Abstract
Left ventricular free-wall rupture is one of the most fatal complications after acute myocardial infarction. Surgical treatment of post-infarction left ventricular free-wall rupture has evolved over time. Direct closure of the ventricular wall defect (linear closure) and resection of the infarcted myocardium (infarctectomy), with subsequent closure of the created defect with a prosthetic patch, represented the original techniques. Recently, less aggressive approaches, either with the use of surgical glues or the application of collagen sponge patches on the infarct area to cover the tear and achieve haemostasis, have been proposed. Despite such modifications in the therapeutic strategy and surgical treatment, however, postoperative in-hospital mortality may be as high as 35%. In extremely high-risk or inoperable patients, a non-surgical approach has been reported.
Keywords: Cardiac rupture, myocardial infarction, surgical treatment, mechanical complication
Introduction
Left ventricular free-wall rupture (LVFWR) may represent a dramatic and life-threatening event, occurring in up to 2% of patients with acute myocardial infarction (AMI).1,2 The clinical presentation varies from a catastrophic blowout type characterized by cardiogenic shock and eventually cardiac arrest, to the oozing type with haemodynamic instability and pericardial effusion.3
Prompt diagnosis and management can lead to successful treatment for LVFWR. Conservative strategies have been described,4,5 but the poor results of this treatment make surgical intervention generally mandatory. Despite different surgical approaches having been proposed, in-hospital mortality continues to be high6,7 and the most appropriate surgical management remains still controversial, particularly in terms of rupture recurrence or other type of post-operative complications.
This review describes the approaches reported in the literature regarding the management of this mechanical complication of AMI.
History
The first free-wall rupture of the heart after AMI was described by William Harvey in 1647.8 In 1769, Morgagni collected and reported 11 cases of myocardial rupture discovered postmortem9 (Figure 1). Ironically, Morgagni himself later died of ventricular rupture. Duaine, in 1871, reported the first large series of patients with cardiac rupture and concluded that rupture never occurs spontaneously.10 However, it was not until 1925, in the report of Krumbhaar and Crowell, that the relationship of LVFWR to myocardial infarction was first pointed out.11
Figure 1.
Morgagni’s description of a case of ventricular wall rupture.
Frontispiece of De sedibus, et causis morborum per anatomen indagatis (JB Morgagni, 1765), with a portrait of Morgagni and Morgagni’s description (‘Epistola anatomico-medica XXVII’) of a case of ventricular wall rupture (by courtesy of the Medical Library of Spedali Civili, Brescia, Italy).
Hatcher and associates, from Emory University, in 1970 described the first successful operation for free-wall rupture of the right ventricle.12 Two years later, Fitzgibbon et al. reported the successful repair of LVFWR associated with ischaemic heart disease.13 Cardiac rupture was treated, on cardiopulmonary bypass (CPB), with infarctectomy and closure of the myocardial defect. In the same year, Montegut described the history of a 58-year-old man who was successfully treated for post-infarction LVFWR with direct suture.10
In 1973, Calick et al. reported the surgical repair of ruptured myocardium with a Dacron® patch placed over the area of perforation.14 Ten years later, Nunez and colleagues described post-AMI free-wall ventricular rupture in seven patients who underwent surgical intervention.15 The control of haemorrhage was obtained by covering the ventricular tear and the surrounding infarcted myocardium with a Teflon® patch fixed on epicardium by a continuous Prolene® suture.15
The first attempt to repair LVFWR with a patch fixed with glue over the area of rupture was reported by Löfström and associates in 1972, but the Dacron® patch loosened soon after the operation, causing the patient’s death.16 In 1993, the first series of patients successfully treated with a sutureless epicardial patch technique was described.3 Padró and colleagues successfully treated 13 patients with subacute LVFWR using a Teflon® patch fixed onto the heart surface with only surgical glue.3
Operative strategies
Clinical presentation and course are variable, depending on the location, the size and the time-course of the rupture.17 Although acute LVFWR is often fatal, some patients with subacute or contained rupture present with a window of opportunity for intervention.18,19 Thereby, the diagnosis or even a high suspicion of cardiac rupture represents an indication for emergency surgery without further delay.
The most important diagnostic method for LVFWR is transthoracic echocardiography: the presence of reduced myocardial wall thickness, haemopericardium or epicardial clots and cardiac tamponade are the most relevant findings.20 In suitable patients, cardiac magnetic resonance can complement the diagnosis by identifying contained ventricular rupture.21 Pericardiocentesis prior to surgery, which confirms a haemorrhagic effusion, may further support the diagnosis, but the definitive diagnosis is usually made at surgery.
Rarely, the rupture of the free-wall of the left ventricle is contained by epicardial clots or pericardial adhesions, leading to the formation of a pseudoaneurysm. Left ventricular pseudoaneurysm (LVPA) that occurs a few days after AMI may be unstable and tends to rupture.22 Since the clinical presentation of this condition is variable, ranging from uneventful condition to congestive heart failure, or even sudden death,23 a high index of suspicion for this serious complication of AMI is paramount. Diagnosis can be made by several imaging techniques, including echocardiography, computed tomography, ventriculography and magnetic resonance imaging (MRI). Echocardiography is the first-line imaging modality in suspected LVPA. Colour-Doppler can help to demonstrate the discontinuity of the ventricular wall and highlight the distinctive bi-directional flow between the extracardiac echo-free space and the left ventricle.24 Contrast ventriculography has the likelihood of establishing a definitive diagnosis23 and can help along with coronary angiography in surgical planning; however, it is an invasive procedure that might precipitate ventricular rupture. Owing to its ability to visualize the heart in precise detail, MRI can provide essential information to confirm diagnosis and clearly elucidate the characteristics of the pseudoaneurysm and guide surgical intervention.25 However, MRI can be performed only in patients with LVPA who remain haemodynamically stable. When the diagnosis of LVPA is established, surgical correction is usually mandatory because onset of rupture is unpredictable.
