Abstract
The production of poly-β-hydroxybutyrate (PHB) under varying environmental conditions (pH, temperature and carbon sources) was examined in the cyanobacterium Scytonema geitleri Bharadwaja isolated from the roof-top of a building. The S. geitleri produced PHB and the production of PHB was linear with the growth of cyanobacterium. The maximum PHB production (7.12% of dry cell weight) was recorded when the cells of S. geitleri were at their stationary growth phase. The production of PHB was optimum at pH 8.5 and 30 °C, and acetate (30 mM) was the preferred carbon source.
Keywords: carbon source, cyanobacteria, PHB, Scytonema geitleri
1. Introduction
Synthetic polymers are potent environmental toxic pollutants which are non-degradable and have accumulated in the environment. Therefore, the efforts of scientists have been focused on alternative environmental biopolymers. Among the biodegradable polymers, polyhydroxyalkanoates (PHAs), including polyhydroxybutyrate (PHB), have been recently recognized polyesters due to their features as biodegradable thermoplastics [1].
The PHB is mostly produced by prokaryotes and accumulated intracellularly as an energy and carbon storage material [2]. Pure PHB is of biological origin and is thermoplastic, stereo-specific and biodegradable [3,4]. It has wide applications in environmental, agricultural and biomedical fields [5,6,7].
The PHB production has been reported from cyanobacteria such as Chloroglea fritschii [8], Spirulina platensis [9], Oscillatoria limosa and Spirulina sp. [10,11,12], Synechocystis sp. PCC 6803 [13,14], Nostoc muscorum [15,16], and Aulosira fertilissima [7]. Kamravanesh et al. [17] found that a mutant strain of Synechocystis sp. PCC 6714 showed 2.5-fold higher productivity than their wild type under nitrogen and phosphorus starvation. Similarily, Troschl et al. [18] produced poly-β-hydroxybutyrate from Synechocytis sp. CCALA192 at pilat-scale with carbon dioxide as a sole carbon source in a photobioreactor. Several microorganisms including Gram positive like Bacillus megaterium, Bacillus subtilis and Corynebacterium glutamicum, Gram negative bacteria like groups of Pseudomonas and Alcaligenes eutrophus, have been reported to produce different types of polyhydroxyalkanoates (PHAs) [19]. However, studies related PHB productions by cyanobacterial members under different environmental conditions are very much limited and no reports are available for Scytonema geitleri Bharadwaja. Further, it is unknown whether different environmental drivers, such as pH, nitrogen source, temperature, moisture, light intensity, etc., affects PHB production rate by cyanobacteria. In this study, using a temperature and desiccation tolerant cyanobacterium S. geitleri Bharadawaja (isolated from the roof-top), the production of PHB in response to different environmental drivers such as pH, temperature and carbon sources (sucrose and acetate), was examined. Evidence of the presence of PHB was also examined by using spectrophotometric and gas chromatographic assays.
2. Materials and Methods
2.1. Organism and Culture Conditions
The cyanobacterium S. geitleri Bharadwaja was isolated from the roof-top of the Botany Department, Banaras Hindu University, Varanasi, India. The organism was purified using standard microbiological procedures and identified morphologically as per Desikachary [20]. Cultures of S. geitleri were grown in 250 mL Erlenmeyer flasks containing 100 mL of Chu-10 medium (pH 7.5) [21] and maintained in a culture room at 28 ± 1 °C under 14:10 h light-dark rhythm. The cultures were illuminated with a combination of day and white light fluorescent lamp giving an average light intensity of 95 µmoL m−2 s−1 on the surface of the vessels.
2.2. Growth Measurement
The growth of S. geitleri was measured by measuring the increase in its chlorophyll a (chl a) content. Cyanobacterial culture (3 mL) was harvested by centrifugation and the resulting pellet was suspended in an equal volume of 80% acetone. After thorough vortexing, the suspension was kept at 4 °C for 24 h. Thereafter, it was centrifuged and absorbance of the cell-free extract was recorded at 663 nm using 80% acetone as a blank. Chl-a was quantified following Mackinney [22]. Oven dried cyanobacterial pellets having constant weight were used to measure the dry weight.
2.3. Extraction of Polyhydroxybutyrate
The cells of S. geitleri (28 day old) were harvested by centrifugation at 5000× g for 10 min and lyophilized. The lyophilized cells were digested with 4% (w/v) sodium hypochlorite (Fisher Scientific, Ottawa, ON, Canada) at 37 °C for 15 h [23]. The residual material was centrifuged at 10,000× g for 10 min and the residue was washed thrice with 10 mL each of water, acetone, ethanol, and ether. The resulting residue was air dried; subjected to warm chloroform extraction and filtered. The supernatant containing PHB was concentrated using a vacuum rotary evaporator, precipitated using 10 mL of ice-cold methanol and centrifuged. The precipitate thus obtained was air dried and used for further analysis of PHB [8,24].
