Abstract
Background:
Serotonin transporter blockers, like citalopram, dose-dependently bind to the serotonin transporter. Pharmacological magnetic resonance imaging (phMRI) can be used to non-invasively monitor effects of serotonergic medication. Although previous studies showed that phMRI can measure the effect of a single dose of serotoninergic medication, it is currently unclear whether it can also detect dose-dependent effects.
Aims:
To investigate the dose-dependent phMRI response to citalopram and compared this with serotonin transporter occupancy, measured with single photon emission computed tomography (SPECT).
Methods:
Forty-five healthy females were randomized to pre-treatment with placebo, a low (4 mg) or clinically standard (16 mg) oral citalopram dose. Prior to citalopram, and 3 h after, subjects underwent SPECT scanning. Subsequently, a phMRI scan with a citalopram challenge (7.5 mg intravenously) was conducted. Change in cerebral blood flow in response to the citalopram challenge was assessed in the thalamus and occipital cortex (control region).
Results:
Citalopram dose-dependently affected serotonin transporter occupancy, as measured with SPECT. In addition, citalopram dose-dependently affected the phMRI response to intravenous citalopram in the thalamus (but not occipital cortex), but phMRI was less sensitive in distinguishing between groups than SPECT. Serotonin transporter occupancy showed a trend-significant correlation to thalamic cerebral blood flow change.
Conclusion:
These results suggest that phMRI likely suffers from higher variation than SPECT, but that these techniques probably also assess different functional aspects of the serotonergic synapse; therefore phMRI could complement positron emission tomography/SPECT for measuring effects of serotonergic medication.
Keywords: Citalopram, phMRI, serotonin, SPECT
Introduction
The serotonin transporter (SERT) plays a key role in regulating extracellular levels of serotonin. It has been implicated in a number of neuropsychiatric disorders and is an important target for many antidepressants and psychotropic medications, such as selective serotonin reuptake inhibitors (SSRIs) (Vaswani et al., 2003). SSRIs block the reuptake of serotonin from the synapse. Molecular imaging techniques, such as positron emission tomography (PET) and single photon emission computed tomography (SPECT), are invaluable imaging tools to assess the SERT occupancy by SSRIs. For instance, PET and SPECT studies have demonstrated that the treatment response to SSRIs in depressed patients is associated with SERT occupancy; a therapeutic dose of SSRIs coincided with approximately 80% SERT occupancy. For SSRIs like paroxetine or citalopram, a curve–linear relationship was observed, with subclinical doses showing less SERT occupancy, whereas higher doses quickly reached a plateau (Meyer et al., 2004).
Longitudinal and prospective measurements, and drug response monitoring, are important to facilitate personalized treatment strategies for patients with neuropsychiatric disorders such as major depression. In this respect, PET and SPECT imaging techniques are not optimal due to their use of radioactive ligands, particularly in children or other vulnerable populations. These considerations have prompted researchers to develop non-ionizing imaging alternatives to study neurotransmitters in vivo. One such alternative, that can be used repeatedly and is easily accessible, is pharmacological magnetic resonance imaging (phMRI). phMRI is thought to provide an index of neurotransmitter function based on changes in brain cerebral haemodynamics, such as the blood oxygen level-dependent (BOLD) signal or cerebral blood flow (CBF), after administration of a specific pharmacological challenge (Jenkins, 2012).
A number of preclinical and clinical studies have demonstrated the potential of phMRI to examine the serotonin system in living brain. For instance, a preclinical study has shown that an increase in serotonin release induced by the serotonin-releaser fenfluramine evokes region-specific changes in the BOLD response in rats (Preece et al., 2009). Conversely, a depletion of the serotonin pool with p-chlorophenylalanine attenuated the phMRI response to fenfluramine (Preece et al., 2009), showing the sensitivity of phMRI to detect extracellular serotonin fluctuations in the brain. PhMRI signal changes following a serotonin challenge also corresponded with results from autoradiographic measures of CBF and glucose metabolism (Houston et al., 2001). Finally, persistent changes in serotonergic function could be assessed following prolonged SSRI exposure with phMRI in rodents (Klomp et al., 2012b). A few research groups have further observed similar region-specific changes in humans using either BOLD (McKie et al., 2005) or arterial spin labelling (ASL) (Chen et al., 2011; Schouw et al., 2012).
These findings suggest that phMRI can be used as a non-invasive tool to study serotonergic function. This novel technique may also yield a wealth of information on how serotonin-related drugs, such as SSRIs, modulate brain function, and how drug responses are altered in neuropsychiatric or developmental disorders. So far, however, the relationship between neurovascular responses and SERT occupancy has not been studied in humans. The ability to measure differences in serotonin function with phMRI is of particular interest, as it enables us to non-invasively monitor treatment effects of serotonergic medication in patients. This would thus enable optimization of dose regimens on an individual basis and in doing so also reduce side effects.
In this randomized, placebo-controlled [123I]N-ω-fluoropropyl-2β-carbomethoxy-3β-(4iodophenyl)nortropane ([123I]FP-CIT) SPECT and phMRI study, we measured both thalamic SERT occupancy and the haemodynamic response to different doses of the SSRI citalopram, as we previously showed that extrastriatal [123I]FP-CIT binding preferentially reflects SERT binding (Booij et al., 2007). After baseline SPECT assessment, we administered an oral dose of citalopram (one of three doses randomized), after which we conducted a follow-up SPECT assessment to measure SERT occupancy. Following the SPECT procedures, a phMRI scan was conducted in which subjects received an intravenous citalopram dose (all subjects received the same dose to saturate SERT binding). We expected to replicate previous studies and show a dose-dependent effect of citalopram on SERT occupancy using SPECT. Moreover, we expected that the phMRI response to the intravenous dose was also dependent on the received oral pre-treatment dose. More specifically, we expected that the phMRI response would be greatest when SERT is unoccupied by citalopram, and that higher SERT occupancy by citalopram would be associated with a lower phMRI response.