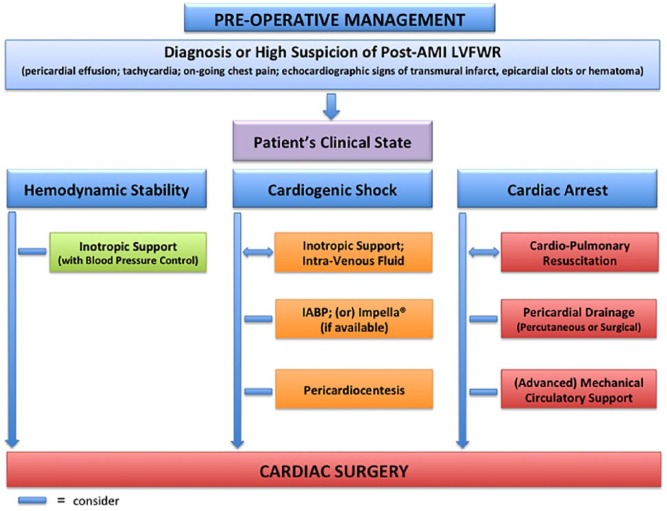
On the way to surgery, restoration of a satisfactory haemodynamic state may require inotropic support, intra-venous fluid, intra-aortic balloon pump (IABP) and pericardial drainage, as guided by clinical status (Figure 2). Pericardiocentesis may relieve cardiac tamponade and improve circulation in a critical situation; however, it is often unhelpful because much of the pericardial space is taken up by non-drainable clots. Decompression of the pericardium may also be obtained by subxiphoid drainage or by a classic sternotomy. In the presence of refractory cardiac arrest, extracorporeal membrane oxygenation might provide a chance to perform definitive surgical treatment.26
Figure 2.
Pre-operative management.
An algorithm that can be applied in cases of left ventricular free-wall rupture.
AMI: acute myocardial infarction; LVFWR: left ventricular free-wall rupture; IABP: intra-aortic balloon pump.
Different opinions are published about the opportunity to perform coronary angiograms or avoid this investigation in order to ‘save time’.17,27 Some authors believe that coronary angiography should be promptly performed as soon as pericardial effusion is noted in AMI patients, before they deteriorate.28,29 The knowledge of coronary status is of great help in deciding where and how to place the sutures during surgery; in addition, a proper revascularization of the diseased vessels supplying the non-infarcted area at the time of LVFWR repair (concomitant coronary artery bypass grafting) has been shown to exert a positive impact on survival and freedom from angina.29,30
Patients being operated for post-AMI LVFWR are generally unstable, so exposure of the femoral vessels at the groin and circuit preparation for rapid institution of CPB should be considered before thoracic incision. The use of CPB support, however, is a matter of controversy. Some authors, in fact, believe that in certain circumstances (anterior defect, haemodynamic stability, oozing type rupture, sub-acute course), and with the application of particular surgical techniques (epicardial patch covering, sutureless repair), LVFWR can be safely treated without utilizing CPB.28
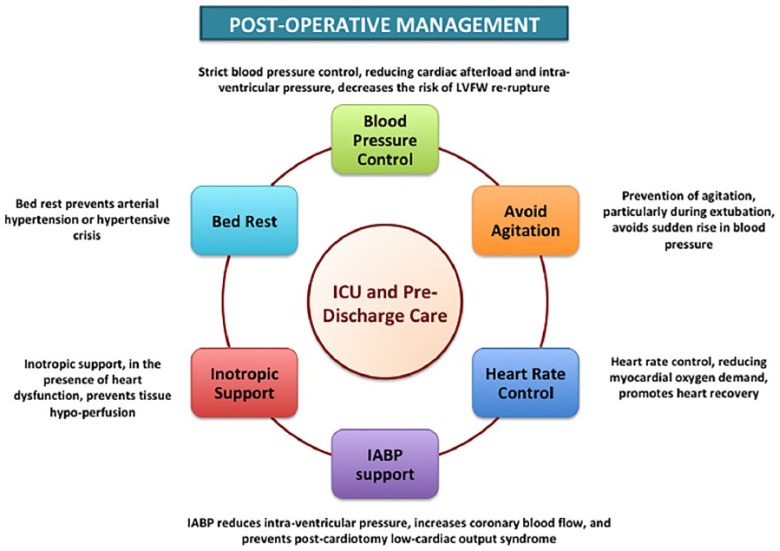
The post-operative use of IABP, and other mechanical circulatory support, should always be taken into consideration because they can reduce the intra-cavitary pressure of the left ventricle, increase coronary blood flow and limit or prevent the development of low cardiac output syndrome.17,31 Timóteo and colleagues reported that the use of IABP in patients with post-AMI mechanical complications was associated with improved in-hospital outcome.32 However, the European Society of Cardiology/European Association for Cardio-Thoracic Surgery Guidelines on myocardial revascularization allocate short-term mechanical circulatory support, in the presence of post-AMI mechanical complications, in Class of recommendation IIb.33 Further investigations are required to better evaluate the efficacy and safety of mechanical devices in the setting of post-infarction LVFWR repair. General principles of post-operative care, after LVFWR repair, are shown in Figure 3.
Figure 3.
Post-operative management.
General principles of post-operative care after left ventricular free-wall rupture repair.
LVFW: left ventricular free-wall; ICU: intensive care unit; IABP: intra-aortic balloon pump.
