Abstract
Extra-virgin olive oil (EVOO) is largely appreciated for its proven nutritional properties. Additionally, organic foods are perceived as healthier by consumers. In this context, the aim of the present study was to compare the phenolic profiles of EVOO from olives of the Hojiblanca variety, cultivated under organic and conventional systems. The quantification and identification of individual polyphenols was carried out by liquid chromatography coupled to mass spectrometry in tandem mode (LC-MS/MS). Significantly higher levels (p < 0.05) of phenolic compounds were found in organic EVOOs. The methodology used was able to detect previously unreported differences in bioactive components between organic and conventional EVOOs.
Keywords: phenolic compounds, Hojiblanca, variety, organic, conventional, agriculture, mass spectrometry, oleocanthal, secoiridoids, ripening, NMR
1. Introduction
Extra virgin olive oil (EVOO), a key component of the Mediterranean diet, is highly appreciated for its nutritional and organoleptic attributes. The minor compounds include aliphatic and triterpene alcohols, sterols, hydrocarbons, volatile compounds and antioxidants such as carotenoids and polyphenols, which contribute to the organoleptic characteristics, stability and nutritional value of EVOO [1,2]. The qualitative and quantitative composition of polyphenols in EVOO is affected by many variables, such as the degree of olive ripeness, the technological production process and storage conditions [3,4]. The most important changes in the polyphenol content occur during the crushing and malaxation of olives [5,6] as well as during the storage and filtration of EVOO [7]. Other influential factors include the use of organic and conventional growing systems [8,9].
The market for organic products, generally perceived as healthier and safer than conventional foods, is growing annually [10], despite the higher costs and lower productivity of organic compared to traditional agriculture. In 2016, on a global level, up to 178 countries practiced organic agriculture, on an extension of 57.8 million hectares, with a market size of 89.7 billion US dollars [11]. A key difference between the two growing systems is soil fertility management, which can affect the nutritive composition of plants, including levels of secondary metabolites [12]. In organic agriculture, which is associated with the promotion of biodiversity and biological cycles, crops obtain nitrogen and nutrients from a diverse soil ecosystem. Contrastingly, conventional farming uses fertilizers containing soluble inorganic nitrogen and other nutrients, which are more directly available to plants [13]. Phenolic biosynthesis in plants is known to be strongly affected by the cultivar, the environmental conditions (especially light), as well as the type of fertilization [14,15]. Previous studies demonstrate that the organic fruits have higher phenolic content than conventional ones [16,17,18].
Therefore, the objective of our study was to compare the content of polyphenols (secoiridoids, flavones, phenolic alcohols, phenolic acids and lignans) in Hojiblanca EVOO produced by organic and conventional production systems under the same environmental conditions. Moreover, we applied a quantitative 1H nuclear magnetic resonance (qNMR) method to corroborate the concentration of oleocanthal (OLC) in our EVOOs obtained by LC-MS.
2. Results
2.1. Total Amount of Phenolic Compounds
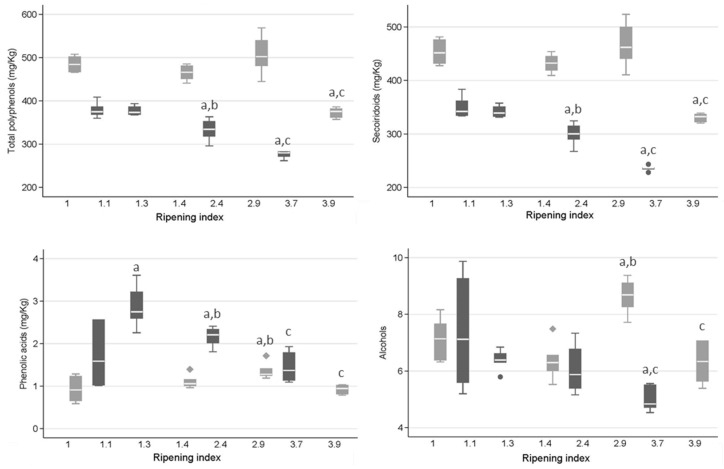
The average of total phenols (TP) in organic and conventional EVOO, with the p-value for the differences assessed by the Mann–Whitney test, are shown in Table 1. In order to control the ripening factor when assessing the differences in phenolic concentrations between the two types of EVOO, generalized linear models adjusting for ripening index (RI) were used (Table S1). The mean TP content of organic and conventional EVOO was 456.89 ± 56.74 and 338.19 ± 42.96 mg·kg−1, respectively, being 26% higher in EVOO produced by the organic system (Figure 1).
Table 1.
Phenolic compound contents (mg·kg−1) of conventional and organic EVOO made from Hojiblanca olives. * p-values of U Mann–Whitney test.
| Organic | Conventional | p * | |
|---|---|---|---|
| Total Phenols | 456.89 ± 56.74 | 338.19 ± 42.96 | <0.001 |
| Secoiridoids | 420.72 ± 59.42 | 306.48 ± 48.09 | <0.001 |
| Oleuropein | 0.82 ± 0.02 | 0.81 ± 0.02 | 0.2 |
| Oleuropein derivatives | |||
| Oleuropein der I | 22.77 ± 3.01 | 34.83 ± 4.44 | <0.001 |
| Oleuropein der II | 3.21 ± 0.54 | 1.67 ± 0.25 | <0.001 |
| Oleuropein der III | 3.63 ± 0.58 | 2.33 ± 0.33 | <0.001 |
| me-3,4-DHPEA-EA | 1.46 ± 0.25 | 0.97 ± 0.07 | <0.001 |
| Hydroxy oleuropein aglycone I (HOA I) | 1.44 ± 0.34 | 1.17 ± 0.18 | 0.007 |
| Hydroxy oleuropein aglycone II (HOA II) | 2.18 ± 0.77 | 1.67 ± 0.41 | 0.02 |
| HDCM OA | 9.13 ± 3.53 | 6.37 ± 1.94 | 0.01 |
| 3,4-DHPEA-EA I | 7.18 ± 0.98 | 4.74 ± 0.42 | <0.001 |
| 3,4-DHPEA-EA II | 5.82 ± 0.94 | 3.02 ± 0.74 | <0.001 |
| Lactone | 0.19 ± 0.04 | 0.33 ± 0.20 | <0.001 |
| Ligstroside derivatives | |||
| Ligstroside I | 20.45 ± 2.77 | 12.32 ± 2.47 | <0.001 |
| Ligstroside II | 41.61 ± 3.68 | 24.41 ± 4.02 | <0.001 |
| Ligstroside III | 54.59 ± 10.63 | 34.75 ± 8.00 | <0.001 |
| Oleocanthal | 186.72 ± 40.61 | 132.10 ± 37.02 | <0.001 |
| Elenolic acid | 55.35 ± 8.10 | 40.37 ± 7.39 | <0.001 |
| Elenolic acid derivatives | |||
| Hydroxyelenolic acid | 3.41 ± 1.42 | 3.19 ± 1.70 | 0.5 |
| Flavones | 28.21 ± 5.55 | 25.53 ± 5.85 | 0.09 |
| Luteolin | 22.69 ± 5.09 | 19.35 ± 5.38 | 0.03 |
| Apigenin | 5.51 ± 0.69 | 6.17 ± 0.78 | 0.008 |
| Phenolic alcohols | 7.11 ± 1.15 | 6.21 ± 1.37 | 0.07 |
| Hydroxytyrosol | 4.47 ± 1.10 | 3.65 ± 1.32 | 0.01 |
| Dihydroxytyrosol | 1.73 ± 0.09 | 1.78 ± 0.10 | 0.001 |
| 3,4-DHPEA-AC | 0.91 ± 0.02 | 0.91 ± 0.03 | 0.51 |
| Lignans | 0.47 ± 0.06 | 0.79 ± 0.09 | <0.001 |
| Pinoresinol | 0.47 ± 0.06 | 0.79 ± 0.09 | <0.001 |
| Phenolic acids | 1.08 ± 0.26 | 2.05 ± 0.71 | <0.001 |
| Ferulic acid | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.003 |
| p-coumaric acid | 0.67 ± 0.15 | 1.04 ± 0.36 | <0.001 |
| Vanillic acid | 0.35 ± 0.12 | 0.93 ± 0.37 | <0.001 |
Figure 1.