Methods
Participants
The medical ethics committee of the Academic Medical Center in Amsterdam approved the study procedures and written informed consent was obtained from all subjects and carried out in accordance with the standards of the Declaration of Helsinki. Participants were recruited through online advertisements and flyers at local universities and colleges. Forty-five healthy female volunteers (mean age = 21.6 years, age range 18–28) were included. Exclusion criteria were a history of a chronic neurological/psychiatric disorder, family history of sudden heart failure, current use of psychostimulant medication, abnormal electrocardiogram, excessive consumption of alcohol (>21 units/week), caffeine (>8 cups/day) or nicotine (>15 cigarettes/day) and contra-indications for MRI or SPECT. The absence of psychiatric disorders and drug abuse was checked with the Mini-International Neuropsychiatric Interview Plus. All participants were required to be on hormonal contraceptives to minimize confounding effects of hormonal cycle.
Study design and procedures
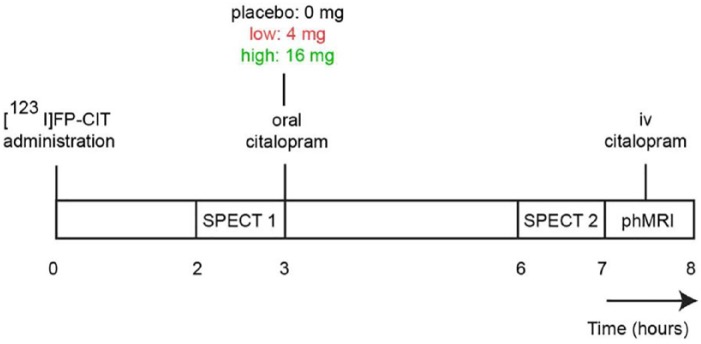
To study the dose-dependent effects of citalopram on SERT occupancy and phMRI response, a double-blind, dose–response design was used (Figure 1). Participants received potassium-iodide tablets prior to the [123I]FP-CIT administration to block thyroid uptake of free radioactive iodide. The first SPECT scan was conducted 2 h post-injection to assess baseline SERT availability. Following the first SPECT scan, participants were randomized into one of the following three groups: those receiving placebo (‘placebo’ n=15), those receiving a low dose (4 mg; ‘low group’ n=15) and those receiving a clinical dose (16 mg; ‘high group’ n=15) of oral citalopram (solution, 16 mg equivalent to 20 mg in tablet form (due to bioavailability differences), Lundbeck). These doses have been shown to correspond to 0%, ~40% and ~80% SERT occupancy, respectively (Klein et al., 2006). The citalopram was dissolved in lemonade and administered immediately after the first SPECT scan. The placebo condition consisted of lemonade only. After 3 h, participants underwent a second SPECT scan. The change in SERT binding between SPECT-1 and SPECT-2 was used to calculate SERT occupancy. Then, phMRI was performed, in which all participants received a 7.5 mg intravenous challenge with citalopram in line with previous studies (McKie et al., 2005; Schouw et al., 2012). This intravenous dose was intended to induce saturated occupancy in all groups, regardless of oral dose. Therefore, the phMRI response that we would measure was the difference between initial SERT occupancy (following oral dose) compared with maximal SERT occupancy (following intravenous (i.v.) dose). Citalopram plasma was assessed at baseline, 3 h after citalopram administration and following the MRI scan (i.e. 30 min after i.v. administration; for practical reasons it was chosen not to obtain plasma directly after the administration).
Figure 1.
Study day.
SPECT: single photon emission computed tomography; phMRI: pharmacological magnetic resonance imaging; iv: intravenous
Subjective ratings
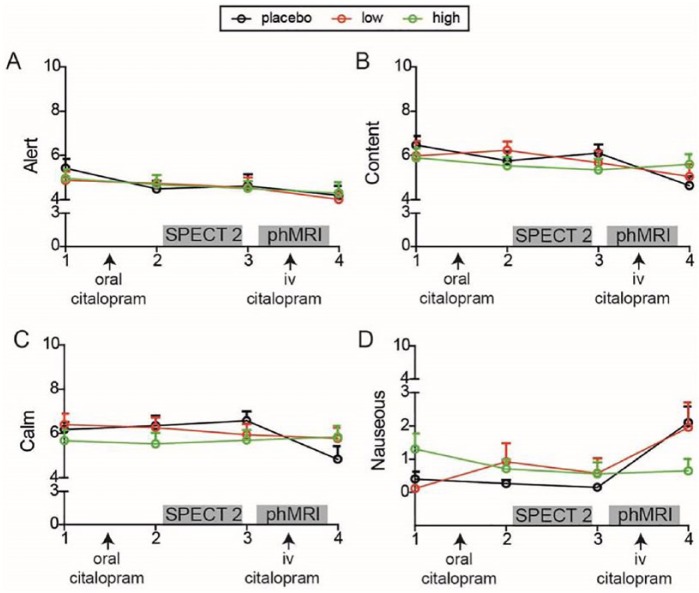
Subjective effects of citalopram were assessed at four time points: 1) at baseline, 2) 3 h after oral citalopram, 3) 4 h after oral citalopram, 4) after i.v. citalopram. To assess these effects we used Visual Analogue Scales (VAS) measuring mood and subjective well-being, including the following variables: nausea, alertness, contentment and calmness. Subjects were asked to mark a cross on the line that represented their feeling at that moment in time. Linear mixed models were used for analysing VAS scores over time between groups using restricted maximum likelihood and a compound symmetry covariance structure.
