Abstract
Background: Chronic obstructive pulmonary disease (COPD) is currently the fourth largest fatal disease in the world, and is expected to rise to third place by 2020. Frequent acute exacerbations lead to increased mortality. Some suggestions for prophylactic use of macrolides in preventing COPD exacerbations have been raised, but there are still several issues that need to be addressed, such as target population, the course of treatment, therapeutic dose, and so on.
Objective: To evaluate, via exploratory meta-analysis, the efficacy of long-term macrolide therapy at low doses in stable COPD.
Methods: A systematic literature search was performed in PubMed, Embase, and Cochrane database from inception to March 28, 2019. Randomized controlled trials (RCT) which reported long-term use of macrolides in prevention of COPD were eligible.
Results: A total of 10 articles were included in this study. It was found that there was a 23% relative risk reduction in COPD exacerbations among patients taking macrolides compared to placebo (P<0.01). The median time to first exacerbation was effectively prolonged among patients taking macrolides vs placebo (P<0.01). Sub-group analysis showed erythromycin was advantageous and older patients were less responsive to macrolides.
Conclusions: Long-term low dose usage of macrolides could significantly reduce the frequency of the acute exacerbation of COPD. The treatment was well tolerated, with few adverse reactions, but it was not suitable for the elderly. It is recommended that this treatment regimen could be used in patients with GOLD grading C or D, because they have a higher risk of acute exacerbation and mortality. It needs to be further discussed whether this treatment should last for 12 months or longer.
Keywords: chronic obstructive pulmonary disease, low dose, long-term, exacerbation, macrolide, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease. Frequent acute exacerbations lead to a sharp decline in lung function, resulting in high disability, mortality, and mental problems, such as anxiety and depression, which seriously affects patients’ quality-of-life.1 In the meantime, acute exacerbations of chronic obstructive pulmonary disease (AECOPD) also consume a large amount of medical and health resources. According to statistics, the medical cost of AECOPD accounts for more than 50% of COPD treatment.2
To date, there are two main methods to prevent AECOPD. One is non-drug therapy, including smoking cessation, flu vaccination, pulmonary function training, and lung volume reduction surgery. The other is drug therapy, such as long-acting bronchodilators, glucocorticoids, phosphodiesterse-4 inhibitors, and N-acetylcysteine.3 However, these measures reduce the AECOPD by up to 25–30%.4,5 How to effectively reduce the acute exacerbation of COPD is a very meaningful proposition.
It is known to us that over 50% of acute exacerbations are caused by bacterial infections, accompanied by increased airway inflammation.6,7 As macrolides have anti-inflammatory, anti-viral, and immunomodulatory effects,8 scholars have carried out clinical trials to use it to prevent exacerbations of COPD. However, this method is not generally accepted. We, therefore, try to explore the efficacy of macrolides in preventing the acute exacerbation of COPD by statistical methods.
Methods
Search strategies
We systematically searched PubMed, Embase, and the Cochrane Library from their inception until March 28, 2019 using the following search term: chronic obstructive pulmonary disease AND macrolides or azithromycin or erythromycin or clarithromycin AND randomized controlled trials or controlled clinical trials. There was no language restriction.
