Abstract
Epidemiology of opportunistic infections (OI) after kidney allograft transplantation in the modern era of immunosuppression and the use of OI prevention strategies are poorly described. We retrospectively analyzed a single-center cohort on kidney allograft adult recipients transplanted between January 2008 and December 2013. The control group included all kidney recipients transplanted in the same period, but with no OI. We analyzed 538 kidney transplantations (538 patients). The proportion of OI was 15% (80 and 72 patients). OI occurred 12.8 (6.0–31.2) months after transplantation. Viruses were the leading cause (n = 54, (10%)), followed by fungal (n = 15 (3%)), parasitic (n = 6 (1%)), and bacterial (n = 5 (0.9%)) infections. Independent risk factors for OI were extended criteria donor (2.53 (1.48–4.31), p = 0.0007) and BK viremia (6.38 (3.62–11.23), p < 0.0001). High blood lymphocyte count at the time of transplantation was an independent protective factor (0.60 (0.38–0.94), p = 0.026). OI was an independent risk factor for allograft loss (2.53 (1.29–4.95), p = 0.007) but not for patient survival. Post-kidney transplantation OIs were mostly viral and occurred beyond one year after transplantation. Pre-transplantation lymphopenia and extended criteria donor are independent risk factors for OI, unlike induction therapy, hence the need to adjust immunosuppressive regimens to such transplant candidates.
Keywords: kidney transplantation, opportunistic infection, allograft survival, BK virus nephropathy
1. Introduction
Kidney allograft recipients are exposed to a broad range of infectious pathogens that give rise to infections with unusual and more severe presentations [1]. Opportunistic infections (OIs) include infections caused by uncommon pathogens and those caused by common pathogens but with unusual and more severe forms [2]. The reported incidence of OIs is variable, from 10% to 25% [3,4]. Currently, prevention strategies against cytomegalovirus (CMV), herpes simplex viruses (HSV), and Pneumocystis spp. are recommended and result in a significant reduction of post-transplantation OIs [5] and 50% decrease in the risk of death due to infectious causes. However, infections remain the most common cause of non-cardiovascular deaths (15–20%) [5,6].
After solid-organ transplantation (SOT), OIs flourish in the first 12 months boosted by the immunosuppressive status [2] since less than 20% of SOT recipients receive no induction therapy and up to 60% of kidney transplant recipients receive a T-cell depleting agent [7,8]. Anti-thymocyte globulin primarily induces rapid, profound, and long-lasting depletion of T-lymphocytes in peripheral blood and lymphoid organs, and apparently it does not spare B-cell and NK cell populations [9,10]. Thanks to such therapies, patient and kidney allograft survival after kidney transplantation have markedly improved and acute allograft rejection has decreased [11,12,13]. On the other hand, one could argue that the long duration of immunosuppression might be the culprit for the increased incidence of OIs.
The epidemiology of OIs after SOT was previously described in two large cohorts on transplant recipients. The first one was conducted 10 years ago and included SOT recipients treated with alemtuzumab [4]. They showed that receiving lung or intestinal transplants was independent risk factors for OIs [4]. Published in the era of modern immunosuppression and after the wide use of prevention strategies, the second study included abdominal SOT recipients (kidney, pancreas, and liver), hence the heterogeneous patient profiles and immunosuppressive regimens [3]. The authors highlighted the delayed onset of OIs where most infections occurred after six months without any impact on recipient’s survival and graft function [3]. A recent pediatric cohort on kidney allograft recipients has confirmed the absence of impact of viral OIs (CMV, Epstein Barr virus (EBV), and BK virus (BKV)) on kidney allograft survival [14]. In other studies on kidney allograft recipients, only selected OIs, secondary to specific pathogens (Nocardia, Aspergillus, Cryptococcus neoformans), have been reported [15,16,17].
Given the lack of clinical and epidemiological data on OIs after kidney allograft transplantation, we conducted a large monocentric cohort study on all kidney allograft recipients in our center to analyze the epidemiology of OIs and their impact on kidney recipient survival and allograft function.
2. Materials and Methods
2.1. Study Design and Patients
We conducted a single center retrospective cohort enrolling all adult kidney allograft recipients registered between January 2008 and December 2013. We excluded cases with primary allograft non-function happening within seven days after transplantation. Expanded criteria donor (ECD) was defined as donors older than 60 years or between 50 and 60 years, with two of the three following criteria: (i) hypertension; (ii) pre-retrieval serum creatinine > 1.50 mg/dL; and (iii) cerebrovascular cause of brain death [18]. Glomerular filtration rate was estimated (eGFR) using MDRD formula [19]. Acute rejection episodes were classified according to updated Banff classification [20]. Allograft loss was considered if eGFR was below 15 mL/min/1.73 m2. All recipients were followed at least one year after transplantation unless death or graft loss occurred earlier.
2.2. Infectious Prophylaxis
The management for CMV prophylaxis followed international recommendations [21]. Prophylaxis involved the administration of oral valganciclovir to high (D+/R-) and intermediate (R+ treated with thymoglobulin) risk patients. Duration of prophylaxis was 6 months in high risk patients and 3 months in intermediate ones.
Participants with past history of tuberculosis were treated with isoniazid for three months after transplantation. Pneumocystis jirovecii prophylaxis included trimethoprim-sulfamethoxazole (400 mg) or pentacarinat aerosol for 12 months after transplantation and till CD4 count dropped to <200/µL.
2.3. Opportunistic Infections
OIs were defined according to current literature [1] and international guidelines [22,23]. All episodes were retrospectively and blindly validated (review of all medical reports without the patient name and the final conclusion (clinical and biological data) of infections that happened in kidney-transplant recipients included in the study) by an infectious disease specialist part of the study group. The following OIs were considered:
-Bacteria: Mycobacterium sp., Listeria monocytogenes and Nocardia sp.
-Virus: CMV, active replication of HSV, Varicella-Zoster virus (VZV), Human Herpes Virus-8 (HHV8), BKV, Norovirus, and JC virus.
We included BKV infection, as BK virus, highly seroprevalent in humans, appears to cause clinical disease only in immunocompromised patients and almost all after kidney transplantation (tubulointerstitial nephritis called BKV-induced nephropathy directly related to plasma viral load) [24]. In our center, during the first year after kidney transplantation, BK viruria tests were performed at 1, 2, 3, 6, 9, and 12 months. BK viremia was checked once BK viruria was positive. If BK viruria (associated with BK viremia or not) was positive, a blood test was performed every two weeks.
We also considered Kaposi sarcoma, as one of the four types was organ transplant-associated and usually regresses with reduction in immunosuppression [25].
-Fungi: Candida spp, Cryptococcus spp., invasive molds, and Pneumocystis jirovecii.
-Parasites: Toxoplasma gondii, Microsporidium sp, Cryptosporidium sp, Leishmania sp.
2.4. Endpoints
Clinical endpoints were an OI episode, death, and allograft loss. Recipients with at least one episode of OI were compared with the control group which included all other kidney allograft recipients engrafted at the same time period.