SPECT acquisition and analysis
Subjects underwent SPECT imaging 2 h and 6 h after intravenous administration of approximately 110 MBq [123I]FP-CIT (specific activity > 750 MBq/nmol; radiochemical purity > 98%, produced according to Good Manufacturing Practice criteria at GE Healthcare, Eindhoven, The Netherlands). The radioligand [123I]FP-CIT binds with high affinity to the dopamine transporter (DAT) primarily in the striatum, and to the SERT primarily in extrastriatal brain areas, such as the thalamus and midbrain (Booij et al., 2007). SPECT scans were acquired using a brain-dedicated InSPira-HD SPECT camera (Neurologica, Boston, USA) with the following parameters: matrix = 121 × 121; slice thickness = 4 mm; acquisition time per slice = 180 s; energy window = 159 keV (with 20% lower and upper boundaries). 3D images were reconstructed (using an iterative expectation maximization algorithm, correction using a computed tomography template and spatial smoothing (3 mm)). SPECT images were co-registered with the individual 3D T1-weighted (T1w) magnetic resonance image using SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK).
A region of interest (ROI) analysis was performed to determine SERT binding in the thalamus. The thalamus was chosen as ROI because it is richly innervated by serotonergic neurons located in the raphe nucleus and our radiotracer shows reliable thalamic SERT binding (Booij et al., 2007). Moreover, the thalamus is thought to be a crucial region in brain circuits mediating therapeutic actions of SSRIs (Geday et al., 2005). Thalamic masks (mean (SD) ROI size = 15.2 (1.2) cm3) were extracted from individual T1w scans using Freesurfer (Figure 2(a)). The cerebellum was used as a reference region to assess non-specific binding (mean (SD) ROI size = 131.2 (28.7) cm3) (Booij et al., 2007). Specific relative to non-specific binding ratios (binding potential: BPND) were calculated as follows: ((mean thalamic binding – mean cerebellum binding) / mean cerebellum binding). The reduction in BPND following citalopram administration (representing occupancy) was expressed normalized to the placebo group.
Figure 2.
Single photon emission computed tomography (SPECT) and arterial spin labelling regions of interest. (a) Representative T1-weighted image with co-registered SPECT data (red/yellow) and the individual thalamic mask (green) superimposed. (b) Representative cerebral blood flow (CBF) image masked by the individual grey matter mask with the individual thalamic mask (green) and occipital mask (red) superimposed.
MRI acquisition and analysis
MRI data were acquired using a 3.0T Ingenia scanner (Philips, Best, The Netherlands) with a 32-channel receive-only head coil. For anatomical reference, a high-resolution 3D T1w scan was obtained (1 mm3 resolution). Then, pseudo-continuous ASL (pCASL) data were acquired with a 2D multi-slice echo-planar imaging readout using the following parameters: TR/TE = 4100/14 ms; post-label delay = 1525 ms; label duration = 1650 ms; field of view = 240 mm × 240 mm; 17 contiguous slices of 7 mm, voxel size= 3 mm × 3mm × 7 mm; dynamics = 183. In addition, an M0 scan was obtained using a proton-density scan with the same-readout as the pCASL acquisition. Citalopram was administered as a bolus injection 5 min after baseline imaging (37 dynamics). The bolus of 7.5 mg (dissolved in 45 ml saline) was infused over 7.5 min, followed by 15 ml saline flush (2.5 min). The first and last 37 dynamics were averaged to obtain the pre- and post-citalopram CBF map respectively. ASL post-processing was performed with the ExploreASL toolbox (Mutsaerts et al., 2018). In short, T1w images were segmented into grey matter (pGM) and white matter (pWM) probability maps. Motion was estimated and motion spikes were excluded. Perfusion-weighted images were rigid-body registered to the pGM images. CBF was quantified using a single compartment model, making use of the individual M0 image (Alsop et al., 2014). The pGM and pWM maps were spatially normalized and CBF maps were transformed to Montreal Neurological Institute space. Mean CBF was extracted from ROIs in the thalamus and an occipital ROI (Figure 2(b)). The occipital cortex is an area with very low SERT density, albeit higher than the cerebellum (Kish et al., 2005). However, our field of view did not always incorporate the cerebellum and therefore we chose the occipital cortex as control region. ROIs were based on the Harvard Oxford Atlas and multiplied by pGM masks of the individual subject to obtain individual ROI masks (thalamus: mean (SD) ROI size = 17.2 (2.3) cm3; occipital cortex: mean (SD) ROI size = 168.9 (22.9) cm3). Heart rate (HR) was measured during the scan using a peripheral pulse unit and phase-contrast MRI was used to obtain blood flow (2D-flow) to the brain both before and after i.v. citalopram administration.
Statistical analysis
All data were analysed with SPSS version 22 (IBM Corp., Armonk, USA), with the significance level set at p<0.05 (two-sided). Baseline differences between groups were assessed using univariate analysis of variance (ANOVA). Repeated measures AN(C)OVA were used for the dependent variables thalamic BPND, thalamic CBF, blood plasma, HR and 2D-flow with time as within-subjects variable and citalopram dose as a between-subjects variable. For phMRI, baseline CBF values were added as covariate, because the baseline CBF scan was measured after the oral citalopram dose. For the ANOVAs, partial eta-squared are calculated as a measure of effect size. Linear contrasts were used to assess the dose-dependency of this effect. In addition, correlation analyses were used to assess the association between the change in SERT SPECT, change in phMRI and blood plasma levels.