Boxplots for concentrations (mg·kg−1) of total phenols (TP) and polyphenolic groups in extra-virgin olive oil (EVOO) by ripening index (RI) and growing method. (a means p < 0.05 vs. 1st RI, b means p < 0.05 vs. 2nd RI, c means p < 0,05 vs. 3rd RI within the same growing.
2.2. Concentrations of Phenolic Groups and Selected Phenolic Compounds
The major phenolic compounds in EVOO were secoiridoids (SEC), whereas lignans, phenolic acids and flavones were present in low concentrations. The SEC represented 91–92% of the phenolic compounds, with higher levels in the organic than conventional EVOO (420.72 ± 59.42 and 306.48 ± 48.09 mg·kg−1, respectively). OLC was the predominant ligstroside derivative found in organic and conventional EVOO samples (186.72 ± 40.61 and 132.10 ± 37.02 mg·kg−1, respectively), being 30% higher in the former. Also, the elenolic acid concentration was positively affected by the organic system (55.35 ± 8.10 mg·kg−1), being 27% lower in conventional EVOO (40.37 ± 7.39 mg·kg−1).
Luteolin was the predominant flavonoid in both organic and conventional EVOO (22.69 ± 5.09 and 19.35 ± 5.38 mg·kg−1, respectively) and the apigenin represented 20–24% of the total flavonoids and its content was not affected by the agronomic conditions (6.17 ± 0.78 and 5.51 ± 0.69 mg·kg−1, conventional and organic, respectively).
The concentration of the total phenolic alcohols was not affected by the organic or conventional growing systems (7.11 ± 1.15 and 6.21 ± 1.37 mg·kg−1, respectively). However, the content of hydroxytyrosol was higher under the organic than the conventional system (4.47 ± 1.10 and 3.65 ± 1.32 mg·kg−1, respectively).
The content of lignans and phenolic acids, which are important phenolic components of EVOO, were higher under the conventional system (0.79 ± 0.09 and 2.05 ± 0.71 mg·kg−1, respectively). The only lignan found was pinoresinol and the phenolic acids were p-coumaric, ferulic and vanillic acid (1.04 ± 0.36, 0.07 ± 0.01, and 0.93 ± 0.37 mg·kg−1, respectively).
2.3. EVOO Phenolic Profile and Olive Fruit Ripening
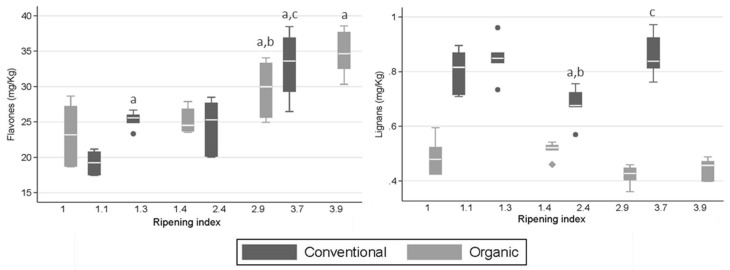
The concentration of total SEC, phenolic acids, flavones and lignans in EVOO samples extracted from olives of the Hojiblanca cultivar, grown in conventional and organic conditions and harvested at different RI, are presented in Figure 1. To assess the effect of the RI of the olives on the content of phenolic compounds in conventional and organic EVOO, regression models were fitted (Table 2).
Table 2.
Linear regressions (polyphenol content vs. RI) by type of cultivation.
| Organic | Conventional | |||
|---|---|---|---|---|
| Coefficient | p | Coefficient | p | |
| Total phenols | −27.4 | 0.004 | −40.2 | <0.001 |
| Secoiridoids | −31.2 | 0.001 | −44.7 | <0.001 |
| Phenolic alcohols | 0.05 | 0.81 | −0.76 | 0.002 |
| Phenolic acids | 0.02 | 0.74 | −0.29 | 0.04 |
| Flavones | 3.77 | <0.001 | 4.23 | <0.001 |
| Lignans | −0.02 | 0.01 | 0.003 | 0.9 |
The concentration of TP and SEC decreased during ripening in both conventional and organic EVOO (Table 2). Conversely, an increase in the content of flavones was correlated with ripeness in both organic and conventional EVOO (p < 0.001). The total phenolic acids and phenolic alcohols were affected by the olive ripening stage only in the conventional system, showing lower levels with later harvests. Lignans were not affected by the RI in any system.
2.4. Analysis of Oleocanthal by NMR
The qNMR showed that the level of OLC was higher (168.96 mg·kg−1) in the EVOO made from organic vs. conventionally cultivated olives (118.21 mg·kg−1) (Table 3). Thus, the significant variation in OLC concentrations among the samples of the Hojiblanca EVOO is in accordance with previous studies recently reviewed [19] and the results of our OLC analysis by mass spectrometry (MS).
Table 3.
Concentration of oleocanthal (OLC) measured by qNMR in EVOOs of Hojiblanca variety.