2.5. Statistical Analysis
Continuous variables are presented as mean (± Standard Deviation (SD)) or median (Interquartile Range (IQR)). Categorical variables are presented as counts (%). Baseline donor, recipient, and kidney transplant characteristics were compared between OI and control groups using Student t-test or Wilcoxon test for continuous variables, and Chi-2 or Fisher’s exact tests for categorical variables, as appropriate. Time-to-event survival analyses were conducted to determine predictors of OI occurrence, patient overall survival, and allograft survival. Survival curves were plotted using Kaplan–Meier method and logrank tests to assess significance upon group comparison. Time varying Cox proportional hazard models were built for each endpoint, and hazard ratios (HR) along with their 95% confidence intervals (95% CI) were calculated. Factors yielding p < 0.2 in the univariate analyses were then considered in the multivariate analyses’ models, using a stepwise backward approach by sequentially removing variables not significant at p < 0.1 until the final model was reached. Variables with available repeated data over time were entered both as time-fixed (value at the time of transplantation) and as time-varying (all available time points) variables into the Cox model. No imputation of missing data was done. Competing risk survival analysis (e.g., Fine–Gray methodology) cannot be directly applied on time-varying variables, therefore only results from Cox models are reported for allograft survival. All tests were two-tailed, and the significance level was reached with p value < 0.05. The analysis was performed using Stata SE v15.1 (College Station, TX, USA).
3. Results
3.1. Whole Cohort
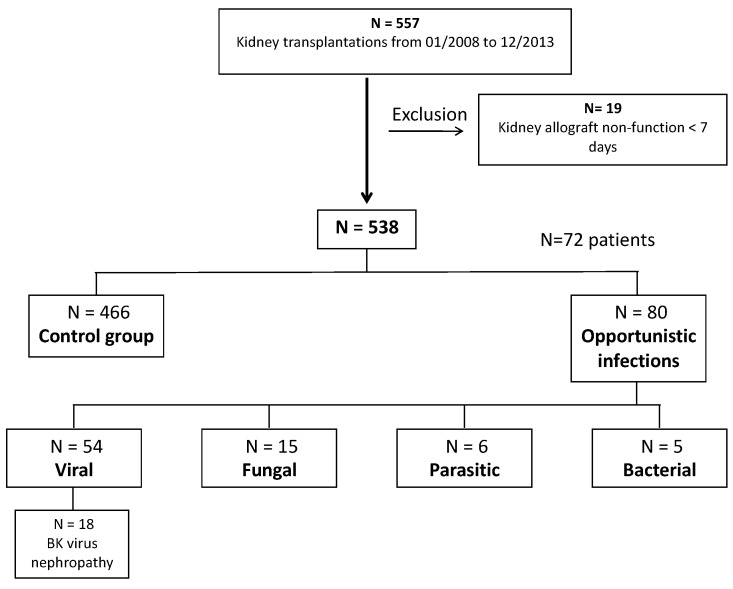
A flow-chart of the study population is presented in Figure 1. Between January 2008 and December 2013, 557 kidney transplantations were performed in 557 patients (n), of whom 19 showed early primary allograft non-function. Overall, only 538 transplantations in 538 patients were included. Mean age was 52 ± 14 years. Mean follow-up was 55 ± 24 months. At the end of follow-up period, patient survival was 88% with 65 deaths, allograft survival was 87% with 72 allograft losses, and mean eGFR was 48 ± 20 mL/min/1.73 m2. Table 1 and Table 2 described the whole cohort.
Figure 1.
Flow chart of the study population. Between January 2008 and December 2013, 557 kidney transplantations were performed in n = 557 patients. Nineteen patients were excluded because of primary allograft non-function within the first week after transplantation. The final cohort included 538 transplantations in 538 patients.
Table 1.
Baseline characteristics of the patients included in the study.
| Variables | Whole Cohort | Opportunistic Infections Group | Control Group | p-Value |
|---|---|---|---|---|
| n = 538 (100%) | n = 72 (13%) | n = 466 (87%) | ||
| Recipients characteristics | ||||
| Age, years, mean (SD) | 52 ± 14 | 55 ± 15 | 51 ± 13 | 0.06 |
| Sex, Female, n (%) | 200 (37) | 25 (35) | 175 (38) | 0.64 |
| Initial nephropathy | ||||
| Glomerulopathy, n (%) | 140 (26) | 17 (24) | 123 (26) | 0.62 |
| Unknown, n (%) | 104 (19) | 15 (21) | 89 (19) | 0.73 |
| Diabetes Mellitus, n (%) | 88 (16) | 12 (17) | 76 (16) | 0.94 |
| Hypertension, n (%) | 54 (10) | 8 (11) | 46 (10) | 0.75 |
| Chronic interstitial nephropathy, n (%) | 32 (6) | 7 (10) | 25 (5) | 0.15 |
| Genetic, n (%) | 78 (15) | 6(8) | 72 (15) | 0.11 |
| Urologic, n (%) | 29 (5) | 6 (8) | 23 (5) | 0.23 |
| Other, n (%) | 13 (3) | 2(3) | 12 (3) | 1.00 |
| Diabetes before transplantation, n(%) | 120 (22) | 16 (22) | 104 (22) | 0.97 |
| Dialysis, n (%) | 488 (91) | 67 (93) | 421 (90) | 0.46 |
| Hemodialysis, n (%) | 448 (83) | 387 (83) | 61 (85) | 0.72 |
| HIV +, n (%) | 25 (5) | 4 (6) | 21 (5) | 0.69 |
| HCV +, n (%) | 38 (7) | 5 (7) | 33 (7) | 0.97 |
| CMV +, n (%) | 443 (82) | 58 (81) | 385 (83) | 0.67 |
| Donor characteristics | ||||
| Living donor, n (%) | 43 (8) | 3 (4) | 40 (9) | 0.20 |
| Extended criteria donor, n (%) | 245 (46) | 48 (67) | 197 (42) | 0.0001 |
| Age, years, mean (SD) | 55 ± 16 | 59 ± 14 | 54 ± 16 | 0.02 |
| eGFR, mL/min/1.73 m², median (IQR) | 80 (58–103) | 72 (56–94) | 81 (58–103) | 0.26 |
| CMV +, n (%) | 291 (54) | 39 (54) | 252 (54) | 0.99 |
| Sensitization risk factors | ||||
| Former kidney transplantation, n (%) | 39 (7) | 6 (8) | 33 (7) | 0.70 |
| Anti-HLA antibodies, n (%) | 285 (53) | 38 (53) | 247 (53) | 0.93 |
| Donor specific anti-HLA antibodies, n (%) | 77 (14) | 7 (10) | 70 (15) | 0.23 |
| Kidney transplant characteristics | ||||
| Cold ischemia time, hours, median (IQR) | 16 (12–20) | 16 (13–20) | 16 (12–20) | 0.50 |
| Immunosuppressive therapy | ||||
| Induction, n (%) | 521 (97) | 72 (100) | 449 (96) | 0.10 |
| Basiliximab, n (%) | 265 (49) | 39 (54) | 226 (48) | 0.37 |
| Antithymocyte globulin, n (%) | 257 (48) | 34 (47) | 223 (48) | 0.92 |
| Rituximab, n (%) | 47 (9) | 6 (8) | 41 (9) | 0.90 |