Sample size estimation
A previous [123I]β-CIT SPECT study observed statistically significant reductions in thalamic SERT binding of about 72% following an oral pre-treatment with 20 mg citalopram even in six subjects (de Win et al., 2005). Furthermore, statistically significant citalopram-induced changes were detected using ASL-based phMRI in 10 MDMA users when compared with seven control subjects (Schouw et al., 2012). Furthermore, the sample size needed to detect a reproducible effect of 16 mg oral citalopram with phMRI in the thalamus (which is also the ROI in this study) was 11 subjects (Klomp et al., 2012a). In the current study, we also wanted to assess smaller effects of ~40% SERT occupancy. Therefore, we set our sample size to N = 15 per group.
Results
Subjective ratings
There was no significant interaction effect of group and time on being alert (F(6,43)=0.36; p=0.90), content (F(6,42)=0.08, p=0.66) or calm (F(6,42)=1.35; p=0.26), but there was a significant interaction effect on being nauseous (F(6,39)=2.42, p=0.04), as a result of the placebo and low groups displaying some slight increase in nausea after the intravenous citalopram (Figure 3). Furthermore, a main effect of time was found on being content (F(3,41)=6.30, p=0.001), and alert (F(3,43)=2.98, p=0.042), which both decreased over the day. In summary, we observed slightly more nausea in the placebo and low groups after intravenous citalopram administration compared with the high group, in which the SERT was already blocked to a large extent.
Figure 3.
Subjective ratings. Line graphs of subjective ratings over the four time points for each group separately.
SPECT: single photon emission computed tomography; phMRI: pharmacological magnetic resonance imaging; iv: intravenous
Citalopram plasma levels
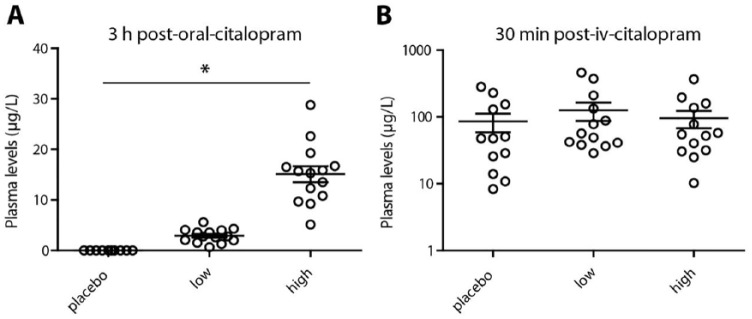
The oral dosage of citalopram was well tolerated by all subjects, and positively associated with blood plasma levels (Figure 4). Three hours after oral citalopram, a Kruskall–Wallis test showed a difference between the groups (H=33.03; p<0.001) and post-hoc Mann–Whitney U tests showed that all groups differed from each other (p<0.001). No difference between groups in citalopram plasma levels was found after intravenous citalopram (H=1.48; p=0.48).
Figure 4.
Blood plasma levels. Blood samples were collected before the second single photon emission computed tomography (SPECT) scan and after the pharmacological magnetic resonance imaging (phMRI) scan. Citalopram plasma levels (μg/L) were determined using mass spectrometry. (a) Citalopram plasma levels prior to SPECT 2 at 3 h post-oral-citalopram. (b) Citalopram plasma levels after the phMRI scan at 30 min post-iv- citalopram on a log scale.
*Analysis of variance: p<0.05
iv: intravenous
SPECT
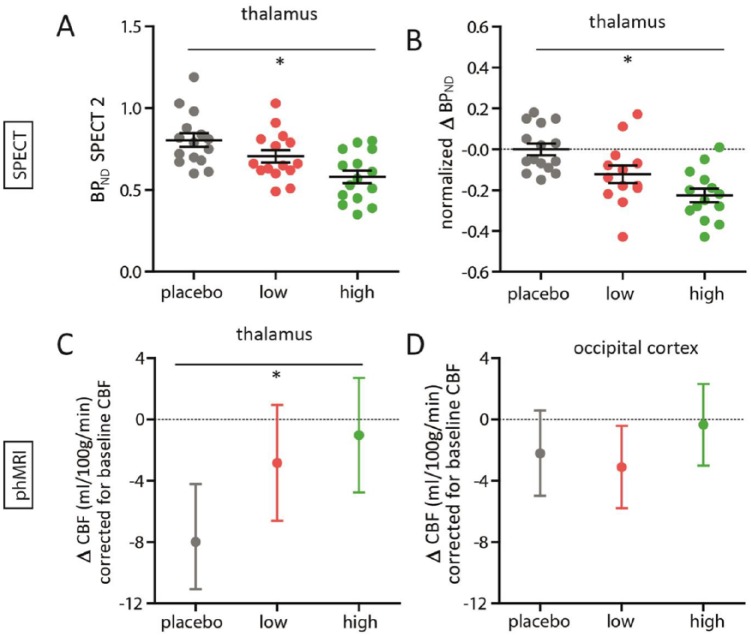
SPECT data for three subjects (n=2 from the low group and n=1 from the high group) were not suitable for further analysis due to technical errors during image reconstruction. At baseline, no significant differences in thalamic BPND between the groups was observed (F(2,39)=0.42; p=0.66). Following citalopram administration, at SPECT-2, the groups significantly differed in thalamic BPND (F(2,39)=7.57; p=0.002) (Figure 5(a)). We observed a significant time*group interaction (F(2,39)=11.22; p<0.001; ηp2=0.37) (Figure 5(b)) as a result of SERT displacement by citalopram. The post-hoc tests showed that the high group showed significantly higher displacement (representing SERT occupancy) compared with the placebo group (–40.5%; p<0.001), and the same for the low group compared with the placebo group (–24.5%; p=0.02). Moreover, the low and high groups also significantly differed (p=0.04), and this dose-dependency was confirmed by a linear contrast (p<0.001).