| Integration | Concentration (mg·kg−1) | |
|---|---|---|
| Conventional | 0.477 | 118.21 |
| Organic | 0.684 | 168.96 |
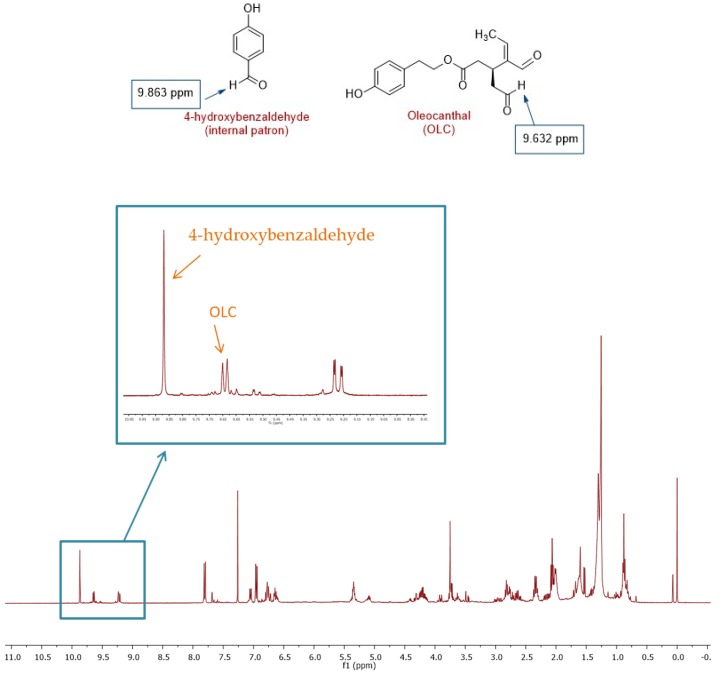
As 1D 1H NMR typically provides an excellent linear response to component concentrations, it was envisaged as a simple and reliable methodology to validate the UPLC-MS monitoring of OLC levels. The aldehydic proton region of the target compound in the 1H NMR spectrum of EVOO acetonitrile extracts, when recorded in CDCl3, presented a well-resolved set of peaks, making feasible the integration of one of the aldehydic protons and its comparison with the peak of the internal standard. OLC was quantified by integrating the singlet at 9.632 ppm (Figure 2).
Figure 2.
1H NMR spectrum of EVOO acetonitrile extracts recorded in CDCl3 and using 4-hydroxybenzaldehyde as the internal standard. The signals of the OLC and internal standard are shown in the expanded spectrum.
3. Discussion
3.1. Total Amount of Phenolic Compounds
Recent studies found a higher polyphenol content in organic EVOO [20] and a different acid composition, as well as a higher degree of bitterness (cv. Leccino and Frantoio) and pungency (cv. Frantoio) and less sweetness (cv. Frantoio) [21]. A similar enhancing effect of organic cultivation on TP content was observed in EVOO extracted from olives of the Casaliva variety, both unripe (51% increase) and ripe (40% increases), whereas in a multi-varietal organic EVOO this effect was only observed with unripe olives [22]. However, in other studies, agronomic factors did not play a clear role in the TP content of EVOO made from olives of different cultivars, which instead was mainly affected by the year of the harvest [8], or water availability [23]. The type of farming (organic or conventional) only becomes a major factor in the TP content of tomatoes [24], pepper [25] and fruits [26] when these are grown under similar environmental conditions.
3.2. Concentrations of Phenolic Groups and Selected Phenolic Compounds
Organic agriculture is associated with a natural increase in the amount of defense substances, as the plant is exposed to greater stress in the absence of synthetic pesticides. In addition, without synthetic fertilizers there is less bioavailable nitrogen, with concomitant lower plant growth rates and an enhanced production of secondary metabolites such as phenolic compounds [27,28].
Previous studies have established that SEC (oleuropein and oleuropein, ligstroside and elenolic acid derivatives) are the most complex and abundant family of polyphenols in EVOO polar fractions [29,30,31] and are the principal contributors to organoleptic traits [32]. SEC are synthesized through the secologanin pathway, which does not depend on nitrogen or phosphorus, so their production is not impeded with the low nitrogen and phosphorus availability of organic soil [33]. A recent study demonstrated that the foliar fertilization with a biofertilizer rich in calcium increased oleuropein aglycone and OLC levels in EVOO, which in contrast, decreased significantly with the use of a biofertilizer rich in nitrogen, phosphorus and potassium [34]. Therefore, conventional practices could explain the lower content of most of the SEC compounds (oleuropein derivatives and ligstrosides derivatives) compared to organic practices. The ester breakdown of SEC leads to the formation of elenolic acid and derivatives [35]. In our study, the elenolic acid concentration was in accordance with previously reported results in different EVOO (16.8–58.6 mg·kg−1) [36,37]. Since the content of SEC was higher in organic EVOO, it is also logical to have higher content of elenolic acid (Table 1), because the more SEC, the more elenolic acid is released from SEC breakdown.
The main flavonoids present in EVOO are luteolin and apigenin [38,39] and a high luteolin content is of great interest, as it has been associated with health-promoting and antioxidant properties of foods [40,41]. The luteolin concentration reported here (Table 1) is higher than in other studies (3.12 and 7.57 mg·kg−1) [36,42], including one in which EVOO was also extracted from Hojiblanca olives (3.69–6.67 mg·kg−1) [43]. These differences among studies are not surprising, since it has been shown that the concentration of luteolin depends considerably on the olive variety, geographical area, season, environmental conditions and cultivation method [36,44]. In another study, the use of a biofertilizer rich in nitrogen, phosphorus and potassium led to a significant increase in the apigenin content of EVOO (1.66 ± 0.32 mg·kg−1), whereas luteolin levels increased when the biofertilizer was supplemented with calcium (2.12 ± 0.39 mg·kg−1) [34].
In our work, the concentration of the total phenolic alcohols is similar to that of another study comparing organic and conventional EVOO made from the same type of olives (8.33–11.0 and 10.5–16.3 mg·kg−1, respectively) [43]. Other authors reported a decrease in hydroxytyrosol concentration in EVOO when olive trees were fertilized with nitrogen, phosphorus and potassium, as occurred with SEC [34]. The phenolic alcohols are derived from the SEC, so their biosynthesis does not depend on the nitrogen or the phosphorus [33].
Both lignans and phenolic acids are synthesized through the phenylpropanoid pathway [45,46] and depend on the shikimic pathway, in which nitrogen and phosphorus take part [47]. The greater availability of nitrogen and phosphorus in conventional farming could thus explain the lower lignan and phenolic acid concentration in the organic EVOO.
3.3. EVOO Phenolic Profile and Olive Fruit Ripening
During ripening, the chemical structure and concentrations of compounds in olives can be modified by chemical reactions and the enzymatic activity of glycosidases, phenol oxidases and phenol polymerases [48,49]. The amount of these enzymes depends on the cultivar and maturation stage [41]. Thus, the degree of olive fruit ripeness is a crucial parameter in EVOO quality [50]. Previous studies have reported a reduction in TP, beginning at a maturation index of 2.5–3 [51], or a significant gradual decrease from the first to the fifth harvest [23]. It has been suggested that the TP content depends more on the olive cultivar than an early or late harvest [52].
The amount of SEC decreases significantly with ripeness, both in organic and conventional systems, as reported in the literature [48,53,54]. The SEC concentration was found to decrease by 31% (92.1–63.0 mg·kg−1) between the first and last harvests (maturation index of 2.4–5.6), due to oleuropein degradation during ripening [55]. Also, Gutierrez-Rosales et al. [56] showed that high contents of oleuropein aglycone at the initial stage of ripening were caused by a high activity of β-glucosidase. This indicates that oleuropein biosynthesis combines with enzymatic hydrolysis to produce the aglycone form. Thus, when the olive is in a green stage, the level of β-glucosidase activity increases proportionally with the amount of oleuropein and ligstroside, whereas in the black stage, when the phenolic glycoside concentration is reduced, the glucosidase activity is low [49].