Surgical materials
In the past, surgical treatment of LVFWR was generally achieved using only Prolene® sutures, buttressed or not with Teflon® felt. In recent years, however, cardiac procedures have employed patches and surgical glues. Different materials have been proposed in patch-based repairs. The most common applied are: Teflon®,15 Goretex®,34 Dacron®35 and pericardium,36 either autologous or xenograft. Collagen sponges, or fleeces, have been utilized in other surgical specialties, ranging from gynaecology, urology and liver surgery.37,38 The use of collagen sponges in cardiac surgery is not new; its safety has been assessed in different cardio-thoracic procedures.39 However, the indication for using TachoComb® or TachoSil® patches in LVFWR repair has become more common only in recent years, based on preliminary favourable results.40,41
Adhesives reported to be successful in the treatment of LVFWR have been of several types and include the biologic glues (fibrin based or gelatin hydrogels) as well as the synthetic cyanoacrylate monomers. Fibrin glues function by reproducing the normal clotting cascade and result in a stable fibrin matrix after the degradation of exogenous fibrinogen. The main advantage is their lack of toxicity and complete biocompatibility.42 Gelatin-based glues have greater bonding strength than fibrin glues; however, cytotoxic effect has been shown, due to the release of formaldehyde during degradation, raising concerns regarding potential long-term complications.43 The main limitation of both these biologic glues is that they are effective only in the absence of bleeding. Synthetic glues, such as cyanoacrylate, are monomers that polymerize in an exothermic reaction when brought into contact with fluid. Although they are cytotoxic and potentially mutagenic, acetylation has greatly reduced these concerns.44 Using Histoacryl® to secure a patch of Teflon® onto the myocardium, Padró and coworkers reported a 100% survival rate among 13 patients treated for subacute LVFWR.3
Surgical techniques
As previously mentioned, several different techniques can be applied to treat post-infarction LVFWR. Depending on the optional use of sutures to treat the rupture of the ventricular wall, two different kinds of surgical approach can be proposed: sutureless and sutured repair. Initially, the sutured techniques were the only ones used. Such approaches included: i) linear closure, ii) infarctectomy and closure, and iii) patch covering. More recently, the availability of tissue adhesive materials and surgical glues have allowed the wide diffusion of the sutureless technique.
Principles of surgical treatment of LVFWR are to relieve tamponade, close the tear and/or stop the bleeding, anchor the repair on healthy tissue and minimize distortion of heart geometry, while preventing recurrence of rupture or pseudoaneurysm formation. The chosen method for surgical repair is usually dictated by the type of rupture, its surrounding tissues and the presence of concomitant lesions.
Linear closure
The first successful repairs of LVFWR with linear closure were reported by Montegut10 and Cobbs and colleagues.45 In this technique, the ventricular tear is closed with Prolene® horizontal mattress sutures buttressed by Teflon® felt. The sutures should be along the non-ischaemic area to avoid suture in the necrotic myocardium, which usually generates myocardial tearing and increased bleeding. Additionally, an over and over suture can be taken, approximating the edges of the Teflon® felts, to achieve a satisfactory haemostasis45 (Figure 4). Better results have been reported using linear closure between strips of Teflon® felt, if the ventricular tear and surrounding infarct area are covered with a patch secured to the heart surface with stitches, sutures or surgical glue.
Figure 4.
Linear closure.
The ventricular tear is closed with Prolene® horizontal mattress sutures with two supporting Teflon® felt; an over and over suture is taken to achieve haemostasis.
This technique is usually not feasible in the presence of a large necrotic area, based on the potential distortion and excessive reduction of the residual left ventricular cavity.
Infarctectomy and closure
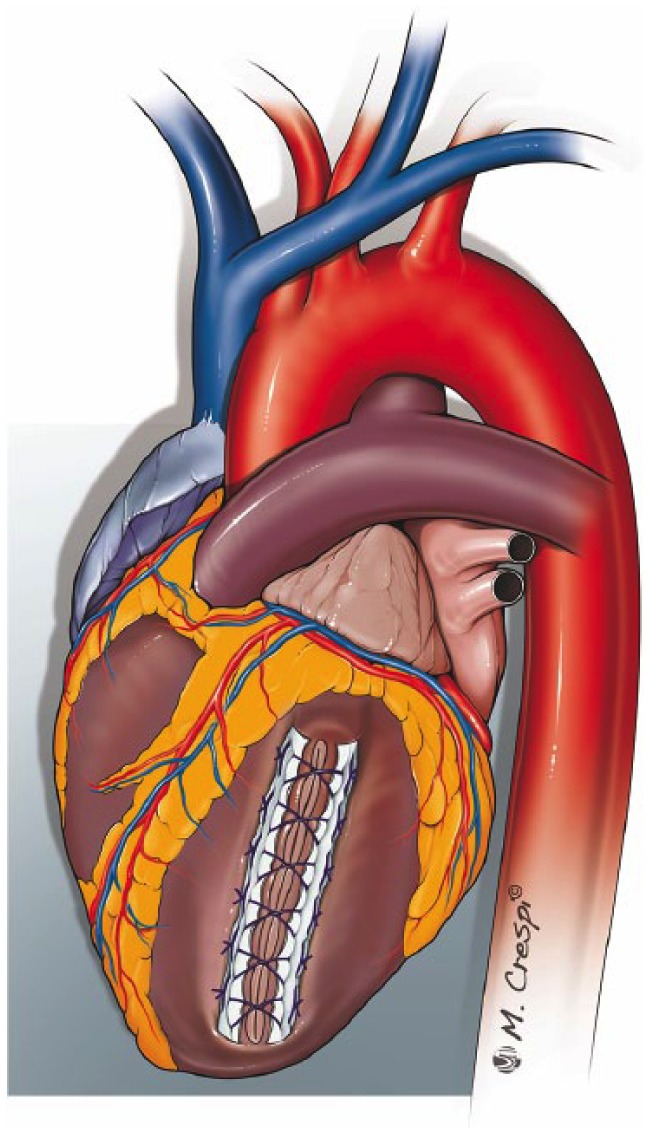
Infarct excision and closure of the defect, with either direct closure or prosthetic patch, has fallen into disuse as a result of the increased application of biological glues. These procedures are possibly now reserved for the treatment of acute massive ruptures (blow-out type rupture) or in the presence of concomitant lesions such as a post-infarction ventricular septal defect.