Selection criteria
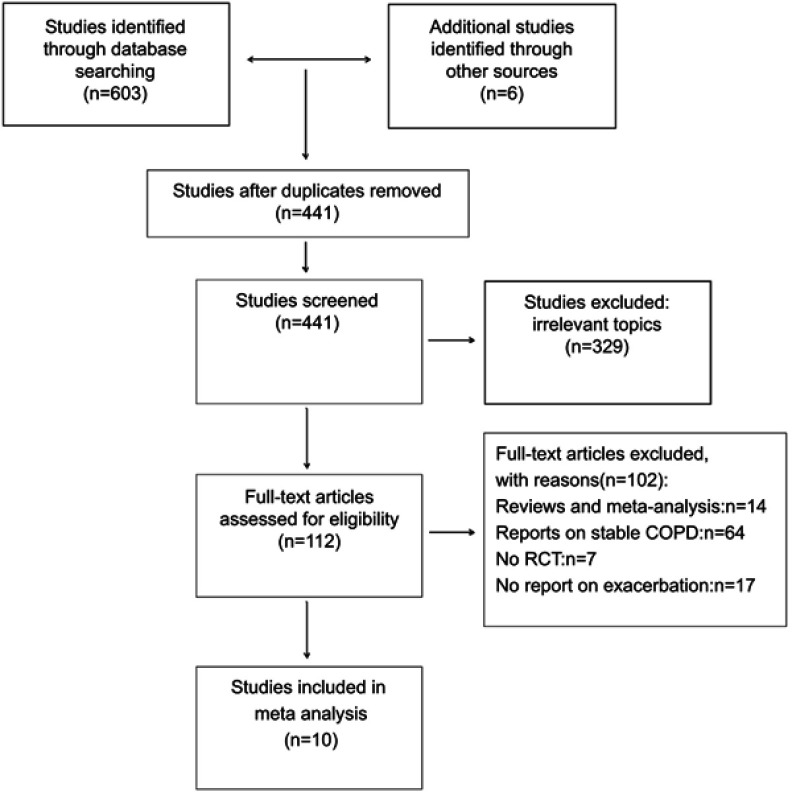
Studies were considered eligible for inclusion if they were RCTs that enrolled patients with a diagnosis of stable COPD.9 The initial search strategy yielded 609 studies. Two investigators (Cao and Wu) independently performed the literature search and the study selection. Dr Yao mediated disagreement regarding any specific study. Ten studies were ultimately selected for inclusion in the meta-analysis (Figure 1).9–18
Figure 1.
Flow diagram depicting the number of studies included at each stage of the selection process.Abbreviation: RCT, randomized controlled trials.
Data extraction and quality assessment
Of the studies selected, relevant data were directly derived from the paper including study design, the demographic data, macrolides dosing, duration of treatment, and outcomes assessment. COPD exacerbations are defined as an acute worsening of respiratory symptoms (dyspnea, increased quantity of sputum, or purulent sputum) that result in additional therapy. The primary endpoint was the total number of COPD exacerbations, median time to first exacerbation, and the rate of exacerbations per patient per year. Secondary endpoints included mortality, hospitalization rates, St George Respiratory Questionnaire (SGRQ) score, adverse events, and drug resistance.
Statistical analysis
Results from the intention-to-treat data were extracted, respectively, and summarized as risk ratio (RR) and corresponding 95% confidence interval (95% CI) by meta-analysis. The between-study heterogeneity was assessed by the I2statistic and Q-test. The fixed effect model was used for combining of results if P for heterogeneity test ≥0.1, or random-effects model was adopted otherwise. Sensitivity analyses were performed on age, sex, smoking history, type, dose, and duration of macrolides administered. Funnel plot was used to evaluate publication bias, and P<0.05 was considered statistically significant. Data were analyzed with R software Version3.4 (The R Foundation for Statistical Computing).
Results
Ten articles were retained in this study after manual curation. All the patients were in stable phase and had a history of acute exacerbations before enrollment. A total of 1,521 patients were randomly allocated to the macrolides treatment group, and 1,418 were randomly allocated to the control group. The study duration lasted from 3 months to 12 months. Three of the 10 RCTs were not blinded (Table 1).
Table 1.
Major study characteristics from the ten RCTs included in the meta-analysis
| Literature | Year | Treated Patients | Placebo | Therapy strategy | Duration of therapy | Jadad Scale |
|---|---|---|---|---|---|---|
| Suzuki et al9 |
2001 | 55 | 54 | Erythromycin 200–400 mg once daily |
12 months | 2 |
| Banerjee et al10 | 2004 | 31 | 36 | Clarithromycin 500 mg once daily |
3 months | 3 |
| Seemungal et al11 | 2008 | 53 | 56 | Erythromycin 250 mg twice daily |
12 months | 5 |
| He et al12 | 2010 | 18 | 18 | Erythromycin 125 mg three times daily |
6 months | 4 |
| Albert et al13 | 2011 | 558 | 559 | Azithromycin 250 mg once daily |
12 months | 3 |
| Berkhof et al14 | 2013 | 42 | 42 | Azithromycin 250 mg three times a week |
3 months | 5 |
| Uzun et al15 | 2014 | 47 | 45 | Azithromycin 500 mg three days a week |
12 months | 5 |
| Simpson et al16 | 2014 | 15 | 15 | Azithromycin 250 mg once daily |
12 months | 5 |
| Shafuddin et al17 | 2015 | 97 | 94 | Roxithromycin 300 mg once daily |
3 months | 5 |
| Ra et al18 | 2017 | 504 | 499 | Azithromycin 250 mg once daily |
12 months | 4 |
Abbreviation: RCTs, randomized controlled trials.