2.4. Assay of Polyhydroxybutyrate
Dried PHB was digested with 10 mL concentrated H2SO4 in a water bath at 100 °C for 20 min. After cooling and thorough mixing, the absorbance of the crotonic acid (formed after H2SO4 digestion of PHB) was recorded at 235 nm using H2SO4 as a blank [25]. The presence of PHB was further confirmed spectrophotometrically by recording the absorption spectra of the sample as well as the standard poly-β-hydroxybutyric acid (Sigma-Aldrich., St. Louis, MO, USA) from 200–500 nm.
2.5. Quantification of Polyhydroxybutyrate
In small screw-cap glass tubes (Borosil, Mumbai, India), 1 mL of PHB solution (dissolved in chloroform) was taken and to that, 0.85 and 0.15 mL of methanol and sulfuric acid were added respectively. The tubes were placed in a thermostat-equipped water bath for 140 min at 100 °C for degradation of PHB to its constituent p-hydroxybutyric acid methyl esters after methanolysis. Following this, 0.5 mL of double distilled water was added to the tubes and the tubes were shaken vigorously for 1 min. The bottom layer (organic phase composed of methyl esters) was removed, transferred to a small screw-cap glass vial and stored at −70 °C for further analysis.
The PHB was analyzed using gas chromatograph (CP-3800 GC, Varian, Inc., Mitchell Drive Walnut Creek, CA, USA) fitted with a flame ionization detector (FID). The sample (2 µL) was injected by a Hamilton syringe into a CP-Sil 8 CB column (5% diphenyl and 95% dimethyl polysiloxane, 30 m by 0.32 mm inner diameter, 1µm film thickness). The column temperature was maintained initially at 80 °C for 2 min followed by subsequent increment of 10 °C in temperature up to 200 °C for another 2 min, and finally maintained at 250 °C for 10 min. The injector and detector temperatures were kept at 200 and 250 °C respectively. While N2 was used as carrier gas with a flow rate of 30 mL min−1, H2 and O2 were, however, used as flame gases with a flow rate of 30 mL min−1 and 300 mL min−1 respectively under split mode (30:100). PHB was identified using standard poly 3-hydroxybutyric acid (Sigma-Aldrich, USA) following the method of Brandl et al. [26].
3. Results and Discussion
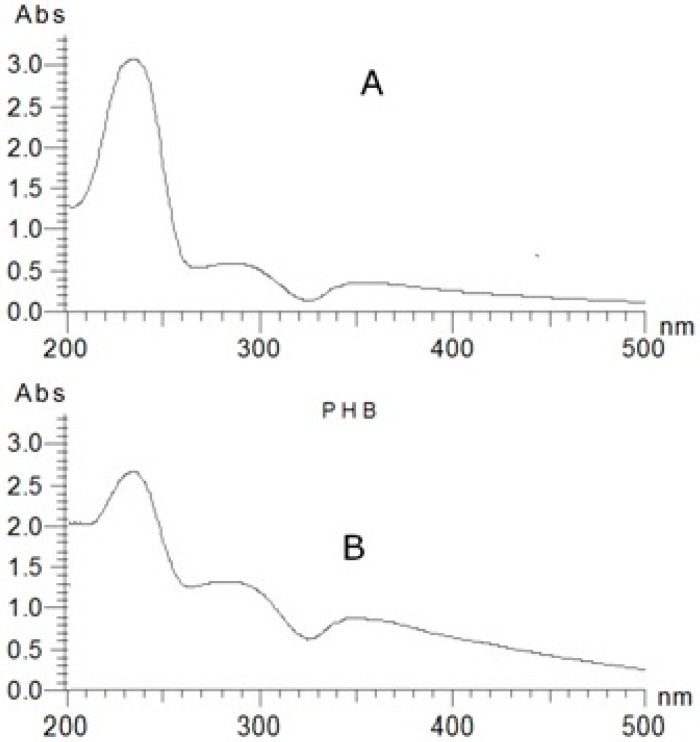
For the extraction of PHB, 28-day-old S. geitleri were used. A tough, translucent and grey film of PHB was recovered from the cyanobacterium. To ascertain whether the synthesized product was PHB or not, the synthesized product and standard poly 3-hydroxybutyric acid were digested with concentrated H2SO4 for their conversion to crotonic acid and their respective absorption spectra were recorded from 200–500 nm. Interestingly, both the synthesized product and standard poly 3-hydroxybutyric acid exhibited similar crotonic acid peaks with absorption maxima at 235 nm (Figure 1), thus confirming the synthesis and production of PHB in S. geitleri. Further, to have more conclusive evidence regarding the synthesis of PHB by S. geitleri, the degradation products of PHB of S. geitleri and standard poly 3-hydroxybutyric acid after methanolysis (p-hydroxybutyric acid methyl esters) were analyzed and quantified using gas chromatograph (data not shown). The appearance of the peaks at the retention time of 7.33 min for both PHB of S. geitleri and standard poly 3-hydroxybutyric acid again confirmed the synthesis of PHB by S. geitleri. Synthesis of PHB in microbial biomass has also been detected using gas chromatograph [26,27,28].