Figure 5.
Single photon emission computed tomography (SPECT) and pharmacological magnetic resonance imaging (phMRI) results. (a) Scatter dot plots of the thalamic binding potential (BPND) for each group for SPECT 2, and (b) the difference in thalamic BPND, normalized to the placebo group, from pre- to post-oral citalopram for each group. (c) Estimated marginal mean with 95% confidence interval of thalamic cerebral blood flow (CBF) (corrected for baseline thalamic CBF) for each group from pre- to post-intravenous citalopram, and (d) of occipital CBF (corrected for baseline occipital CBF).
*Analysis of variance: p<0.05
phMRI
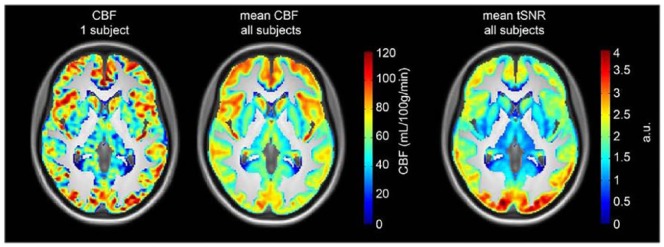
For one subject, ASL data were missing due to nausea (n=1 from the placebo group). Figure 6 (left) shows a representative CBF map of a 5-min ASL acquisition for one subject, and Figure 6 (centre) shows the mean CBF map from all subjects. In addition, Figure 6 (right) shows the temporal signal to noise ratio (tSNR) for a 5-min ASL acquisition. As reported in previous studies, it can be appreciated that both CBF and tSNR are higher in cortical than in subcortical areas. Before the intravenous citalopram challenge, pre-treatment conditions did not affect thalamic CBF significantly (F(2,41)=2.57; p=0.09), nor did it affect occipital CBF (F(2,41)=0.52; p=0.60). However, following the intravenous citalopram we found a significant difference in phMRI response (ΔCBF) in the thalamus between the three groups (F(2,40)=3.84; p=0.03, ηp2=0.16), corrected for baseline thalamic CBF (Figure 5(c)). Baseline thalamic CBF significantly correlated with thalamic ΔCBF (r= −0.66; p<0.001), indicating that higher baseline thalamic CBF was associated with more decrease in CBF following i.v. citalopram. Furthermore, the difference between placebo and a low and high dose showed a linear relation (linear contrast: p=0.01). Post-hoc tests showed significant differences between the placebo and high group (p=0.01), a trend between the placebo and low group (p=0.06), but no difference between the low and high group (p=0.51). On the contrary, we did not find significant differences between groups in ΔCBF in our control region, the occipital cortex (F(2,40)=1.14; p=0.33; ηp2=0.05) (Figure 5(d)).
Figure 6.
Arterial spin labelling (ASL) data quality. Left: a representative cerebral blood flow (CBF) map of one subject for a 5-min ASL acquisition. Centre: the mean CBF map averaged over all subjects. Right: the mean temporal signal to noise ratio (tSNR) map averaged over all subjects. All maps are masked with a grey matter mask (>25% probability of being grey matter) and overlaid on the mean T1 image of all subjects.
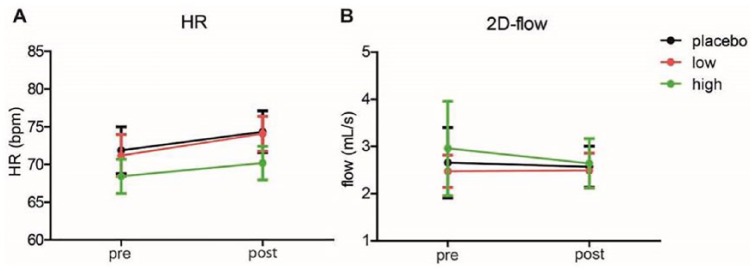
Cardiovascular effects
No differences in HR were observed prior to the ASL scan (i.e. before the i.v. citalopram, but after the oral citalopram) (F(2,36)=0.41; p=0.66). A main effect of citalopram on HR was found (F(1,36)=11.47; p=0.002), but no interaction was found with group (F(2,36=0.23; p=0.80) (Figure 7(a)). There was no baseline effect of group on 2D-flow (F(2,41)=1.31; p=0.28), nor a main effect of citalopram on 2D-flow (F(1,39)=0.15; p=0.70), nor an interaction with group was observed (F(2,39)=1.01; p=0.37) (Figure 7(b)).
Figure 7.
Cardiovascular effects. (a) Heart rate (HR) was measured during the arterial spin labelling (ASL) scan session. Pre- and post-intravenous citalopram averages (over the same 5 min over which the cerebral blood flow was calculated) are shown in beats per minute. (b) 2D-flow was measured just before and straight after the ASL scan. Pre- and post-blood flow to the brain in the intracranial arteries is shown in mL/s. Data represent mean ± standard error of the mean.
