Abstract
A chemical investigation on 70% EtOH extract from the bark of Phellodendron chinense Schneid (Rutaceae) led to six new methyl apiofuranosides (1–6), and ten known compounds (7–16). All these compounds were characterized by the basic analysis of the spectroscopic data including extensive 1D-, 2D-NMR (HSQC, HMBC), and high-resolution mass spectrometry, and the absolute configurations were determined by both empirical approaches and NOESY. Inhibitory effects of compounds 1–9 and 11–16 on nitric oxide production were investigated in lipopolysaccharide (LPS)-mediated RAW 264.7 cells, as a result, most of these isolates inhibited nitric oxide (NO) release, and among them 9, 11, and 12 displayed the strongest inhibition on NO release at the concentration of 12.5 μM.
Keywords: Phellodendron chinense Schneid, methyl apiofuranoside, nitric oxide
1. Introduction
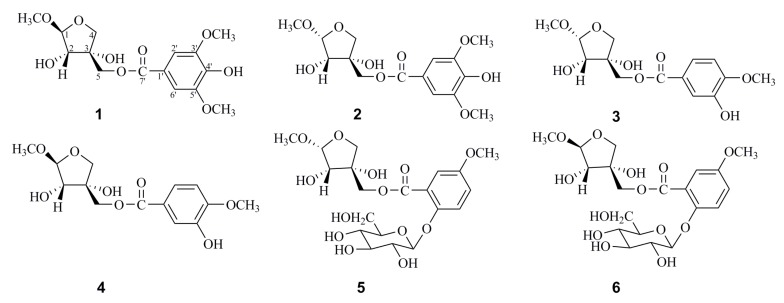
Phellodendron chinense Schneid is a deciduous tree also known as “Huang Bai” and found widely in the east and northeast of Asia [1]. The genus Phellodendron, belonging to the family Rutaceae, comprises of approximately 10 species. Among them, the bark of P. chinense Schneid, well-known as an oriental folk medicine, has been used for the treatment of meningitis, bacillary dysentery, pneumonia, tuberculosis, and liver cirrhosis for centuries, and is also an important natural source of berberine [2,3]. Previous phytochemical studies on this plant revealed the presence of alkaloids, which mainly belong to isoquinoline alkaloids, berberine-type, and aporphine-type [4]. In addition, some flavonoids, triterpenoids, and coumarins were also reported [5,6]. Pharmacology studies have demonstrated that its crude extracts and active compounds possess wide pharmacological activities, especially hypoglycemic, anti-inflammatory, and antibacterial activities [7,8]. The reports of methyl apiofuranosides are relatively rare [9,10]; during the course of our investigation on the chemical components of P. chinense Schneid, six new methyl apiofuranosides (1–6) (Figure 1) and ten phenolic acids (7–16) were obtained. In this report, their isolation, structural characterization, and inhibitory effects on nitric oxide production are described.
Figure 1.
Structures of compounds 1–6.
2. Results
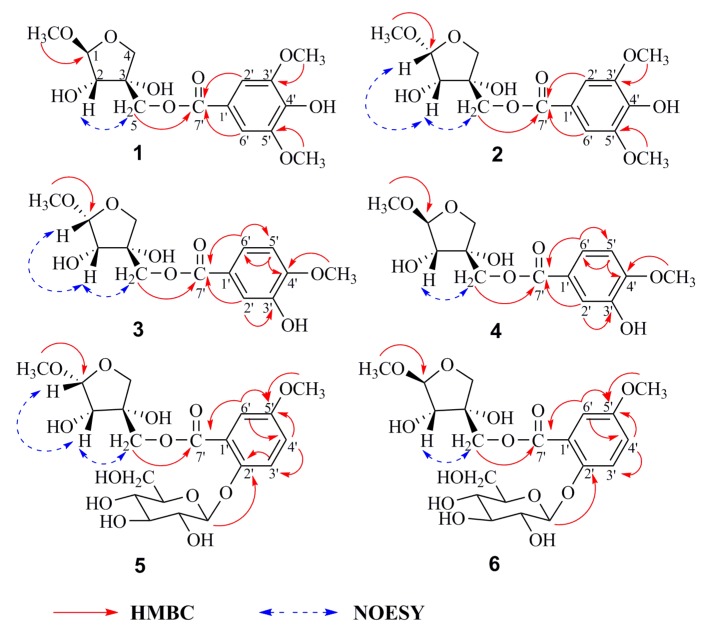
Methyl 5-O-(4′-hydroxy-3′,5′-dimethoxy-benzoyl)-β-d-erythro-apiofuranoside (1), afforded a molecular formula of C15H20O9 based on the (−)-high-resolution electrospray ionization mass spectra (HRESIMS) ion peak at m/z 343.1032 [M − H]− (calcd for C15H19O9, 343.1029), indicating six degrees of hydrogen deficiency. The characteristic IR absorptions demonstrated the presence of hydroxy (3412 cm−1) and benzene ring (1621, 1112, and 615 cm−1) groups. The 1H-NMR spectrum of 1 exhibited typical signals for three methoxyl groups (δH 3.89, s, 3H; 3.89, s, 3H; 3.37, s, 3H) and one set of protons of the aromatic system (δH 7.35, 2H, s) (Table 1). In addition, signals in the region of δH 3.50–5.50 mainly came from one sugar unit, characterized by an anomeric proton signal at δH 4.86 (1H, d, J = 2.5 Hz). In the 13C-NMR spectrum, apart from six characteristic carbon signals for a benzene ring at δC 121.2, 108.5, 149.1, 142.3, 149.1, and 108.5, nine carbon resonances were observed and ascribed to one ester carbonyl at δC 167.9, five oxygen bearing carbons at δC 111.6, 78.9, 