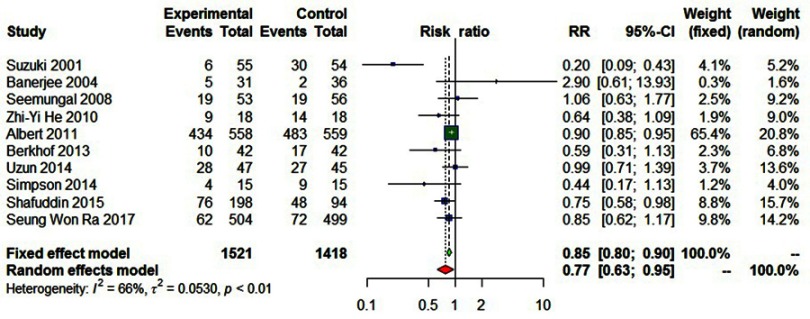
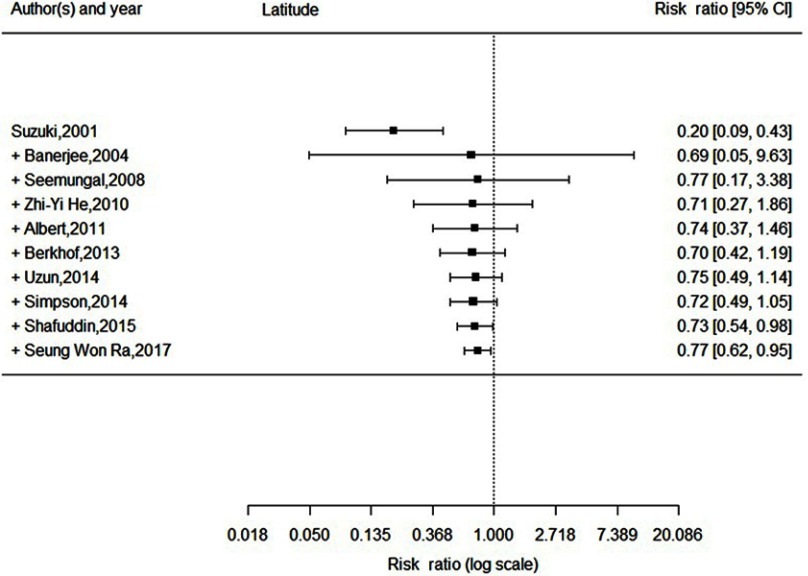
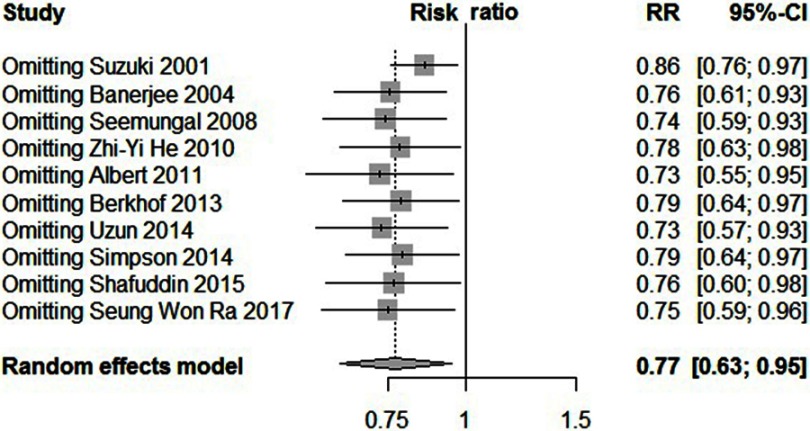
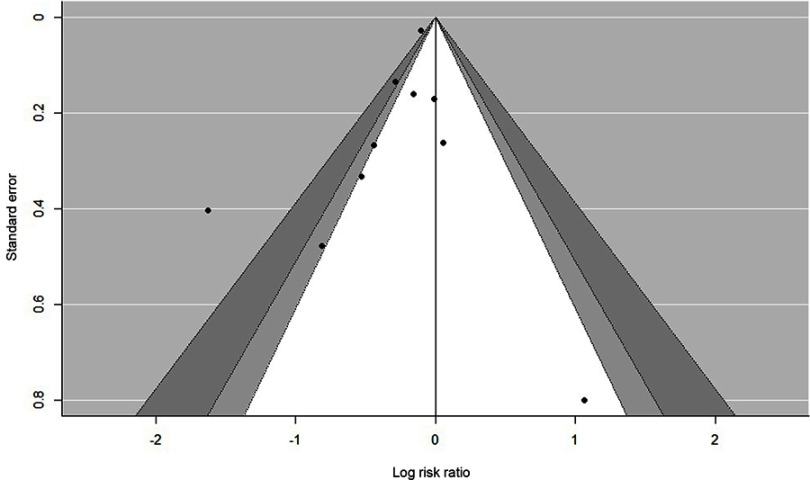
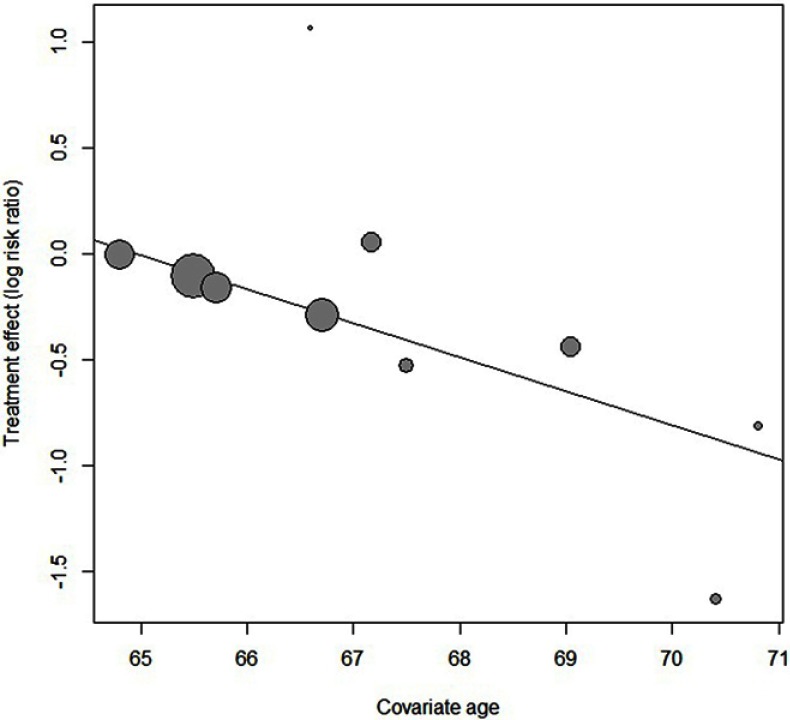
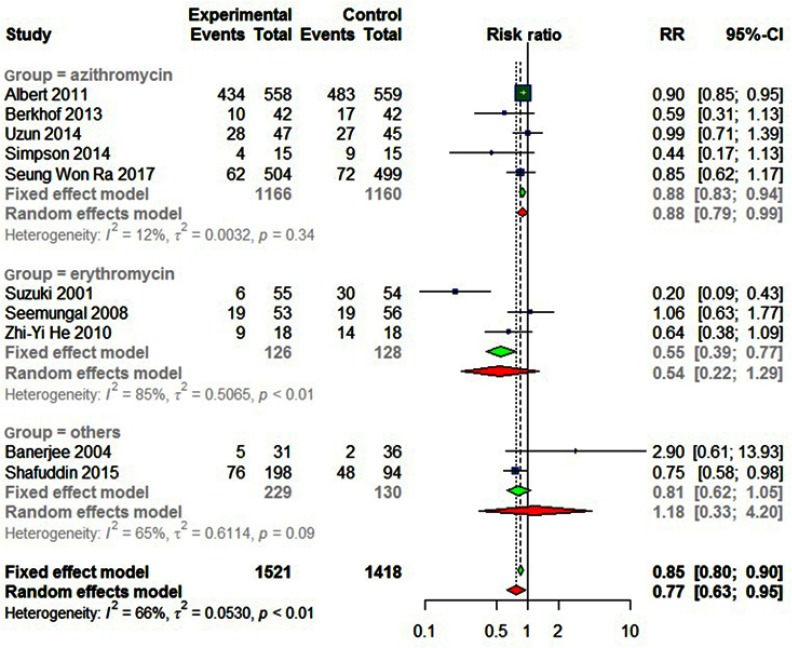
Statistically significant heterogeneity was observed among the recruited 10 studies (I2=65.9%, P=0.0018). A random effects model was used, which resulted in a 23% decrement in exacerbations among patients taking macrolides treatment vs placebo group (Figure 2). Further, by adding the studies sequentially following the chronological order, the meta-analysis showed stable estimates of RR, with steadily shrunk confidence intervals due to cumulated evidence (Figure 3). Furthermore, to evaluate the impact of individual studies on the primary result, we repeated meta-analyses by knocking one study out each time, which showed a consistently significant result (Figure 4). There was no significant publication bias observed among these 10 studies (Figure 5). In addition, to evaluate whether the patients demographical or clinical characteristics affect the efficacy of treatment, meta regression was performed. There was a negative correlation between the treatment effectiveness and the age of patients ((Figure 6). Sub-group analysis by drug was also performed, and erythromycin showed a certain superiority (P<0.01; Figure 7). However, no significant benefit was observed in other agents.