Figure 1.
Crotonic acid peak (at 235 nm) of standard poly-β-hydroxybutyric acid (A) and polyhydroxybutyrate (PHB) isolated from Scytonema geitleri (B).
3.1. Relationship between Growth and Polyhydroxybutyrate Production
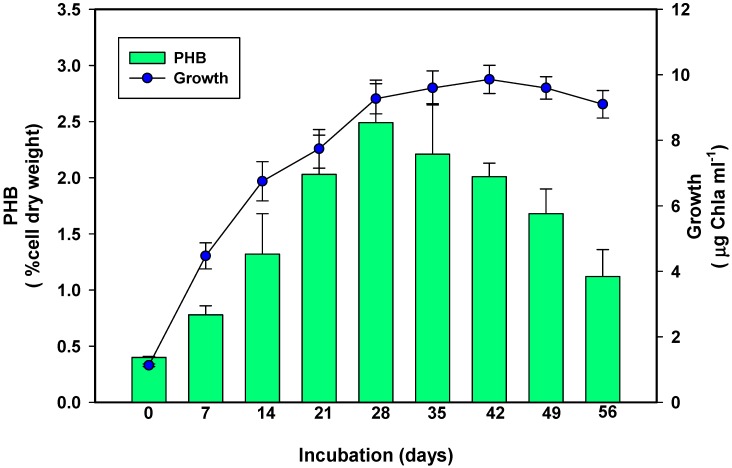
To ascertain whether any relationship exists between growth of S. geitleri and its PHB production, both the activities were measured simultaneously up to 56 days (Figure 2). It is evident from the data of Figure 2 that S. geitleri started producing PHB at the early growth phase and attained its optimal level (≈2.7% cell dry weight) on the 28th day of incubation. However, further increases in the incubation period resulted in a significant decrease in PHB production, attaining its minimum level on the 56th day of incubation. Similar results were also found by Ansari and Fatma [29] where they found that in Nostoc muscorum NCCU-442, PHB accumulation started at the early growth stage while its maximum level was recorded on the 21st day of incubation. The above contention was also supported by the observations made for other cyanobacteria [30,31,32]. PHB serves as carbon reserve and energy source for cyanobacteria [33]. The production of PHB by S. geitleri at its early growth phase and decrease in its production due to prolonged incubation may be explained by the facts that at early growth phases when nutrients were available in abundance and not limiting the growth medium, the cells of S. geitleri accumulated excess carbon in the form of PHB. However, during prolonged incubation of the cells, when the nutrients were exhausted and the resources became limiting, the cells of S. geitleri used PHB as an energy and carbon source to maintain its metabolic activities.
Figure 2.
Production of PHB in S. geitleri in relation to growth and the incubation period. Values are the mean (n = 3) ± standard deviation (SD).
