 Hz, CH(CH)), 2.46 (dd, 1H, = 15.2 Hz, = 7.1 Hz, CHaHb), 2.54 (dd, 1H, = 15.2 Hz, = 7.4 Hz, CHaHb), 2.92–3.03 (m, 1H, CH(CH)), 6.13 (dd, 1H, = 15.6 Hz, = 7.5 Hz, CH = CH), 7.15 (d, 1H, J = 15.6 Hz, CH = CH), 7.53–7.37 (m, 5H, HAr), 7.72 (d, 1H, J = 8.2 Hz, HAr), 7.81 (d, 1H, J = 8.7 Hz, HAr), 8.06 (d, 1H, J = 8.5 Hz, HAr), 9.28 (s, 1H, ); C NMR (125 MHz, CDCl), [ppm]: 20.25 (CH(CH)), 34.14 (CH(CH)), 41.50 (CH), 125.86 (CH = CH), 137.16 (CH = CH), 178.47 (C = O), CAr: 123.70, 123.84, 125.57, 125.65, 126.46, 127.54, 128.41, 131.13, 133.52, 135.15; IR (cm): 2600, 1704, 1591, 1507, 1451, 1406, 1394, 1374, 1288, 1230, 1170, 1140, 1089, 1069, 1013, 965, 911, 857, 791, 773, 730; HR-MS (ESI-TOF) calculated for CHO, m/z [M + Na]: 263.104794; experimental value: 263.105649.
3-methyl-5-phenylpent-4-enoic acid (4b): The product was obtained as a colorless oil with a yield of 96.5%; = 1.5434; = 0.15 (EtOAc:hexane 1:5); H NMR (CDCl, 500 MHz), [ppm]: 1.17 (d, 3H, J = 6.8 Hz, CH(CH)), 2.39 (dd, 1H, = 15.3 Hz, = 7.3 Hz, CHaHb), 2.48 (dd, 1H, = 15.3 Hz, = 7.2 Hz, CHaHb), 2.80–2.91 (m, 1H, CH(CH)), 6.14 (dd, 1H, = 15.9 Hz, = 7.5 Hz, CH = CH), 6.41 (d, 1H, J = 15.9 Hz, CH = CH), 7.16–7.37 (m, 5H, HAr), 10.14 (s, 1H, ); C NMR (125 MHz, CDCl), [ppm]: 20.17 (CH(CH)), 33.66 (CH(CH)), 41.39 (CH), 127.09 (CH = CH), 133.84 (CH = CH), 178.68 (C = O), CAr: 126.14, 128.50, 129.05, 137.30; IR (cm): 2630, 1702, 1598, 1494, 1446, 1408, 1374, 1347, 1288, 1250, 1218, 1173, 1144, 1096, 1074, 1024, 965, 931, 909, 859, 841, 746, 689, 664; HR-MS (ESI-TOF) calculated for CHO, m/z [M + Na]: 213.089144; experimental value: 213.088930.
5-(4-fluorophenyl)-3-methylpent-4-enoic acid (4c): The product was obtained as a colorless oil with a yield of 98.0%; = 1.5205, = 0.16 (EtOAc:hexane, 1:5); H NMR (CDCl, 500 MHz), [ppm]: 1.17 (d, 3H, J = 6.8 Hz, CH(CH)), 2.40 (dd, 1H, = 15.3 Hz, = 7.2 Hz, CHaHb), 2.47 (dd, 1H, = 15.3 Hz, = 7.2 Hz, CHaHb), 2.79–2.90 (m, 1H, CH(CH)), 6.05 (dd, 1H, = 15.9 Hz, = 7.5 Hz, CH = CH), 6.38 (d, 1H, J = 15.9 Hz, CH = CH), 6.93–7.02 (m, 2H, HAr), 7.27–7.32 (m, 2H, HAr), 11.06 (s, 1H, ); C NMR (125 MHz, CDCl), [ppm]: 20.19 (CH(CH)), 33.65 (CH(CH)), 41.36 (CH), 127.97 (CH = CH), 133.56 (CH = CH), 178.60 (C = O), CAr: 115.37 (d, = 21.6 Hz), 127.61 (d, = 7.8 Hz), 133.44 (d, = 3.3 Hz), 162.11 (d, = 246.20 Hz); IR (cm): 2622, 1698, 1593, 1508, 1461, 1431, 1411, 1378, 1354, 1314, 1261, 1221, 1190, 1160, 1128, 1100, 1079, 1012, 974, 960, 952, 935, 855, 812, 770, 703; HR-MS (ESI-TOF) calculated for CHFO, m/z [M + Na]: 231.079723; experimental value: 231.080843.
3.4. The Chlorolactonization of Unsaturated Carboxylic Acids 4a–c
The mixture of an unsaturated carboxylic acid (0.005 mol) and N-chlorosuccinimide (0.009 mol) was dissolved in 60 mL of THF. Acetic acid was added dropwise and the reaction mixture was stirred at room temperature for 48 h. When the reaction was completed, the mixture was dissolved in diethyl ether, washed with saturated and dried over anhydrous magnesium sulfate. The crude product was purified by column chromatography on silica gel using a mixture of acetone and hexane (1:10) [28]. Spectral data are given below. The chlorolactonization of 4a gave chlorolactones 5a and 6a with a total yield of 51.19% (5a:6a 81.55:18.45). The chlorolactonization of 4b gave chlorolactones 5b and 6b with a total yield of 82.21% (5b:6b 84.42:15.58). The chlorolactonization of 4c gave chlorolactones 5c and 6c with a total yield of 61.67% (5c:6c 81.02:18.98).
Trans,trans-5-chloro-tetrahydro-4-methyl-6-(naphthalen-1-yl)pyran-2-one (5a): The product was obtained as a colorless solid; mp = 157–158 C; = 0.19 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.29 (d, 3H, J = 6.4 Hz, CH(CH)), 2.47–2.61 (m, 2H, CH(CH), CHaHb), 3.07 (dd, 1H, = 18.5 Hz, = 10.0 Hz, CHaHb), 4.18 (t, 1H, J = 9.7 Hz, CHCl), 5.99 (d, 1H, J = 10.0 Hz, CHAr), 7.47–7.61 (m, 4H, HAr), 7.90 (d, 2H, J = 8.3 Hz, HAr), 8.09 (d, 1H, J = 8.5 Hz, HAr); C NMR (125 MHz, CDCl), [ppm]: 19.70 (CH(CH)), 35.98 (CH(CH)), 37.15 (CH), 62.72 (CHCl), 81.97 (CHAr), 169.03 (C = O), CAr: 123.01, 125.00, 125.85, 125.97, 126.62, 129.09, 130.10, 131.18, 131.85, 133.87; IR (cm): 1727, 1596, 1512, 1453, 1408, 1375, 1350, 1324, 1286, 1263, 1239, 1216, 1175, 1105, 1085, 1062, 1022, 997, 950, 900, 860, 834, 805, 775, 732, 674, 640; HR-MS (ESI-TOF) calculated for , m/z [M + K]: 313.039760; experimental value: 313.041132.