Figure 2.
Forest plot, examining the effectiveness of the long-term macrolide therapy for preventing acute exacerbation among COPD patients vs placebo.
Figure 3.
Forest plot, showing the results from a cumulative meta-analysis of 10 studies examining the effectiveness of the macrolide therapy to prevent exacerbation for COPD.
Figure 4.
Forest plot, showing the results from knock-one-out meta-analysis of 10 studies.
Figure 5.
Funnel plot, showing no significant publication bias among the included trials regarding overall results.
Figure 6.
Bubble diagram, showing that there was a highly statistically significant negative correlation between the age of patients and response to macrolides (ORage=0.817).
Figure 7.
Forest plot, showing different genera of macrolides in prevention exacerbation for COPD.
Seven studies involving 2,939 patients showed that there was a 23% relative risk reduction in exacerbations among patients taking macrolide antibiotics compared to those taking placebo (RR=0.7708; 95% CI=0.6265–0.9484; P<0.01, I2=65.9%; Figure 2). A sensitivity analysis was conducted because Albert et al’s13 study accounted for a large percentage of the overall effect, showing a 27% relative risk reduction of exacerbations among patients taking macrolide antibiotics (RR=0.73, 95% CI=0.55–0.95, P=0.02, I2=61.8%).
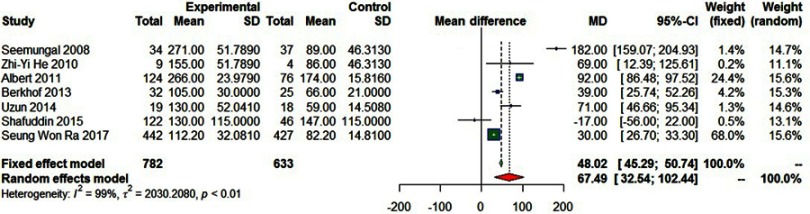
Seven studies involving 1,415 participants reported the median time to first exacerbation, and showed the prolongation of remission after prophylactic use of macrolide antibiotics (MD=67.49, 95% CI=32.54–102.44, P<0.01, I2=99%; Figure 8).
Figure 8.
Forest plot of the median time to the first exacerbation.
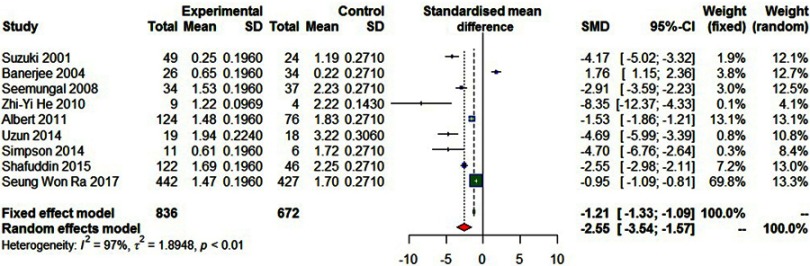
Nine studies involving 1,508 participants reported the rate of exacerbations per patient per year, showing a reduction in the rate of exacerbations in the macrolides group (SMD=−2.55, 95% CI=−3.54–−1.57, P<0.01, I2=97%; Figure 9).
Figure 9.
Forest plot of risk ratios for exacerbations per patient per year treated with macrolides compared with the control.
Six studies reported changes of SGRQ score before and after macrolide antibiotics treatment. Three studies showed an improvement in the SGRQ score after macrolides treatment, but the difference was not statistically significant. The number of hospitalizations were decreased in the macrolides group compared to the control group in four studies.12,14–16 However, there was also no difference in the two groups.