3.2. Relative Contribution of Carbon Sources on Polyhydroxybutyrate Production
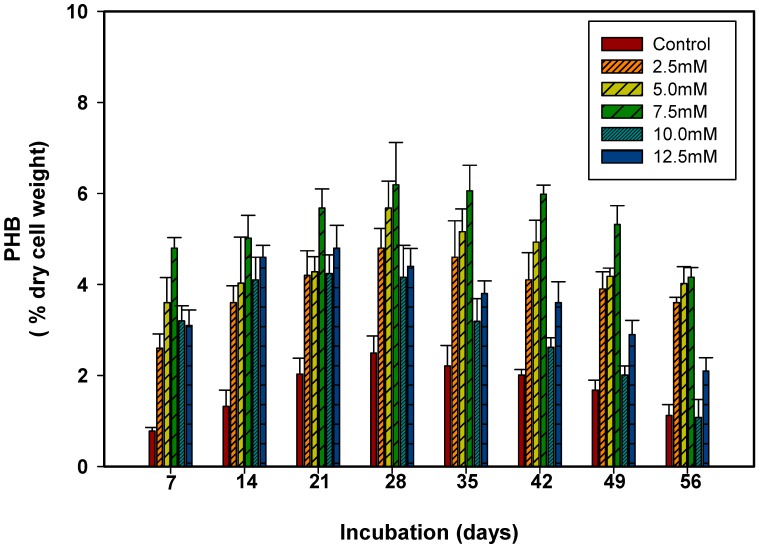
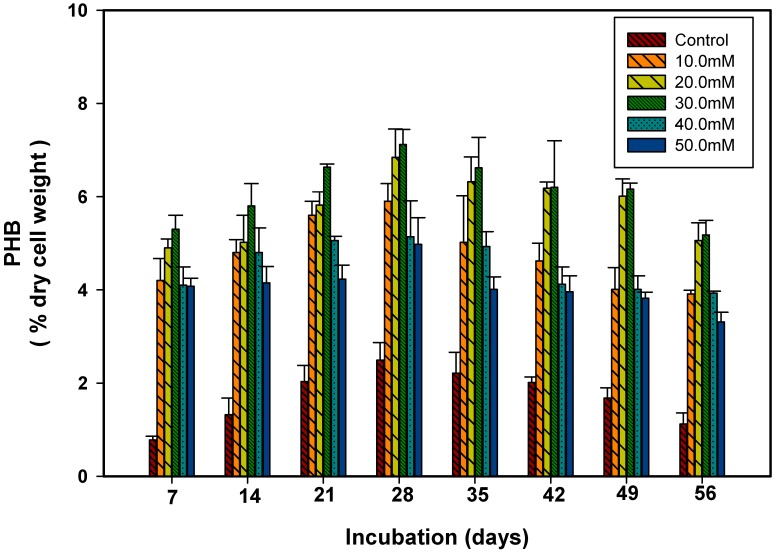
To ascertain the relative contribution of carbon sources (sucrose and acetate) on PHB production in S. geitleri, PHB content was measured in the cells of S. geitleri grown in the presence of sucrose (2.5–12.5 mM) and acetate (10–50 mM) for 7–56 days (Figure 3 and Figure 4). The PHB production in S. geitleri increased with an increase in sucrose and acetate concentrations, and attained its maximal levels of 6.19 and 7.12% of cells dry weight (cdw) at 7.5 mM sucrose and 30 mM acetate respectively on the 28th day of incubation. Similar results were also supported by Bhati et al. [34] who used the cyanobacterium Nostoc muscoroum Agardh. Further increases in sucrose and acetate concentrations, however, resulted in a significant decrease in PHB production. Panda et al. [35] also showed that acetate is directly used as a precursor for PHB synthesis. The PHB is synthesized in bacteria by the condensation of two moles of acetyl-CoA to acetoacetyl-CoA and subsequently to β-hydroxybutyryl-CoA [36]. The PHB synthesis is stimulated by high intracellular concentration of dihydronicotinamide adenine dinucleotide phosphate (NADPH) and a high ratio of NADPH/nicotinamide adenine dinucleotide phosphate (NADP). Many microorganisms directly utilize acetate to increase their intracellular acetyl-CoA pool for the synthesis of PHB [14,37,38]. The stimulation of PHB production in sucrose supplemented cultures of S. geitleri may be due to the production of NADPH necessarily required for the activity of acetoacetyl CoA reductase in PHB biosynthetic pathway [39,40]. The inhibitory effect of high concentrations of acetate and sucrose on PHB production in S. geitleri may be due to their high concentration causing an increase in osmotic pressure [41]. The maximum stimulation of PHB production was also reported in acetate and glucose supplemented cultures which were possibly due to the easy access to the precursor, i.e., acetate and NADPH [16,39]. Besides sucrose and acetate, other carbon and nitrogen sources have also been used to see the stimulation of PHB in cyanobacteria and bacteria [40,42]. Nishioka et al. [43] obtained 55% (dcw) PHB as carbon dioxide as the sole carbon source from the mutant strain of Synechococcus sp. MA-19 under phasphate limited conditions.
Figure 3.
Production of PHB in S. geitleri in response to sucrose. Values are the mean (n = 3) ± SD.
Figure 4.
Production of PHB in S. geitleri in response to acetate. Values are the mean (n = 3) ± SD.