Cis,trans-5-chloro-tetrahydro-4-methyl-6-(naphthalen-1-yl)pyran-2-one (6a): The product was obtained as a colorless solid; mp = 98–99.5 C; = 0.28 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 0.98 (d, 3H, J = 6.5 Hz, CH(CH)), 2.03–2.11 (m, 1H, CH(CH)), 2.67 (dd, 1H, = 18.7 Hz, = 11.4 Hz, CHaHb), 2.75 (dd, 1H, = 18.7 Hz, = 6.3 Hz, CHaHb), 4.51 (t, 1H, J = 1.9 Hz, CHCl), 6.55 (d, 1H, J = 1.9 Hz, CHAr), 7.44–7.59 (m, 4H, HAr), 7.89 (d, 2H, J = 8.2 Hz, HAr), 8.07 (d, 1H, J = 8.4 Hz, HAr); C NMR (125 MHz, CDCl), [ppm]: 17.81 (CH(CH)), 27.12 (CH(CH)), 33.06 (CH), 62.19 (CHCl), 84.33 (CHAr), 169.24 (C = O), CAr: 121.76, 123.56, 125.22, 126.30, 127.35, 129.46, 129.51, 130.12, 132.99, 133.83; IR (cm): 1733, 1598, 1507, 1455, 1393, 1342, 1281, 1218, 1169, 1112, 1074, 1038, 997, 945, 909, 886, 863, 802, 777, 737, 703, 615; HR-MS (ESI-TOF) calculated for , m/z [M + Na]: 297.065822; experimental value: 297.067366.
Trans,trans-5-chloro-tetrahydro-4-methyl-6-phenylpyran-2-one (5b): The product was obtained as a colorless solid; mp = 105–106.5 C, = 0.18 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.25 (d, 3H, J = 6.4 Hz, CH(CH)), 2.35–2.47 (m, 2H, J = 17.2 Hz, CHaHb), 2.92–3.01 (m, 1H, CHaHb), 3.76 (t, 1H, J = 9.8Hz, CHCl), 5.18 (d, 1H, J = 10.0 Hz, CHAr), 7.33–7.43 (m, 5H, HAr); C NMR (125 MHz, CDCl), [ppm]: 19.62 (CH(CH)), 35.22 (CH(CH), 37.10 (CH), 63.27 (CHCl), 85.05 (CHAr), 168.95 (C = O), CAr: 127.51, 128.52, 129.29, 136.44; IR (cm): 1730, 1496, 1460, 1401, 1376, 1345, 1288, 1240, 1218, 1184, 1107, 1074, 1055, 1031, 1020, 995, 930, 906, 893, 850, 800, 757, 670, 651, 618; HR-MS (ESI-TOF) calculated for , m/z [M + Na]: 247.050173; experimental value: 247.051037.
Cis,trans-5-chloro-tetrahydro-4-methyl-6-phenylpyran-2-one (6b): The product was obtained as a colorless oil; = 1.5453; = 0.27 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.05 (d, 3H, J = 6.6 Hz, CH(CH)), 2.24–2.36 (m, 1H, CH(CH)), 2.59 (dd, 1H, = 18.4 Hz, = 10.3 Hz, CHaHb), 2.64 (dd, 1H, = 18.4 Hz, = 6.9 Hz, CHaHb), 4.33 (t, 1H, J = 2.5 Hz, CHCl), 5.79 (d, 1H, J = 2.5 Hz, CHAr), 7.25–7.28 (m, 2H, HAr), 7.32–7.47 (m, 3H, HAr); C NMR (125 MHz, CDCl), [ppm]: 17.65 (CH(CH)), 27.07 (CH(CH), 33.54 (CH), 63.32 (CHCl), 85.20 (CHAr), 168.97 (C = O), CAr: 125.30, 128.67, 129.00, 137.80; IR (cm): 1736, 1949, 1453, 1415, 1370, 1333, 1286, 1243, 1220, 1167, 1112, 1071, 1031, 1004, 949, 909, 881, 860, 830, 802, 757, 710, 699, 606; HR-MS (ESI-TOF) calculated for , m/z [M + Na]: 247.050173; experimental value: 247.050609.
Trans,trans-5-chloro-6-(4-fluorophenyl)-tetrahydro-4-methylpyran-2-one (5c): The product was obtained as a colorless solid; mp = 80–81 C; = 0.16 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.25 (d, 3H, J = 6.3 Hz, CH(CH)), 2.34–2.49 (m, 2H, CHaHb, CH(CH)), 2.94–3.05 (m, 1H, CHaHb), 3.71 (t, 1H, J = 9.8 Hz, CHCl), 5.17 (d, 1H, J = 10.0 Hz, CHAr), 7.03–7.13 (m, 2H, HAr), 7.32–7.42 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 19.62 (CH(CH)), 35.51 (CH(CH), 37.04 (CH), 63.25 (CHCl), 84.32 (CHAr), 168.71 (C = O), CAr: 115.53 (d, = 21.9 Hz), 129.35 (d, = 8.4 Hz), 132.33 (d, = 3.2 Hz), 163.10 (d, = 248.4 Hz); IR (cm): 1731, 1609, 1514, 1453, 1435, 1427, 1381, 1352, 1334, 1260, 1221, 1192, 1160, 1105, 1083, 1065, 1035, 1010, 960, 919, 883, 844, 825, 810, 786, 719, 687; HR-MS (ESI-TOF) calculated for , m/z [M + Na]: 265.040751; experimental value: 265.041057.
Cis,trans-5-chloro-6-(4-fluorophenyl)-tetrahydro-4-methylpyran-2-one (6c): The product was obtained as a colorless oil; = 1.5453, = 0.27 (acetone:heksane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.10 (d, 3H, J = 6.6 Hz, CH(CH)), 2.27–2.37 (m, 1H, CH(CH)), 2.62 (dd, 1H, = 18.4 Hz, = 9.7 Hz, CHaHb), 2.66 (dd, 1H, = 18.4 Hz, = 7.0 Hz, CHaHb), 4.30 (t, 1H, J = 2.8 Hz, CHCl), 5.75 (d, 1H, J = 2.7 Hz, CHAr), 7.09–7.15 (m, 2H, HAr), 7.23–7.33 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 17.42 (CH(CH)), 27.41 (CH(CH), 33.71 (CH), 63.02 (CHCl), 84.46 (CHAr), 168.69 (C = O), CAr: 116.02 (d, = 21.9 Hz), 127.29 (d, = 8.3 Hz), 133.56 (d, = 3.1 Hz), 163.65 (d, = 248.4 Hz); IR (cm): 1737, 1605, 1510, 1453, 1414, 1372, 1358, 1340, 1286, 1272, 1218, 1176, 1155, 1110, 1096, 1078, 1060, 1040, 1000, 950, 911, 888, 860, 834, 796, 745, 723, 692, 631; HR-MS (ESI-TOF) calculated for , m/z [M + Na]: 265.040751; experimental value: 265.041871.
The bromolactonization of unsaturated carboxylic acids 4a–c: The mixture of an unsaturated carboxylic acid (0.005 mol) and N-bromosuccinimide (0.01 mol) was dissolved in 60 mL of THF. Acetic acid was added dropwise and reaction mixture was stirred at room temperature for 48 h. When the reaction was completed, the mixture was dissolved in diethyl ether, washed with saturated NaHCO and dried over anhydrous magnesium sulfate. The crude product was purified by column chromatography on silica gel using a mixture of acetone and hexane (1:10) [28]. Spectral data are given below. The bromolactonization of 4a gave bromolactones 7a and 8a with a total yield of 72.23% (7a:8a 82.30:17.70). The bromolactonization of 4b gave bromolactones 7b and 8b with a total yield of 71.94% (7b:8b 78.15:21.85). The bromolactonization of 4c gave bromolactones 7c and 8c with a total yield of 77.72% (7c:8c 76.27:23.73).
