Abstract
Solar urticaria is a rare type of photodermatosis that significantly reduces the quality of life of the subjects affected, with a risk of anaphylaxis should the entire body be exposed to the sun. Patients are forced to modify and limit their normal activities, and since the symptoms are triggered by exposure to sun, which is difficult if not impossible to avoid in everyday life, a safe and effective therapy appears to be essential.
Unfortunately, traditional therapies in a discrete number of patients are unable to provide adequate and safe answers.
We describe the case of an 18-year-old woman who began to manifest the first symptoms walking under the spring sunshine. A few minutes after sun exposure, itching and burning sensation began, followed immediately by erythema appearance in the photoexposed skin areas.
She was treated with non-sedating H1-blocking antihistamines and leukotriene antagonist with no success, so we decided to treat with omalizumab.
The satisfactory response after the failure of previous standard therapeutic strategies, confirms the effectiveness of this molecule in the treatment of solar urticaria, which despite what reported in several studies, has not yet been recognized and authorized by the competent Health Authorities in the treatment of this condition.
Keywords: Allergy, Solar urticaria, Omalizumab, IgE, Translational immunology
1. Introduction
Solar urticaria is a rare form of photodermatosis, which commonly occurs within minutes or hours after artificial light irradiation or sun exposure and fades within 24 hours.
It is often confined to sun-exposed body surfaces, but can sometimes develop under clothing. Duration, intensity, and wavelength of irradiation can determine the severity of solar urticaria. All skin types and ethnic groups are affected worldwide with it slightly more common in women than men. It can occur throughout an individuals life span but the mean age of onset is in the fourth decade. We do not have a precise data of prevalence and incidence, but literature reported figures range from 2.3% to 17.8% [1].
The disease can be controlled with phototherapy, antihistamines, leukotriene antagonist, cyclosporin A, intravenous immunoglobulin, or plasmapheresis alone or in combination, in most patients [2,3]. A total or partial failure of standard therapies is observed in a subset of patients that necessitate a different pharmacological approach. In recent years, omalizumab, a recombinant DNA-derived humanized anti IgE monoclonal antibody approved for severe-moderate asthma and chronic idiopathic urticaria, seems to be safe and effective also in the treatment of solar urticaria [4].
2. Case report
We describe the case of an 18 year old woman that suddenly, while strolling in the sun during the spring period, began to experience itching, burning sensation, and redness in the sun-exposed areas of the skin (Fig. 1 a) She had no significant co-morbidities, no atopic family members, nor anyone suffering from similar disorders, and did not take any drugs. The treatment with non-sedating H1-blocking antihistamines and leukotriene antagonist was unsuccessful. She was treated with rupatadine 10 mg/die for one month that up-dosed to 10 mg twice a day for two months. The following month, a leukotriene antagonist was added (montelukast 10 mg/die). She did not tolerate further dose increases of rupatadine for drowsiness and headache.
Figure 1.
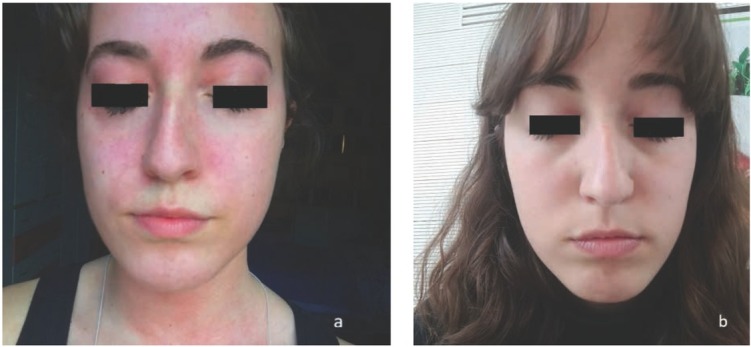
a) urticaria after solar exposure before omalizumab treatment and b) no reaction to solar exposure after omalizumab treatment.
Despite the rupatadine increase in dose and the association with montelukast, sun exposure still induced the appearance of skin lesions and itching. Given the lack of response to standard therapy and the risk that this condition entails, we considered a different therapeutic approach necessary.
The assessment with Urticaria Control Test (UTC) and Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) of her disease showed an uncontrolled condition and a significantly reduced quality of life before the treatment and a quick response to therapy.
The patient was advised to use total sun protection, avoid expose to artificial sun light, and to use adequate textile light protection.
The CU diagnosis was based on the criteria of the EAACI/GA2LEN/EDF/WAO Guidelines (2017 revision and update) for the definition, classification, diagnosis, and management of urticaria [5].