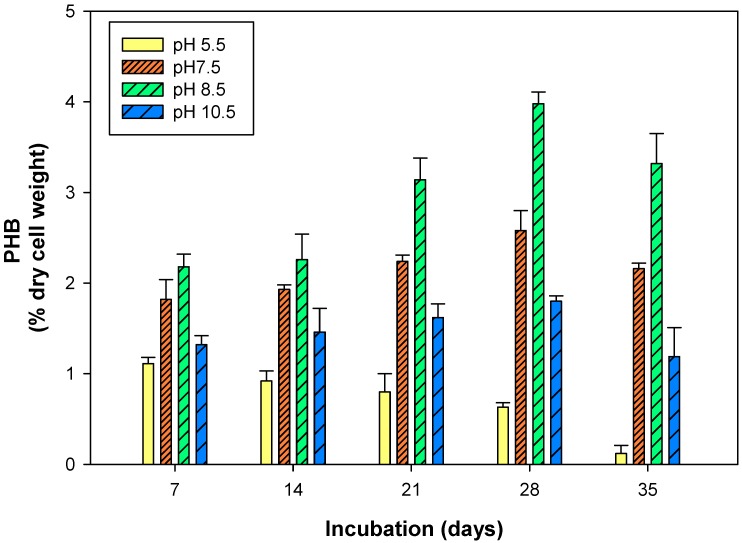
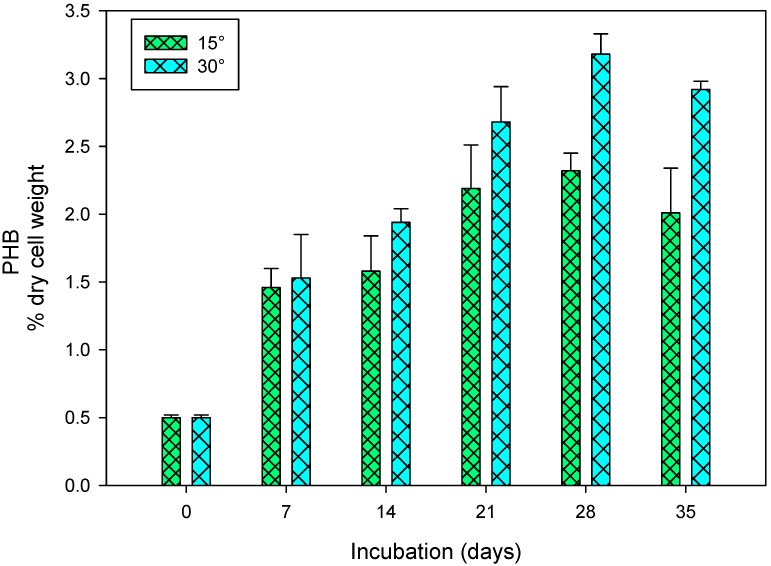
3.3. Polyhydroxybutyrate Production in Response to pH and Temperature
The pH and temperature are the key factors affecting the cyanobacterial metabolism. To ascertain the response of pH and temperature on PHB production in S. geitleri, the cells of S. geitleri were grown at different pH (5.5–10.5) and temperatures (15 and 30 °C) for 7–35 days and PHB production was measured (Figure 5 and Figure 6). Sodium acetate (50 mM, pH 5.5), potassium phosphate (50 mM, pH 7.5) and glycine-NaOH (50 mM, pH 8.5–10.5) buffers were used to maintain the different pH. It is evident from the data of Figure 5 that the cells of S. geitleri grown at pH 8.5 produced the maximum amount of PHB (3.98% of dry cell wt) on the 28th day of incubation. Further, an increase or decrease in pH from pH 8.5 resulted in a significant decrease in PHB production. At pH 8.5, the maximum PHB accumulations were also recorded in Synechocystis sp. PCC6803 [35]. Ansari and Fatma [29] also found similar results and suggested that alkaline pH (8–9.5) favored high PHB accumulation compared to acidic pH (5–7). Likewise, S. geitleri grown at 30 °C produced higher amounts of PHB (3.18% cell dry wt.) compared to that grown at 15 °C (Figure 6). The cells of S. geitleri, however, produced an equal amount of PHB on the seventh day at both the temperatures. Nostoc muscorum NCCU-442 also accumulated maximum PHB at 30 °C when compared to 15, 20, 25, 40, 45 °C [29]. In case of Synechocystis sp. PCC6803, maximum PHB accumulation occurred at 28 °C [35]. It is a well-established fact that cyanobacteria grows well at relatively high temperatures (25–27 °C) and in alkaline conditions. Furthermore, enzyme activity proceeds with a maximum rate at optimum temperatures which varies with organisms. In the present study, the ability of S. geitleri to produce maximum PHB at pH 8.5 and 30 °C was also supported by the findings of other workers [44]. Karbasi et al. [45] also studied the PHB production in four different bacterial species, viz. Azotobacter beijinckii DSMZ 1041, Cupriavidus necator DSMZ 545, Hydrogenophaga pseudoflava DSMZ 1034, and Azohydromonas lata DSMZ 1123 and found that maximum PHB accumulation occurred at 30 °C.
Figure 5.
Production of PHB in S. geitleri in response to pH. Values are the mean (n = 3) ± SD.