Correlational analyses
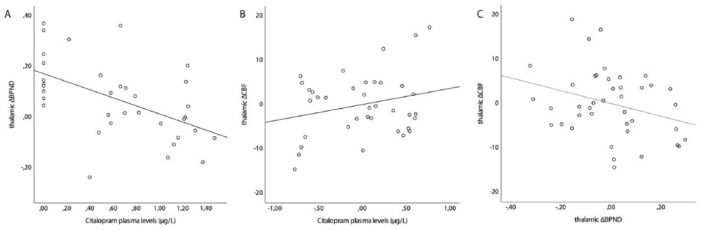
Citalopram plasma levels prior to the second SPECT scan correlated significantly with change (Δ) in thalamic BPND (r= −0.54; p=0.001) and trend significantly with thalamic ΔCBF (r=0.32; p=0.057), but not with thalamic ΔCBF controlling for baseline thalamic CBF (partial correlation; r=0.25; p=0.15). The thalamic ΔBPND also showed a trend significant correlation with thalamic ΔCBF, controlling for baseline thalamic CBF (partial correlation; r= −0.30; p=0.06) (Figure 8).
Figure 8.
Correlation plots. (a) Correlation plot between citalopram plasma levels (μg/L) and thalamic binding potential (ΔBPND). (b) Partial correlation plot between citalopram plasma levels (μg/L) and thalamic ΔCBF (taking into account the baseline thalamic cerebral blood flow (CBF)). (c) Partial correlation plot between thalamic ΔBPND and thalamic ΔCBF (taking into account the baseline thalamic CBF).
Discussion
In this study we investigated whether phMRI could detect dose-related haemodynamic responses related to oral pre-dosing with the SSRI citalopram. In addition to replicating dose-dependent SERT occupancy by using SPECT, we demonstrate a linear relation between group and phMRI response. We show that a clinically standard oral dose of citalopram prior to the phMRI scan is associated with reduced phMRI response following an intravenous citalopram challenge, whereas the placebo group showed a clear decrease in thalamic CBF following the i.v. citalopram; the phMRI response of the subclinical dose group fell in between those of the placebo and high dose groups. However, the difference between the groups was not as distinct using phMRI compared with SPECT. Moreover, the phMRI signal changes did not strongly correlate with changes in SPECT measurements.
SPECT
Previous PET and SPECT studies using the selective SERT radiotracers [11C]DASB and [123I]ADAM have shown dose-dependent associations of SERT occupancy with both dose and plasma levels of SSRIs (Klein et al., 2006; Meyer et al., 2004). Our results are comparable to these studies, because we also show a time*group interaction and found an association between citalopram plasma levels and SERT occupancy. However, although typical therapeutic doses (16–32 mg for liquid citalopram) are associated with an ~80% SERT occupancy, we only found a ~40% displacement of [123I]FP-CIT at 16 mg. This could be due to various reasons, the most likely one being our administration of citalopram after the [123I]FP-CIT injection, implying that citalopram is required to displace the radioligand. As in-vivo [123I]FP-CIT binding is associated with a slow Koff (Booij et al., 1999), this probably resulted in lower than expected differences between the placebo and high dose groups. However, Ziebell et al. (Ziebell et al., 2010) also administered citalopram after [123I]FP-CIT and did obtain a 63% displacement, but they used a higher dose which was administered i.v. Additionally, [123I]FP-CIT does not selectively bind to the SERT, but also binds to the DAT, and therefore at least some of the binding in the thalamus could result from DAT binding, which then cannot be displaced by citalopram. Nevertheless, when comparing plasma concentrations with a previous study (Meyer et al., 2004), our mean citalopram concentration of 15.1 µg/L in the high group corresponds with an approximately 40% SERT occupancy. Moreover, we found, similar to previous studies, a positive relationship between plasma levels and SERT displacement.
phMRI and its comparison with SPECT
This study assessed whether the phMRI response is dependent on different doses of SERT blockade. PhMRI literature has shown in both animal models and human studies that single-dose SSRI administration induced a haemodynamic response. For example, administration of both the SSRIs fluoxetine (Klomp et al., 2012b) and citalopram (Sekar et al., 2011) elicited increased BOLD responses in rats. In humans, citalopram i.v. also increased the BOLD response in the striatum, amygdala, hippocampus and thalamus (McKie et al., 2005). In contrast, data from studies using ASL and PET imaging suggest that thalamic CBF and metabolism are actually decreased after intravenous SSRI administration (Geday et al., 2005; Schouw et al., 2012). The discrepancies between BOLD and ASL/PET studies could be due to the fact that the BOLD signal is sensitive to CBF, cerebral blood volume and oxygen consumption and therefore less specific, whereas ASL measures CBF only. Nevertheless, to further elucidate the mechanisms underlying these haemodynamic signal changes, future studies measuring both BOLD and CBF simultaneously during pharmacological stimulation are necessary, using, for example, dual-echo sequences (Merola et al., 2017).
In line with our negative findings in the occipital cortex in this study, Chen et al. (Chen et al., 2011) also demonstrated decreased CBF (assessed with ASL) in serotonin-innervated areas, but did not show an effect in non-serotonergic brain regions. These findings are in agreement with research showing that acute administration of SSRIs decreases extracellular serotonin concentrations in serotonin projection areas, which could decrease neuronal activity and the demand for oxygen-rich blood (Nord et al., 2013). Indeed, prolonged treatment has been associated with increased serotonin concentrations in projection areas, which is probably a result of desensitization of the serotonin 1A autoreceptors (Nord et al., 2013). These neurobiological adaptations coincide with the therapeutic response lag of a couple of weeks for SSRIs.