Trans,trans-5-bromo-tetrahydro-4-methyl-6-(naphthalen-1-yl)pyran-2-one (7a): The product was obtained as a colorless solid; mp = 197–198 C; = 0.13 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.33 (d, 3H, J = 6.6 Hz, CH(CH)), 2.55 (dd, 1H, = 17.4 Hz, = 10.1 Hz, CHaHb), 2.59–2.72 (m, 1H, CH(CH)), 3.08 (dd, 1H, = 17.4 Hz, = 5.7 Hz, CHaHb), 4.28 (dd, 1H, = 10.3 Hz, = 9.6 Hz, ), 6.12 (d, 1H, J = 10.3 Hz, CHAr), 7.49–7.53 (m, 2H, HAr), 7.53–7.59 (m, 4H, HAr), 7.88–7.93 (m, 2H, HAr), 8.10 (d, 1H, J = 8.6 Hz, HAr); C NMR (125 MHz, CDCl), [ppm]: 21.13 (CH(CH)), 36.34 (CH(CH)), 37.49 (CH), 54.75 (), 82.12 (CHAr), 169.22 (C = O), CAr: 123.06, 125.01, 125.89, 126.08, 126.65, 129.14, 130.19, 131.14, 132.24, 133.90; IR (cm): 1724, 1596, 1514, 1453, 1413, 1400, 1376, 1345, 1324, 1285, 1236, 1187, 1170, 1105, 1062, 1013, 994, 947, 906, 893, 860, 834, 800, 773, 735, 660, 631; HR-MS (ESI-TOF) calculated for CHBrO, m/z [M + Na]: 341.015305; experimental value: 341.016902.
Cis,trans-5-bromo-tetrahydro-4-methyl-6-(naphthalen-1-yl)pyran-2-one (8a): The product was obtained as a yellow oil, = 1.5140, = 0.22 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 0.96 (d, 3H, J = 6.4 Hz, CH(CH)), 2.04–2.12 (m, 1H, CH(CH)), 2.66 (dd, 1H, = 18.5 Hz, = 11.4 Hz, CHaHb), 2.72 (ddd, 1H, = 18.5 Hz, = 6.3 Hz, = 1.0 Hz, CHaHb), 4.62 (m, 1H, ), 6.65 (d, 1H, J = 1.7 Hz, CHAr), 7.33–7.71 (m, 4H, HAr), 7.83 (d, 1H, J = 8.2 Hz, HAr), 7.88 (d, 1H, J = 8.2 Hz, HAr), 7.92–7.96 (m, 1H, HAr); C NMR (125 MHz, CDCl), [ppm]: 19.26 (CH(CH)), 27.13 (CH(CH)), 34.27 (CH), 56.19 (), 84.74 (CHAr), 169.10 (C = O), CAr: 121.81, 123.52, 125.22, 126.32, 127.41, 129.15, 129.47, 129.52, 133.60, 133.85; IR (cm): 1740, 1596, 1507, 1453, 1390, 1340, 1270, 1236, 1200, 1153, 1107, 1076, 1037, 994, 947, 904, 886, 861, 800, 780, 746, 683, 606; HR-MS (ESI-TOF) calculated for CHBrO, m/z [M + K]: 356.989243; experimental value: 356.990538.
Trans,trans-5-bromo-tetrahydro-4-methyl-6-phenylpyran-2-one (7b): The product was obtained as a colorless solid; mp = 111–112 C; = 0.16 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.28 (d, 3H, J = 6.5 Hz, CH(CH)), 2.44 (dd, 1H, = 17.2 Hz, = 10.0 Hz, CHaHb), 2.47–2.59 (m, 1H, CH(CH)), 2.99 (dd, 1H, = 17.2 Hz, = 5.6 Hz, CHaHb), 3.86 (dd, 1H, = 10.2 Hz, = 9.7 Hz, ), 5.32 (d, 1H, J = 10.3 Hz, CHAr), 7.34–7.44 (m, 5H, HAr); C NMR (125 MHz, CDCl), [ppm]: 21.04 (CH(CH)), 35.84 (CH(CH), 37.39 (CH), 55.44 (), 85.36 (CHAr), 169.19 (C = O); CAr: 127.59, 128.55, 129.36, 136.70; IR (cm): 1720, 1497, 1458, 1372, 1345, 1284, 1248, 1184, 1170, 1103, 1061, 1006, 956, 906, 889, 845, 775, 753, 696, 631, 617; HR-MS (ESI-TOF) calculated for CHBrO, m/z [M + Na]: 290.999656; experimental value: 291.000717.
Cis,trans-5-bromo-tetrahydro-4-methyl-6-phenylpyran-2-one (8b): The product was obtained as a yellow oil; = 1.5140, = 0.23 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.04 (d, 3H, J = 6.5 Hz, CH(CH)), 2.05–2.14 (m, 1H, CH(CH)), 2.59 (dd, 1H, = 18.4 Hz, = 10.8 Hz, CHaHb), 2.66 (dd, 1H, = 18.4 Hz, = 6.0 Hz, CHaHb), 4.46 (t, 1H, J = 2.5 Hz, ), 5.89 (d, 1H, J = 2.5 Hz, CHAr), 7.27–7.45 (m, 5H, HAr); C NMR (125 MHz, CDCl), [ppm]: 19.07 (CH(CH)), 27.18 (CH(CH), 34.69 (CH), 57.01 (), 85.53 (CHAr), 168.80 (C = O), CAr: 125.35, 128.67, 129.01, 138.31; IR (cm): 1742, 1598, 1451, 1370, 1338, 1281, 1270, 1239, 1162, 1105, 1067, 1035, 1001, 954, 920, 850, 800, 764, 740, 701, 683; HR-MS (ESI-TOF) calculated for CHBrO, m/z [M + K]: 306.973594; experimental value: 306.974724.
Trans,trans-5-bromo-6-(4-fluorophenyl)-tetrahydro-4-methylpyran-2-one (7c): The product was obtained as a colorless solid; mp = 123–124 C; = 0.12 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.28 (d, 3H, J = 6.5 Hz, CH(CH)), 2.44 (dd, 1H, = 17.2 Hz, = 10.0 Hz, CHaHb), 2.47–2.61 (m, 1H, CH(CH)), 2.99 (dd, 1H, = 17.2 Hz, = 5.6 Hz, CHaHb), 3.80 (dd, 1H, = 10.4 Hz, = 9.7 Hz, ), 5.31 (d, 1H, J = 10.4 Hz, CHAr), 7.04–7.16 (m, 2H, HAr), 7.32–7.42 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 21.07 (CH(CH)), 35.88 (CH(CH), 37.33 (CH), 55.53 (), 84.64 (CHAr), 168.93 (C = O), CAr: 115.56 (d, = 21.8 Hz), 129.48 (d, = 8.5 Hz), 132.77 (d, = 3.3 Hz), 163.14 (d, = 248.6 Hz); IR (cm): 1729, 1607, 1512, 1454, 1424, 1372, 1331, 1261, 1220, 1190, 1160, 1035, 1004, 850, 918, 877, 840, 823, 790, 773, 719, 660; HR-MS (ESI-TOF) calculated for CHBrO, m/z [M + Na]: 308.990234; experimental value: 308.991651.