Figure 6.
Production of PHB in S. geitleri in response to temperature. Values are the mean (n = 3) ± SD.
4. Conclusions
Cyanobacterium S. geitleri produced high quality (in the form of tough, translucent and grey film) PHB. The PHB production showed a linear relationship with the growth pattern of the cyanobacterium. The S. geitleri physiologically adapted to varying environmental conditions and produced maximum PHB at pH 8.5 and 30 °C temperatures, and acetate (30 mM) was the preferred carbon source. Compared to other microbial sources, cyanobacterial PHB is supposed to be economical and can be used as an alternative for biodegradable bioplastics on a large scale. In the current scenario of increasing pollution loads, S. geitleri explores a new alternative of conventional petrochemical polymers to reduce microplastics pollution and marine littering.
Acknowledgments
Authors thank the Head of the Department of Botany, Banaras Hindu University for providing necessary facilities. This manuscript is dedicated to our mentor, the Late Prof. Surendra Singh, who passed away due to prolong illness while the manuscript was under preparation. The authors respectfully thank S.P. Singh, Centre of Advanced Study in Botany, Banaras Hindu University, Varanasi-221005, India for editing and improving the English language in the manuscript.
Author Contributions
M.K.S. performed the experimental work, P.K.R. and A.R. analyzed the data and wrote the manuscript, J.S.S. reviewed and edited the manuscript, S.S. prepared the design of experimental work and the lab facility.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Singh M., Patel S.K., Kalia V.C. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb. Cell Factories. 2009;8:38. doi: 10.1186/1475-2859-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liebergesell M., Sonomoto K., Madkour M., Mayer F., Steinbüchel A. Purification and Characterization of the Poly(Hydroxyalkanoic Acid) Synthase from Chromatium vinosum and Localization of the Enzyme at the Surface of Poly(Hydroxyalkanoic Acid) Granules. JBIC J. Boil. Inorg. Chem. 1994;226:71–80. doi: 10.1111/j.1432-1033.1994.tb20027.x. [DOI] [PubMed] [Google Scholar]
- 3.Stubbe J., Tian J., He A., Sinskey A.J., Lawrence A.G., Liu P. Nontemplate-Dependent Polymerization Processes: Polyhydroxyalkanoate Synthases as a Paradigm. Annu. Biochem. 2005;74:433–480. doi: 10.1146/annurev.biochem.74.082803.133013. [DOI] [PubMed] [Google Scholar]
- 4.Tian J., Stubbe J. Polyhydroxyalkanoate (PHA) homeostasis: The role of the PHA synthase. Nat. Prod. Rep. 2003;20:445. doi: 10.1039/b209687k. [DOI] [PubMed] [Google Scholar]
- 5.Fukada E., Ando Y. Piezoelectric properties of poly-β-hydroxybytyrate and copolymers of β-hydroxybutyrate and β-hydroxyvalerate. Int. J. Biol. Macromol. 1986;8:361–366. doi: 10.1016/0141-8130(86)90056-5. [DOI] [Google Scholar]
- 6.Duvernoy O., Malm T., Ramström J., Bowald S. A Biodegradable Patch used as a Pericardial Substitute after Cardiac Surgery: 6- and 24-Month Evaluation with CT. Thorac. Cardiovasc. 1995;43:271–274. doi: 10.1055/s-2007-1013226. [DOI] [PubMed] [Google Scholar]
- 7.Samantaray S., Mallick N. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 2012;24:803–814. doi: 10.1007/s10811-011-9699-7. [DOI] [Google Scholar]
- 8.Carr N. The occurrence of poly-β-hydroxybutyrate in the blue-green alga, Chlorogloea fritschii. Biochim. Biophys. Acta. 1966;120:308–310. doi: 10.1016/0926-6585(66)90353-0. [DOI] [PubMed] [Google Scholar]
- 9.Campbell J., Stevens S.E., Bankwill D.L. Accumulation of poly-β-hydroxybutyrate in Spirulina platensis. J. Bacteriol. 1982;149:361–366. doi: 10.1128/jb.149.1.361-363.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stal L.J., Heyer H., Jacobs G. Occurrence and Role of Poly-Hydroxy-Alkanoate in the Cyanobacterium Oscillatoria Limosa. In: Dawes E.A., editor. Novel Biodegradable Microbial Polymers. Volume 186. Springer; Dordrecht, The Netherlands: 1990. pp. 435–438. (NATO ASI Series (Series E: Applied Sciences)). [Google Scholar]