Reports in the literature on the influence of ripening on flavonoid content are contradictory. The content of flavones (luteolin and apigenin) in olives was observed to increase up to a maturation index close to 4, decreasing thereafter [38], whereas elsewhere this tendency was found at an index of 0.76–1.27 [54]. Furthermore, an increase in flavonoid concentration has been reported in EVOO made from olives at an intermediate ripening stage [57,58]. In Hojiblanca EVOO, a higher content of luteolin was obtained by harvesting medium-ripe olives (6.10 and 6.59 mg·kg−1, conventional and organic EVOO, respectively), whereas an early harvest resulted in increased apigenin (3.32 and 3.65 mg·kg−1, conventional and organic EVOO, respectively) [43]. The association of a higher concentration of luteolin with an intermediate ripening stage could be because apigenin is the substrate for a hydroxylase enzyme in the flavonoid pathway, giving rise to luteolin [53].
With respect to phenolic acids, our results are in agreement with those of Jimenez et al., who reported that p-coumaric and ferulic acid contents were higher in EVOO extracted from olives at an early RI, decreasing progressively thereafter, and that the concentration of vanillic acid was apparently not affected by the ripening process [59]. Another study found an increase in phenolic acids in EVOO extracted from more mature olives, which may be due to the activity of hydrolytic enzymes on the complex phenols [53].
3.4. Analysis of Oleocanthal by NMR
The various extraction procedures and analytical methods developed for the quantification of EVOO phenolic compounds have generated ambiguous results that are difficult to compare. The most commonly used methods are liquid chromatography (LC), followed by UV-Vis or detection by MS [60,61]. However, OLC can react with different solvents and consequently both liquid–liquid extraction and chromatographic analysis may interfere with its determination by LC-MS, leading in some cases to broader or multiple peaks in MS detection. [62]. A promising new method has been published recently by Sánchez de Medina et al. [63], but further studies are needed to assess its accuracy and reliability. For this reason, the chromatographic results obtained with this method were validated by acetonitrile extraction from random samples of EVOO and directly measured OLC levels by qNMR, as described by Karkoula et al. [64]. The interest of qNMR is due to the repeatability and reproducibility of measurements, as well as its rapidity compared to more classical methods, and its reliability [65]. Other advantages include simple sample preparation, low sample consumption and non-destructive measurement.
This validation study was performed by NMR using CDCl3 as the solvent, avoiding both the undesired interactions of other solvents like methanol or water with the target compound, and the overlap of the aldehydic proton peaks. We chose 4-hydroxybenzaldehyde as the internal standard due to its price, stability, and solubility in our deuterated solvent, and the simplicity of the resulting 1H NMR spectrum. Moreover, the aldehydic proton peaks of this compound and OLC do not overlap.
4. Material and Methods
4.1. Chemicals
OLC was purchased from PhytoLab GmbH (Vestenbergsgreuth, Germany); oleuropein, lutein, m-coumaric acid, pinoresinol, lariciresinol, isolariciresinol, secoisolariciresinol and taxifolin were obtained from Sigma-Aldrich (Madrid, Spain). p-Coumaric acid, vanillic acid, ferulic acid and apigenin were obtained from Fluka (Buchs, Switzerland), hydroxytyrosol from Extrasynthese (Genay, France), and verbascoside from HWI ANALYTIK GmbH (Rülzheim, Germany). Hexane, methanol, acetonitrile, and chloroform-d were purchased from Sigma-Aldrich and cyclohexane from Carlo Erba (Madrid, Spain).
4.2. Olive Fruit Samples
Olive fruits were collected from olive trees of the Hojiblanca cultivar, which were cultivated using organic and conventional agricultural practices without irrigation. The orchard was located on the experimental farm of the Agricultural Research Training Centre in Cabra in the province of Cordoba, at an altitude of approximately 547 m. The soil pH was 8 and its composition was limestone and sand. The climate was continental Mediterranean, with hot summers and cold winters, and the average temperature between October of 2017 and January of 2018 was 12.7 °C, with an average relative humidity of 64.4%. Ten trees were selected per cultivation system. The olives were harvested on 4 different days with 2 weeks of difference between every picking.
The RI of each harvest was determined according to the methodology proposed by Uceda and Frías [66], which is based on the color of the skin and the pulp. 100 olives were randomly selected and the following formula was applied: RI = (A × 0 + B × 1 + C × 2 + D × 3 + E × 4 + F × 5 + G × 6 + H × 7) /100. Where A, B, C, D, E, F, G, H are the number of olives with the 8 different ripening stages. Those are: stage 0: intense green skin; stage 1: yellowish green skin; stage 2: green skin with red spots, in less than half of the fruit; stage 3: reddish or purple skin in more than half of the fruit; stage 4: black skin and white pulp; stage 5: black skin and pulp purple; stage 6: black skin and more than half of the pulp purple; stage 7: black skin and totally purple pulp. The fruit RI in organic and conventional system was 1 to 3.945 and 1.06 to 3.68., respectively.
4.3. Oil Samples
Three representative olive samples, each weighing a minimum of one kilogram, were processed and the corresponding EVOOs were obtained using an Abencor milling system (Abengoa S.A., Seville, Spain). This system reproduced the industrial process on a laboratory scale. The apparatus consisted of three elements: a hammer mill, a thermobeater and a pulp centrifuge. The olive fruits (6 kg) were milled using a stainless-steel hammer mill equipped with a 5-mm sieve that was operated at 3000 rpm. The resulting olive paste was immediately kneaded in a mixer at 50 rpm for 30 min at 30 °C, with hot water added at 20 min. Centrifugation of the kneaded olive paste was performed in a basket centrifuge at 3500 rpm for 1 min. After centrifugation, the oil was decanted and stored in amber glass bottles at 4 °C in darkness and without headspace until analysis.
4.4. Polyphenol Analysis by Liquid Chromatography
The liquid–liquid extraction of phenolic compounds was performed with the method proposed by Capriotti et al. [67]. 1 g of EVOO was dissolved in hexane (oil/hexane 1:1, w/v) in a 10 mL centrifuge tube and shaken for 30 s. The polyphenols were extracted with 2 mL of MeOH and stirred for 30 s; the emulsion was then centrifuged at 3000 rpm and 4 °C for 3 min. The supernatant (methanolic extract) was subjected to a second cleaning with hexane, and the hexane extract was subjected to a second extraction of polyphenols with MeOH. All extracts were shaken for 30 s and centrifuged at 3000 rpm and 4 °C for 3 min. The methanolic extracts were recovered and cleaned up by dispersing 50 mg of C18. The samples were evaporated and reconstituted with 800 μL of MeOH:H2O (80:20 v/v), filtered with (Polytetrafluoroethylene) PTFE syringe filters (0.2 µm), transferred to an amber glass vial and stored at −80 °C until analysis. The internal standard was added to the EVOO to obtain a final concentration of 5 ppm after the reconstitution. The experiment was done in triplicate.