| Intravenous immunoglobulins, n (%) | 89 (16) | 9 (13) | 80 (17) | 0.32 |
| Maintenance | ||||
| Calcineurin inhibitors, n (%) | 534 (99) | 72 (100) | 462 (99) | 0.43 |
| Mycophenolate mofetil, n (%) | 538 (100) | 72 (100) | 466 (100) | 1.00 |
| Steroids, n (%) | 537 (99,8) | 71 (99) | 466 (100) | 0.13 |
| Belatacept | 6 (1) | 0 (0) | 6 (1) | 0.33 |
| Combined transplant | ||||
| Heart, n (%) | 4 (1) | 1 (1) | 3 (1) | 0.67 |
| Pancreas, n (%) | 10 (2) | 1 (1) | 9 (2) | 0.67 |
| Liver, n (%) | 19 (3) | 4 (6) | 15 (3) | 0.67 |
| White blood cells at the time of transplantation | ||||
| Leucocytes (G/L), median (IQR) | 6.3 (5.2–7.9) | 6.3 (5.3–8.2) | 6.3 (5.2–7.8) | 0.66 |
| Neutrophils (G/L), median (IQR) | 4.2 (3.1–5.4) | 4.6 (3.2–6.2) | 4.1 (3.0–5.4) | 0.17 |
| Lymphocytes (G/L), median (IQR) | 1.3 (1.0–1.7) | 1.2 (0.9–1.6) | 1.3 (1.0–1.8) | 0.04 |
| CD4 T-cells (/µL), median (IQR) | 525 (373–704) | 493 (340–637) | 526 (389–704) | 0.15 |
| CD4 T-cells (%), median (IQR) | 45.1 (37.8–52.9) | 46.1 (37.2–52.3) | 45.1 (38.0–53.0) | 0.51 |
| CD8 T-cells (/µL), median (IQR) | 312 (200–451) | 284 (242–411) | 314 (198–466) | 0.79 |
| CD8 T-cells (%), median (IQR) | 26.9 (20.3–33.0) | 27.7 (23.6–36.7) | 26.6 (19.8–32.7) | 0.11 |
Table 2.
Follow-up of the patients included in the study.
| Variables | Whole Cohort | Opportunistic Infections Group | Control Group | p-Value |
|---|---|---|---|---|
| n = 538 (100%) | n = 72 (13%) | n = 466 (87%) | ||
| New onset diabetes after transplantation, n (%) | 34 (6) | 5 (7) | 29 (6) | 0.82 |
| Acute rejection, n (%) | 136 (25) | 23 (32) | 113 (24) | 0.19 |
| T-cell mediated, n (%) | 87 (16) | 15 (21) | 72 (16) | 0.54 |
| Antibody-mediated, n (%) | 34 (6) | 6 (8) | 28 (6) | 0.54 |
| Mixed, n (%) | 15 (3) | 2 (3) | 13 (3) | 0.54 |
| Time from transplantation, months (median, IQR) | 5 (2–18) | 7 (3–35) | 4 (2–14) | 0.14 |
| Before opportunistic infection, n (%) | 127 (24) | 15 (21) | 112 (24) | 0.66 |
| Viral Infections | ||||
| BK viruria | 163 (30) | 26 (36) | 137 (29) | 0.25 |
| Time from transplantation, months (median, IQR) | 6 (3–17) | 7 (3–12) | 6 (3–21) | 0.83 |
| Before opportunistic infection, n (%) | 149 (28) | 16 (22) | 133 (28) | 0.32 |
| BK viremia | 58 (11) | 22 (31) | 36 (8) | 0.0001 |
| Time from transplantation, months (median, IQR) | 5 (3–8) | 6 (3–12) | 4 (3–6) | 0.14 |
| Before opportunistic infection, n (%) | ||||
| CMV viremia | 178 (33) | 30 (42) | 148 (32) | 0.10 |
| Time from transplantation, CMV viremia (months, median, IQR) | 4 (2–7) | 5 (2–11) | 3 (2–7) | 0.22 |
| Before opportunistic infection, n (%) | 161 (30) | 17 (24) | 144 (31) | 0.27 |
| 12-month follow-up | ||||
| eGFR mL/min/1.73 m² (median, IQR) | 48 (36–60) | 41 (31–53) | 48 (37–61) | 0.003 |
| Allograft loss, n (%) | 19 (4) | 5 (7) | 14 (3) | 0.09 |
| Time from transplantation, months (median, IQR) | 7 (5–11) | 10 (7–11) | 6 (3–10) | 0.252 |
| Death, n (%) | 16 (3) | 3 (4) | 13 (3) | 0.52 |
| Time from transplantation, months (median, IQR) | 6 (2–9) | 6 (5–7) | 5 (2–9) | 0.95 |
| Last follow-up | ||||
| Time from transplantation, months (median, IQR) | 52 (38–75) | 48 (34–68) | 53 (38–77) | 0.045 |
| eGFR mL/min/1.73 m² (median, IQR) | 45 (36–60) | 38 (29–52) | 46 (34–62) | 0.0009 |
| Allograft loss, n (%) | 68 (13) | 13 (18) | 55 (12) | 0.14 |
| Time from transplantation, months (median, IQR) | 34 (13–54) | 31 (110–48) | 34 (14–55) | 0.81 |
| Death, n (%) | 65 (12) | 10 (14) | 55 (12) | 0.64 |
| Time from transplantation, months (median, IQR) | 34 (13–51) | 29.9 (6–51) | 34 (14–55) | 0.58 |
3.2. Opportunistic Infections
Eighty OI episodes were reported in 15% of patients (n = 72). The median time to post-transplantation OI was 12.8 (6.0–31.2) months, and in 39 patients (48.8%), OI occurred over the first post-transplantation year.
Viruses were the leading cause of OI episodes, n = 54 (68%), representing 10% of the whole cohort. Median time to viral OI onset was 14 (7–31) months after transplantation. Of those viral OIs, we recorded 21 (39%) shingles (4%-whole cohort), 18 (33%) BKV nephropathy (BKVN) (3%-whole cohort), 6 (11%) Kaposi sarcoma (1%-whole cohort), 3 (6%) CMV disease (0.5%-whole cohort), 3 (6%) norovirus gastroenteritis (0.5%-whole cohort), and 1 (2%) of each of the following: JC virus causing progressive multifocal leukoencephalopathy (PML) (0.2%-whole cohort), VZV retinitis (0.2%-whole cohort), and HSV-1 esophagitis (0.2%-whole cohort).
Fungal infections were the second most common OIs, registered in 15 patients (19%) (3%-whole cohort), in the first 6 (2–25) months after transplantation, which is significantly earlier than viral infections (p = 0.04). We counted five (33%) invasive candidiasis (0.9%-whole cohort), four (27%) invasive aspergillosis (IA) (0.7%-whole cohort), three (20%) cryptococcosis (0.5%-whole cohort), two (13%) Pneumocystosis pneumonia (PCP) (0.3%-whole cohort), and one (7%) disseminated Trichophyton Rubrum infection (0.2%-whole cohort).