- 11.Vincenzini M., Sili C., De Philippis R., Ena A., Materassi R. Occurrence of poly-β-hydroxybutyrate in Spirulina species. J. Bacteriol. 1990;172:2791–2792. doi: 10.1128/jb.172.5.2791-2792.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrivastav A., Mishra S.K., Mishra S. Polyhydroxyalkanoates synthesis by Spirulina subsalsa from Gujrat coast of India. Int. J. Biol. Macromol. 2010;46:255–260. doi: 10.1016/j.ijbiomac.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Wu G., Wu Q., Shen Z. Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 2001;76:85–90. doi: 10.1016/S0960-8524(00)00099-7. [DOI] [PubMed] [Google Scholar]
- 14.Panda B., Mallick N. Enhanced poly-β-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 2007;44:194–198. doi: 10.1111/j.1472-765X.2006.02048.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharma L., Mallick N. Accumulation of poly-β-hydroxybutyrate in Nostoc muscorum: Regulation by pH, light-dark cycles, N and P status and carbon sources. Bioresour. Technol. 2005;96:1304–1310. doi: 10.1016/j.biortech.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Sharma L., Mallick N. Enhancement of poly-β-hydroyxybutyrate accumulation in Nostoc muscorum under mixotrophy, chemoheterotrophy and limitation of gas exchange. Biotechnol. Lett. 2005;27:59–62. doi: 10.1007/s10529-004-6586-1. [DOI] [PubMed] [Google Scholar]
- 17.Kamravamanesh D., Kovacs T., Pflügl S., Druzhinina I., Kroll P., Lackner M., Herwig C. Increased poly-β-hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: Mutant generation and characterization. Bioresour. Technol. 2018;266:34–44. doi: 10.1016/j.biortech.2018.06.057. [DOI] [PubMed] [Google Scholar]
- 18.Troschl C., Meixner K., Fritz I., Leitner K., Romero A.P., Kovalcik A., Sedlacek P., Drosg B. Pilot-scale production of poly-β-hydroxybutyrate with the cyanobacterium Synechocytis sp. CCALA192 in a non-sterile tubular photobioreactor. Algal. Res. 2018;34:116–125. doi: 10.1016/j.algal.2018.07.011. [DOI] [Google Scholar]
- 19.Hassan M.A., Bakhiet E.K., Ali S.G., Hussien H.R. Production and characterization of polyhydroxybutyrate (PHB) produced by Bacillus sp. isolated from Egypt. J. Appl. Pharm. Sci. 2016;6:046–051. doi: 10.7324/JAPS.2016.60406. [DOI] [Google Scholar]
- 20.Desikachary T.V. Cyanophyta. Indian Council of Agricultural Research; New Delhi, India: 1959. [Google Scholar]
- 21.Chu S.P. The influence of the mineral composition of the medium on the growth of planktonic algae. I. Method and culture media. J. Ecol. 1942;30:284–325. doi: 10.2307/2256574. [DOI] [Google Scholar]
- 22.Mackinney G. Absorption of light by chlorophyll solution. J. Biol. Chem. 1941;140:315–322. [Google Scholar]
- 23.Williamson D.H., Wilkinson J.F. The isolation and estimation of the poly-β-hydroxy- butyrate inclusions of Bacillus species. J. Gen. Microbiol. 1958;19:198–209. doi: 10.1099/00221287-19-1-198. [DOI] [PubMed] [Google Scholar]
- 24.Huang R., Reusch R.N. Poly (β-hydroxybutyrate) is associated with specific protein in the cytoplasm and membrane of Escherichia coli. J. Biol. Chem. 1996;271:22196–22202. doi: 10.1074/jbc.271.36.22196. [DOI] [PubMed] [Google Scholar]
- 25.Law J.H., Slepecky R.A. Assay of Poly-Β-Hydroxybutyric Acid. J. Bacteriol. 1961;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandl H., Gross R.A., Lenz R.W., Fuller R.C. Pseudomonas oleovorans as a Source of Poly(β-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl. Environ. Microbiol. 1988;54:1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braunegg G., Sonnleimer B., Lafferty R.M. A rapid gas chromatographic method for the determination of Poly-β-hydroxybutyric acid in microbial biomass. Eur. J. Appl. Microbiol. Biotechnol. 1978;6:29–37. doi: 10.1007/BF00500854. [DOI] [Google Scholar]
- 28.Cui Y., Barford J.P., Renneberg R. Gas Chromatographic Determination of Poly(3-hydroxybutyrate) with Alkaline Hydrolysis and Acid Esterification. Anal. Lett. 2007;40:2915–2924. doi: 10.1080/00032710701603959. [DOI] [Google Scholar]