The identification and quantification of phenolic compounds was performed using an AcquityTM UPLC (Waters; Milford, MA, EUA) coupled to an API 3000 triple-quadruple mass spectrometer (PE Sciex) with a turbo ion spray source. Separation of compounds was achieved using an Acquity UPLC® BEH C18 Column (2.1 × 50 mm, i.d., 1.7 µm particle size) and Acquity UPLC® BEH C18 Pre-Column (2.1 × 5 mm, i.d., 1.7 µm particle size) (Waters Corporation®, Ireland) (See supporting information). The mobile phases were H2O with 0.2% acetic acid (A) and ACN (B). An increasing linear gradient (v/v) of B was used (t (min), %B), as follows: (0, 5); (2.5, 5); (12.5, 40); (12.6, 100); (13.5, 100); (13.6,5); (15,5), at a constant flow rate of 0.4 mL/min. The injection volume was 10 µL and the column temperature 40 °C.
The quantification of OLC was performed using a methodology proposed by Sánchez de Medina et al. with some modifications. Separation was achieved using an Acquity UPLC® BEH C18 Column (2.1 × 50 mm, i.d., 1.7 µm particle size) and Acquity UPLC® BEH C18 Pre-Column (2.1 × 5 mm, i.d., 1.7 µm particle size) (Waters Corporation®, Ireland). The mobile phases were MeOH (A) and H2O (B), both with 0.1% of formic acid. An increasing linear gradient (v/v) of B was used (t (min), %B), as follows: (0, 100); (2, 100); (4.75, 46.4); (4.9, 0); (5.9, 0); (6.100); (6.5, 100), at a constant flow rate of 0.6 mL·min−1. The injection volume was 5 µL and the column temperature 50 °C. The MS potentials were optimized for the compound (Supporting Table S2). Method suitability was evaluated by submitting random samples to a comparative NMR study.
Ionization was achieved using an electrospray interface operating in the negative mode [M–H] and all the compounds were monitored in the multiple monitoring mode (MRM) with the following settings: capillary voltage, −3500 V; nebuliser gas (N2), 10 (arbitrary units); curtain gas (N2), 12 (arbitrary units); and drying gas (N2) heated to 450 °C. The declustering potential, focusing potential, collision energy and entrance potential were optimized to detect phenolic compounds with the highest signals, following the method described by Suárez et al. [39]. The system was controlled by Analyst version 1.4.2 software supplied by Applied Biosystems.
The calibration curves were prepared in refined oil and were linear over the concentration ranges 0–20 mg·mL−1 using oleuropein, hydroxytyrosol, p-coumaric acid, m-coumaric acid, vanillic acid, ferulic acid, apigenin, luteolin, pinoresinol, lariciresinol, isolariciresinol, secoisolariciresinol, verbascoside and OLC.
4.5. Analysis of Oleocanthal by NMR
The OLC extraction and sample preparation for NMR analysis were carried out using the methodology proposed by Karkoula et al. [30]. Olive oil (8.0 g) was mixed with cyclohexane (32 mL) and ACN (40 mL). The mixture was homogenized using a vortex mixer for 30 s and centrifuged at 4000 rpm for 5 min. The ACN phase (40 mL) was collected, mixed with 1.6 mL of 4-hydroxybenzaldehyde solution (0.5 mg·mL−1) in ACN, and evaporated under reduced pressure using a rotary evaporator (Buchi, Model R-200 with dry ice and acetone cold-trap condenser, Switzerland).
The residue of the above procedure was dissolved in CDCl3 (750 μL), and an accurately measured volume of the solution (550 μL) was transferred to a 5 mm NMR tube. 1H NMR spectra were recorded at 400 MHz using an NMR spectrometer (Varian VNMRS 400 MHz). Typically, 128 scans were collected into 32K data points over a spectral width of 16 ppm (6410 Hz), with a relaxation delay of 1 s and an acquisition time of 2.5 s. The spectra were phase corrected and integrated automatically using MNova. Accurate integration was performed manually for the peaks of interest.
4.6. Statistical Analysis
Significant differences between organic and conventional samples were assessed by the Mann–Whitney test (Table 1). The relationship between categorical exposure variables (organic vs conventional cultivation) and concentration of polyphenols was assessed by Generalized Linear Models adjusting for RI (Table 2). Regression models were also fitted to assess associations between polyphenol concentration as a dependent variable and RI as an independent variable. Statistical analyses were conducted using STATA software (version 14.0; StataCorp, College Station, TX, USA). p values < 0.05 were considered statistically significant.
5. Conclusions
The TP in EVOO made from Hojiblanca olives were analyzed, comparing organic and conventional growing systems under the same environmental conditions, and levels were significantly higher in organic samples (p < 0.05). The concentration of SEC, which are synthesized through the secologanin pathway without the need for nitrogen or phosphorus, was higher in oils from olives cultivated under organic conditions. These included oleocanthal, which was satisfactorily analyzed by LC-MS/MS, as demonstrated by results obtained by qNMR.
In contrast, the concentrations of lignans and phenolic acids were higher under the conventional system, as their synthesis is through the phenylpropanoid pathway via shikimic acid, which requires nitrogen and phosphorus. In the case of phenolic alcohols and flavones, there were no significant differences associated with the cultivation method.
When the effect of the ripening stage of the olive fruit was assessed, both the TP and SEC concentrations were found to decrease with maturation in both production systems, whereas the flavone content increased. Olive maturation was also associated with a decline in certain compounds: lignans in organic EVOO and phenolic acids and phenolic alcohols in conventional samples.
It should be emphasized that long-term experiments are required to eliminate the effect of seasonality. There is also a need for more randomized, controlled dietary intervention trials to corroborate the potentially greater beneficial effects of organic food on human health compared to those produced conventionally. However, organic food may be recommended, not only for its health benefits, but also because its production has less of an environmental impact.
Acknowledgments
A.L.-Y. thanks the National Council for Science and Technology (CONACYT) of Mexico for the doctoral scholarship. J.L.-C. thanks the Ministry of Science Innovation and Universities for the FPI contract. P.Q.-R. is grateful for the Sara Borrell postdoctoral program from the Instituto de Salud Carlos III (ISCIII). A.T.-R. thanks the Ministry of Science Innovation and Universities for the Juan de la Cierva-formación contract A.V.-Q. thanks the Ministry of Science Innovation and Universities for the Ramon y Cajal contract. The authors wish to thank the CCiT-UB for the mass spectrometry equipment.
Supplementary Materials
The following are available online, Table S1: GLM model adjusted for ripeness (conventional vs. organic); Table S2: Multiple reaction Monitoring conditions for the polyphenols.