79.1, 75.0, and 67.7 and three methoxy carbons at δC 57.0, 57.0, and 56.0 with the aid of an HSQC experiment. These characteristic signals, in combination with the HRESIMS data, implied that 1 is a methyl apiofuranose [11]. According to previous reports, apiose unit with 1-OH and 2-OH in trans configuration presents constant coupling J1,2 0–2 Hz, whereas cis configuration is characterized by J1,2 3–4 Hz [12,13]. It should be noted that there was no apiose authentic sample at hand, and this branched chain sugar can occur in four isomeric forms. The β-d-apiofuranose moiety was characterized on the basis of the carbon chemical shift and the coupling constant of the anomeric proton, in combination with the NOE correlation between H-2 and H-5 [9,14,15,16]. The methyl apiose unit was therefore identified as a methyl β-d-apiofuranoside based on the anomeric proton singlet at δH 4.86 (1H, d, J = 2.5 Hz), the chemical shift of C-1 at 111.6, and on the NOESY correlation between H-2 (δH 3.97, d, J = 2.5 Hz) and H-5 (δH 4.32, s, 2H) by empirical approaches reported by Ishii [9]. Furthermore, the observable HMBC correlations (Figure 2) of 3′-OCH3/C-3′; 5′-OCH3/C-5′; H-2′ and H-6′/C-7′; 1-OCH3/C-1 were used to establish 4′-hydroxy-3′,5′-dimethoxy-benzoyl. HMBC correlations from H-5 (δH 4.32) to the ester carbonyl at δC 167.9 (C=O) suggested that the benzoyl group was attached to C-5 of the methyl apiose unit (Figures S1-1–S1-9). Consequently, the structure of 1 was determined to be methyl 5-O-(4′-hydroxy-3′,5′-dimethoxy-benzoyl)-β-d-erythro-apiofuranoside, and elucidated as shown in Figure 1.
Table 1.
1H-NMR (600 MHz) and 13C-NMR (150 MHz) spectroscopic data for compounds 1–4.
| 1 a | 2 a | 3 a | 4 a | |||||
|---|---|---|---|---|---|---|---|---|
| Position | δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) |
| Api-1 | 111.6 | 4.86 (1H, d, J = 2.5 Hz) | 104.7 | 4.90 (1H, d, J = 4.6 Hz) | 104.7 | 4.90 (1H, d, J = 4.6 Hz) | 111.6 | 4.86 (1H, d, J = 2.6 Hz) |
| 2 | 78.9 | 3.97 (1H, d, J = 2.5 Hz) | 74.5 | 3.99 (1H, d, J = 4.6 Hz) | 74.4 | 3.99 (1H, d, J = 4.6 Hz) | 78.8 | 3.96 (1H, d, J = 2.6 Hz) |
| 3 | 79.1 | 76.6 | 76.7 | 79.1 | ||||
| 4 | 75.0 | 4.04 (1H, d, J = 9.8 Hz) | 75.4 | 4.07 (1H, d, J = 9.9 Hz) | 75.4 | 4.07 (1H, d, J = 9.8 Hz) | 75.0 | 4.03 (1H, d, J = 9.8 Hz) |
| 3.89 (1H, d, J = 9.8 Hz) | 3.91 (1H, d, J = 9.9 Hz) | 3.91 (1H, d, J = 9.8 Hz) | 3.89 (1H, d, J = 9.8 Hz) | |||||
| 5 | 67.7 | 4.32 (2H, m) | 68.7 | 4.29 (2H, m) | 68.4 | 4.28 (2H, m) | 67.5 | 4.31 (2H, m) |
| 1′ | 121.2 | 121.2 | 125.4 | 125.4 | ||||
| 2′ | 108.5 | 7.35 (1H, s) | 108.5 | 7.35 (1H, s) | 116.1 | 7.59 (1H, d, J = 1.9 Hz) | 116.1 | 7.59 (1H, d, J = 1.9 Hz) |
| 3′ | 149.1 | 149.1 | 148.9 | 148.9 | ||||
| 4′ | 142.3 | 142.3 | 153.2 | 153.2 | ||||
| 5′ | 149.1 | 149.1 | 113.9 | 6.84 (1H, d, J = 8.8 Hz) | 113.8 | 6.85 (1H, d, J = 8.0 Hz) | ||
| 6′ | 108.5 | 7.35 (1H, s) | 108.5 | 7.35 (1H, s) | 122.4 | 7.57 (1H, dd, J = 8.8, 1.9 Hz) | 122.4 | 7.58 (1H, dd, J = 8.0, 1.9 Hz) |
| 7′ | 167.9 | 167.9 | 168.0 | 168.0 | ||||
| 3′-OCH3 | 57.0 | 3.89 (3H, s) | 57.0 | 3.88 (3H, s) | ||||
| 4′-OCH3 | 56.6 | 3.90 (3H, s) | 56.6 | 3.90 (3H, s) | ||||
| 5′-OCH3 | 57.0 | 3.89 (3H, s) | 57.0 | 3.88 (3H, s) | ||||
| 1-OCH3 | 56.0 | 3.37 (3H, s) | 56.0 | 3.43 (3H, s) | 55.7 | 3.43 (3H, s) | 55.7 | 3.38 (3H, s) |
a Data were recorded in CD3OD.
Figure 2.
Key HMBC and NOESY correlations of compounds 1–6.