Phototesting was performed confirming the diagnosis. Laboratory tests results showed white blood cell 6x103/μl, hemoglobin 14.1 gr/dl, platelet 228x103/μl, AST 23 U/l, ALT 18 U/l, creatinine 0.80 mg/dl, blood urea nitrogen (BUN) 46 mg/dl, erythrocyte sedimentation rate 7 mm, C reactive protein 3.00 mg/l, complement C3 133 mg/dl, C4 24 mg/dl, total IgE 144 KUA/l, D-dimer 0.17 mg/l, TSH 1.30 mUI/ml, Ab-anti-thyroperoxidase 15.70 IU/ml, rheumatoid factor 5 UI/ml, anti-nuclear antibodies negative (IFA), anti-double stranded DNA negative (IFA), anti-extractable nuclear antigens 0,100 U/ml (ELIA ImmunoCAP), ANCA-p 0,100 U/ml (ELIA ImmunoCAP), ANCA-c 0,010 U/ml (ELIA ImmunoCAP). Serum protein electrophoresis was in the normal range and porphyrin profile, autologous serum skin test, and most common viral bacterial and parasitic infection were negative(EBV, CMV, HBV, HCV, Coxachie mix, HSV, HP, toxoplasma, borrelia, troponema, anisakis, giardia, entamoeba, enterobius).
After receiving the patient’s informed consent, in compliance with the ethical standards in the field and the norms established by the Internal Review Board of the University of L’Aquila (Comitato Etico di Ateneo D.R. n. 206/2013 and D.R. n. 46/2017), omalizumab was administered off-label (300 mg via subcutaneous injection per month, for six months) at room temperature. The dose of omalizumab administered to our patient was the same recommended in the treatment of chronic spontaneous urticaria. Rupatadine was gradually reduced and discontinued on the second month of omalizumab therapy. No side effects were observed at the injection site.
The patient reported a significant improvement after the first injection with the complete disappearance of symptoms, and she was free of symptoms after the end of the therapy. She spent her summer on the beach without complaining about any disturbance (Fig. 1 b).The patient provided her written informed consent for the publication of this report.
3. Discussion
Solar urticaria is a rare form of physical urticaria and its pathophysiology is still largely unknown, despite the likely significant role played by the mast cells activated by immunoglobulins E [1,2].
It is an immediate hypersensitivity reaction, which might be IgE-mediated, occurring after exposure to the sun. The radiation may activate a precursor substance in the skin or serum that acts as a chromophore, turning it into an immunologically active photoallergen. Specific IgE antibodies producted against it induces the degranulation of mast cells, resulting in lesions of urticaria. Occasionally, solar urticaria is triggered by exogenous substance such as some medications like atorvastatin, chlorpromazine, tetracycline, or oral contraceptives [6].
What is certain is that, also in this case, a key role is played by the activated mast cells and the consequent release of inflammatory mediators, such as histamine [2].
As for the pathogenesis, the therapy for solar urticaria and above all its failures, have some dark sides that need an adequate knowledge.
An effective and safe therapy that can improve the quality of patients’ life and ensure protection from the serious risks they might incur is essential.
Omalizumab is an anti-IgE antibody, which has demonstrated robust clinical efficacy, and seems to be promising in a wide range of other allergic diseases [7,8]. Although it is not yet clear how omalizumab specifically acts in the various diseases in which it has proven effective, its primarily action is to neutralize free serum IgE, therefore it also reduces the surface levels of IgE on FcεRI-expressing cells, including mast cells and basophils. As IgE surface levels decline on these allergic effector cells, they lose the ability to bind allergen and to undergo IgE-dependent activation, and the allergic cascade is interrupted.
The suppression of these cells leads to a reduction of the pro-inflammatory cytokine levels and of dendritic cells, lymphocytes and eosinophils in tissues, and eventually, to a decrease in the inflammatory state of the whole immune system [9].
Unfortunately, traditional therapies are often unable to provide adequate and safe responses in solar urticaria, leading to a reduce quality of life in individuals affected, who are forced to significantly limit their normal activities [19, 20, 21].
And since the symptoms are triggered by the exposure to sun, which is difficult if not altogether impossible to avoid in everyday life, a safe and effective therapy appears to be essential.
Solar urticaria of all chronic induced urticarias sub-types, has the highest number of publications on omalizumab use and the majority show that omalizumab successfully control the disease [10, 11, 12, 13, 14, 15, 16, 17]. Initial doses range from 150 to 375 mg, although in some patients a higher dose of 450 mg is necessary to achieve complete symptom control. Patients often relapse 2 to 8 weeks after discontinuing treatment but then respond well to the next omalizumab administration.