In the conventional approach, the excision of necrotic myocardial tissue is followed by replacement using a prosthetic patch, carefully fashioned to reestablish the geometry of the left ventricular chamber17 (Figure 5), or by direct closure of the myocardial defect with interrupted mattress sutures reinforced with Teflon® felt.46 As described by Reardon et al., once the prosthetic patch is sutured with pledgeted interrupted sutures, the edge can be oversewn to the myocardium with a continuous Prolene® running suture to achieve haemostasis.17
Figure 5.
Infarctectomy and closure of the defect with a prosthetic patch.
After infarctectomy, a prosthetic patch is fashioned to fit this space and is sutured with pledgeted interrupted sutures.
Such surgical techniques are demanding. Transmural stitches are placed in a healthy but friable myocardial tissue, so a tear could occur, and the suture line, placed along the non-ischaemic myocardium, can lead to heart geometry distortion and further deterioration of ventricular function, due to new iatrogenic myocardial infarction areas. Furthermore, heparinization associated with CPB increases the risk of bleeding.
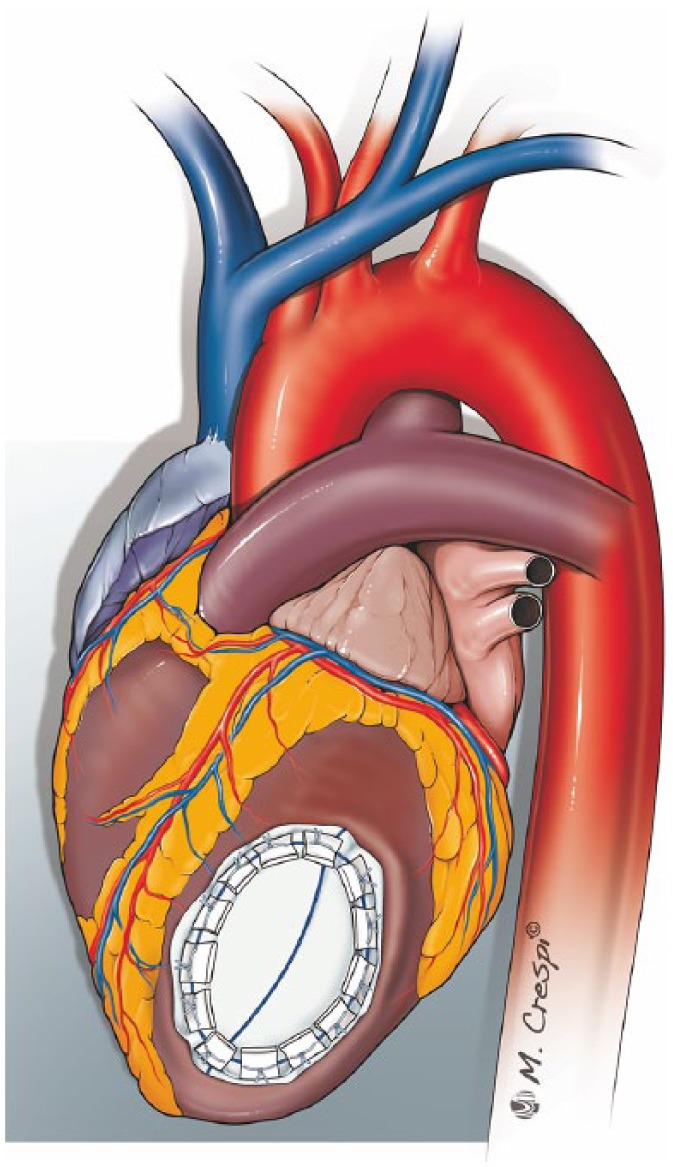
Patch covering
Some authors considered this technique the method of choice when LVFWR shows no active bleeding (or direct tear).47 In this method, ventricular rupture and the surrounding infarcted muscle are covered with a patch grafted to the healthy epicardium by Prolene® running sutures (Figure 6). Meticulous attention should be paid to avoiding coronary involvement. Glue, injected underneath the patch, is a mandatory adjunct to increase the compression strength on the myocardium and prevent blood leakage to the epicardial surface along the suture line.28,29
Figure 6.
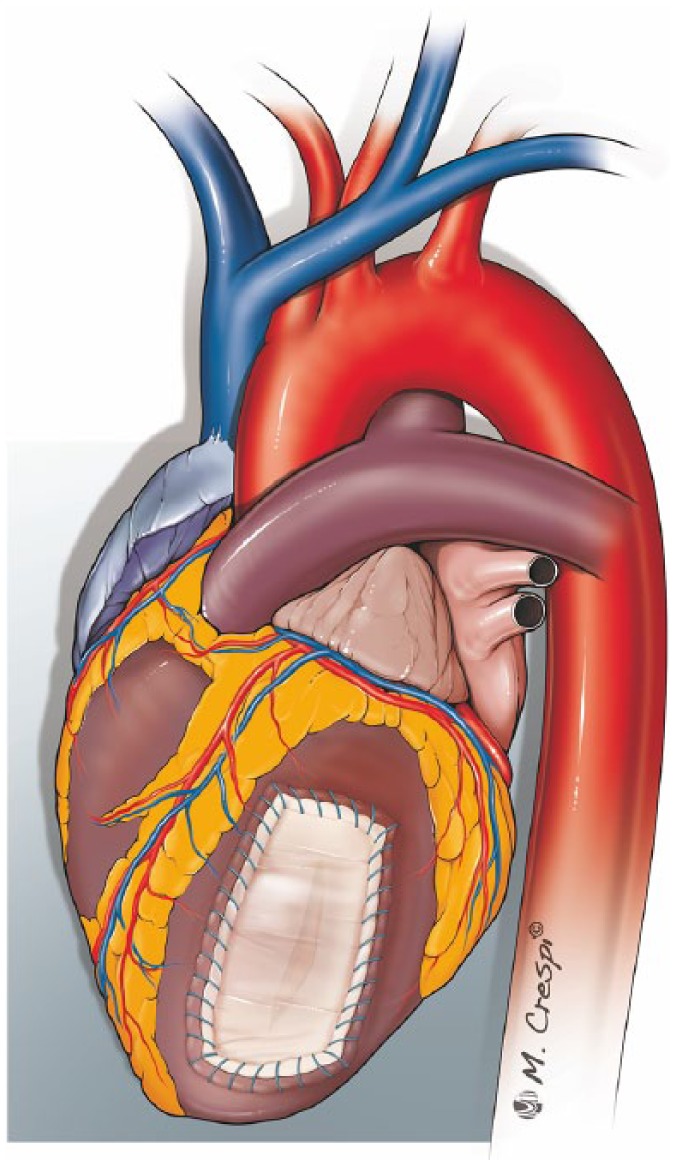
Patch covering technique.