Among the six (7%) parasitic infections (1%-whole cohort) occurring 16 (5–23) months after transplantation, four were cryptosporidiosis (0.7%-whole cohort) and two microsporidiosis with gastrointestinal involvement (0.3%-whole cohort). Finally, five (6%) bacterial infections (0.9%-whole cohort) were described, of which two (40%) were tuberculosis (0.3%-whole cohort), two (40%) were nocardiosis (0.3%-whole cohort), and one (20%) was disseminated atypical mycobacteria infection (0.15%-whole cohort). Time to post-transplantation infection was 11 (9–34) months. Seven (10%) recipients had more than one post-transplantation OI episode.
The comparison between OI and control groups is shown in Table 1 and Table 2. Donors were significantly older in OI group than in control group (p = 0.02), with a similar statistical trend in recipients (p = 0.056). At the time of transplantation, blood lymphocytes count was significantly lower in OI group (p = 0.04). Numbers and percentages of CD4 and CD8 T-cells were similar in both groups; the same was found for the immunosuppressive treatments after transplantation (induction and maintenance).
The estimated GFR in OIs group was significantly lower than in control group at any given time (i.e., at 12-months or last available follow-up data). Acute rejection incidence and CMV viremia were similar in both groups. At the end of follow-up, event rates, allograft loss, and time to death after transplantation were similar in both groups.
In time-to-event analysis, the univariate risk factors for OIs after kidney transplantation (Table 3) were older recipient age (HR 1.02 (1–1.04), p = 0.03), older donor age (1.02 (1.01–1.04), p = 0.02), and ECD (2.76 (1.68–4.54), p < 0.0001). Higher CD4+ T-cells during follow-up and higher blood lymphocyte count at the time of transplantation were protective factors against OI (0.31 (0.11–0.83) and 0.61 (0.40–0.95), respectively). At the time of transplant, blood lymphocytes count was significantly lower in patients with OI (Table 1 (OI) Median 1.2 (IQR 0.9–1.6) vs. (Controls) 1.3 (1.0–1.8); p = 0.04) while CD4/CD8 numbers (%) were similar in both groups (Table 1) or using time-to-event analysis (Table 3). Induction and maintenance immunosuppressive regimens, acute rejection episode, and CMV viremia were not OI risk factors.
Table 3.
Opportunistic infection risk factors, univariate analysis.
| Whole Cohort | With No BK Virus Nephropathy | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Recipient characteristics | ||||||
| Female | 0.88 | 0.54–1.43 | 0.60 | 0.97 | 0.56–1.69 | 0.91 |
| Age at transplantation | 1.02 | 1.00–1.04 | 0.03 | 1.03 | 1.01–1.06 | 0.003 |
| Dialysis | 1.76 | 0.64–4.83 | 0.27 | 2.71 | 0.66–1.11 | 0.17 |
| Hemodialysis | 1.21 | 0.62–2.37 | 0.57 | 1.32 | 0.59–2.92 | 0.50 |
| HIV+ | 0.95 | 0.30–3.03 | 0.94 | 1.30 | 0.41–4.18 | 0.66 |
| CMV+ | 0.90 | 0.50–1.61 | 0.72 | 0.95 | 0.48–1.89 | 0.88 |
| HCV+ | 1.01 | 0.41–2.51 | 0.98 | 1.09 | 0.39–3.01 | 0.87 |
| Initial nephropathy | ||||||
| Hypertension | 1.11 | 0.53–2.31 | 0.78 | 1.53 | 0.72–3.25 | 0.27 |
| Unknown origin | 1.13 | 0.64–2.00 | 0.68 | 1.23 | 0.65–2.35 | 0.52 |
| Diabetes | 1.11 | 0.60–2.07 | 0.73 | 1.00 | 0.47–2.11 | 0.99 |
| Genetic | 0.48 | 0.21–1.12 | 0.09 | 0.54 | 0.21–1.35 | 0.19 |
| Glomerulopathy | 0.91 | 0.53–1.57 | 0.74 | 0.76 | 0.39–1.47 | 0.41 |
| Tubular and interstitial | 1.64 | 0.71–3.78 | 0.25 | 1.85 | 0.74–4.65 | 0.19 |
| Urologic | 1.56 | 0.68–3.60 | 0.30 | 1.39 | 0.50–3.86 | 0.52 |
| Other | 1.03 | 0.25–4.20 | 0.97 | * | * | * |
| Diabetes before transplantation | 1.02 | 0.57–1.81 | 0.95 | 0.91 | 0.45–1.82 | 0.78 |
| Donor characteristics | ||||||
| Age | 1.02 | 1.01–1.04 | 0.01 | 1.03 | 1.01–1.05 | 0.002 |
| Expanded Criteria Donor | 2.76 | 1.68–4.54 | <0.0001 | 3.74 | 2.03–6.90 | <0.0001 |
| eGFR | 1.00 | 0.99–1.00 | 0.31 | 1.00 | 0.99–1.00 | 0.41 |
| Living donor | 0.47 | 0.15–1.49 | 0.20 | 0.20 | 0.03–1.48 | 0.12 |
| CMV+ | 1.00 | 0.63–1.60 | 0.99 | 1.05 | 0.61–1.80 | 0.86 |
| Sensitization risk factors | ||||||
| Anti HLA antibodies | 0.99 | 0.62–1.59 | 0.98 | 0.97 | 0.56–1.66 | 0.90 |
| Former kidney transplantation | 1.02 | 0.41–2.54 | 0.96 | 1.10 | 0.40–3.04 | 0.86 |
| Donor specific anti-HLA antibodies | 0.69 | 0.32–1.51 | 0.36 | 0.39 | 0.12–1.25 | 0.11 |
| Combined Transplants | ||||||
| Pancreas | 0.69 | 0.10–5.00 | 0.72 | 0.92 | 0.13–6.65 | 0.93 |
| Liver | 1.87 | 0.68–5/12 | 0.23 | 1.90 | 0.59–6.09 | 0.28 |
| Heart | * | * | * | * | * | * |