Comparable to previous studies, we observed small non-significant differences in mean baseline CBF (i.e. after oral dosing). This corroborates previous findings from our group reporting on the small effect size and relatively high variation of oral citalopram phMRI (Klomp, 2012a). Nevertheless, we did observe that there was a strong dependency of the subsequent phMRI response on baseline CBF. This shows that it is important to take baseline CBF into account when analysing the phMRI response; particularly in this case, as the subjects had already been administered the oral citalopram dose. As hypothesized, we found significant group differences in the phMRI response to the i.v. citalopram challenge, using baseline CBF as a covariate. The group with the highest pre-dose of citalopram showed no change in CBF, whereas the placebo group showed the largest change. This indicates that phMRI can indeed detect a therapeutically relevant blockade of SERT. However, the mean phMRI response to a subclinical dose (low group) was more comparable to the high group than to the placebo group. This suggests that also SERT occupancy lower than 80% can be detected. However, the lack of difference between the low and high groups, despite a linear effect of dose on phMRI response, implies that currently it is difficult to distinguish different oral dosages of citalopram with phMRI. This could be a result of low power, because despite our a priori sample size calculations we only find small to moderate effect sizes. Moreover, we did not find a strong correlation between our SPECT and phMRI measurements. Together, this demonstrates more variability in the phMRI than in the SPECT measurements. An alternative, but not necessary incompatible, explanation, is that these techniques presumably measure different aspects of the serotonergic system: SPECT measures transporter binding directly, whereas phMRI is an indirect measure. PhMRI measures the haemodynamic response in the whole synapse, including responses of both pre- and postsynaptic transporters and receptors as well as serotonin release. The SPECT displacement explained a small portion of the variance of the phMRI response, implying that part of the response is indeed regulated by binding to the SERT. Some preclinical studies have shown that the phMRI response is indeed linked to neurotransmitter release (Preece et al., 2009) while pre- and post-synaptic processes were shown to make up the lion’s share of the synapse energy expenditure, therefore likely inducing the strongest changes in CBF. Thus, the phMRI response is probably a combination of the effects of intravenous citalopram binding to the SERT, which increases serotonin in the synaptic cleft, which can then bind to post-synaptic receptors and transmit the neuronal signal. Another explanation for the low correlation is that [123I]FP-CIT is not a selective SERT tracer and our measurements could have also included some DAT binding (Booij et al., 2007).
Methodological considerations
One of the limitations of our study is that we included only female volunteers, and our results can thus not be extrapolated to males. Nevertheless, previous studies have shown considerable sex effects of [123I]FP-CIT on thalamic binding (Koch et al., 2014) and by including only women variability in our study was reduced. Moreover, results cannot be generalized to older people and patient samples, given that we included only relatively young and healthy subjects. In addition, we chose to focus on the thalamus, as this area is rich in SERT (with 20 times more SERT than DAT (Koch et al., 2014)), making this ROI the ideal candidate to address our research question with the radiotracer [123I]FP-CIT. Conversely, it is a limitation of [123I]FP-CIT SPECT that we cannot measure other SERT-rich brain regions (e.g. the striatum) with this non-selective ligand, which therefore limits the use of this ligand for SERT imaging in a clinical context. To that end, more SERT-selective PET and SPECT radiotracers, such as [11C]DASB and [123I]ADAM, that could assess the raphe nuclei and a number of different serotonergic projections, including the striatum, would be preferable (Meyer, 2007). For the phMRI results we also analysed the data using an ROI-based approach; first of all in order to compare the results with the SPECT data, but also because ASL has a relatively low intrinsic signal to noise ratio and our sample size does not allow meaningful voxel-based approaches in regions such as the thalamus.
So as to preclude possible global effects of citalopram on CBF we analysed a number of control conditions. First, we assessed general cardiovascular effects of citalopram using HR and flow measurements. Although HR was slightly increased after intravenous citalopram, this affected groups equally, and did not affect blood flow towards the brain (measured with 2D-flow). Furthermore, we assessed changes in CBF in the occipital cortex, a region low in SERT (Figure 5(c)) and found no effect; suggesting that the effects of intravenous citalopram are indeed due to SERT binding, rather than non-specific effects on global CBF. It is important to note that the occipital cortex still contains (very) low amounts of SERT protein (Kish et al., 2005), whereas the cerebellum is almost devoid of SERT and might have been a better control region. However, we could not capture the full cerebellum in our ASL scan, and the occipital cortex has a more optimal tSNR. Therefore, the occipital cortex can be considered as a reliable control region.
Conclusion
We show a linear relation between citalopram dose (placebo>low>high) and the phMRI response. However, we did not find significant differences between the low and high citalopram group in terms of phMRI response, which warrants further developments in improving the sensitivity of phMRI. Furthermore, in comparison with SPECT, phMRI might assess different functional aspects of serotonergic neurotransmission. Taken together, we conclude that phMRI allows to study biologically relevant variations and abnormalities in the serotonergic system, for example, in response to SSRI treatment, and can complement molecular imaging approaches like PET and SPECT.
Acknowledgments
LR, JB and AS conceived and designed the study. AS, MS, HS and WB collected the data. AS and SA analysed the data. HM and PL assisted in the data interpretation. All authors participated in the writing and approved of the final manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by ERA-NET PrioMedChild (grant no. 40-41800-98-026).