The same molecular formula, C15H20O9, for 2 (methyl 5-O-(4′-hydroxy-3′,5′-dimethoxy-benzoyl)-α-d-erythro-apiofuranoside) was established based on its (−)-HRESIMS ion peak at m/z 343.1031 (calcd for C15H19O9, 343.1029). Analysis of the NMR spectroscopic data suggests that 2 shares the same methyl apiofuranoside skeleton as 1, except for two differences in the chemical shift of C-1 and J1,2. Their respective chemical shift of C-1 exhibited 104.7 in 2 but 111.6 in 1, furthermore, J1,2 = 4.6 Hz in 2 and J1,2 = 2.5 Hz in 1 (Table 1). The methyl apiose unit was therefore identified as a methyl α-d-apiofuranoside based on the anomeric proton singlet at δH 4.90 (1H, d, J = 4.6 Hz), the chemical shift of C-1 at δC 104.7, and on the NOESY correlation among H-2 (δH 3.99, d, J = 4.6 Hz), H-5 (δH 4.29, m, 2H), and H-1 (δH 4.90, d, J = 4.6 Hz) [14]. In comparison of the NOESY spectrum of 2 and 1, there are correlations between H-2/H-1 and H-5/H-1 in 2, while H-2/H-5 has the correlation in 1 only (Figures S2-1–S2-9). The structure of compound 2 was proposed as shown.
Methyl 5-O-(2′-hydroxyl-5′-methoxyl-benzoyl)-α-d-erythro-apiofuranoside (3), afforded a molecular formula of C14H18O8 based on the (−)-HRESIMS ion peak at m/z 313.0921 [M − H]− (calcd for C14H17O8, 313.0923), corresponding to six degrees of hydrogen deficiency. The characteristic IR absorptions demonstrated the presence of hydroxy (3415 cm−1) and benzene ring (1604 and 1458 cm−1) groups. The 1H-NMR spectrum indicated that compound 3 had two methoxyl groups (δH 3.90, s, 3H and 3.43, s, 3H) and one set of protons of the aromatic system at δH 7.59 (1H, d, J = 1.9 Hz, H-2′), 7.57 (1H, dd, J = 8.8, 1.9 Hz, H-6′) and 6.84 (1H, d, J = 8.8 Hz, H-5′), revealing three aromatic protons coupled in an ABX pattern. Analysis of 1H-NMR and 13C-NMR spectroscopic data suggests that 3 shares the same methyl apiofuranose-type skeleton as 2 (Figures S3-1–S3-9, Supporting Information). In addition, the observable HMBC correlations (Figure 2) of 3′-OCH3/C-3′; H-5′/C-4′ and C-6′; H-6′/C-5′ and C-7′; and H-2′/C-3′ and C-7′ were used to establish the 2′-hydroxyl-5′-methoxyl-benzoyl moiety. The structure of compound 3 was assigned as shown.
The same molecular formula, C14H18O8, for 4 was established based on its (−)-HRESIMS ion peak at m/z 313.0927 (calcd for C14H17O8, 313.0923), respectively. Analysis of their NMR spectroscopic data suggests that 4 shares the same methyl apiofuranoside skeleton as 3, except for two differences in the chemical shift of C-1 and J1,2. Their respective chemical shift of C-1 exhibited 111.6 in 4 and 104.7 in 3, J1,2 = 2.6 Hz in 4 and J1,2 = 4.6 Hz in 3 (Table 1). In addition, NOESY data facilitated the determination of the absolute configuration of methyl 5-O-(3′-hydroxyl-4′-methoxyl-benzoyl)-β-d-erythro-apiofuranoside (Figures S4-1–S4-9).
Methyl 5-O-(2′-O-β-d-glucosyl-5′-methoxyl-benzoyl)-α-d-erythro-apiofuranoside (5) afforded a molecular formula of C20H28O13 based on the HRESIMS ion peak at m/z 521.1513 [M + COOH]− (calcd for C21H29O15, 521.1506), corresponding to seven degrees of hydrogen deficiency. Its 1H-NMR data revealed the signals for two methoxyl groups (δH 3.90, s, 3H; 3.43, s, 3H), one set of protons of the aromatic system at δH 7.64 (1H, d, J = 2.0 Hz, H-6′), 7.68 (1H, dd, J = 8.5, 2.0 Hz, H-4′) and 7.22 (1H, d, J = 8.5 Hz, H-3′) revealing three aromatic protons coupled in an ABX pattern, two sugar anomeric proton signals at δH 4.90 (1H, d, J = 4.5 Hz) and 5.03 (1H, d, J = 7.6 Hz) (Table 2). The 13C-NMR spectroscopic data suggests that the presence of an ester carbonyl, a benzene ring, a methyl apioforanose, a glucose and two methoxyl groups in 5, with the aid of an HSQC experiment. The acid hydrolysis of 5 followed by derivatization with l-cysteine methyl ester liberated d-glucose, which were identified by applying HPLC systems equipped with UV detectors and C18 reversed-phase columns [17,18]. In addition, the observable HMBC correlations (Figure 2) of H-1′′/C-2′; H-6′/C-4′, C-5′, C-7′; H-3′/C-2′, C-4′; H-4′/C-3′, C-5′; 5′-OCH3/C-5′ and H-5/C-7′ were used to establish 2′-O-β-D-glucosyl-5′-methoxyl-benzoyl. Similarly, the configuration of 5 was deduced by the analysis of the NOESY spectrum (Figures S5-1–S5-9).
Table 2.