| Kidney Transplant Characteristics | ||||||
| Cold ischemia time | 1.02 | 0.99–1.05 | 0.28 | 1.03 | 0.99–1.07 | 0.18 |
| Induction Immunosuppressive regimen | ||||||
| Basiliximab | 1.21 | 0.76–1.93 | 0.43 | 1.51 | 0.87–2.61 | 0.15 |
| Antithymocyte globulin | 1.01 | 0.63–1.61 | 0.96 | 0.84 | 0.48–1.45 | 0.52 |
| Intravenous Immunoglobulin | 0.74 | 0.37–1.48 | 0.39 | 0.42 | 0.15–1.16 | 0.09 |
| Rituximab | 1.03 | 0.44–2.37 | 0.95 | 0.45 | 0.11–1.83 | 0.26 |
| Maintenance immunosuppressive regimen | ||||||
| Calcineurin inhibitors | * | * | * | * | * | * |
| Mycophenolate Mophetil | * | * | * | * | * | * |
| Steroids | * | * | * | * | * | * |
| Belatacept | * | * | * | * | * | * |
| White blood cells at the time of transplantation | ||||||
| Leucocytes (/1000) | 1.01 | 0.92–1.11 | 0.84 | |||
| Neutrophils (/1000) | 1.05 | 0.95–1.15 | 0.37 | |||
| Lymphocytes (/1000) | 0.61 | 0.40–0.95 | 0.028 | |||
| TCD4 cells (/1000) | 0.34 | 0.08–1.38 | 0.13 | |||
| TCD4 cells (%) | 0.98 | 0.96–1.01 | 0.25 | |||
| TCD8 cells (/1000) | 1.28 | 0.31–5.24 | 0.73 | |||
| TCD8 cells (%) | 1.02 | 1.00–1.05 | 0.06 | |||
| White blood cells as time-varying variables during follow-up | ||||||
| Leucocytes (/1000) | 0.94 | 0.84–1.05 | 0.28 | 0.89 | 0.78–1.02 | 0.09 |
| Neutrophils (/1000) | 0.98 | 0.87–1.10 | 0.72 | 0.92 | 0.79–1.07 | 0.28 |
| Lymphocytes (/1000) | 0.74 | 0.50–1.11 | 0.15 | 0.70 | 0.43–1.13 | 0.14 |
| TCD4 cells (/1000) | 0.31 | 0.11–0.83 | 0.02 | 0.45 | 0.15–1.33 | 0.15 |
| TCD4 cells (%) | 0.97 | 0.95–0.99 | 0.004 | 0.98 | 0.96–1.00 | 0.11 |
| TCD8 cells (/1000) | 0.70 | 0.23–2.10 | 0.52 | 0.90 | 0.27–2.98 | 0.86 |
| TCD8 cells (%) | 1.01 | 0.99–1.03 | 0.42 | 1.00 | 0.98–1.02 | 0.94 |
| Follow up | ||||||
| CMV viremia before OI | 1.29 | 0.76–2.18 | 0.34 | 1.53 | 0.85–2.76 | 0.16 |
| BK viruria before OI | 1.94 | 1.15–3.28 | 0.01 | 0.34 | 0.12–0.96 | 0.04 |
| Acute rejection before OI | 1.48 | 0.83–2.63 | 0.19 | 1.38 | 0.70–2.71 | 0.35 |
| High calcineurin inhibitors level in the month before OI | 2.06 | 0.97–4.35 | 0.06 | 3.87 | 1.53–9.80 | 0.004 |
| Mycophenolate Mofetil at the time of OI | 1.134 | 0.45–2.83 | 0.79 | 0.41 | 0.10–1.78 | 0.24 |
| mTOR at the time of OI | 0.49 | 0.15–1.56 | 0.23 | 0.56 | 0.17–1.80 | 0.33 |
* data non analyzed.
Independent risk factors for OI according to multivariate analysis were ECD (2.53 (1.48–4.31), p = 0.0007), and BK viremia (6.38 (3.62–11.23), p < 0.0001). High blood lymphocyte count at the time of transplantation was an independent protective risk factor (0.60 (0.38–0.94), p = 0.026). The multivariable analysis conducted only on patients with available pre-transplantation CD4 T-cell counts (n = 456) showed that ECD (2.92 (1.62–5.27), p = 0.0004) and BK viremia (5.11 (2.72–9.57), p < 0.0001) were independent risk factors for OI. In contrast, a higher CD4 T-cell percentage during follow-up (time-varying variable) (0.98 (0.96–0.99), p = 0.015) and, to a lesser extent, a higher lymphocyte count at the time of transplantation (0.68 (0.44–1.07), p = 0.09) were independent protective factors.
3.3. Patients and Allograft Survival
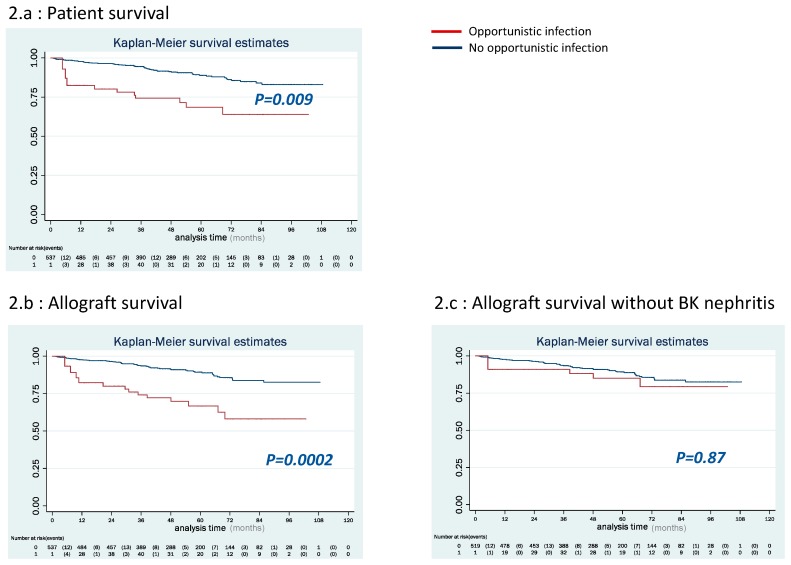
In OI group, patient survival was significantly lower than in control group (Figure 2a, p = 0.009). After OI episode, 10 patients (14%) died, of whom three (30%) deaths were related to an OI episode (one PML, one PCP, and one IA). Other causes of death included cardio-vascular disease (n = 3), hemorrhagic shock (n = 1), traumatism (n = 1), bacterial infections (n = 1), and neoplasia (n = 1). OI was not an independent risk factor for death as shown by the multivariable analysis (Table 4). OI lost its statistical significance after multivariable adjustment for recipient age at transplantation, TCD8 cells (/1000) during follow-up, neutrophils (/1000) during follow-up, HCV+ status, former kidney transplantation and diabetes. Consequently, and in accordance with our statistical analysis strategy (section #2), OI was left out of Table 4 showing only results from the final multivariable model after a stepwise backward approach was applied.
Figure 2.
Patient, allograft, and event-free survival in both groups (Kaplan–Meier survival analysis): (a) in OI group, patient survival was significantly lower than in control group (p = 0.009); (b) allograft survival was significantly lower in OI group (p = 0.0002); and (c) allograft survival without BK virus nephropathy was not significantly lower in OI group (p = 0.87).
Table 4.