Cis,trans-5-bromo-6-(4-fluorophenyl)-tetrahydro-4-methylpyran-2-one (8c): The product was obtained as a colorless oil; = 1.5140, = 0.21 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.07 (d, 3H, J = 6.5 Hz, CH(CH)), 2.08–2.12 (m, 1H, CH(CH)), 2.60 (dd, 1H, = 18.4 Hz, = 10.3 Hz, CHaHb), 2.67 (ddd, 1H, = 18.4 Hz, = 6.1 Hz, = 0.9 Hz, CHaHb), 4.41 (td, 1H, = 3.1 Hz, = 0.8 Hz, ), 5.84 (dd, 1H, = 3.1 Hz, = 0.5 Hz CHAr), 7.08–7.13 (m, 2H, HAr), 7.24–7.29 (m, 2H, HAr); C NMR (125Hz, CDCl), [ppm]: 18.84 (CH(CH)), 27.54 (CH(CH), 34.84 (CH), 56.58 (), 84.80 (CHAr), 168.63 (C = O), CAr: 116.06 (d, = 21.9 Hz), 127.38 (d, = 8.3 Hz), 134.02 (d, = 3.2 Hz), 162.67 (d, = 248.6 Hz); IR (cm): 1740, 1602, 1507, 1453, 1413, 1370, 1359, 1338, 1273, 1227, 1190, 1160, 1115, 1096, 1074, 1062, 1033, 1004, 951, 911, 855, 832, 802, 790, 750, 716, 670; HR-MS (ESI-TOF) calculated for CHBrO, m/z [M + Na]: 308.990234; experimental value: 308.991301.
The iodolactonization of unsaturated carboxylic acids 4a–c: The mixture of an unsaturated carboxylic acid (0.005 mol), diethyl ether and saturated aqueous was stirred for 30 min and a solution of iodine in potassium iodide was dropwise added. The reaction mixture was refluxed for 24 h. When the reaction was completed, the mixture was dissolved in diethyl ether and washed with saturated solution. The organic layer was washed with saturated and dried over anhydrous magnesium sulfate. The crude product was purified by column chromatography on silica gel using a mixture of acetone and hexane (1:15) [29]. Spectral data are given below. The iodolactonization of 4a gave -hydroxylactones 12a and 13a with a total yield of 84.59% (12a:13a 10.24:89.76). The iodolactonization of 4b gave -iodolactones 9b, 10b, -iodoactone 11b and -hydroxylactone 12b, 13b with total yield of 75.28% (9b:10b:11b:12b:13b 41.71:33.19:7.00:13.79:4.65). The iodolactonization of 4c gave -iodolactones 9c, 10c, -iodoactone 11c and -hydroxylactone 12c, 13c with total yield of 68.55% (9c:10c:11c:12c:13c 30.26:37.42:8.00:7.23:18.08).
Trans-dihydro-5-((S)-hydroxy(naphthalen-1-yl)methyl)-4-methylfuran-2(3H)-one (12a): The product was obtained as a yellow oil; = 1.427; = 0.08 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 0.56 (d, 3H, J = 6.8 Hz, CH), 2.11 (dd, 1H, = 17.5 Hz, = 6.6 Hz, CHaHb), 2.72–2.79 (m, 1H, CH(CH)), 2.85 (dd, 1H, = 17.5 Hz, = 9.3 Hz, CHaHb), 4.53 (dd, 1H, = 5.5 Hz, = 2.8 Hz, COCH), 5.92 (d, 1H, J = 2.3 Hz, CHOH), 7.50–7.59 (m, 3H, HAr), 7.80–7.86 (m, 2H, HAr), 7.90 (d, 1H, J = 8.1 Hz, HAr), 7.96 (d, 1H, J = 8.1 Hz, HAr); C NMR (125 MHz, CDCl), [ppm]: 19.74 (CH), 28.38 (CH(CH)), 37.34 (CH), 69.97 (CHOH), 88.20 (COCH), 175.91 (C = O), CAr: 122.11, 123.65, 125.49, 125.82, 126.64, 128.69, 129.18, 129.88, 133.56, 133.69; IR (cm): 3422, 1774, 1593, 1510, 1458, 1417, 1379, 1359, 1327, 1291, 1254, 1209, 1166, 1112, 1092, 1078, 1037, 1008, 975, 936, 920, 881, 854, 823, 802, 782, 741, 694, 660, 628, 615; HR-MS (ESI-TOF) calculated for CHO, m/z [M + Na]: 279.099709; experimental value: 279.100786.
Cis-dihydro-5-((S)-hydroxy(naphthalen-1-yl)methyl)-4-methylfuran-2(3H)-one (13a): The product was obtained as a brown solid; mp = 123–124 C; = 0.06 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.31 (d, 3H, J = 7.0 Hz, CH), 1.57 (s, 1H, ), 2.57 (dd, 1H, = 17.0 Hz, = 8.8 Hz, CHaHb), 2.64 (dd, 1H, = 17.0 Hz, = 8.6 Hz, CHaHb), 2.72–2.83 (m, 1H, CH(CH)), 4.86 (dd, 1H, = 7.2 Hz, = 3.4 Hz, COCH), 5.72 (t, 1H, J = 3.5 Hz, CHOH), 7.47–7.58 (m, 3H, HAr), 7.71 (d, 1H, J = 6.9 Hz, HAr), 7.84 (d, 1H, J = 8.2 Hz, HAr), 7.90 (d, 1H, J = 8.0 Hz, HAr), 8.02 (d, 1H, J = 7.9 Hz, HAr); C NMR (125 MHz, CDCl), [ppm]: 14.30 (CH), 33.02 (CH(CH)), 36.87 (CH), 68.97 (CHOH), 83.96 (COCH), 177.11 (C = O), CAr: 122.28, 122.95, 125.49, 125.76, 126.58, 129.02, 129.24, 130.36, 133.88, 134.90; IR (cm): 3492, 1747, 1593, 1507, 1453, 1415, 1374, 1350, 1327, 1286, 1275, 1257, 1214, 1189, 1173, 1153, 1110, 1096, 1037, 1026, 1000, 942, 918, 852, 834, 787, 773, 755, 730, 655, 644, 622; HR-MS (ESI-TOF) calculated for CHO, m/z [M + K]: 295.073647; experimental value: 295.074508.
Trans,trans-tetrahydro-5-iodo-4-methyl-6-phenylpyran-2-one (9b): The product was obtained as a colorless solid; mp = 107–108 C; = 0.15 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.29 (d, 3H, J = 6.6 Hz, CH(CH)), 2.41 (dd, 1H, = 17.4 Hz, = 9.8 Hz, CHaHb), 2.48–2.61 (m, 1H, CH(CH)), 2.93 (dd, 1H, = 17.4 Hz, = 5.9 Hz, CHaHb), 3.97 (dd, 1H, = 10.9 Hz, = 10.0 Hz, CHI), 5.45 (d, 1H, J = 10.9 Hz, CHAr), 7.32–7.44 (m, 5H, HAr); C NMR (125 MHz, CDCl), [ppm]: 23.57 (CH(CH)), 36.00 (CH(CH), 37.12 (CH), 38.88 (CHI), 86.82 (CHAr), 169.49 (C = O), CAr: 127.74, 128.50, 129.43, 137.53; IR (cm): 1719, 1499, 1453, 1413, 1372, 1340, 1290, 1245, 1211, 1140, 1100, 1070, 996, 950, 933, 907, 887, 850, 769, 743, 695; HR-MS (ESI-TOF) calculated for CHIO, m/z [M + Na]: 338.985797 experimental value: 338.986432.