1H-NMR (600 MHz) and 13C-NMR (150 MHz) spectroscopic data for compounds 5–6.
| 5 a | 6 a | |||
|---|---|---|---|---|
| Position | δ C | δH (J in Hz) | δ C | δH (J in Hz) |
| Api-1 | 104.7 | 4.90 (1H, d, J = 4.5 Hz) | 111.6 | 4.86 (1H, d, J = 2.6 Hz) |
| 2 | 74.5 | 4.00 (1H, d, J = 4.5 Hz) | 78.8 | 3.96 (1H, d, J = 2.6 Hz) |
| 3 | 76.6 | 79.1 | ||
| 4 | 75.4 | 4.07 (1H, d, J = 10.0 Hz) | 75.0 | 4.03 (1H, d, J = 9.8 Hz) |
| 3.92 (1H, d, J = 10.0 Hz) | 3.90 (1H, d, J = 9.8 Hz) | |||
| 5 | 68.7 | 4.31 (2H, m) | 67.8 | 4.33 (2H, m) |
| 1′ | 125.3 | 125.3 | ||
| 2′ | 152.5 | 152.5 | ||
| 3′ | 116.6 | 7.22 (1H, d, J = 8.5 Hz) | 116.6 | 7.22 (1H, d, J = 8.5 Hz) |
| 4′ | 124.9 | 7.68 (1H, dd, J = 8.5, 2.0 Hz) | 124.9 | 7.67 (1H, dd, J = 8.5, 2.0 Hz) |
| 5′ | 150.6 | 150.6 | ||
| 6′ | 114.5 | 7.64 (1H, d, J = 2.0 Hz) | 114.5 | 7.64 (1H, d, J = 2.0 Hz) |
| 7′ | 167.6 | 167.5 | ||
| 5′-OCH3 | 56.9 | 3.90 (3H, s) | 56.9 | 3.91 (3H, s) |
| 1-OCH3 | 55.7 | 3.43 (3H, s) | 56.0 | 3.38 (3H, s) |
| Glc-1′′ | 102.1 | 5.03 (1H, d, J = 7.6 Hz) | 102.1 | 5.03 (1H, d, J = 7.6 Hz) |
| 2′′ | 74.9 | 3.53 (1H, m) | 75.0 | 3.53 (1H, m) |
| 3′′ | 78.6 | 3.47 (1H, m) | 78.5 | 3.47 (1H, m) |
| 4′′ | 71.4 | 3.40 (1H, m) | 71.4 | 3.42 (1H, m) |
| 5′′ | 78.0 | 3.49 (1H, m) | 78.0 | 3.49 (1H, m) |
| 6′′ | 62.6 | 3.70 (1H, dd, J = 12.0, 5.6 Hz) | 62.6 | 3.69 (1H, dd, J = 12.0, 5.6 Hz) |
| 3.87 (1H, m) | 3.88 (1H, m) | |||
a Data were recorded in CD3OD.
The HRESIMS of 6 displayed an ion peak at m/z 521.1516 [M + COOH]− (calcd for C21H29O15, 521.1506), suggesting that this compound shares the same molecular formula (C20H28O13) as that of 5. The resonances in its NMR data (Figures S4-4–S4-7) demonstrated the planar structure of 4 was similar to that of 5 except for two differences in the chemical shift of C-1 and J1,2. However, the NOESY correlations among H-2 (δH 3.96, d, J = 2.6 Hz) and H-5 (δH 4.33, m, 2H) were indicative of a β-d-erythro-apiofuranoside in 6, which was opposite that of 5, and further supported by the 1H-NMR and downfield-shifted resonance of H-1 at δH 4.86 (1H, d, J = 2.6 Hz) and chemical shift of C-1 exhibited 111.6 in 13C-NMR (Figures S6-1–S6-9). The structure of compound 6 (methyl 5-O-(2′-O-β-d-glucosyl-5′-methoxyl-benzoyl)-β-d-erythro-apiofuranoside) was proposed as shown.
Ten known compounds were identified as p-coumaric acid (7) [19], trans-ferulic acid (8) [20], 3,4-dimethoxycinnamic acid (9) [21], methyl-p-coumarate (10) [22], caffeic acid methyl ester (11) [23], ferulic acid methyl ester (12) [24], (−)-5-O-feruloylquinic acid methyl ester (13) [25], methyl 4-hydroxybenzoate (14) [26], ethyl 3,4-dihydroxybenzoate (15) [27], and 4-hydroxy-3,5-dimethoxy benzoic acid methyl ester (16) [28] based on their obtained spectroscopic data.