A patch of pericardium is sealed on the infarcted area using surgical glue and fixed with a running Prolene® suture on the surrounding healthy myocardium.
Because the anchoring sutures are placed only in the epicardium, myocardial damage is minimal. Furthermore, in active bleeding cardiac ruptures, fixing the patch with running sutures appears to be more effective to prevent re-rupture than a sutureless technique. Although this type of repair can be performed avoiding CPB, some surgeons prefer to perform it on CPB with aortic cross clamp to reduce the tension on the epicardial tissue and cardiac movement, thereby favouring a quicker and more effective running suture and reducing the risk of further myocardial tearing.48
Pocar and colleagues reported a modified patch covering technique in which a Tachosil® fleece was applied to widely cover the ventricular tear and the adjacent infarcted tissue; thereafter, a generous pericardial patch was fixed with a few separate Prolene® stitches and fibrin glue was injected to seal the two layers.40
Sutureless repair
Availability of tissue adhesive materials has allowed a sutureless patch technique of repair for the oozing type of ventricular rupture. A prosthetic patch, adequately fashioned to cover the area of haematoma and muscle necrosis, its borders placed on healthy myocardium in the peri-necrotic area, is fixed onto the myocardial surface with surgical glue. The glued patch must be held in place for 1 to 3 min (depending on the type of glue used), until it becomes firmly adherent and haemostatic. As reported above, a wide range of synthetic and biological glues can be used for this purpose. The obvious limitation of the biological glues is that they are effective only in the absence of active bleeding. So glues should preferably be used if the tear is sealed or the lesion is of the oozing type.
Lachapelle et al. suggested that even patients with actively bleeding lesions can be treated using sutureless repair, provided they are on CPB with total decompression of the heart.49 Nevertheless, concerns remain over the safety of this technique when applied to treat blow-out ruptures. In this setting, the risk of recurrent re-rupture and pseudoaneurysm formation is high, since it is very unlikely that a simply glued patch can withstand the intraventricular pressure when there is a direct communication between the left ventricular cavity and pericardial space.
Recent reports are likely to shift toward the use of collagen sponge patch (Figure 7). A TachoComb® or Tachosil® patch is placed and pressed with wet gauze to the dry surface of the ventricle for a few minutes. When a simple patch is not large enough to cover the entire damage surface, a second patch is applied. Again, surrounding healthy myocardium not involved in the necrotic process is important to allow better anchoring and avoid re-rupture. This surgical approach, particularly when reserved to treat patients with oozing type rupture, has shown satisfactory clinical results.50,51
Figure 7.
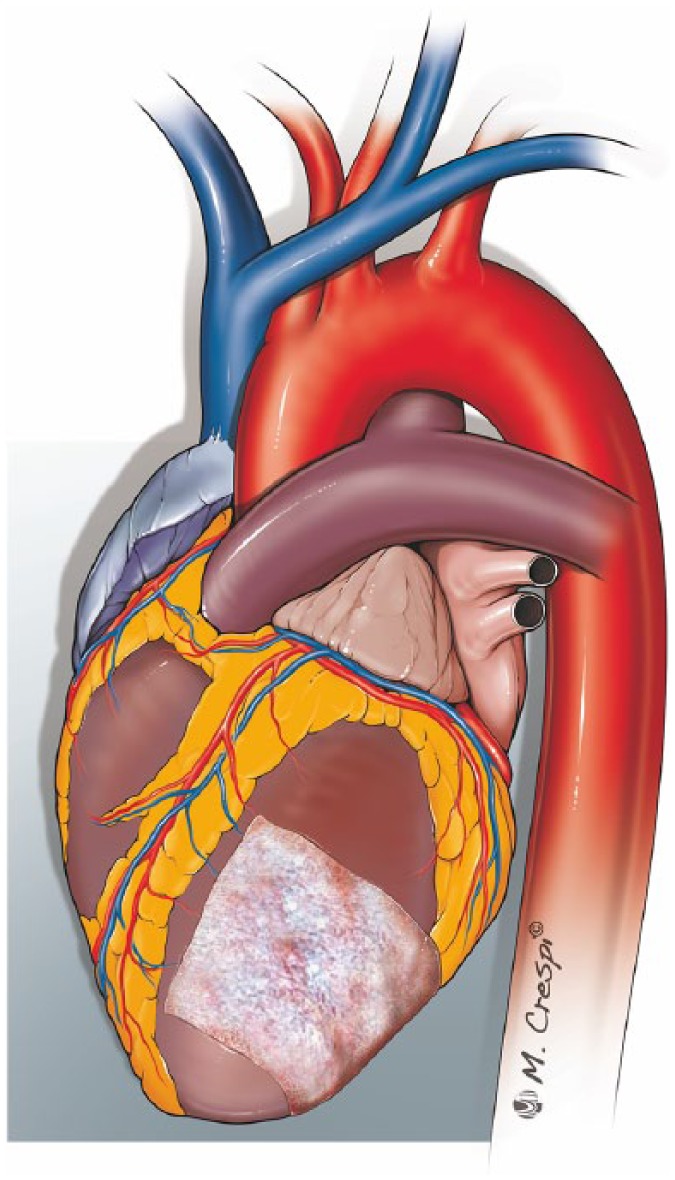
Sutureless repair.