Cis,trans-tetrahydro-5-iodo-4-methyl-6-phenylpyran-2-one (10b): The product was obtained as a colorless oil, = 1.5227, = 0.19 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 0.97 (d, 3H, J = 6.4 Hz, CH(CH)), 1.27–1.31 (m, 1H, CH(CH)), 2.49 (dd, 1H, = 18.5 Hz, = 10.5 Hz, CHaHb), 2.67 (ddd, 1H, = 18.5 Hz, = 5.6 Hz, = 1.1 Hz, CHaHb), 4.60 (td, 1H, = 3.1 Hz, = 1.1 Hz, CHI), 5.91 (d, 1H, J = 3.1 Hz, CHAr), 7.25–7.43 (m, 5H, HAr); C NMR (125 MHz, CDCl), [ppm]: 21.03 (CH(CH)), 27.34 (CH(CH), 36.36 (CH), 38.08 (), 86.32 (CHAr), 168.32 (C = O), CAr: 125.06, 128.18, 128.51, 138.42; IR (cm): 1735, 1495, 1451, 1407, 1380, 1368, 1354, 1334, 1322, 1285, 1265, 1233, 1190, 1132, 1103, 1061, 1030, 998, 950, 903, 883, 852, 796, 763, 735, 695, 664; HR-MS (ESI-TOF) calculated for CHIO, m/z [M + K]: 354.959735 experimental value: 354.958974.
Cis-dihydro-5-(iodo(phenyl)methyl)-4-methylfuran-2(3H)-one (11b): The product was obtained as a colorless solid, mp = 86–87 C; = 0.15 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.17 (d, 3H, J = 7.0 Hz, CH), 2.32 (dd, 1H, = 17.0 Hz, = 0.5 Hz, CHaHb), 2.87 (dd, 1H, = 17.0 Hz, = 7.4 Hz, CHaHb), 2.96–3.03 (m, 1H, CH(CH)), 4.96 (d, 1H, J = 11.2 Hz, CHI), 5.11 (dd, 1H, = 11.2 Hz, = 4.5 Hz, COCH), 7.34–7.39 (m, 3H, HAr), 7.39–7.44 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 12.82 (CH), 28.38 (CHI), 34.00 (CH(CH)), 38.88 (CH), 84.45 (COCH), 176.00 (C = O), CAr: 127.79, 128.61, 128.85, 140.09; IR (cm): 1765, 1500, 1452, 1414, 1372, 1341, 1290, 1247, 1212, 1141, 1100, 1071, 997, 950, 932, 908, 886, 851, 769, 742, 695; HR-MS (ESI-TOF) calculated for CHIO, m/z [M + Na]: 338.985797 experimental value: 338.986737.
Trans-dihydro-5-(hydroxy(phenyl)methyl)-4-methylfuran-2(3H)-one (12b): The product was obtained as a colorless solid, mp = 86–87 C; = 0.11 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 0.81 (d, 3H, J = 6.9 Hz, CH), 2.10 (dd, 1H, = 17.6 Hz, = 6.7 Hz, CHaHb), 2.57–2.76 (m, 1H, CH(CH)), 2.73 (dd, 1H, = 17.6 Hz, = 9.2 Hz, CHaHb), 4.30 (dd, 1H, = 5.5 Hz, = 3.3 Hz, COCH), 5.09 (t, 1H, J = 3.6 Hz, CHOH), 7.29–7.34 (m, 3H, HAr), 7.36–7.42 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 19.77 (CH), 28.80 (CH(CH)), 37.06 (CH), 72.29 (CHOH), 89.74 (COCH), 176.99 (C = O), CAr: 126.00, 128.12, 128.63, 138.34; IR (cm): 3442, 1767, 1600, 1496, 1451, 1415, 1379, 1356, 1324, 1275, 1225, 1214, 1202, 1162, 1103, 1085, 1060, 1008, 983, 936, 915, 879, 859, 818, 762, 707, 694, 676, 617; HR-MS (ESI-TOF) calculated for CHO, m/z [M + Na]: 229.084059 experimental value: 229.084576.
Cis-dihydro-5-(hydroxy(phenyl)methyl)-4-methylfuran-2(3H)-one (13b): The product was obtained as a colorless solid, mp = 89–91 C; = 0.11 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.32 (d, 3H, J = 6.9 Hz, CH), 1.58 (s, 1H, ), 2.20 (dd, 1H, = 17.2 Hz, = 4.8 Hz, CHaHb), 2.77 (dd, 1H, = 17.3 Hz, = 8.4 Hz, CHaHb), 2.80–2.89 (m, 1H, CH(CH)), 4.85 (dd, 1H, = 7.2 Hz, = 4.0 Hz, COCH), 5.11 (d, 1H, J = 3.9 Hz, CHOH), 7.27–7.35 (m, 2H, HAr), 7.37–7.48 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 19.20 (CH), 32.85 (CH(CH)), 35.03 (CH), 69.70 (CHOH), 84.59 (COCH), 175.49 (C = O), CAr: 125.67, 128.29, 128.78, 138.37; IR (cm): 3419, 1751, 1587, 1497, 1434, 1403, 1387, 1361, 1301, 1255, 1243, 1202, 1167, 1075, 1067, 1054, 1011, 983, 933, 887, 828, 785, 752, 732, 697; HR-MS (ESI-TOF) calculated for CHO, m/z [M + Na]: 229.084059 experimental value: 229.084781.
Trans,trans-6-(4-fluorophenyl)-tetrahydro-5-iodo-4-methylpyran-2-one (9c): The product was obtained as a colorless solid, mp = 136–138 C, = 0.16 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.28 (d, 3H, J = 6.5 Hz, CH(CH)), 2.36 (dd, 1H, = 17.2 Hz, = 10.0 Hz, CHaHb), 2.51–2.57 (m, 1H, CH(CH)), 2.92 (dd, 1H, = 17.5 Hz, = 6.0 Hz, CHaHb), 3.90 (dd, 1H, = 10.8 Hz, = 10.1 Hz, CHI), 5.42 (d, 1H, J = 10.8 Hz, CHAr), 7.05–7.09 (m, 2H, HAr), 7.32–7.36 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 23.6 (CH(CH)), 27.03 (CH(CH), 31.66 (CH), 37.69 (CHI), 86.43 (CHAr), 169.68 (C = O), CAr: 115.53 (d, = 21.7 Hz), 129.58 (d, = 8.4 Hz), 133.78 (d, = 3.3 Hz), 163.09 (d, = 248.60 Hz); IR (cm): 1720, 1601, 1515, 1459, 1421, 1411, 1366, 1334, 1300, 1285, 1223, 1207, 1176, 1162, 1110, 1090, 1071, 1053, 1004, 952, 909, 896, 858, 844, 822, 778, 775, 717; HR-MS (ESI-TOF) calculated for CHFIO, m/z [M + Na]: 356.976375 experimental value: 356.977702.
Cis,trans-6-(4-fluorophenyl)-tetrahydro-5-iodo-4-methylpyran-2-one (10c): The product was obtained as a yellow oil; = 1.5227, = 0.24 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.04 (d, 3H, J = 6.5 Hz, CH(CH)), 1.39–1.48 (m, 1H, CH(CH)), 2.52 (dd, 1H, = 18.4 Hz, = 9.8 Hz, CHaHb), 2.71 (ddd, 1H, = 18.4 Hz, = 5.5 Hz, = 1.0 Hz, CHaHb), 4.55 (td, 1H, = 3.9 Hz, = 0.9 Hz, CHI), 5.86 (d, 1H, J = 3.9 Hz, CHAr), 7.04–7.13 (m, 2H, HAr), 7.22–7.29 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 21.13 (CH(CH)), 28.37 (CH(CH), 30.90 (CH), 36.88 (CHI), 85.92 (CHAr), 168.53 (C = O), CAr: 115.96 (d, = 21.8 Hz), 127.56 (d, = 8.3 Hz), 134.62 (d, = 3.0 Hz), 162.62 (d, = 248.8 Hz); IR (cm): 1735, 1605, 1508, 1453, 1411, 1384, 1366, 1354, 1340, 1328, 1283, 1263, 1225, 1184, 1157, 1133, 1095, 1073, 1056, 998, 946, 907, 890, 851, 829, 802, 782, 750, 714; HR-MS (ESI-TOF) calculated for CHFIO, m/z [M + Na]: 356.976375 experimental value: 356.977461.