The satisfactory response to omalizumab in our patient, after the failure of previous standard therapeutic strategies, confirms the effectiveness of this molecule in the treatment of solar urticaria which, despite what reported in several studies, has not yet been recognized and authorized by the competent Health Authorities in the treatment of this condition. Recently Snast et al. [4] reported that omalizumab provides clinical benefits in approximately 80% of patients with solar urticarial and that those who fail to respond on standard doses may improve with higher monthly dosages. Based on our studies, and what is reported in recent scientific publications, omalizumab could be an effective and safe therapeutic alternative in the treatment of solar urticaria.
Despite the effectiveness shown by omalizumab in many cases of solar urticaria refractory to conventional therapies, a certain proportion of subjects remain who do not respond satisfactorily [18, 19, 20, 21], and it is therefore necessary to study more thoroughly the pathogenetic mechanism of the disease in order to be able to select the patients and establish with confidence the most appropriate treatment.
In addition to clarifying the potential of omalizumab in the treatment of the resistant forms of solar urticaria, these investigations will also serve to pave the way for further, indispensable therapeutic pathways.
4. Learning points
-
-
Solar urticaria is a form of physical urticaria characterized by a localized or systemic eruption of papules and itching triggered by electromagnetic wavelengths ranging from 290 to 760 nm.
-
-
The symptoms of solar urticaria have a significant negative impact on the quality of life of patients (seriously affect QoL) and there is a risk of anaphylaxis should the entire body be exposed.
-
-
Avoidance of sun exposure, sunscreens and textile light protection are the firstline prophylactic measures, albeit which is difficult to achieve in everyday life.
-
-
Second-generation H1 antihistamines are the first line of treatment in solar urticaria and most patients require higher than standard doses. Phototherapy, leukotriene antagonist, cyclosporin A, intravenous immunoglobulin, plasmapheresis, alone or in combination represent further management options.
-
-
A total or partial failure of standard therapies is observed in a subset of patients.
-
-
Omalizumab could be an adequate, effective and safe therapeutic option.
Acknowledgments
The authors would like to thank Novartis Farma Italy who provides unconditional support for publishing expenses.
Footnotes
Conflict of interest
Conflict of interests: The authors do not declare a conflict of interest
References
- [1].Goetze S, Elsner P. Solar Urticaria. J Dtsch Dermatol Ges. 2015;13(12):1250–1253. doi: 10.1111/ddg.12809. [DOI] [PubMed] [Google Scholar]
- [2].Maurer M, Metz M, Brehler R, Hillen U, Jakob T, Mahler V, Pföhler C, Staubach P, Treudler R, Wedi B, Magerl M. Omalizumab treatment in patients with chronic inducible urticaria: A systematic review of published evidence. J All Clin Immunol. 2018;141(2):638–649. doi: 10.1016/j.jaci.2017.06.032. [DOI] [PubMed] [Google Scholar]
- [3].Arasi S, Crisafulli G, Caminiti L, Guarneri F, Aversa T, Porcaro F, Pajno GB. Treatment with omalizumab in a 16-year-old Caucasian girl with refractory solar urticaria. Pediatr Allergy Immunol. 2015;26:583–585. doi: 10.1111/pai.12413. [DOI] [PubMed] [Google Scholar]
- [4].Snast I, Kremer N, Lapidoth M, Enk CD, Tal Y, Rosman Y, Confino-Cohen R, Hodak E, Levi A. Omalizumab for the treatment of solar urticarial: case series and systematic review of the literature. J Allergy Clin Immunol Pract. 2018;4:1198–1204. doi: 10.1016/j.jaip.2018.02.032. [DOI] [PubMed] [Google Scholar]
- [5].Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, Bernstein JA, Bindslev-Jensen C, Brzoza Z, BuenseBedrikow R, Canonica GW, Church MK, Craig T, Danilycheva IV, Dressler C, Ensina LF, Giménez-Arnau A, Godse K, Gonçalo M, Grattan C, Hebert J, Hide M, Kaplan A, Kapp A, Katelaris CH, Kocatürk E, Kulthanan K, Larenas-Linnemann D, Leslie TA, Magerl M, Mathelier-Fusade P, Meshkova RY, Metz M, Nast A, Nettis E, Oude-Elberink H, Rosumeck S, Saini SS, Sánchez-Borges M, Schmid-Grendelmeier P, Staubach P, Sussman G, Toubi E, Vena GA, Vestergaard C, Wedi B, Werner RN, Zhao Z, Maurer M.. The 2017 Revision and Update. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397. The EAACI/GA2LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. [DOI] [PubMed] [Google Scholar]