A TachoSil® patch is applied to widely cover the ventricular wall rupture and the adjacent infarcted tissues.
In conclusion, the sutureless technique is simple, fast and can be done without CPB; moreover, it preserves left ventricular geometry and, leaving the necrotic tissue untouched, provides complete haemostasis. However, before considering this approach to be safe and suitable for all types of LVFWR, further investigations are required.
Percutaneous intra-pericardial fibrin-glue injection therapy
Recently, a new therapeutic option, namely percutaneous intra-pericardial fibrin-glue injection therapy (PIFIT), has been introduced to the clinical setting. The use of PIFIT to enhance haemostasis in post-infarction LVFWR was first described by Ogiwara et al. in 1995.52 Fibrin-glue, comprising fibrinogen and thrombin, when topically applied, exerts a local haemostatic and sealant effect. It is widely used in various surgical procedures.53 PITIF for LVFWR was reported in several case reports with reasonable short- and medium-term clinical outcomes.54,55 The use of intra-pericardial thrombin injection, as an alternative sealant, has also been reported.56 Despite an initial concern about uncontrolled pericardial adhesion after fibrin-glue application, reports, using serial echocardiographic follow-up, have not shown development of left ventricular restriction.54 However, Terashima et al. reported an in-hospital mortality of 25%,54 thereby, at present, such a technique should be applied only to patients with LVFWR and high surgical risk due to poor general condition.
Conservative management
Conservative management, in patients with sub-acute LVFWR who have recovered from cardiac tamponade (with or without pericardiocentesis) and have a prohibitive surgical risk, or when emergency surgical repair is not available, has been described. Management includes maintenance of fluid infusion and inotropic support as needed, with early attempts at weaning within the ensuing 24 h. This is followed by institution of beta-blockade therapy and strict blood pressure control. Insertion of IABP, reducing left ventricular wall stress and intra-cavitary pressure, could limit infarct extension and avoid re-rupture. Prolonged bed rest has also been included in the conservative management to prevent arterial hypertension or hypertensive crisis, often considered the precipitating causes of re-rupture.57 Subjects who survive generally have small leaks that might close spontaneously by epicardial fibrin deposits. Obviously, this kind of treatment should be considered as unusual and non-standard. Blinc et al. conducted a retrospective cohort study of 107 patients who developed LVFWR; survival for the conservatively treated group was only 10%.58 By contrast, surgically treated patients had acceptable long-term survival (50%). So, surgical repair has to be considered undoubtedly the treatment of choice.
Conclusion
Despite the first successful LVFWR repair dating back to the year 1972, this surgical procedure continues to be associated with high in-hospital mortality rates.6
Different techniques have been developed over the years for the management of LVFWR. Nevertheless, the optimal surgical treatment for this post-AMI mechanical complication remains controversial. Although the technical strategy varies, a basic principle that appears to remain unchanged is that surgeons should put the stitches, or fixed patches, in the healthy myocardial tissue.
To date, sutured techniques should be considered the procedure of choice for surgical repair of LVFWR with active bleeding at the site of rupture. In the presence of oozing rupture, the most common operative finding, the sutureless technique represents a safe and effective alternative option, demonstrating satisfactory outcome results. This field still has room for cardiac surgeons to improve surgical strategies and techniques.
Footnotes
Conflict of interest: RL is the Principal Investigator of PERSIST-AVR Trial sponsored by LivaNova and he is consultant for Medtronic.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Nishiyama K, Okino S, Andou J, et al. Coronary angioplasty reduces free wall rupture and improves mortality and morbidity of acute myocardial infarction. J Invasive Cardiol 2004; 16: 554–548. [PubMed] [Google Scholar]
- 2. Qian G, Wu C, Chen YD, et al. Predictive factors of cardiac rupture in patients with ST-elevation myocardial infarction. J Zhejiang Univ Sci B 2014; 15: 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Padró JM, Mesa JM, Silvestre J, et al. Subacute cardiac rupture: Repair with a sutureless technique. Ann Thorac Surg 1993; 55: 20–23. [DOI] [PubMed] [Google Scholar]
- 4. Nasir A, Gouda M, Khan A, et al. Is it ever possible to treat left ventricular free wall rupture conservatively? Interact Cardiovasc Thorac Surg 2014; 19: 488–493. [DOI] [PubMed] [Google Scholar]
- 5. Terashima M, Fujiwara S, Yaginuma GY, et al. Outcome of percutaneous intrapericardial fibrin-glue injection therapy for left ventricular free wall rupture secondary to acute myocardial infarction. Am J Cardiol 2008; 101: 419–421. [DOI] [PubMed] [Google Scholar]
- 6. Formica F, Mariani S, Singh G, et al. Postinfarction left ventricular free wall rupture: A 17-year single-centre experience. Eur J Cardiothorac Surg 2018; 53: 150–156. [DOI] [PubMed] [Google Scholar]