Cis-5-((4-fluorophenyl)iodomethyl)-dihydro-4-methylfuran-2(3H)-one (11c): The product was obtained as a colorless solid, mp = 118–119 C; = 0.16 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.16 (d, 3H, J = 7.1 Hz, CH), 2.33 (dd, 1H, = 17.1 Hz, = 0.5 Hz, CHaHb), 2.87 (dd, 1H, = 17.1 Hz, = 7.3 Hz, CHaHb), 2.95–3.00 (m, 1H, CH(CH)), 4.94 (d, 1H, = 11.2 Hz, CHI), 5.04 (dd, 1H, = 11.2 Hz, = 4.5 Hz, COCH), 6.97–7.10 (m, 2H, HAr), 7.32–7.43 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 12.85 (CH), 28.45 (CHI), 34.05 (CH(CH)), 38.91 (CH), 84.30 (COCH), 176.11 (C = O), CAr: 116.24 (d, = 22.1 Hz), 127.64 (d, = 8.0 Hz), 133.62 (d, = 3.0 Hz), 162.38 (d, = 246.3 Hz). IR (cm): 1760, 1600, 1512, 1460, 1420, 1412, 1361, 1335, 1301, 1284, 1226, 1203, 1174, 1161, 1108, 1091, 1070, 1052, 1001, 950, 906, 894, 857, 842, 821, 779, 776, 714; HR-MS (ESI-TOF) calculated for CHFIO, m/z [M + Na]: 356.976375 experimental value: 356.976932.
Trans-5-((4-fluorophenyl)(hydroxy)methyl)-dihydro-4-methylfuran-2(3H)-one (12c): The product was obtained as a colorless solid, mp = 118–119 C, = 0.10 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 0.83 (d, 3H, J = 6.9 Hz, CH), 2.11 (dd, 1H, = 17.7 Hz, = 6.9 Hz, CHaHb), 2.53–2.64 (m, 1H, CH(CH)), 2.74 (dd, 1H, = 17.7 Hz, = 9.2 Hz, CHaHb), 4.26 (dd, 1H, = 5.3 Hz, = 3.4 Hz, COCH), 5.07 (d, 1H, J = 3.2 Hz, CHOH), 6.98–7.12 (m, 2H, HAr), 7.33–7.43 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 19.74 (CH), 28.84 (CH(CH)), 37.03 (CH), 72.70 (CHOH), 89.65 (COCH), 176.93 (C = O), CAr: 115.57 (d, = 21.5 Hz), 127.72 (d, = 8.1 Hz), 134.17 (d, = 3.1 Hz), 163.46 (d, = 246.6 Hz); IR (cm): 3431, 1772, 1593, 1509, 1456, 1412, 1378, 1361, 1329, 1287, 1257, 1215, 1169, 1114, 1093, 1075, 1041, 1008, 975, 936, 920, 881, 854, 826, 802, 782, 749, 715; HR-MS (ESI-TOF) calculated for CHFO, m/z [M + Na]: 247.074638 experimental value: 247.075208.
Cis-5-((4-fluorophenyl)(hydroxy)methyl)-dihydro-4-methylfuran-2(3H)-one (13c): The product was obtained as a colorless solid, mp = 118–119 C; = 0.08 (acetone:hexane 1:7); H NMR (CDCl, 500 MHz), [ppm]: 1.34 (d, 3H, J = 6.8 Hz, CH), 1.59 (s, 1H, ), 2.24 (dd, 1H, = 17.3 Hz, = 4.8 Hz, CHaHb), 2.78 (dd, 1H, = 17.3 Hz, = 8.5 Hz, CHaHb), 2.81–2.89 (m, 1H, CH(CH)), 4.81 (dd, 1H, = 7.3 Hz, = 4.1 Hz, COCH), 5.09 (d, 1H, J = 4.1 Hz, CHOH), 7.14–7.24 (m, 2H, HAr), 7.29–7.38 (m, 2H, HAr); C NMR (125 MHz, CDCl), [ppm]: 19.22 (CH), 32.86 (CH(CH)), 35.05 (CH), 69.72 (CHOH), 84.57 (COCH), 175.47 (C = O), CAr: 116.26 (d, = 22.1 Hz), 127.69 (d, = 8.0 Hz), 133.63 (d, = 3.0 Hz), 162.34 (d, = 246.4 Hz); IR (cm): 3439, 1751, 1592, 1507, 1459, 1410, 1380, 1365, 1327, 1285, 1259, 1216, 1169, 1115, 1094, 1077, 1044, 1010, 973, 937, 920, 882, 853, 827, 801, 782, 748, 716; HR-MS. HR-MS (ESI-TOF) calculated for CHFO, m/z [M + Na]: 247.074638 experimental value: 247.075284.
3.5. Cytotoxic Activity
Cell cultures: The L929 mouse fibroblasts and human gastric adenocarcinoma cell line AGS were used for in vitro cytotoxicity assays. The cells were maintained under standard conditions (37 C, 5% CO) in 25 tissue culture flasks in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and antibiotics: 100 U/mL penicillin and 100 g/mL streptomycin. The cell suspension containing 10 cells in 1 mL, was obtained by treatment of confluent monolayers with 0.25% trypsin solution, washed and subcultured. Cell cultures were supplemented with medium to maintain them in log phase growth. The viability of the cells was assessed by exclusion of trypan blue dye.
Measurements of cellular metabolic activity and global growth inhibition: L929 cells were seeded into 96-well plates (2 × 10 cells/well) for 24 h, at 37 C, 5% . Tested compounds 5a–c, 6a–c, 7a–c, 8a–c, 9b,c, 10b,c, 12a and 13a were diluted serially in RPMI-1640 medium in concentrations of 50, 20, 10, 5, 0.5 and 0.1 g/mL, added to the cells (100 L/well), and incubated under standard conditions for 24 h [43]. The potential harmful effect of lactones on L929 and AGS cells was assessed by the ability of the cells to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan. The solution of MTT (5 mg/mL in PBS), was added to each well and the plates were incubated for 4h at 37 C. Formazan crystals were dissolved with acidic isopropanol (0.1 M HCl in absolute isopropanol) and the plates were read in a plate reader (Victor 2) at 570 nm. The percentage of MTT reduction was calculated by comparing the absorbance of treated cells to that of untreated control cells. Results are expressed as the mean percentage of viable cells ± standard deviation (SD) of four independent experiments. Statistical significance was accepted at a p-value < 0.05.