- 29.Ansari S., Fatma T. Cyanobacterial Polyhydroxybutyrate (PHB): Screening, Optimization and Characterization. PLoS ONE. 2016;11:0158168. doi: 10.1371/journal.pone.0158168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stal L.J. Poly(hydroxyalkanoates) in cyanobacteria: A review. FEMS Microbiol. Rev. 1992;103:169–180. doi: 10.1111/j.1574-6968.1992.tb05835.x. [DOI] [Google Scholar]
- 31.Mallick N. Polyhydroxyalkanoates in cyanobacteria: Progress and prospects. In: Santra S.C., Mallik A., editors. New Frontiers of Environmental Biotechnological Applications. ENVIS Centre on Environmental Biotechnology; Kolkata, India: 2011. pp. 56–80. [Google Scholar]
- 32.Samantaray S., Nayak J.K., Mallick N. Wastewater Utilization for Poly-β-Hydroxybutyrate Production by the Cyanobacterium Aulosira fertilissima in a Recirculatory Aquaculture System. Appl. Environ. Microbiol. 2011;77:8735–8743. doi: 10.1128/AEM.05275-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dawes E.A., Senior P.J. The Role and Regulation of Energy Reserve Polymers in Micro-organisms. Adv. Microb. Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- 34.Bhati R., Mallick N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: Process optimization and polymer characterization. Algal Res. 2015;7:78–85. doi: 10.1016/j.algal.2014.12.003. [DOI] [Google Scholar]
- 35.Panda B., Jain P., Sharma L., Mallick N. Optimization of cultural and nutritional conditions for accumulation of poly-β-hydroxybutyrate in Synechocystis sp. PCC 6803. Bioresour. Technol. 2006;97:1296–1301. doi: 10.1016/j.biortech.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Poirier Y., Nawrath C., Somerville C. Production of Polyhydroxyalkanoates, a Family of Biodegradable Plastics and Elastomers, in Bacteria and Plants. Nat. Biotechnol. 1995;13:142–150. doi: 10.1038/nbt0295-142. [DOI] [PubMed] [Google Scholar]
- 37.Gibson J. Movement of acetate across the cytoplasmic membrane of the unicellular cyanobacteria Synechococcus and Aphanocapsa. Arch. Microbiol. 1981;130:175–179. doi: 10.1007/BF00411073. [DOI] [PubMed] [Google Scholar]
- 38.Khatipov E. Accumulation of poly-β-hydroxybutyrate by Rhodobacter sphaeroides on various carbon and nitrogen substrates. FEMS Microbiol. Lett. 1998;162:39–45. doi: 10.1016/S0378-1097(98)00099-8. [DOI] [Google Scholar]
- 39.Lee I.Y., Kim M.K., Chang H.N., Park Y.H. Regulation of poly-β-hydroxybutyrate biosynthesis by nicotinamide nucleotides in Alcaligenes eutrophus. FEMS Microbiol. Lett. 1995;131:35–39. [Google Scholar]
- 40.Sangkharak K., Prasertsan P. Nutrient optimization for production of polyhydroxybutyrate from halotolerant photosynthetic bacteria cultivated under aerobic-dark condition. Electron. J. Biotechnol. 2008;11:83–94. doi: 10.2225/vol11-issue3-fulltext-2. [DOI] [Google Scholar]
- 41.Tabandeh F., Vasheghani-Farahani E. Biosynthesis of poly-β-hydroxybutyrate as a biodegradable polymer. Iran Polym. J. 2003;12:37–42. [Google Scholar]
- 42.Mallick N., Gupta S., Panda B., Sen R. Process optimization for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer production by Nostoc muscorum. Biochem. Eng. J. 2007;37:125–130. doi: 10.1016/j.bej.2007.04.002. [DOI] [Google Scholar]
- 43.Nishioka M., Nakai K., Miyake M., Asada Y., Taya M. Production of poly-β-hydroxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate-limited conditions. Biotechnol. Lett. 2001;23:1095–1099. doi: 10.1023/A:1010551614648. [DOI] [Google Scholar]
- 44.Suzuki T., Tsygankov A.A., Miyake J., Tokiwa Y., Asada Y. Accumulation of polyhydroxybutyrate by a non-sulphur photosynthetic bacterium Rhodobacter spheroids RV at different pH. Biotech. Lett. 1995;17:395–400. doi: 10.1007/BF00130796. [DOI] [Google Scholar]
- 45.Karbasi F., Ardjmand M., Yunesi H., Kordi A.S., Yaghmaei S. Investigation of optimum fermentation condition for PHA production by four species: Hydrogenophaga pseudoflava DSMZ 1034, Azohydromonas lata DSMZ 1123, Cupriavidus necator DSMZ 545, Azotobacter beijinckii DSMZ 1041. World Appl. Sci. J. 2011;14:36–47. [Google Scholar]