Patient and allograft survival independent risk factors (time varying Cox model).
| Patient Overall Survival | |||
| Variables | HR | 95% CI | p-Value |
| Recipient age at transplantation | 1.08 | 1.05–1.11 | <0.0001 |
| TCD8 cells (/1000), time-varying during follow-up | 0.26 | 0.07–1.01 | 0.052 |
| Neutrophils (/1000), time-varying during follow-up | 1.12 | 0.99–1.27 | 0.08 |
| HCV+ | 3.02 | 1.39–6.55 | 0.005 |
| Former kidney transplantation | 3.18 | 1.35–7.50 | 0.008 |
| Diabetes | 1.83 | 1.04–3.22 | 0.04 |
| Allograft survival | |||
| Variables | HR | 95% CI | p-Value |
| Donor age | 1.02 | 1.00–1.04 | 0.03 |
| TCD4 cells (/1000), time-varying during follow-up | 0.23 | 0.08–0.67 | 0.007 |
| Acute rejection before opportunistic infection | 3.28 | 1.93–5.57 | <0.0001 |
| Opportunistic infection episode | 2.53 | 1.29–4.95 | 0.007 |
| Donor specific anti-HLA antibodies before transplantation | 1.92 | 0.97–3.78 | 0.06 |
| CMV+ donor | 1.83 | 1.05–3.19 | 0.03 |
| Diabetes | 1.97 | 1.15–3.39 | 0.014 |
Allograft survival was significantly lower in OI group (Figure 2b, p = 0.0002). After OI episode, allograft loss occurred in 13 (18%) patients, around 31 (5–63) months after transplantation. Causes of allograft loss were five (38%) BKVN, five (38%) chronic allograft dysfunction, two (16%) refractory acute rejection, and one (8%) unknown cause. OI episode was an independent risk factor for allograft loss with HR = 2.53 (1.29–4.95) (p = 0.007) (Table 4).
3.4. Analysis Excluding BKVN
As BKVN is well-known to cause a chronic destructive infection [24], we performed another analysis excluding BKVN events (Table 3). ECD and low blood lymphocytes count at the time of transplantation remained the two independent risk factors for OI episode (4.09 (2.06–8.09), p < 0.0001 and 0.64 (0.38–1.06), p = 0.08, respectively). OI was not found to be a risk factor for allograft loss (p = 0.87; Figure 2c and Table S1).
4. Discussion
We present here the results of a monocentric cohort analysis conducted on more than 500 kidney allograft recipients. We showed that, in the era of modern immunosuppression and the wide use of infectious disease prophylactic strategies, OIs occurred more than one year after transplantation and that pre-transplantation lymphopenia was an independent risk factor for OI episode, which was not the case for induction therapy. Moreover, OIs were an independent risk factor for allograft loss but had no effect on patient survival.
Although OIs are well defined in the setting of HIV [23], no classification of post-SOT OIs is currently available [2]. However, we tried in our work to carefully apply the current OI definitions on post-SOT settings taking into account the standardized immunosuppressive regimen and the type of SOT. On this point, former studies on allograft recipients were quite heterogenous concerning the infections considered and the type of SOT [3,4,14]. To our knowledge, no study evaluating the risk factors for OIs versus more severe common infections in engrafted patients has been published. Therefore, no conclusion regarding physiopathology and risk factors is available. In our cohort, we used HIV classification to define OI updated with BKVN, an immunosuppression-induced infection after kidney transplantation [23,24]. This selection process allowed us to provide reliable data on incidence and spectrum of OI after kidney transplantation and could be routinely used by clinicians to customize the prevention strategies to the patient condition.
OI proportion in our cohort was significantly lower than the most recently published incidence rate of around 25% [3]. Several explanations may account for this low incidence. First, the post-transplantation CMV, PCP, and bacterial prophylaxis strategies we use in our center are in fulfilment with the international recommendations (e.g., trimethoprim-sulfamethoxazole for Nocardia) [5,21]. Secondly, the immunocompromised recipients were exposed to a lower level of CNI, a strategy previously described to significantly decrease OI incidence [26]. At last, solid-organs failure before transplantation induced variable degrees of immune suppression. For instance, liver cirrhosis is associated with dysfunction of the defensive mechanisms against infections and higher incidence of sepsis [27] unlike end-stage renal failure [28]. Therefore, fungal infections risk is lower after kidney transplantation compared with other SOT populations [29].
Thus, we updated the description of post- kidney transplantation OIs to align it with the new strategies of immunosuppressive therapy. In our cohort, the incidence of CMV disease was significantly lower than previously described, probably because of the application of the regularly-updated prevention recommendations [2]. However, viral infections remained the first cause of OIs, mainly cutaneous shingles and BKVN. No prevention strategy is currently recommended for shingles. BKVN is clearly problematic after kidney transplantation since it thrives in immune suppression status, has a great impact on kidney allograft survival, and there is no curative treatment for it [24]. IA incidence is also lower in our cohort [29], whereas other OIs incidence was in the previously described range [16] after kidney transplantation.
Interestingly, time to OI onset was long, more than one year after transplantation. The latest review has reported a peak of OI at 6–12 months after transplantation [2]. Again, prevention strategies could probably postpone post-transplantation infections onset. However, post-transplantation fungal infection developed significantly earlier as in former studies, which confirmed that those infections flourish by the peak of immunosuppression [29]. No prevention strategy is currently recommended for those infections as well as PCP.
Thereafter, we aimed to identify independent risk factors for post-kidney transplantation OI. We found that ECD and low pre-transplantation lymphocyte count were independent risk factors; the type of induction immunosuppressive treatments and the recipient age were not. In kidney allograft recipients, older donor age, irrespective of recipient age, increases the rate of acute allograft rejection and infections [30,31]. The underlying immune system seems to be more important than immunosuppressive therapy. Aged transplanted mice could have an impaired anti-infectious response with accumulation of memory CD4+ T-cells and reduced Th1 anti-donor immune response [32,33]. These immunological effects could significantly decrease anti-infectious response in recipients transplanted from ECD. High CD4+ T-cells count was significantly a protective factor, but there was no effect of CD8+ T-cells count while CD4/ CD8 numbers (%) at the time of transplant were similar in both groups. The total count in lymphocytes had a superior predictive value for OI than the separate levels of CD4/CD8. However, the study population for analyses on CD4/CD8 was slightly decreased due to missing information on these variables, thus possibly resulting in a moderate loss of statistical power. High late stage differentiated CD28+CD57+CD4+ T-cells rates at the time of transplantation is independently associated with a decreased risk of OI [28]. Analysis of naive CD4+ T-cells remains to be determined since such phenotype has been associated with a high risk of infection in patients with common variable immunodeficiency [34]. Surprisingly, immunosuppressive induction using depletive monoclonal agents was not associated with OI incidence. Comparing the risk of infection with depletive and non-depletive therapies yielded controversial data although the most recent work shows that thymoglobulin was not associated with higher infection risk [35,36,37]. Almost all of our patients were treated with induction therapy. No induction therapy in immunocompromised kidney allograft recipients could be an option [38]. Whether the absence of induction could be associated with a significantly lower incidence of OI need to be elucidated.
How lymphopenia before transplant could influence OI occurring more than one year after transplantation remains unknown. Again, the wide use of prophylaxis (trimethoprim-sulfamethoxazole and valganciclovir) prevents early infection (mostly PCP, Nocardia, and CMV disease). Considering late infection, we believed that lymphopenia before transplantation could be a cumulative effect of immunosuppressive therapies in older patients.
Our data confirm that OI is not an independent risk factor for death [3,4]. In a recent large Finnish cohort, OI rarely caused deaths after kidney transplantation, but the most common cause of infection-related mortality was common bacterial infections, e.g. septicemia and pneumonia [6]. The lack of OI-related effect on mortality compared with the role of common bacterial infections needs deeper analyses of causes and risk factors for common infections; this should enable us to adjust prevention strategies to different contexts. Additionally, recent data suggest that infections could be the first cause of death after transplantation [39].