3.6. Bactericidal Activity
The bacterial cultures: Escherichia coli ATCC 8739 and Staphylococcus aureus ATCC 65389 were grown on Luria–Bertani Agar for 18 h at 37 C. The strain suspension was prepared in 0.85% saline, with an optical density equivalent to a 0.5 McFarland standard. Next, the following mixtures were prepared: 180 L of sterile enriched broth (BTL), 10 L of the bacterial suspension and 10 L of the tested compound (range 0.1–50 g/mL) solubilized in DMSO. As a negative control, only DMSO was added. As a sterility control, 180 L of BTL and 20 L saline were used. The microtiter plate was incubated at 37 C for 18 h. The colony forming unit (CFU) method was used to evaluate the microbial population. The experiment was repeated three times. Gentamicin was used as the reference antibiotic [23].
4. Conclusions
A four-step synthesis gave sixteen new -halo--lactones, two -iodo--lactones and unexpectedly six -hydroxy--lactones. The stereoselectivity of the iodolactonization reaction is significant. The yield of trans,trans chloro- and bromo--lactone was in the range of 76–82%. The iodolactonization of unsaturated carboxylic acids gave cis -hydroxy--lactones with a yield of 90%. All obtained lactones were tested for antimicrobial activity against Escherichia coli strains ATCC 8739 and Staphylococcus aureus ATCC 65389. The -iodolactone 10c and -hydroxy--lactones 12a and 13a showed high bactericidal activity against E. coli. The highest bactericidal activity against S. aureus was observed for lactone 5a, which reduced the number of CFU by 87%. Significant bactericidal activity was observed for cis,trans--chloro--lactone 6c (83%) and cis,trans--bromo--lactone 8a (83%). Synthesized lactones were also tested for cytotoxic activity against two cell lines, mouse fibroblasts L929 and gastric cancer cells AGS. The strongest cell growth inhibition against L929 fibroblasts was observed for cis,trans--iodo--lactones 10b,c and cis,trans--chloro--lactone 6b. The AGS cells were highly sensitive to trans,trans--chloro--lactone 5c, and trans,trans--iodo--lactones 9b,c. Their cytotoxic activity against gastric cancer AGS cells was much higher than doxorubicin, thus these lactones could be promising candidates for anticancer formulations.
Acknowledgments
Special thanks to Division of Biophysics, Faculty of Physics, University of Warsaw for the X-ray diffraction measurements. The Rigaku Oxford Diffraction SuperNova diffractometer was cofinanced by the European Regional Development Fund (POIG.02.01.00-14-122/09).
Abbreviations
The following abbreviations are used in this manuscript:
| NBS | N-bromosuccinimide |
| NCS | N-chlorosuccinimide |
| CFU | the colony forming unit |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| SD | standart deviations |
| the half maximal inhibitory concentration | |
| NMR | nuclear magnetic resonance |
| FTIR | Fourier-transform infrared spectroscopy |
| HR-MS-MS | high-resolution electrospray ionization mass spectra |
| TLC | thin layer chromatography |
Author Contributions
Conceptualization, investigation and writing—original draft preparation, A.K.; supervision and project administration, B.G.; investigation, Ł.L., A.B., S.E.K., W.G., and M.C.; and data curation, writing—review and editing, M.U.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cho J.Y., Baik K.U., Jung J.H., Park M.H. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur. J. Pharmacol. 2000;398:399–407. doi: 10.1016/S0014-2999(00)00337-X. [DOI] [PubMed] [Google Scholar]
- 2.Choi H.G., Lee D.S., Li B., Choi Y.H., Lee S.H., Kim Y.C. Santamarin, a sesquiterpene lactone isolated from Saussurea lappa, represses LPS-induced inflammatory responses via expression of heme oxygenase-1 in murine macrophage cells. Int. Immunopharmacol. 2012;13:271–279. doi: 10.1016/j.intimp.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Fuqua C., Parsek M.R., Greenberg E.P. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 4.Kelly D.R. When is a butterfly like an elephant? Chem. Biol. 1996;3:595–602. doi: 10.1016/S1074-5521(96)90125-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S., Won Y.K., Ong C.N., Shen H.M. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem. Anticancer. Agents. 2005;5:239–249. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- 6.Babaei G., Aliarab A., Abroon S., Rasmi Y., Aziz S.G. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018;106:239–246. doi: 10.1016/j.biopha.2018.06.131. [DOI] [PubMed] [Google Scholar]
- 7.Mereyala H.B., Joe M. Cytotoxic activity of styryl lactones and their derivatives. Curr. Med. Chem. Anticancer. Agents. 2001;1:293–300. doi: 10.2174/1568011013354606. [DOI] [PubMed] [Google Scholar]
- 8.Woerdenbag H.J., Merfort I., Passreiter C.M., Schmidt T.J., Willuhn G., van Uden W., Pras N., Kampinga H.H., Konings A.W. Cytotoxicity of flavonoids and sesquiterpene lactones from Arnica species against the GLC4 and the COLO 320 cell lines. Planta Med. 1994;60:434–437. doi: 10.1055/s-2006-959526. [DOI] [PubMed] [Google Scholar]
- 9.Sabitha G., Reddy C.N., Raju A., Yadav J.S. Stereoselective total synthesis of the cytotoxic lactone (-)-spicigerolide. Tetrahedron Asymmetry. 2011;22:493–498. doi: 10.1016/j.tetasy.2011.02.010. [DOI] [Google Scholar]
- 10.Bach S.M., Fortuna M.A., Attarian R., de Trimarco J.T., Catalán C.A., Av-Gay Y., Bach H. Antibacterial and cytotoxic activities of the sesquiterpene lactones cnicin and onopordopicrin. Nat. Prod. Commun. 2011;6:163–166. doi: 10.1177/1934578X1100600202. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann J., Cheng T.Y., Aggarwal A., Park A.S., Zeiler E., Raju R.M., Akopian T., Kandror O., Sacchettini J.C., Moody D.B., et al. An Antibacterial β-Lactone Kills Mycobacterium tuberculosis by Disrupting Mycolic Acid Biosynthesis. Angew. Chem. Int. Ed. Engl. 2018;57:348–353. doi: 10.1002/anie.201709365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gören N., Jakupovic J., Topal S. Sesquiterpene lactones with antibacterial activity from Tanacetum argyrophyllum var. argyrophyllum. Phytochemistry. 1990;29:1467–1469. doi: 10.1016/0031-9422(90)80102-M. [DOI] [Google Scholar]
- 13.Castelo-Branco P.A., Rubinger M.M., de Alves L.C., de Barros P.M., Pereira S.G., de Melo V.J., Pilo-Veloso D., Zambolim L. Synthesis and Antifungal Activity of Aromatic Bis-γ-lactones Analogous to Avenaciolide. Chem. Biodivers. 2007;4:2745–2754. doi: 10.1002/cbdv.200790223. [DOI] [PubMed] [Google Scholar]