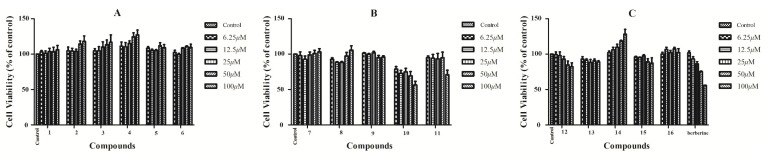
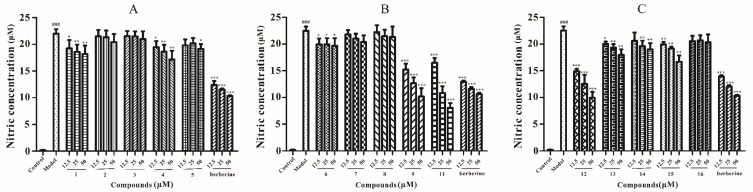
Nitric oxide (NO) is a diatomic free radical that is extremely short lived in biological systems. It is generated from l-arginine by nitric oxide synthase (NOS) and plays an important role in the regulation of physiological responses. The generation of NO is closely associated with inflammation, tumors, and immunoregulation [29,30,31]. In this study, all compounds isolated from the bark of P. chinense Schneid were examined for their inhibitory effects on NO production induced by lipopolysaccharide (LPS) in RAW 264.7 cells. In order to exclude the inhibition of NO production caused by cell cytotoxicity, cell viability was evaluated by the MTT method [32]. RAW 264.7 cell was treated with various concentrations of isolates for 24 h and the cell viability was tested by MTT assay as described in Section 2. As shown in Figure 3, the results revealed that no obvious cytotoxicity (over 85% cell survival) for most of compounds at the concentrations range of 6.25–100 μM was observed except for compound 10. Thus, the NO levels were detected in the RAW 264.7 cells after treated with 12.5 μM, 25 μM, and 50 μM of the tested compounds in subsequent experiments. Most of the isolated compounds inhibited NO release, as shown in Figure 4, and among them methyl apiofuranosides 1, 4, and 6 exhibited the inhibition on NO release at the concentration of 12.5 μM, while 2, 3, and 5 did not affect NO production. Meanwhile, compounds 9, 11, and 12 displayed the strongest inhibition on NO release, compared with the positive control berberine [33]. Together, three new methyl apiofuranosides including 1, 4, and 6 need to be further investigated on the pharmacological properties of NO inhibition.
Figure 3.
Effects of compounds 1–6 (A), compounds 7–11 (B), compounds 12–16 and Berberine (C) on RAW 246.7 cell viability (6.25–100 μM) compared to the control group (without compound group). Data are represented as mean ± SD of three independent experiments.
Figure 4.
Nitric oxide (NO) inhibitory activity of compounds 1–5 (A), compounds 6–9, 11 (B) and compounds 12–16 (C) on RAW 264.7 cell (12.5 μM, 25 μM, 50 μM). ### p < 0.001, compared to the control group (without lipopolysaccharide (LPS)-treated group); * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to the model group (with LPS-treated group). Data are represented as mean ± SD of three independent experiments.
3. Materials and Methods
3.1. General Experimental Procedure
Optical rotations were measured with a AUTOPOL Ⅳ polarimeter (Rudolph, Hackettstown, NJ, USA). The UV spectra were determined by a UV-2450 visible spectrophotometer (Shimadzu, Tokyo, Japan). Infrared spectra were collected using KBr disks on a Tensor 27 infrared spectrometer (Bruker Beijing Scientific Tech Co., Beijing, China). NMR spectra were recorded on a Bruker ARX-600 spectrometer (600 MHz for 1H and 150 MHz for 13C, Bruker Beijing Scientific Tech Co., Beijing, China) in CD3OD with tetramethylsilane as an internal standard. Chemical shifts were expressed in δ (ppm), and coupling constants (J) were reported in Hz. High-resolution electrospray ionization mass spectra (HRESIMS) were acquired on a Waters Xevo G2-S UPLC-Q/TOF mass spectrometer (Water, Milford, MA, USA). Preparative HPLC was performed with an ODS column (C-18, 250 × 20 mm, Inertsil Pak, Tokyo, Japan) in a Waters 600 liquid chromatograph apparatus equipped with a Waters 490 UV detector (Water, Milford, MA, USA). Methanol was HPLC grade. Sigel 60 (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China), Sephadex LH-20 (Advanced Technology Industrial Co., Ltd., Hongkong, China), and ODS (40–75 µm, FujiSilysia Chemical Ltd., Kyoto, Japan) were used as column chromatography stationary phases. TLC was carried out on a silica gel 60 plate 20 × 20 cm (Merck, Berlin, Germany). RP-HPLC was performed on an Agilent 1260 Series instrument with an RP-C18 column (20 × 200 mm i.d., Shim-pack, Shimadzu, Tokyo, Japan).
3.2. Plant Material
The dried bark of P. chinense Schneid was collected in Anguo, Hebei Province, People’s Republic of China. A voucher specimen (AP-2014-62) was identified by Professor Li-Juan Zhang and deposited at the School of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine.
3.3. Extraction and Isolation
The plant material (9.0 kg) was cut into small pieces and heated at reflux with 70% aqueous EtOH (3 × 90 L). The resulting EtOH extract was concentrated in vacuo at 40 °C, suspended in H2O (5 L). A total of 4% dilute sulfuric acid adjusted the pH to 4–5, followed by enrichment of alkaloids by 732 cation-exchange resins to obtain alkaloid fraction (20 g) and non-alkaloid fraction (435 g). The non-alkaloids were detected by TLC, and the Dragendorff’s reagent reaction was negative. The non-alkaloid fraction (435 g) was first fractionated by macroporous adsorptive resin D101, eluted with a step gradient system of EtOH/H2O (v/v, 0–100%), to afford six subfractions (N1–N6). The N5 (32 g) was subjected to silica gel column chromatography (10 cm × 120 cm) using a gradient mixture of CH2Cl2-MeOH (100:0, 100:1, 70:1, 50:1, 30:1, 10:1, 5:1, 1:1, 0:100) as eluent to give eight fractions (N51–N58). N51 was eluted with CH2Cl2-MeOH (1:1) on Sephadex LH-20 and then further purified by ODS column chromatography eluted with MeOH/H2O (20:80, 50:50, 80:20) and by repeated RP-18 HPLC preparation to give 1 (11.0 mg), 2 (22.0 mg), 3 (10.6 mg), 4 (8.6 mg), 5 (9.6 mg), and 6 (10.4 mg).