- [6].Badri T., Schlessinger J. Urticaria solar StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2018-2017 Jun 27. [Google Scholar]
- [7].Sirufo MM, De Martinis M, Ginaldi L.. Omalizumab an effective and safe alternative therapy in severe refractory atopic dermatitis: A case report. Medicine (Baltimore) 97(24):e10897. doi: 10.1097/MD.0000000000010897. 2018Jun; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sirufo MM, Ginaldi L, De Martinis M. Successful treatment with omalizumab in a child with asthma and urticaria: a clinical case report. Front Pediatr. 2019. in press. [DOI] [PMC free article] [PubMed]
- [9].De Martinis M, Sirufo MM, Ginaldi L.. A stadium urticaria. Cold Urticaria is still a mostly unknown disease, with a wide spectrum of severity degrees and few therapeutic certainties: is omalizumab one of these? Reflections from a clinical case report. Iran Red Crescent Med J. 2019;21(1):e84250. doi: 10.5812/ircmj.84250. [DOI] [Google Scholar]
- [10].Terrani I, Bircher AJ. Scherer Hofmeier K Solar urticaria induced by visible light: successful treatment with omalizumab. Clin Exp Dermatol. 2016;41:890–892. doi: 10.1111/ced.12951. [DOI] [PubMed] [Google Scholar]
- [11].Brüning JH, Ziemer M, Pemler S, Simon JC, Treudler R.. Successful treatment of solar urticaria with omalizumab. J Dtsch Dermatol Ges. 2016;14:936–937. doi: 10.1111/ddg.13017. [DOI] [PubMed] [Google Scholar]
- [12].Moncourier M, Assikar S, Matei I, Souyri N, Couture M, Rigot E, Delauménie S, Bédane C. Visible light-induced solar urticaria is improved by omalizumab. Photodermatol Photoimmunol Photomed. 2016;32:314–316. doi: 10.1111/phpp.12271. [DOI] [PubMed] [Google Scholar]
- [13].Ghazanfar MN, Sand C, Thomsen SF. Effectiveness and safety of omalizumab in chronic spontaneous or inducible urticaria: evaluation of 154 patients. Br J Dermatol. 2016;175:404–6. doi: 10.1111/bjd.14540. [DOI] [PubMed] [Google Scholar]
- [14].Guzelbey O, Ardelean E, Magerl M, Zuberbier T, Maurer M, Metz M. Successful treatment of solar urticaria with anti-immunoglobulin E therapy. Allergy. 2008;63:1563–1565. doi: 10.1111/j.1398-9995.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- [15].Levi A, Tal Y, Dranitzki Z, Shalit M, Enk CD. Successful omalizumab treatment of severe solar urticaria in a 6-year-old child. Pediatr Allergy Immunol. 2015;26:588–90. doi: 10.1111/pai.12441. [DOI] [PubMed] [Google Scholar]
- [16].Baliu-Piqué C, Aguilera Peiró P.. Three cases of solar urticaria successfully treated with omalizumab. J Eur Acad Dermatol Venereol. 2016;30:704–706. doi: 10.1111/jdv.13001. [DOI] [PubMed] [Google Scholar]
- [17].Aubin F, Avenel-Audran M, Jeanmougin M, Adamski H, Peyron JL, Marguery MC, Léonard F, Puyraveau M, Viguier M. Société Française de Photodermatologie. Omalizumab in patients with severe and refractory solar urticaria: a phase II multicentric study. J Am Acad Dermatol. 2016;74:574–575. doi: 10.1016/j.jaad.2015.11.021. [DOI] [PubMed] [Google Scholar]
- [18].Duchini G, Bäumler W, Bircher AJ, Scherer K. Failure of omalizumab (Xolair®) in the treatment of a case of solar urticaria caused by ultraviolet A and visible light. Photodermatol Photoimmunol Photomed. 2011;27:336–7. doi: 10.1111/j.1600-0781.2011.00624.x. [DOI] [PubMed] [Google Scholar]
- [19].Müller S, Schempp CM, Jakob T. Failure of omalizumab in the treatment of solar urticaria. J Eur Acad Dermatol Venereol. 2016;30:524–525. doi: 10.1111/jdv.12922. [DOI] [PubMed] [Google Scholar]
- [20].Waibel KH, Reese DA, Hamilton RG, Devillez R. Partial improvement of solar urticaria after omalizumab. J Allergy Clin Immunol. 2010;125:490–491. doi: 10.1016/j.jaci.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [21].Kowalzick L, Thiel W, Bielfeld C, Ziegler H, Eickenscheidt L. Partial response of solar urticaria to omalizumab therapy. Hautarzt. 2017;68:492–6. doi: 10.1007/s00105-016-3913-0. [DOI] [PubMed] [Google Scholar]