ORCID iDs: Anouk Schrantee  https://orcid.org/0000-0002-4035-4845
https://orcid.org/0000-0002-4035-4845
Henk-Jan MM Mutsaerts  https://orcid.org/0000-0003-0894-0307
https://orcid.org/0000-0003-0894-0307
References
- Alsop DC, Detre JA, Golay X, et al. (2014) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij J, de Jong J, de Bruin K, et al. (2007) Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: A double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med 48: 359–366. [PubMed] [Google Scholar]
- Booij J, Hemelaar TG, Speelman JD, et al. (1999) One-day protocol for imaging of the nigrostriatal dopaminergic pathway in Parkinson’s disease by [123I]FPCIT SPECT. J Nucl Med 40: 753–761. [PubMed] [Google Scholar]
- Chen Y, Wan HI, O’Reardon JP, et al. (2011) Quantification of cerebral blood flow as biomarker of drug effect: Arterial spin labeling phMRI after a single dose of oral citalopram. Clin Pharmacol Ther 89: 251–258. [DOI] [PubMed] [Google Scholar]
- de Win MML, Habraken JB a, Reneman L, et al. (2005) Validation of [(123)I]beta-CIT SPECT to assess serotonin transporters in vivo in humans: A double-blind, placebo-controlled, crossover study with the selective serotonin reuptake inhibitor citalopram. Neuropsychopharmacology 30: 996–1005. [DOI] [PubMed] [Google Scholar]
- Geday J, Hermansen F, Rosenberg R, et al. (2005) Serotonin modulation of cerebral blood flow measured with positron emission tomography (PET) in humans. Synapse 55: 224–229. [DOI] [PubMed] [Google Scholar]
- Houston GC, Papadakis NG, Carpenter TA, et al. (2001) Mapping of brain activation in response to pharmacological agents using fMRI in the rat. Magn Reson Imaging 19: 905–919. [DOI] [PubMed] [Google Scholar]
- Jenkins BG. (2012) Pharmacologic magnetic resonance imaging (phMRI): Imaging drug action in the brain. Neuroimage 62: 1072–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Furukawa Y, Chang L-J, et al. (2005) Regional distribution of serotonin transporter protein in postmortem human brain: Is the cerebellum a SERT-free brain region? Nucl Med Biol 32: 123–128. [DOI] [PubMed] [Google Scholar]
- Klein N, Sacher J, Geiss-Granadia T, et al. (2006) In vivo imaging of serotonin transporter occupancy by means of SPECT and [123I]ADAM in healthy subjects administered different doses of escitalopram or citalopram. Psychopharmacology 188: 263–272. [DOI] [PubMed] [Google Scholar]
- Klomp A, Caan M, Denys D, et al. (2012. a) Feasibility of ASL-based phMRI with a single dose of oral citalopram for repeated assessment of serotonin function. NeuroImage 63: 1695–1700. [DOI] [PubMed] [Google Scholar]
- Klomp A, Tremoleda JL, Wylezinska M, et al. (2012. b) Lasting effects of chronic fluoxetine treatment on the late developing rat brain: Age-dependent changes in the serotonergic neurotransmitter system assessed by pharmacological MRI. Neuroimage 59: 218–226. [DOI] [PubMed] [Google Scholar]
- Koch W, Unterrainer M, Xiong G, et al. (2014) Extrastriatal binding of [123I]FP-CIT in the thalamus and pons: Gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging 41: 1938–1946. [DOI] [PubMed] [Google Scholar]
- McKie S, Del-Ben C, Elliott R, et al. (2005) Neuronal effects of acute citalopram detected by pharmacoMRI. Psychopharmacology 180: 680–686. [DOI] [PubMed] [Google Scholar]
- Merola A, Germuska MA, Warnert EA, et al. (2017) Mapping the pharmacological modulation of brain oxygen metabolism: The effects of caffeine on absolute CMRO2 measured using dual calibrated fMRI. Neuroimage 155: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH. (2007) Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci 32: 86–102. [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Meyer JH, Wilson AA, et al. (2004) Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: An [11C]DASB positron emission tomography Study. Am J Psychiatry 161: 826–835. [DOI] [PubMed] [Google Scholar]
- Mutsaerts HJ, Petr J, Thomas DL, et al. (2018) Comparison of arterial spin labeling strategies in the multi-center GENetic Frontotemporal dementia Initiative (GENFI). J Mag Reson Imaging 47: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord M, Finnema SJ, Halldin C, et al. (2013) Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int J Neuropsychopharmacol 16: 1577–1586. [DOI] [PubMed] [Google Scholar]
- Preece M, Taylor MJ, Raley J, et al. (2009) Evidence that increased 5-HT release evokes region-specific effects on blood-oxygenation level-dependent functional magnetic resonance imaging responses in the rat brain. Neuroscience 159: 751–759. [DOI] [PubMed] [Google Scholar]
- Schouw MLJ, Gevers S, Caan MWA, et al. (2012) Mapping serotonergic dysfunction in MDMA (ecstasy) users using pharmacological MRI. Eur Neuropsychopharmacol 22: 537–545. [DOI] [PubMed] [Google Scholar]
- Sekar S, Verhoye M, Van Audekerke J, et al. (2011) Neuroadaptive responses to citalopram in rats using pharmacological magnetic resonance imaging. Psychopharmacology 213: 1–11. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. (2003) Role of selective serotonin reuptake inhibitors in psychiatric disorders: A comprehensive review. Progr Neuro-psychopharmacol Biol Psychiatry 27: 85–102. [DOI] [PubMed] [Google Scholar]
- Ziebell M, Holm-Hansen S, Thomsen G, et al. (2010) Serotonin transporters in dopamine transporter imaging: A head-to-head comparison of dopamine transporter SPECT radioligands 123I-FP-CIT and 123I-PE2I. J Nucl Med 51: 1885–1891. [DOI] [PubMed] [Google Scholar]