Author Contributions
Methodology, A.L.-Y., J.L.-C., P.Q.-R.; formal analysis A.L.-Y., J.L.-C., A.O.-C. and A.T.-R.; investigation, A.L.-Y., J.L.-C., M.P. and A.V.-Q.; writing—original draft preparation, A.L.-Y., J.L.-C., M.P., A.T.-R. and A.V.-Q.; writing—review and editing, A.L.-Y., J.L.-C., M.P., A.T.-R. and A.V.-Q.; visualization, M.P. and A.V.-Q.; supervision, M.P. and A.V.-Q.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Servili M., Sordini B., Esposto S., Urbani S., Veneziani G., Di Maio I., Selvaggini R., Taticchi A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants. 2013;3:1–23. doi: 10.3390/antiox3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Servili M., Esposto S., Fabiani R., Urbani S., Taticchi A., Mariucci F., Selvaggini R., Montedoro G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology. 2009;17:76–84. doi: 10.1007/s10787-008-8014-y. [DOI] [PubMed] [Google Scholar]
- 3.Koseoglu O., Unal M.K. The effect of phenolic compounds on the quality and stability of virgin olive oil; Proceedings of the VI International Symposium on Olive Growing; Evora, Portugal. 9–13 September 2008; pp. 655–663. [Google Scholar]
- 4.Di Giovacchino L., Costantini N., Serraiocco A., Surricchio G., Basti C. Natural antioxidants and volatile compounds of virgin olive oils obtained by two or three-phases centrifugal decanters. Eur. J. Lipid Sci. Technol. 2001;103:279–285. doi: 10.1002/1438-9312(200105)103:5<279::AID-EJLT279>3.0.CO;2-I. [DOI] [Google Scholar]
- 5.El Riachy M., Priego-Capote F., León L., Rallo L., Luque de Castro M.D. Hydrophilic antioxidants of virgin olive oil. Part 2: Biosynthesis and biotransformation of phenolic compounds in virgin olive oil as affected by agronomic and processing factors. Eur. J. Lipid Sci. Technol. 2011;113:692–707. doi: 10.1002/ejlt.201100096. [DOI] [Google Scholar]
- 6.Clodoveo M.L. Malaxation: Influence on virgin olive oil quality. Past, present and future—An overview. Trends Food Sci. Technol. 2012;25:13–23. doi: 10.1016/j.tifs.2011.11.004. [DOI] [Google Scholar]
- 7.Lozano-Sánchez J., Cerretani L., Bendini A., Gallina-Toschi T., Segura-Carretero A., Fernández-Gutiérrez A. New filtration systems for extra-virgin olive oil: Effect on antioxidant compounds, oxidative stability, and physicochemical and sensory properties. J. Agric. Food Chem. 2012;60:3754–3762. doi: 10.1021/jf205353b. [DOI] [PubMed] [Google Scholar]
- 8.Ninfali P., Bacchiocca M., Biagiotti E., Esposto S., Servili M., Rosati A., Montedoro G. A 3-year study on quality, nutritional and organoleptic evaluation of organic and conventional extra-virgin olive oils. JAOCS J. Am. Oil Chem. Soc. 2008;85:151–158. doi: 10.1007/s11746-007-1171-0. [DOI] [Google Scholar]
- 9.Vallverdú-Queralt A., Lamuela-Raventós R.M. Foodomics: A new tool to differentiate between organic and conventional foods. Electrophoresis. 2016;37:1784–1794. doi: 10.1002/elps.201500348. [DOI] [PubMed] [Google Scholar]
- 10.Hurtado-Barroso S., Tresserra-Rimbau A., Vallverdú-Queralt A., Lamuela-Raventós R.M. Organic food and impact on human health. Int. J. PharmTech Res. 2016;9:316–324. doi: 10.1080/10408398.2017.1394815. [DOI] [PubMed] [Google Scholar]
- 11.González N., Marquès M., Nadal M., Domingo J.L. Occurrence of environmental pollutants in foodstu ff s: A review of organic vs. conventional food. Food Chem. Toxicol. 2019;125:370–375. doi: 10.1016/j.fct.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Williams C.M. Nutritional quality of organic food: Shades of grey or shades of green? Proc. Nutr. Soc. 2008;61:19–24. doi: 10.1079/PNS2001126. [DOI] [PubMed] [Google Scholar]
- 13.Worthington V. Effect of Agricultural Methods on Nutritional Quality: A Comparison of Organic with Conventional Crops. Altern. Ther. Health Med. 1998;4:58–69. [PubMed] [Google Scholar]
- 14.Häkkinen S.H., Törrönen A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000;33:517–524. doi: 10.1016/S0963-9969(00)00086-7. [DOI] [Google Scholar]
- 15.Romero-Pérez A.I., Lamuela-Raventós R.M., Andrés-Lacueva C., De La Carmen Torre-Boronat M. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J. Agric. Food Chem. 2001;49:210–215. doi: 10.1021/jf000745o. [DOI] [PubMed] [Google Scholar]
- 16.Vallverdú-Queralt A., Medina-Remón A., Casals-Ribes I., Amat M., Lamuela-Raventós R.M. A metabolomic approach differentiates between conventional and organic ketchups. J. Agric. Food Chem. 2011;59:11703–11710. doi: 10.1021/jf202822s. [DOI] [PubMed] [Google Scholar]
- 17.Martí R., Leiva-Brondo M., Lahoz I., Campillo C., Cebolla-Cornejo J., Roselló S. Polyphenol and L-ascorbic acid content in tomato as influenced by high lycopene genotypes and organic farming at different environments. Food Chem. 2018;239:148–156. doi: 10.1016/j.foodchem.2017.06.102. [DOI] [PubMed] [Google Scholar]
- 18.Cuevas F.J., Pradas I., Ruiz-Moreno M.J., Arroyo F.T., Perez-Romero L.F., Montenegro J.C., Moreno-Rojas J.M. Effect of organic and conventional management on bio-functional quality of thirteen plum cultivars (Prunus salicina Lindl.) PLoS ONE. 2015;10:e0136596. doi: 10.1371/journal.pone.0136596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicerale S., Lucas L.J., Keast R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012;23:129–135. doi: 10.1016/j.copbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Barbieri S., Bendini A., Valli E., Gallina Toschi T. Do consumers recognize the positive sensorial attributes of extra virgin olive oils related with their composition? A case study on conventional and organic products. J. Food Compos. Anal. 2015;44:186–195. doi: 10.1016/j.jfca.2015.09.001. [DOI] [Google Scholar]
- 21.Rosati A., Cafiero C., Paoletti A., Alfei B., Caporali S., Casciani L., Valentini M. Effect of agronomical practices on carpology, fruit and oil composition, and oil sensory properties, in olive (Olea europaea L.) Food Chem. 2014;159:236–243. doi: 10.1016/j.foodchem.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Trombetta D., Smeriglio A., Marcoccia D., Giofrè S., Toscano G., Mazzotti F., Giovanazzi A., Lorenzetti S. Analytical evaluation and antioxidant properties of some secondary metabolites in northern Italian mono-and multi-varietal extra virgin olive oils (EVOOs) from early and late harvested olives. Int. J. Mol. Sci. 2017;18:797. doi: 10.3390/ijms18040797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alowaiesh B., Singh Z., Fang Z., Kailis S.G. Harvest time impacts the fatty acid compositions, phenolic compounds and sensory attributes of Frantoio and Manzanilla olive oil. Sci. Hortic. (Amsterdam) 2018;234:74–80. doi: 10.1016/j.scienta.2018.02.017. [DOI] [Google Scholar]