Methyl 5-O-(4′-hydroxy-3′,5′-dimethoxy-benzoyl)-β-d-erythro-apiofuranoside (1): colorless or white acicular crystal; −24° (c 0.1, MeOH); UV (MeOH) λmax (log ε) 216 (4.3), 279 (4.0) nm; IR (KBr) νmax 3412, 2968, 1621, 1112, 1054, 1024, 615 cm−1; 1H and 13C-NMR data (Table 1); HRESIMS m/z 343.1032 [M − H]− (calcd for C15H19O9, 343.1029).
Methyl 5-O-(4′-hydroxy-3′,5′-dimethoxy-benzoyl)-α-d-erythro-apiofuranoside (2): colorless oil; +50° (c 0.1, MeOH); UV (MeOH) λmax (log ε) 217 (4.3), 279 (4.0) nm; IR (KBr) νmax 3416, 2965, 1619, 1463, 1363, 1222, 1045, 778, 612 cm−1; 1H and 13C-NMR data (Table 1); HRESIMS m/z 343.1031 [M − H]− (calcd for C15H19O9, 343.1029).
Methyl 5-O-(2′-hydroxyl-5′-methoxyl-benzoyl)-α-d-erythro-apiofuranoside (3): colorless oil; +56° (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (4.0), 264 (3.8) nm; IR (KBr) νmax 3415, 2941, 1707, 1604, 1275, 1059, 768 cm−1; 1H and 13C-NMR data (Table 1); HRESIMS m/z 313.0921 [M − H]− (calcd for C14H17O8, 313.0923).
Methyl 5-O-(3′-hydroxyl-4′-methoxyl-benzoyl)-β-d-erythro-apiofuranoside (4): colorless oil; −6° (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (4.0), 264 (3.8) nm; IR (KBr) νmax 3414, 2938, 2351, 1717, 1522, 1467, 1324, 1048, 616 cm−1; 1H and 13C-NMR data (Table 1); HRESIMS m/z 313.0927 [M − H]− (calcd for C14H17O8, 313.0923).
Methyl 5-O-(2′-O-β-d-glucosyl-5′-methoxyl-benzoyl)-α-d-erythro-apiofuranoside (5): colorless oil; +14° (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (4.2), 257 (3.8) nm; IR (KBr) νmax 2947, 2351, 1518, 1463, 1375, 1321, 1025, 668 cm−1; 1H and 13C-NMR data (Table 2); HRESIMS m/z 521.1513 [M + COOH]− (calcd for C21H29O15, 521.1506).
Methyl 5-O-(2′-O-β-d-glucosyl-5′-methoxyl-benzoyl)-β-d-erythro-apiofuranoside (6): colorless oil; −24° (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (4.2), 257 (3.8) nm; IR (KBr) νmax 2962, 2351, 1701, 1522, 1467, 1048, 669 cm−1; 1H and 13C-NMR data (Table 2); HRESIMS m/z 521.1516 [M + COOH]− (calcd for C21H29O15, 521.1506).
3.4. Sugar Identification
Compounds 5 and 6 (1.0 mg each) were each hydrolyzed with 2.0 M HCl (4.0 mL), heated for 1.0 h at 85 °C and extracted with ethyl acetate, and the reaction mixture was concentrated [17]. The residue was dissolved in pyridine (1 mL) and reacted with l-cysteine methyl ester (4.0 mg) for 1.0 h at 60 °C, and then ο-tolyl isothiocyanate (10 µL) was added to the mixture and stirred at 60 °C for another 1.0 h. The derivatives were analyzed by HPLC and detected at 250nm [18]. Mobile phase: MeCN-H2O (25:75), flow rate: 1.0 mL/min, tR = 15.54 min (l-glucose), tR = 16.98 min (d-glucose), and the migration time of the derivatives of 5 and 6 was also 16.98 min, which revealed the sugar moiety in compounds 5 and 6 was d-glucose. The d-glucose and l-glucose as reference samples were also derivatized and analyzed applying the same procedures mentioned above.
3.5. Cell Culture
The murine RAW 264.7 macrophage cells were obtained from Cell Bank of the Shanghai Institute of Cell Biology and Biochemistry, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and penicillin-streptomycin (100 U/mL) at 37 °C with 5% CO2.
3.6. Cell Viability Assay
An MTT assay was used to evaluate RAW 264.7 cell viability as previously described [34]. The mitochondrial-dependent reduction of 3-(4,5-dimethylthizaol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) to formazan was used to measure cell respiration as an indicator of cell viability [35]. Briefly, after 24 h incubation with or without compounds (6.25–100 μM), an MTT solution (final concentration 5 mg/mL) was added and the cells were incubated for another 2.5 h at 37 °C. After removing the supernatant, 150 μL of DMSO was added to the cells to dissolve the formazan. The absorbance of each group was measured by using a microplate reader at a wavelength of 490 nm. Results are expressed as a percentage of viable cells when compared with the control group. The viability of RAW 264.7 cells of the control group (with 0.1%DMSO only) is defined as 100%.
3.7. Measurement of NO Release
The accumulated nitrite in the supernatant was evaluated using the Griess reagent [36,37] (Beyotime, Nanjing, China). RAW 264.7 macrophage cells were seeded in 96-well culture plates (5 × 104 cells/well) for 24 h. Then, various concentrations of test compounds were added, after 2.5 h incubation, with the presence of 1 μg/mL LPS. 0.1% DMSO and 1 μg/mL LPS without test compounds were added as model group, only with the presence of 0.1% DMSO and equivalent DMEM as the control group. Twenty hours later, culture supernatant and Griess reagent were mixed and then incubated for 10 min. The optical density of the mixture was read at 540 nm using an automated microplate reader. The NO inhibitory rate was measured in relation to the model group (cells were treated with LPS only). Berberine (purity ≥98%, Yuanye, Shanghai, China) served as the positive control.