Finally, in our cohort, OI was an independent risk factor for allograft loss only if BKVN episodes were considered. The negative impact of BKVN on kidney allograft survival is well-documented [24]. Thus, in one of the analyses, we excluded BKVN from OI episodes and found no impact on kidney allograft survival on the long-term [3]. To decrease BKVN, only m-TOR inhibitors based immunosuppressive combination showed a significant effect, thus should be considered in all patient with standard immunologic risk [40].
Our study presents limits. The first one is being a single center study and retrospective. These results must be confirmed in a prospective multicentric cohort. However, the single center study implies only one way to manage immunosuppression after transplantation. The second one is that we performed an overview of OI without considering specific prognosis of each infection.
In conclusion, our study showed that, in the era of modern immunosuppression and the wide use of infection prophylactic regimens, OIs occurred later, more than one year after kidney transplantation and were mainly viral. Pre-transplantation lymphopenia and ECD were the two independent risk factors for OI, hence the need for customized immunosuppressive regimen in such transplant candidates. BKVN incidence remained high with a clear negative impact on allograft survival. In low-risk recipients, m-TOR based immunosuppressive therapy is the only prophylaxis to prevent BKVN and should be considered more widely. Two more issues need to be further studied: the specific role of pre-transplantation leucocytes subpopulation especially naive T-cells, and the difference between OI and common infections which have been described as the main cause of patient death after kidney transplantation.
Abbreviations
| ABMR | antibody-mediated rejection |
| ATG | antithymocyte globulin |
| BKV | BK virus |
| BKVN | BK virus nephropathy |
| CMV | cytomegalovirus |
| CNI | Calcineurin inhibitor |
| DGF | delayed graft function |
| DSA | donor specific antibodies |
| EBV | Epstein Barr virus |
| ECD | expanded criteria donor |
| eGFR | estimated glomerular filtration rate |
| HIV | Human immunodeficiency virus |
| HSV | herpes simplex virus |
| HHV8 | Human Herpes virus-8 |
| IQR | interquartile range |
| IA | invasive aspergillosis |
| M | month |
| MDRD | modified diet of renal disease |
| N | number |
| OIs | opportunistic infections |
| PML | progressive multifocal leukoencephalopathy |
| PCP | Pneumocystosis pneumonia |
| SD | standard deviation |
| TCMR | T-cell-mediated rejection |
| VZV | varicella-zoster virus |
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/8/5/594/s1, Table S1: Allograft survival risk factors univariable analysis.
Author Contributions
Conceptualization, P.A., G.M., D.B., N.D.C., and M.M.; Methodology, G.M., E.A., and M.M.; Software, E.A.; Validation, all authors; Formal Analysis, P.A., G.M., D.B., E.A., M.M., and P.G.; Investigation, P.A., G.M., and M.M.; Resources, M.M.; Data Curation, V.A., T.S., S.F., G.G., F.B., and V.F.; Writing—Original Draft Preparation, P.A., G.M., and M.M.; Writing—Review and Editing: P.A., G.M., D.B., V.F., S.F., M.M., and P.G.; Visualization, G.M.; and Supervision, M.M.
Conflicts of Interest
The authors declare no conflict of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Fishman J.A. Infection in solid-organ transplant recipients. N. Engl. J. Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 2.Fishman J.A. Opportunistic infections--coming to the limits of immunosuppression? Cold Spring Harb. Perspect. Med. 2013;3:a015669. doi: 10.1101/cshperspect.a015669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helfrich M., Dorschner P., Thomas K., Stosor V., Ison M.G. A retrospective study to describe the epidemiology and outcomes of opportunistic infections after abdominal organ transplantation. Transpl. Infect. Dis. 2017;19 doi: 10.1111/tid.12691. [DOI] [PubMed] [Google Scholar]
- 4.Peleg A.Y., Husain S., Kwak E.J., Silveira F.P., Ndirangu M., Tran J., Shutt K.A., Shapiro R., Thai N., Abu-Elmagd K., et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin. Infect. Dis. 2007;44:204–212. doi: 10.1086/510388. [DOI] [PubMed] [Google Scholar]
- 5.Group KDIGOKTW KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009;9(Suppl. 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 6.Kinnunen S., Karhapää P., Juutilainen A., Finne P., Helanterä I. Secular Trends in Infection-Related Mortality after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2018;13:755–762. doi: 10.2215/CJN.11511017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matas A.J., Smith J.M., Skeans M.A., Thompson B., Gustafson S.K., Schnitzler M.A., Stewart D.E., Cherikh W.S., Wainright J.L., Snyder J.J., et al. OPTN/SRTR 2012 Annual Data Report: Kidney. Am. J. Transplant. 2014;14(Suppl. 1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 8.Yeung M.Y., Gabardi S., Sayegh M.H. Use of polyclonal/monoclonal antibody therapies in transplantation. Expert Opin. Biol. Ther. 2017;17:339–352. doi: 10.1080/14712598.2017.1283400. [DOI] [PubMed] [Google Scholar]
- 9.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 10.Zand M.S., Vo T., Huggins J., Felgar R., Liesveld J., Pellegrin T., Bozorgzadeh A., Sanz I., Briggs B.J. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. 2005;79:1507–1515. doi: 10.1097/01.TP.0000164159.20075.16. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe R.A., Roys E.C., Merion R.M. Trends in organ donation and transplantation in the United States, 1999–2008. (4 Pt 2)Am. J. Transplant. 2010;10:961–972. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 12.Hill P., Cross N.B., Barnett A.N., Palmer S.C., Webster A.C. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst. Rev. 2017;1:CD004759. doi: 10.1002/14651858.CD004759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients 2010 data report. Am. J. Transplant. 2012;12(Suppl. 1):1–156. doi: 10.1111/j.1600-6143.2011.03886.x. [DOI] [PubMed] [Google Scholar]
- 14.Jordan C.L., Taber D.J., Kyle M.O., Connelly J., Pilch N.W., Fleming J., Meadows H.B., Bratton C.F., Nadig S.N., McGillicuddy J.W., et al. Incidence, risk factors, and outcomes of opportunistic infections in pediatric renal transplant recipients. Pediatr. Transplant. 2016;20:44–48. doi: 10.1111/petr.12625. [DOI] [PubMed] [Google Scholar]