- 24.Vallverdú-Queralt A., Medina-Remón A., Casals-Ribes I., Lamuela-Raventos R.M. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012;130:222–227. doi: 10.1016/j.foodchem.2011.07.017. [DOI] [Google Scholar]
- 25.Hallmann E., Rembial kowska E. Characterisation of antioxidant compounds in sweet bell pepper (Capsicum annuum L.) under organic and conventional growing systems. J. Sci. Food Agric. 2012;92:2409–2415. doi: 10.1002/jsfa.5624. [DOI] [PubMed] [Google Scholar]
- 26.Mditshwa A., Magwaza L.S., Tesfay S.Z., Mbili N. Postharvest quality and composition of organically and conventionally produced fruits: A review. Sci. Hortic. (Amsterdam) 2017;216:148–159. doi: 10.1016/j.scienta.2016.12.033. [DOI] [Google Scholar]
- 27.Assumpção C.F., Nunes I.L., Mendonça T.A., Bortolin R.C., Jablonski A., Flôres S.H., De Oliveira Rios A. Bioactive Compounds and Stability of Organic and Conventional Vitis labrusca Grape Seed Oils. JAOCS J. Am. Oil Chem. Soc. 2016;93:115–124. doi: 10.1007/s11746-015-2742-0. [DOI] [Google Scholar]
- 28.Hunter D., Foster M., Mcarthur J.O., Ojha R., Petocz P., Samman S. Evaluation of the micronutrient composition of plant foods produced by organic and conventional agricultural methods. Crit. Rev. Food Sci. Nutr. 2011;51:571–582. doi: 10.1080/10408391003721701. [DOI] [PubMed] [Google Scholar]
- 29.Miho H., Díez C.M., Mena-Bravo A., Sánchez de Medina V., Moral J., Melliou E., Magiatis P., Rallo L., Barranco D., Priego-Capote F. Cultivar influence on variability in olive oil phenolic profiles determined through an extensive germplasm survey. Food Chem. 2018;266:192–199. doi: 10.1016/j.foodchem.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Karkoula E., Skantzari A., Melliou E., Magiatis P. Quantitative measurement of major secoiridoid derivatives in olive oil using qNMR. Proof of the artificial formation of aldehydic oleuropein and ligstroside aglycon isomers. J. Agric. Food Chem. 2014;62:600–607. doi: 10.1021/jf404421p. [DOI] [PubMed] [Google Scholar]
- 31.García-Rodríguez R., Belaj A., Romero-Segura C., Sanz C., Pérez A.G. Exploration of genetic resources to improve the functional quality of virgin olive oil. J. Funct. Foods. 2017;38:1–8. doi: 10.1016/j.jff.2017.08.043. [DOI] [Google Scholar]
- 32.Campestre C., Angelini G., Gasbarri C., Angerosa F. The compounds responsible for the sensory profile in monovarietal virgin olive oils. Molecules. 2017;22:1833. doi: 10.3390/molecules22111833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen S.R., Franzyk H., Wallander E. Chemotaxonomy of the oleaceae: Iridoids as taxonomic markers. Phytochemistry. 2002;60:213–231. doi: 10.1016/S0031-9422(02)00102-4. [DOI] [PubMed] [Google Scholar]
- 34.Dabbaghi O., Tekaya M., Flamini G., Zouari I., El-Gharbi S., M’barki N., Laabidi F., Cheheb H., Attia F., Aïachi Mezghani M., et al. Modification of Phenolic Compounds and Volatile Profiles of Chemlali Variety Olive Oil in Response to Foliar Biofertilization. J. Am. Oil Chem. Soc. 2019;96:585–593. doi: 10.1002/aocs.12201. [DOI] [Google Scholar]
- 35.Figueiredo-González M., Reboredo-Rodríguez P., González-Barreiro C., Carrasco-Pancorbo A., Cancho-Grande B., Simal-Gándara J. The involvement of phenolic-rich extracts from Galician autochthonous extra-virgin olive oils against the α-glucosidase and α-amylase inhibition. Food Res. Int. 2019;116:447–454. doi: 10.1016/j.foodres.2018.08.060. [DOI] [PubMed] [Google Scholar]
- 36.Kelebek H., Kesen S., Selli S. Comparative study of bioactive constituents in Turkish olive oils by LC-ESI/MS/MS. Int. J. Food Prop. 2015;18:2231–2245. doi: 10.1080/10942912.2014.968788. [DOI] [Google Scholar]
- 37.Arslan D., Karabekir Y., Schreiner M. Variations of phenolic compounds, fatty acids and some qualitative characteristics of Sariulak olive oil as induced by growing area. Food Res. Int. 2013;54:1897–1906. doi: 10.1016/j.foodres.2013.06.016. [DOI] [Google Scholar]
- 38.de Torres A., Espínola F., Moya M., Alcalá S., Vidal A.M., Castro E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT Food Sci. Technol. 2018;90:22–30. doi: 10.1016/j.lwt.2017.12.003. [DOI] [Google Scholar]
- 39.Suárez M., Macià A., Romero M.P., Motilva M.J. Improved liquid chromatography tandem mass spectrometry method for the determination of phenolic compounds in virgin olive oil. J. Chromatogr. A. 2008;1214:90–99. doi: 10.1016/j.chroma.2008.10.098. [DOI] [PubMed] [Google Scholar]
- 40.Murkovic M., Lechner S., Pietzka A., Bratacos M., Katzogiannos E. Analysis of minor components in olive oil. J. Biochem. Biophys. Methods. 2004;61:155–160. doi: 10.1016/j.jbbm.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Ye J.H., Wijesundera C., Shi M. Effects of Agronomic and Oil Processing Conditions on Natural Antioxidative Phenolics in Olive (Oleaeuropaea L.) Austin J. Nutr. Food Sci. 2014;2:1050. [Google Scholar]
- 42.Collado-González J., Grosso C., Valentão P., Andrade P.B., Ferreres F., Durand T., Guy A., Galano J.M., Torrecillas A., Gil-Izquierdo Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017;235:298–307. doi: 10.1016/j.foodchem.2017.04.171. [DOI] [PubMed] [Google Scholar]
- 43.Jimenez B., Sánchez-Ortiz A., Lorenzo M.L., Rivas A. Effect of organic cultivation of Picual and Hojiblanca olive varieties on the quality of virgin olive oil at four ripening stages. Eur. J. Lipid Sci. Technol. 2014;116:1634–1646. doi: 10.1002/ejlt.201400010. [DOI] [Google Scholar]
- 44.Kalogiouri N.P., Aalizadeh R., Thomaidis N.S. Investigating the organic and conventional production type of olive oil with target and suspect screening by LC-QTOF-MS, a novel semi-quantification method using chemical similarity and advanced chemometrics. Anal. Bioanal. Chem. 2017;409:5413–5426. doi: 10.1007/s00216-017-0395-6. [DOI] [PubMed] [Google Scholar]
- 45.Dean J.F.D., LaFayette P.R., Rugh C., Tristram A.H., Hoopes J.T., Eriksson K.-E.L., Merkle S.A. ACS Symposium Series. Volume 78. American Chemical Society; Washington, DC, USA: 2009. Laccases Associated with Lignifying Vascular Tissues; pp. 96–108. [Google Scholar]