- 14.Barrero A.F., Oltra J.E., Alvarez M., Raslan D.S., Saúde D.A., Akssira M. New sources and antifungal activity of sesquiterpene lactones. Fitoterapia. 2000;71:60–64. doi: 10.1016/S0367-326X(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 15.Ozçelik B., Gürbüz I., Karaoglu T., Yeşilada E. Antiviral and antimicrobial activities of three sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Microbiol. Res. 2009;164:545–552. doi: 10.1016/j.micres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Hwang D.R., Wu Y.S., Chang C.W., Lien T.W., Chen W.C., Tan U.K., Hsu J.T., Hsieh H.P. Synthesis and anti-viral activity of a series of sesquiterpene lactones and analogues in the subgenomic HCV replicon system. Bioorg. Med. Chem. 2006;14:83–91. doi: 10.1016/j.bmc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 17.Nawrot J., Smitalová Z., Holub M. Deterrent activity of sesquiterpene lactones from the Umbelliferae against storage pests. Biochem. Syst. Ecol. 1983;11:243–245. doi: 10.1016/0305-1978(83)90061-3. [DOI] [Google Scholar]
- 18.Szczepanik M., Dams I., Wawrzeńczyk C. Feeding Deterrent Activity of Terpenoid Lactones with the p-Menthane System Against the Colorado Potato Beetle (Coleoptera: Chrysomelidae) Environ. Entomol. 2005;34:1433–1440. doi: 10.1603/0046-225X-34.6.1433. [DOI] [Google Scholar]
- 19.Dufossé L., Latrasse A., Spinnler H.A. Importance des lactones dans les arômes alimentaires: Structures, distribution, propriétés sensorielles et biosynthèse. Sci. Aliments. 1994;14:17–50. [Google Scholar]
- 20.Solhaug A., Eriksen G.S., Holme J.A. Mechanisms of Action and Toxicity of the Mycotoxin Alternariol: A Review. Basic Clin. Pharmacol. Toxicol. 2016;119:533–539. doi: 10.1111/bcpt.12635. [DOI] [PubMed] [Google Scholar]
- 21.Wzorek A., Gawdzik B., Gładkowski W., Urbaniak M., Barańska A., Malińska M., Woźniak K., Kempińska K., Wietrzyk J. Synthesis, characterization and antiproliferative activity of β-aryl-δ-iodo-γ-lactones. J. Mol. Struct. 2013;1047:160–168. doi: 10.1016/j.molstruc.2013.05.010. [DOI] [Google Scholar]
- 22.Gawdzik B., Wzorek A., Kamizela A., Urbaniak M., Gładkowski W., Lis M., Obmińska-Mrukowicz B., Białońska A. β-Aryl-δ-Hydroxy-γ-Lactones: Synthesis, Structural Analysis and Cytotoxic Activity. Curr. Org. Synth. 2016;13:901–906. doi: 10.2174/1570179413666151218201553. [DOI] [Google Scholar]
- 23.Kamizela A., Gawdzik B., Urbaniak M., Lechowicz Ł., Białońska A., Gonciarz W., Chmiela M. Synthesis, Characterization, Cytotoxicity, and Antibacterial Properties of trans-γ-Halo-δ-lactones. ChemistryOpen. 2018;7:543–550. doi: 10.1002/open.201800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaylord N.G., Becker E.I. The reaction of grignard reagents with 3, 4-epoxy-1-butene. I. 1-napthylmagnesium bromide. J. Org. Chem. 1950;15:305–316. doi: 10.1021/jo01148a012. [DOI] [Google Scholar]
- 25.Johnson W.S., Werthemann L., Bartlett W.R., Brocksom T.J., Li T.T., Faulkner D.J., Petersen M.R. Simple stereoselective version of the Claisen rearrangement leading to trans-trisubstituted olefinic bonds. Synthesis of squalene. J. Am. Chem. Soc. 1970;92:741–743. doi: 10.1021/ja00706a074. [DOI] [Google Scholar]
- 26.Klueggea J., Herdtweckb E., Bach T. Iron(II)-catalyzed chlorolactamization of γ,δ-unsaturated carboxylic acids. Synlett. 2004;7:1199–1202. doi: 10.1002/chin.200443109. [DOI] [Google Scholar]
- 27.Snider B.B., Johnston M.I. Regioselectivity of the halolactonization of γ,δ-unsaturated acids. Tetrahedron Lett. 1985;45:5497–5500. doi: 10.1016/S0040-4039(01)80869-8. [DOI] [Google Scholar]
- 28.Gładkowski W., Skrobiszewski A., Mazur M., Siepka M., Pawlak A., Obmińska-Mrukowicz B., Białońska A., Poradowski D., Drynda A., Urbaniak M. Synthesis and anticancer activity of novel halolactones with β-aryl substituents from simple aromatic aldehydes. Tetrahedron. 2013;69:10414–10423. doi: 10.1016/j.tet.2013.09.094. [DOI] [Google Scholar]
- 29.Mori K., Nakazono Y. Synthesis of both the enantiomers of dihydroactinidiolide. A pheromone component of the red imported fire ant. Tetrahedron. 1986;42:283–290. doi: 10.1016/S0040-4020(01)87429-9. [DOI] [Google Scholar]
- 30.Stoddart J.F. Stereochemistry of Carbohydrates. John Wiley & Sons Inc.; Toronto, ON, Canada: 1971. p. 137. [Google Scholar]
- 31.Liberek B., Sikorski A., Melcer A., Konitz A. X-ray diffraction and high-resolution NMR spectroscopy ofmethyl 3-azido-2,3-dideoxy-α-D-lyxo-hexopyranoside. Carbohydr. Res. 2003;338:795–799. doi: 10.1016/S0008-6215(03)00005-3. [DOI] [PubMed] [Google Scholar]
- 32.CCDC1887715. The Data Can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. [(accessed on 12 May 2019)]; Available online: www.ccdc.cam.ac.uk/structures.
- 33.CCDC1887716. The Data Can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. [(accessed on 12 May 2019)]; Available online: www.ccdc.cam.ac.uk/structures.
- 34.CCDC1887758. The Data Can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. [(accessed on 12 May 2019)]; Available online: www.ccdc.cam.ac.uk/structures.
- 35.CCDC1887719. The Data Can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. [(accessed on 12 May 2019)]; Available online: www.ccdc.cam.ac.uk/structures.
- 36.CCDC1887720. The Data Can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. [(accessed on 12 May 2019)]; Available online: www.ccdc.cam.ac.uk/structures.
- 37.Florou D., Patsis C., Ardavanis A., Scorilas A. Effect of doxorubicin, oxaliplatin, and methotrexate administration on the transcriptional activity of BCL-2 family gene members in stomach cancer cells. Cancer Biol. Ther. 2013;14:587–596. doi: 10.4161/cbt.24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai N., Lai C.S., He K., Zhou Z., Zhang L., Quan Z., Zhu N., Zheng Q.Y., Pan M.H., Ho C.T. Sesquiterpene Lactones from Inula britannica and Their Cytotoxic and Apoptotic Effects on Human Cancer Cell Lines. J. Nat. Prod. 2006;69:531–535. doi: 10.1021/np050437q. [DOI] [PubMed] [Google Scholar]
- 39.Mazur M., Gładkowski W., Podkowik M., Bania J., Nawrot J., Białońska A., Wawrzeńczyk C. Lactones 43. New biologically active lactone: β-cyclocitral derivatives. Pest. Manag. Sci. 2014;70:286–294. doi: 10.1002/ps.3557. [DOI] [PubMed] [Google Scholar]
- 40.Gładkowski W., Włoch A., Pawlak A., Sysak A., Białońska A., Mazur M., Mituła P., Maciejewska G., Obmińska-Mrukowicz B., Kleszczyńska H. Preparation of Enantiomeric β-(2,5-Dimethylphenyl)Bromolactones, Their Antiproliferative Activity and Effect on Biological Membranes. Molecules. 2018;23:3035. doi: 10.3390/molecules23113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CrysAlisPro . Rigaku Oxford Diffraction. CrysAlisPro; Yarnton, UK: 2016. [Google Scholar]
- 42.Petříček V., Dušek M., Palatinus L. Crystallographic comp JANA2006r. general features. Z. Kristallogr. 2014;229:345–352. [Google Scholar]
- 43.ISO 10993-5:2009 . Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO; Geneva, Switzerland: 2009. [Google Scholar]