- 15.López-Medrano F., Fernández-Ruiz M., Silva J.T., Carver P.L., van Delden C., Merino E., Pérez-Saez M.J., Montero M., Coussement J., de Abreu Mazzolin M., et al. Multinational case-control study of risk factors for the development of late invasive pulmonary aspergillosis following kidney transplantation. Clin. Microbiol. Infect. 2018;24:192–198. doi: 10.1016/j.cmi.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Lebeaux D., Freund R., van Delden C., Guillot H., Marbus S.D., Matignon M., Van Wijngaerden E., Douvry B., De Greef J., Vuotto F., et al. Outcome and Treatment of Nocardiosis After Solid Organ Transplantation: New Insights from a European Study. Clin. Infect. Dis. 2017;64:1396–1405. doi: 10.1093/cid/cix124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh N., Dromer F., Perfect J.R., Lortholary O. Cryptococcosis in solid organ transplant recipients: Current state of the science. Clin. Infect. Dis. 2008;47:1321–1327. doi: 10.1086/592690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Port F.K., Bragg-Gresham J.L., Metzger R.A., Dykstra D.M., Gillespie B.W., Young E.W., Delmonico F.L., Wynn J.J., Merion R.M., Wolfe R.A., et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281–1286. doi: 10.1097/00007890-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 19.Levey A.S., Eckardt K.U., Tsukamoto Y., Levin A., Coresh J., Rossert J., De Zeeuw D., Hostetter T.H., Lameire N., Eknoyan G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 20.Loupy A., Haas M., Solez K., Racusen L., Glotz D., Seron D., Nankivell B.J., Colvin R.B., Afrouzian M., Akalin E., et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am. J. Transplant. 2017;17:28–41. doi: 10.1111/ajt.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotton C.N., Kumar D., Caliendo A.M., Huprikar S., Chou S., Danziger-Isakov L., Humar A. The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102:900–931. doi: 10.1097/TP.0000000000002191. [DOI] [PubMed] [Google Scholar]
- 22.Humar A., Michaels M. Monitoring AIWGoID. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am. J. Transplant. 2006;6:262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 23.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm. Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 24.Nankivell B.J., Renthawa J., Sharma R.N., Kable K., O’Connell P.J., Chapman J.R. BK Virus Nephropathy: Histological Evolution by Sequential Pathology. Am. J. Transplant. 2017;17:2065–2077. doi: 10.1111/ajt.14292. [DOI] [PubMed] [Google Scholar]
- 25.Penn I. Kaposi’s sarcoma in transplant recipients. Transplantation. 1997;64:669–673. doi: 10.1097/00007890-199709150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Ekberg H., Bernasconi C., Tedesco-Silva H., Vítko S., Hugo C., Demirbas A., Acevedo R.R., Grinyó J., Frei U., Vanrenterghem Y., et al. Calcineurin inhibitor minimization in the Symphony study: Observational results 3 years after transplantation. Am. J. Transplant. 2009;9:1876–1885. doi: 10.1111/j.1600-6143.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- 27.Fagiuoli S., Colli A., Bruno R., Craxì A., Gaeta G.B., Grossi P., Mondelli M.U., Puoti M., Sagnelli E., Stefani S., et al. Management of infections pre- and post-liver transplantation: Report of an AISF consensus conference. J. Hepatol. 2014;60:1075–1089. doi: 10.1016/j.jhep.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Crepin T., Carron C., Roubiou C., Gaugler B., Gaiffe E., Simula-Faivre D., Ferrand C., Tiberghien P., Chalopin J.M., Moulin B., et al. ATG-induced accelerated immune senescence: Clinical implications in renal transplant recipients. Am. J. Transplant. 2015;15:1028–1038. doi: 10.1111/ajt.13092. [DOI] [PubMed] [Google Scholar]
- 29.López-Medrano F., Fernández-Ruiz M., Silva J.T., Carver P.L., van Delden C., Merino E., Pérez-Saez M.J., Montero M., Coussement J., de Abreu Mazzolin M., et al. Clinical Presentation and Determinants of Mortality of Invasive Pulmonary Aspergillosis in Kidney Transplant Recipients: A Multinational Cohort Study. Am. J. Transplant. 2016;16:3220–3234. doi: 10.1111/ajt.13837. [DOI] [PubMed] [Google Scholar]
- 30.Tullius S.G., Milford E. Kidney allocation and the aging immune response. N. Engl. J. Med. 2011;364:1369–1370. doi: 10.1056/NEJMc1103007. [DOI] [PubMed] [Google Scholar]
- 31.Meier-Kriesche H.U., Ojo A.O., Hanson J.A., Kaplan B. Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int. 2001;59:1539–1543. doi: 10.1046/j.1523-1755.2001.0590041539.x. [DOI] [PubMed] [Google Scholar]
- 32.Tesar B.M., Du W., Shirali A.C., Walker W.E., Shen H., Goldstein D.R. Aging augments IL-17 T-cell alloimmune responses. Am. J. Transplant. 2009;9:54–63. doi: 10.1111/j.1600-6143.2008.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denecke C., Bedi D.S., Ge X., Kim I.K., Jurisch A., Weiland A., Habicht A., Li X.C., Tullius S.G. Prolonged graft survival in older recipient mice is determined by impaired effector T-cell but intact regulatory T-cell responses. PLoS ONE. 2010;5:e9232. doi: 10.1371/journal.pone.0009232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouillot G., Carmagnat M., Gérard L., Garnier J.L., Fieschi C., Vince N., Karlin L., Viallard J.F., Jaussaud R., Boileau J., et al. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J. Clin. Immunol. 2010;30:746–755. doi: 10.1007/s10875-010-9424-3. [DOI] [PubMed] [Google Scholar]
- 35.Hellemans R., Hazzan M., Durand D., Mourad G., Lang P., Kessler M., Charpentier B., Touchard G., Berthoux F., Merville P., et al. Daclizumab Versus Rabbit Antithymocyte Globulin in High-Risk Renal Transplants: Five-Year Follow-up of a Randomized Study. Am. J. Transplant. 2015;15:1923–1932. doi: 10.1111/ajt.13191. [DOI] [PubMed] [Google Scholar]
- 36.Brennan D.C., Schnitzler M.A. Long-term results of rabbit antithymocyte globulin and basiliximab induction. N. Engl. J. Med. 2008;359:1736–1738. doi: 10.1056/NEJMc0805714. [DOI] [PubMed] [Google Scholar]
- 37.Thomusch O., Wiesener M., Opgenoorth M., Pascher A., Woitas R.P., Witzke O., Jaenigen B., Rentsch M., Wolters H., Rath T., et al. Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): An open-label, multicentre, randomised controlled trial. Lancet. 2016;388:3006–3016. doi: 10.1016/S0140-6736(16)32187-0. [DOI] [PubMed] [Google Scholar]
- 38.Hellemans R., Bosmans J.L., Abramowicz D. Induction Therapy for Kidney Transplant Recipients: Do We Still Need Anti-IL2 Receptor Monoclonal Antibodies? Am. J. Transplant. 2017;17:22–27. doi: 10.1111/ajt.13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colvin M., Smith J.M., Hadley N., Skeans M.A., Carrico R., Uccellini K., Lehman R., Robinson A., Israni A.K., Snyder J.J., et al. OPTN/SRTR 2016 Annual Data Report: Heart. Am. J. Transplant. 2018;18(Suppl. 1):291–362. doi: 10.1111/ajt.14561. [DOI] [PubMed] [Google Scholar]
- 40.Pascual J., Berger S.P., Witzke O., Tedesco H., Mulgaonkar S., Qazi Y., Chadban S., Oppenheimer F., Sommerer C., Oberbauer R., et al. Everolimus with Reduced Calcineurin Inhibitor Exposure in Renal Transplantation. J. Am. Soc. Nephrol. 2018;29:1979–1991. doi: 10.1681/ASN.2018010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.