- 7. Sakaguchi G, Komiya T, Tamura N, et al. Surgical treatment for postinfarction left ventricular free wall rupture. Ann Thorac Surg 2008; 85: 1344–1346. [DOI] [PubMed] [Google Scholar]
- 8. Harvey W. Complete works. Willis R. (transl.) London: Sydenham Society, 1647, p.127. [Google Scholar]
- 9. Morgagni JB. The seat and causes of disease investigated by anatomy, vol. 1 Alexander B. (transl.). London: Millar A and Caddel T, 1769, pp.811–834. [Google Scholar]
- 10. Montegut FJ., Jr. Left ventricular rupture secondary to myocardial infarction. Report of survival with surgical repair. Ann Thorac Surg 1972; 14: 75–78. [DOI] [PubMed] [Google Scholar]
- 11. Krumbhaar EB, Crowell C. Spontaneous rupture of the heart. Am J Med Sci 1925; 170: 828. [Google Scholar]
- 12. Hatcher CR, Jr, Mansour K, Logan WD, Jr, et al. Surgical complications of myocardial infarction. Am Surg 1970; 36: 163–170. [PubMed] [Google Scholar]
- 13. FitzGibbon GM, Hooper GD, Heggtveit HA. Successful surgical treatment of postinfarction external cardiac rupture. J Thorac Cardiovasc Surg 1972; 63: 622–630. [PubMed] [Google Scholar]
- 14. Calick A, Kerth W, Barbour D, et al. Successful surgical therapy of ruptured myocardium. Chest 1974; 66: 188. [DOI] [PubMed] [Google Scholar]
- 15. Núñez L, de la Llana R, López Sendón J, et al. Diagnosis and treatment of subacute free wall ventricular rupture after infarction. Ann Thorac Surg 1983; 35: 525–529. [DOI] [PubMed] [Google Scholar]
- 16. Löfström B, Mogensen L, Nyquist O, et al. 3. Attempts at emergency surgical treatment. Chest 1972; 61: 10–13. [DOI] [PubMed] [Google Scholar]
- 17. Reardon MJ, Carr CL, Diamond A, et al. Ischemic left ventricular free wall rupture: Prediction, diagnosis, and treatment. Ann Thorac Surg 1997; 64: 1509–1513. [DOI] [PubMed] [Google Scholar]
- 18. Pohjola-Sintonen S, Muller JE, Stone PH, et al. Ventricular septal and free wall rupture complicating acute myocardial infarction: Experience in the Multicenter Investigation of Limitation of Infarct Size. Am Heart J 1989; 117: 809–818. [DOI] [PubMed] [Google Scholar]
- 19. French JK, Hellkamp AS, Armstrong PW, et al. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEX-AMI). Am J Cardiol 2010; 105: 59–63. [DOI] [PubMed] [Google Scholar]
- 20. Lancellotti P, Price S, Edvardsen T, et al. The use of echocardiography in acute cardiovascular care: Recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2015; 4: 3–5. [DOI] [PubMed] [Google Scholar]
- 21. Porto AG, McAlindon E, Ascione R, et al. Magnetic resonance imaging-based management of silent cardiac rupture. J Thorac Cardiovasc Surg 2015; 149: e31–e33. [DOI] [PubMed] [Google Scholar]
- 22. Villanueva C, Milder D, Manganas C. Ruptured left ventricular false aneurysm following acute myocardial infarction: Case report and review of the literature. Heart Lung Circ 2014; 23: e261–263. [DOI] [PubMed] [Google Scholar]
- 23. Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol 1998; 32: 557–561. [DOI] [PubMed] [Google Scholar]
- 24. Roelandt JR, Sutherland GR, Yoshida K, et al. Improved diagnosis and characterization of left ventricular pseudoaneurysm by Doppler color flow imaging. J Am Coll Cardiol 1988; 12: 807–811. [DOI] [PubMed] [Google Scholar]
- 25. Orsborne C, Schmitt M. Left ventricular pseudoaneurysm after myocardial infarction detected by cardiac MRI. BMJ Case Rep 2014; 2014: bcr2014207277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lazzeri C, Bernardo P, Sori A, et al. Venous-arterial extracorporeal membrane oxygenation for refractory cardiac arrest: A clinical challenge. Eur Heart J Acute Cardiovasc Care 2013; 2: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sutherland FW, Guell FJ, Pathi VL, et al. Postinfarction ventricular free wall rupture: Strategies for diagnosis and treatment. Ann Thorac Surg 1996; 61: 1281–1285. [DOI] [PubMed] [Google Scholar]
- 28. Iemura J, Oku H, Otaki M, et al. Surgical strategy for left ventricular free wall rupture after acute myocardial infarction. Ann Thorac Surg 2001; 71: 201–204. [DOI] [PubMed] [Google Scholar]
- 29. Mantovani V, Vanoli D, Chelazzi P, et al. Post-infarction cardiac rupture: Surgical treatment. Eur J Cardiothorac Surg 2002; 22: 777–780. [DOI] [PubMed] [Google Scholar]
- 30. Leva C, Bruno PG, Gallorini C, et al. Complete myocardial revascularization and sutureless technique for left ventricular free wall rupture: Clinical and echocardiographic results. Interact Cardiovasc Thorac Surg 2006; 5: 408–412. [DOI] [PubMed] [Google Scholar]
- 31. Okada K, Yamashita T, Matsumori M, et al. Surgical treatment for rupture of left ventricular free wall after acute myocardial infarction. Interact Cardiovasc Thorac Surg 2005; 4: 203–206. [DOI] [PubMed] [Google Scholar]
- 32. Timóteo AT, Nogueira MA, Rosa SA, et al. ; ProACS Investigators. Role of intra-aortic balloon pump counterpulsation in the treatment of acute myocardial infarction complicated by cardiogenic shock: Evidence from the Portuguese nationwide registry. Eur Heart J Acute Cardiovasc Care 2016; 5: 23–31. [DOI] [PubMed] [Google Scholar]
- 33. Sousa-Uva M, Neumann FJ, Ahlsson A, et al. ; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019; 55: 4–90. [DOI] [PubMed] [Google Scholar]