Abbreviations
| DMEM | dulbecco’s modified eagle medium |
| DMSO | Dimethyl Sulfoxide |
| HMBC | Heteronuclear Multiple Bonding Correlation |
| HSQC | Heteronuclear Single Quantum Correlation |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide |
| NOE | Nuclear Overhauser Enhancement |
| NOESY | Nuclear Overhauser Enhancement Spectroscopy |
| RAW | leukemia cellsin mouse macrophage |
Supplementary Materials
Supplementary Materials are available online.
Author Contributions
P.-F.W. and Y.-P.L. performed the experiments, and wrote the first version of the manuscript; L.-Q.D., S.-J.C. and L.-N.W. designed the experiment; L.N.W. approved and revised the submitted manuscript; F.Q. presented the conception of the project, acquired the funding, supervised the execution of the whole project, and drafted and edited the final manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (No. 81430095).
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Shiao P.G. Photocatalogue of Chinese Traditional Medicine. Volume 10. Taiwan Business Publication Company; Taipei, Taiwan: 1990. p. 86. [Google Scholar]
- 2.Hsu K.J. Chinese Traditional Medicine. Chinese Pharmaceutical Science and Technology Publication Company; Beijing, China: 1996. p. 802. [Google Scholar]
- 3.Chinese Pharmacopoeia Commission . Pharmacopoeia of the People’s Republic of China. Chemical Industry Press; Beijing, China: 2015. pp. 305–306. [Google Scholar]
- 4.Chen M.L., Xian Y.F., Ip S.P., Tsai S.H., Yang J.Y. Chemical and Biological Differentiation of Cortex Phellodendri Chinensis and Cortex Phellodendri Amurensis. Planta Med. 2010;76:1530–1535. doi: 10.1055/s-0030-1249774. [DOI] [PubMed] [Google Scholar]
- 5.Wu T.S., Hsu M.Y., Kuo P.C., Sreenivasulu B., Damu A.G., Su C.R. Constituents from the leaves of Phellodendron amurense var. wilsonii and their bioactivity. J. Nat. Prod. 2003;66:1207–1211. doi: 10.1021/np030034v. [DOI] [PubMed] [Google Scholar]
- 6.Yan C., Zhang Y.D., Wang X.H., Geng S.D., Wang T.Y., Sun M. Tirucallane-type triterpenoids from the fruits of Phellodendron chinense Schneid and their cytotoxic activities. Fitoterapia. 2016;113:132–138. doi: 10.1016/j.fitote.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Cuéllar M.J., Giner R.M., Recio M.C., Máñez S., RíOs J.L. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia. 2001;72:221–229. doi: 10.1016/S0367-326X(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 8.Park S.D., Lai Y.S., Kim C.H. Immunopontentiating and antitumor activities of the purified polysaccharides from Phellodendron chinense SCHNEID. Life Sci. 2004;75:2621–2632. doi: 10.1016/j.lfs.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Ishii T., Yanagisawa M. Synthesis, separation and NMR spectral analysis of methyl apiofuranosides. Carbohydr. Res. 1998;313:189–192. doi: 10.1016/S0008-6215(98)00262-6. [DOI] [Google Scholar]
- 10.Ishiia T., Ono H. NMR ctroscopic analysis of the borate diol esters of methyl apiofuranosides. Carbohydr. Res. 1999;321:257–260. doi: 10.1016/S0008-6215(99)00184-6. [DOI] [Google Scholar]
- 11.Park S., Goo Y.M., Na D.S. Isolation and structure elucidation of a catechin glycoside with phospholipase A2 inhibiting activity from Ulmi cortex. Bull. Korean Chem. Soc. 1996;17:101–103. [Google Scholar]
- 12.Angyal S.J., Bodkin C.L., Mills J.A., Pojer P.M. ChemInform Abstract: Complexes of carbohydrates with metal cations. IX Synthesis of the methyld D-tagatosides, D-psicosides, D-apiosides, and D-erythrosides. Cheminform. 1977;8:1259–1268. doi: 10.1002/chin.197740360. [DOI] [Google Scholar]
- 13.Tronchet J.M.J., Tronchet J. 9-(3-C-hydroxymethyl-α-L-threofuranosyl) adenine (9-(β-Dapio-L-furanosyl) adenine) Carbohydr. Res. 1974;34:263–270. doi: 10.1016/S0008-6215(00)82901-8. [DOI] [Google Scholar]
- 14.Zhang N., Huang W.X., Xia G.Y., Oppong M.B., Ding L.Q., Li P., Qiu F. Methods for determination of absolute configuration of monosaccharides. Chin. Herb. Med. 2018;10:14–22. doi: 10.1016/j.chmed.2017.12.009. [DOI] [Google Scholar]
- 15.Mathias L., Vieira I.J.C., Filho R.B., Filho R.E. A New Isoflavone Glycoside from Dalbergia nigra. J. Nat. Prod. 1998;61:1158–1161. doi: 10.1021/np9800598. [DOI] [PubMed] [Google Scholar]
- 16.Zhou K.L., Zhao F., Liu Z.H., Zhuang Y.L., Chen L.X., Qiu F. Triterpenoids and Flavonoids from Celery (Apium graveolens) J. Nat. Prod. 2009;72:1563–1567. doi: 10.1021/np900117v. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T., Nakashima T., Ueda T. Facile Discrimination of Aldose Enantiomers by Reversed-Phase HPLC. Chem. Pharm. Bull. 2007;55:899–901. doi: 10.1248/cpb.55.899. [DOI] [PubMed] [Google Scholar]