- 46.Ali Ghasemzadeh Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011;5:6697–6703. doi: 10.5897/jmpr11.1404. [DOI] [Google Scholar]
- 47.Fraser C.M., Chapple C. The Phenylpropanoid Pathway in Arabidopsis. Am. Soc. Plant Biol. 2011;9:e0152. doi: 10.1199/tab.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben Brahim S., Kelebek H., Ammar S., Abichou M., Bouaziz M. LC–MS phenolic profiling combined with multivariate analysis as an approach for the characterization of extra virgin olive oils of four rare Tunisian cultivars during ripening. Food Chem. 2017;229:9–19. doi: 10.1016/j.foodchem.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Clodoveo M.L., Hbaieb R.H., Kotti F., Mugnozza G.S., Gargouri M. Mechanical strategies to increase nutritional and sensory quality of virgin olive oil by modulating the endogenous enzyme activities. Compr. Rev. Food Sci. Food Saf. 2014;13:135–154. doi: 10.1111/1541-4337.12054. [DOI] [PubMed] [Google Scholar]
- 50.Amanpour A., Kelebek H., Selli S. Characterization of aroma, aroma-active compounds and fatty acids profiles of cv. Nizip Yaglik oils as affected by three maturity periods of olives. J. Sci. Food Agric. 2019;99:726–740. doi: 10.1002/jsfa.9241. [DOI] [PubMed] [Google Scholar]
- 51.Peres F., Martins L.L., Ferreira-Dias S. Influence of enzymes and technology on virgin olive oil composition. Crit. Rev. Food Sci. Nutr. 2017;57:3104–3126. doi: 10.1080/10408398.2015.1092107. [DOI] [PubMed] [Google Scholar]
- 52.Bilušić T., Žanetić M., Ljubenkov I., Generalić Mekinić I., Štambuk S., Bojović V., Soldo B., Magiatis P. Molecular characterization of Dalmatian cultivars and the influence of the olive fruit harvest period on chemical profile, sensory characteristics and oil oxidative stability. Eur. Food Res. Technol. 2018;244:281–289. doi: 10.1007/s00217-017-2954-7. [DOI] [Google Scholar]
- 53.Amanpour A., Kelebek H., Selli S. LC-DAD-ESI-MS/MS-based phenolic profiling and antioxidant activity in Turkish cv. Nizip Yaglik olive oils from different maturity olives. J. Mass Spectrom. 2018:227–238. doi: 10.1002/jms.4326. [DOI] [PubMed] [Google Scholar]
- 54.Lukić I., Krapac M., Horvat I., Godena S., Kosić U., Brkić Bubola K. Three-factor approach for balancing the concentrations of phenols and volatiles in virgin olive oil from a late-ripening olive cultivar. LWT Food Sci. Technol. 2018;87:194–202. doi: 10.1016/j.lwt.2017.08.082. [DOI] [Google Scholar]
- 55.Lozano-Sánchez J., Amir Y., Fernández-Gutiérrez A., Bakhouche A., Bengana M., Segura-Carretero A., Youyou A. Influence of olive ripeness on chemical properties and phenolic composition of Chemlal extra-virgin olive oil. Food Res. Int. 2013;54:1868–1875. doi: 10.1016/j.foodres.2013.08.037. [DOI] [Google Scholar]
- 56.Gutierrez-Rosales F., Romero M.P., Casanovas M., Motilva M.J., Mínguez-Mosquera M.I. Metabolites involved in oleuropein accumulation and degradation in fruits of Olea europaea L.: Hojiblanca and Arbequina varieties. J. Agric. Food Chem. 2010;58:12924–12933. doi: 10.1021/jf103083u. [DOI] [PubMed] [Google Scholar]
- 57.Jiménez B., Sánchez-Ortiz A., Rivas A. Influence of the malaxation time and olive ripening stage on oil quality and phenolic compounds of virgin olive oils. Int. J. Food Sci. Technol. 2014;49:2521–2527. doi: 10.1111/ijfs.12592. [DOI] [Google Scholar]
- 58.Artajo L.S., Romero M.P., Motilva M.J. Transfer of phenolic compounds during olive oil extraction in relation to ripening stage of the fruit. J. Sci. Food Agric. 2006;86:518–527. doi: 10.1002/jsfa.2384. [DOI] [Google Scholar]
- 59.Jiménez B., Sánchez-Ortiz A., Lorenzo M.L., Rivas A. Influence of fruit ripening on agronomic parameters, quality indices, sensory attributes and phenolic compounds of Picudo olive oils. Food Res. Int. 2013;54:1860–1867. doi: 10.1016/j.foodres.2013.08.016. [DOI] [Google Scholar]
- 60.Bendini A., Cerretani L., Carrasco-Pancorbo A., Gómez-Caravaca A.M., Segura-Carretero A., Fernández-Gutiérrez A., Lercker G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules. 2007;12:1679–1719. doi: 10.3390/12081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanakis P., Termentzi A., Michel T., Gikas E., Halabalaki M., Skaltsounis A.L. From olive drupes to olive Oil. An HPLC-orbitrap-based qualitative and quantitative exploration of olive key metabolites. Planta Med. 2013;79:1576–1587. doi: 10.1055/s-0033-1350823. [DOI] [PubMed] [Google Scholar]
- 62.Di Donna L., Benabdelkamel H., Mazzotti F., Napoli A., Nardi M., Sindona G. High-throughput assay of oleopentanedialdheydes in extra virgin olive oil by the UHPLC-ESI-MS/MS and isotope dilution methods. Anal. Chem. 2011;83:1990–1995. doi: 10.1021/ac200152r. [DOI] [PubMed] [Google Scholar]
- 63.Sánchez de Medina V., Miho H., Melliou E., Magiatis P., Priego-Capote F., Luque de Castro M.D. Quantitative method for determination of oleocanthal and oleacein in virgin olive oils by liquid chromatography–tandem mass spectrometry. Talanta. 2017;162:24–31. doi: 10.1016/j.talanta.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 64.Karkoula E., Skantzari A., Melliou E., Magiatis P. Direct measurement of oleocanthal and oleacein levels in olive oil by quantitative 1H NMR. Establishment of a new index for the characterization of extra virgin olive oils. J. Agric. Food Chem. 2012;60:11696–11703. doi: 10.1021/jf3032765. [DOI] [PubMed] [Google Scholar]
- 65.Godelmann R., Kost C., Patz C.D., Ristow R., Wachter H. Quantitation of compounds in wine using 1H NMR spectroscopy: Description of the method and collaborative study. J. AOAC Int. 2016;99:1295–1304. doi: 10.5740/jaoacint.15-0318. [DOI] [PubMed] [Google Scholar]
- 66.Uceda M., Frías L. Proceeding of II Seminario Olícola Internacional. International Olive-Oil Council; Cordoba, Spain: 1975. Epocas de recolección. Evolución del fruto y de la composición y calidad del aceite. (Seasons of harvest. Changes on fruit oil content, oil composition and oil quality) [Google Scholar]
- 67.Capriotti A.L., Cavaliere C., Crescenzi C., Foglia P., Nescatelli R., Samperi R., Laganà A. Comparison of extraction methods for the identification and quantification of polyphenols in virgin olive oil by ultra-HPLC-QToF mass spectrometry. Food Chem. 2014;158:392–400. doi: 10.1016/j.foodchem.2014.02.130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.