- 34. Canovas SJ, Lim E, Dalmau MJ, et al. Midterm clinical and echocardiographic results with patch glue repair of left ventricular free wall rupture. Circulation 2003; 108(Suppl. 1): II237–II240. [DOI] [PubMed] [Google Scholar]
- 35. Zeebregts CJ, Noyez L, Hensens AG, et al. Surgical repair of subacute left ventricular free wall rupture. J Card Surg 1997; 12: 416–419. [DOI] [PubMed] [Google Scholar]
- 36. Prêtre R, Benedikt P, Turina MI. Experience with postinfarction left ventricular free wall rupture. Ann Thorac Surg 2000; 69: 1342–1345. [DOI] [PubMed] [Google Scholar]
- 37. Simo KA, Hanna EM, Imagawa DK, et al. Hemostatic agents in hepatobiliary and pancreas surgery: A review of the literature and critical evaluation of a novel carrier-bound fibrin sealant (TachoSil). ISRN Surg 2012; 2012: 729086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fuglsang K, Dueholm M, Stæhr-Hansen E, et al. Uterine healing after therapeutic intrauterine administration of TachoSil (hemostatic fleece) in cesarean section with postpartum hemorrhage caused by placenta previa. J Pregnancy 2012; 2012: 635683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maisano F, Kjaergård HK, Bauernschmitt R, et al. TachoSil surgical patch versus conventional haemostatic fleece material for control of bleeding in cardiovascular surgery: A randomised controlled trial. Eur J Cardiothorac Surg 2009; 36: 708–714. [DOI] [PubMed] [Google Scholar]
- 40. Pocar M, Passolunghi D, Bregasi A, et al. TachoSil for postinfarction ventricular free wall rupture. Interact Cardiovasc Thorac Surg 2012; 14: 866–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muto A, Nishibe T, Kondo Y, et al. Sutureless repair with TachoComb sheets for oozing type postinfarction cardiac rupture. Ann Thorac Surg 2005; 79: 2143–2145. [DOI] [PubMed] [Google Scholar]
- 42. Byrne DJ, Hardy J, Wood RA, et al. Effect of fibrin glues on the mechanical properties of healing wounds. Br J Surg 1991; 78: 841–843. [DOI] [PubMed] [Google Scholar]
- 43. Bingley JA, Gardner MA, Stafford EG, et al. Late complications of tissue glues in aortic surgery. Ann Thorac Surg 2000; 69: 1764–1768. [DOI] [PubMed] [Google Scholar]
- 44. Kung H. Evaluation of the undesirable side-effects of the surgical use of histoacryl glue with special regard to possible carcinogenicity. Basel, Switzerland: RCC Institute for Contract Research in Toxicology and Ecology, 1986. [Google Scholar]
- 45. Cobbs BW, Jr, Hatcher CR, Jr, Robinson PH. Cardiac rupture. Three operations with two long-term survivals. JAMA 1973; 223: 532–535. [DOI] [PubMed] [Google Scholar]
- 46. Schwarz CD, Punzengruber C, Ng CK, et al. Clinical presentation of rupture of the left-ventricular free wall after myocardial infarction: Report of five cases with successful surgical repair. Thorac Cardiovasc Surg 1996; 44: 71–75. [DOI] [PubMed] [Google Scholar]
- 47. Coletti G, Torracca L, Zogno M, et al. Surgical management of left ventricular free wall rupture after acute myocardial infarction. Cardiovasc Surg 1995; 3: 181–186. [DOI] [PubMed] [Google Scholar]
- 48. Haddadin S, Milano AD, Faggian G, et al. Surgical treatment of postinfarction left ventricular free wall rupture. J Card Surg 2009; 24: 624–631. [DOI] [PubMed] [Google Scholar]
- 49. Lachapelle K, deVarennes B, Ergina PL, et al. Sutureless patch technique for postinfarction left ventricular rupture. Ann Thorac Surg 2002; 74: 96–101 [DOI] [PubMed] [Google Scholar]
- 50. Raffa GM, Tarelli G, Patrini D, et al. Sutureless repair for postinfarction cardiac rupture: A simple approach with a tissue-adhering patch. J Thorac Cardiovasc Surg 2013; 145: 598–599. [DOI] [PubMed] [Google Scholar]
- 51. Bergman R, Jainandunsing JS, Woltersom BD, et al. Sutureless management of left ventricle wall rupture; a series of three cases. J Cardiothorac Surg 2014; 9: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ogiwara M, Kyo S, Yokote Y, et al. [Clinical result of left ventricular free wall rupture resulting from acute myocardial infarction]. Kyobu Geka 1995; 48: 286–289. [PubMed] [Google Scholar]
- 53. Spotnitz WD. Fibrin sealant: Past, present, and future: A brief review. World J Surg 2010; 34: 632–634. [DOI] [PubMed] [Google Scholar]
- 54. Terashima M, Fujiwara S, Yaginuma GY, et al. Outcome of percutaneous intrapericardial fibrin-glue injection therapy for left ventricular free wall rupture secondary to acute myocardial infarction. Am J Cardiol 2008; 101: 419–421. [DOI] [PubMed] [Google Scholar]
- 55. Joho S, Asanoi H, Sakabe M, et al. Long-term usefulness of percutaneous intrapericardial fibrin-glue fixation therapy for oozing type of left ventricular free wall rupture: A case report. Circ J 2002; 66: 705–706. [DOI] [PubMed] [Google Scholar]
- 56. Lee JK, Tsui KL, Chan KK, et al. Intra-pericardial thrombin injection for post-infarction left ventricular free wall rupture. Eur Heart J Acute Cardiovasc Care 2012; 1: 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Figueras J, Cortadellas J, Evangelista A, et al. Medical management of selected patients with left ventricular free wall rupture during acute myocardial infarction. J Am Coll Cardiol 1997; 29: 512–518. [DOI] [PubMed] [Google Scholar]
- 58. Blinc A, Noc M, Pohar B, et al. Subacute rupture of the left ventricular free wall after acute myocardial infarction. Three cases of long-term survival without emergency surgical repair. Chest 1996; 109: 565–567. [DOI] [PubMed] [Google Scholar]