- 18.Murata T., Endo Y., Miyase T., Yoshizaki F. Iridoid glycoside constituents of Stachys lanata. J. Nat. Prod. 2008;71:1768–1770. doi: 10.1021/np8001805. [DOI] [PubMed] [Google Scholar]
- 19.Johnsson P., Peerlkamp N. Polymeric fractions containing phenol glucosides in flaxseed. Food Chem. 2002;6:207–212. doi: 10.1016/S0308-8146(01)00269-2. [DOI] [Google Scholar]
- 20.Rowe J.W., Bower C.L., Wagner E.R. Extractives of jack pine bark: Occurrence of cis- and trans-pinosylvin dimethyl ether and ferulic acid esters. Phytochemistry. 1969;8:235–241. doi: 10.1016/S0031-9422(00)85819-7. [DOI] [Google Scholar]
- 21.Andrade P.B., Leitão R., Seabra R.M. 3,4-Dimethoxycinnamic acid levels as a tool for differentiation of Coffea canephora var. robusta and Coffea arabica. Food Chem. 1998;61:511–514. doi: 10.1016/S0308-8146(97)00067-8. [DOI] [Google Scholar]
- 22.Gopalakrishnan S., Subbarao G.V., Nakahara K. Nitrification inhibitors from the root tissues of Brachiaria humidicola, a tropical grass. J. Agric. Food. Chem. 2007;55:1385–1388. doi: 10.1021/jf062593o. [DOI] [PubMed] [Google Scholar]
- 23.Vongvanich N., Kittakoop P., Charoenchai P. Antiplasmodial, Antimycobacterial, and Cytotoxic Principles from Camchaya calcarea. Planta Med. 2006;72:1427–1430. doi: 10.1055/s-2006-951711. [DOI] [PubMed] [Google Scholar]
- 24.Xiao H., Parkin K.L. Isolation and identification of potential cancer chemopreventive agents from methanolic extracts of green onion (Allium cepa) Phytochemistry. 2007;68:1059–1067. doi: 10.1016/j.phytochem.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura S., Fujimoto K., Matsumoto T. Acylated sucroses and acylated quinic acids analogs from the flower buds of Prunus mume and their inhibitory effect on melanogenesis. Phytochemistry. 2013;92:128–136. doi: 10.1016/j.phytochem.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Youn U.J., Lee J.H., Lee Y.J. Regulation of the 5-HT3A Receptor-Mediated Current by Alkyl 4-Hydroxybenzoates Isolated from the Seeds of Nelumbo nucifera. Clin. Biochem. 2010;7:2296–2302. doi: 10.1002/cbdv.200900393. [DOI] [PubMed] [Google Scholar]
- 27.Chai W.M., Shi Y., Feng H.L. NMR, HPLC-ESI-MS, and MALDI-TOF MS Analysis of Condensed Tannins from Delonix regia (Bojer ex Hook.) Raf. and Their Bioactivities. J. Agric. Food. Chem. 2012;60:5013–5022. doi: 10.1021/jf300740d. [DOI] [PubMed] [Google Scholar]
- 28.Majumder P.L., Banerjee S., Sen S. Three stilbenoids from the orchid Agrostophyllum callosum. Phytochemistry. 1996;42:847–852. doi: 10.1016/0031-9422(95)00954-X. [DOI] [Google Scholar]
- 29.David A.G., Timothy R.B. Molecular biology of nitric oxide synthases. Cancer Metast. Rev. 1998;17:7–23. doi: 10.1023/a:1005940202801. [DOI] [PubMed] [Google Scholar]
- 30.Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996:1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 31.Bungorn S., Jintana J., Nawarat W., Doosadee H. Anti-inflammatory effect of Streblus asperleaf extract in rats and its modulationon inflammation-associated genes expression in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2009;124:566–570. doi: 10.1016/j.jep.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 32.Guzik T.J., Korbut R., Adamek G.T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003;54:469. [PubMed] [Google Scholar]
- 33.Naohiro O., Tomofumi S., Yuji N., Noriyasu H., Fumiyuki K. Quantitative analysis of the anti-inflammatory activity of orengedokuto II: berberine is responsible for the inhibition of NO production. J. Nat. Med. 2018;72:706–714. doi: 10.1007/s11418-018-1209-7. [DOI] [PubMed] [Google Scholar]
- 34.Sun L.D., Wang F., Dai F., Wang Y.H., Lin D., Zhou B. Development and mechanism investigation of a new piperlongumine derivative as a potent anti-inflammatory agent. Biochem. Pharmacol. 2015;95:156–169. doi: 10.1016/j.bcp.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Xue G.M., Li X.Q., Chen C. Highly Oxidized Guaianolide Sesquiterpenoids with Potential Antiinflammatory Activity from Chrysanthemum indicum. J. Nat. Prod. 2018;81:378–386. doi: 10.1021/acs.jnatprod.7b00867. [DOI] [PubMed] [Google Scholar]
- 36.Zhao F., Wang L., Liu K. In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L. through inhibition on iNOS pathway. J. Ethnopharmacology. 2009;122:457–462. doi: 10.1016/j.jep.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 37.Choi Y.Y., Kim M.H., Han J.M., Hong J.K., Lee T.H., Kim S.H. The anti-inflammatory potential of Cortex Phellodendron in vivo and in vitro: down-regulation of NO and iNOS through suppression of NF-κB and MAPK activation. Int. J. Immunopharmacol. 2014;19:214–220. doi: 10.1016/j.intimp.2014.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.