Four studies explored the pathogen isolated from the respiratory tract, indicating the first three major pathogens of COPD were H. influenzae, S. pneumoniae, Mycobacterium, and catarrhalis.12,13,15,16 Pseudomonas aeruginosa was reported in two studies.15,16 The bacteria numbers were reduced after macrolides treatment, but no difference was found. On the contrary, Uzun et al16 observed that macrolide-resistant bacteria was 6%(3/50) in the treatment group, while it was 24%(11/46) in the placebo group (P=0.036).
Nine papers studied the adverse effects, showing that the incidence of gastrointestinal reaction was 5.68% (52/916) in the treatment group and 4.90% (45/919) in the control group. The difference was not statistically significant (P>0.25). Cardiovascular events were found in five studies, the incidence was 8.27% (63/762) in the treatment group and 7.78% (59/758) in the control group, with no significant difference (P>0.5). Albert et al.14 had found another side-effect that azithromycin could cause hearing impairment, and the incidence was significantly higher than the placebo group (27% vs 21%, P=0.04). In general, the adverse effects in the macrolide antibiotics group were approximately two-times higher than in the control group.
Sub-group analysis
Sub-group analyses by the types of macrolides are shown in Figure 7. Five studies involving 1,166 participants treated with azithromycin showed no statistical difference in the numbers of excerbations between the treated and control groups (P=0.34). Three studies involving 126 participants treated with erythromycin significantly reduced the exacerbations in comparison with the control group (P<0.01). The other two studies treated with clarithromycin or roxithromycin showed no difference (P=0.09).
Several additional study variables, including age, pack-years of smoking, dose, and duration of therapy were prospectively explored further via meta regression. Through meta-regression, there was a highly statistically significant negative correlation between the age of patients and response to macrolides (ORage=0.817).
Discussion
At present, the treatment of COPD can only control and improve the current symptoms of patients, but cannot improve the long-term prognosis. Increased airway inflammation is the core of COPD, and is closely related to the severity of clinical symptoms and the frequency of AECOPD.19 Various inflammatory cells and inflammatory mediators are involved in the inflammatory reaction.20 Therefore, the key to the prevention and treatment of AECOPD is to treat and control the patient’s chronic airway inflammation reaction. There have been two important guidelines on prevention of COPD exacerbations, one is the ERS/ATS guideline, which advises that antibiotics should be used in ambulatory patients with exacerbations, the other is the 2019 Global Initiative for Obstructive Lung Disease (GOLD), which indicated that prophylactic use of azithromycin (250 mg/day or 500 mg three times per week) or erythromycin (500 mg two times per day) for 1 year can reduce the risk of exacerbations. However, there have been several unresolved problems.
According to the GOLD 2019 report, FEV1 is a very important indicator that predicts mortality and hospitalization rates in COPD. Approximately 20% of patients with GOLD stage 2 often have acute exacerbations, while patients with GOLD stage ≥3 have a higher risk of acute exacerbation and mortality. From this perspective, we believe that the use of antibiotics as prophylaxis may be more meaningful for patients with GOLD stage ≥3.
Macrolides is powerful because of its antiviral, antibacterial, and immunomodulatory effects. Macrolide antibiotics have a broad spectrum of antibacterial activity and can cover Gram-positive bacteria, penicillin-resistant Staphylococcus, some Gram-negative bacteria, anaerobic bacteria, and intracellular pathogens such as Mycoplasma, Chlamydia, Legionella, spirulina, and rickettsia. They also have excellent anti-inflammatory effects, can inhibit the adhesion, aggregation, chemotaxis, and oxidative burst of neutrophils,21 and can also promote neutrophil apoptosis, inhibit inflammatory cells (bronchial epithelial cells, macrophages, neutrophils) to release inflammatory mediators such as IL-8, IL-6, IL-12, TNF-α, and intercellular adhesion molecule (ICAM-1), and inhibit the transcription factor NF-ΚB.22 The recruitment of eosinophil can be prevented by inhibiting RANTES and eosinophil chemokines and the release of IL-5, regulating the function of Th2 and reconstructing the balance of Thl/Th2. Further, it can regulate the immune function through dendritic cells and can directly inhibit the expression of vascular cell adhesion molecule −1mRNA and neutrophil lung infiltration. Macrolide antibiotics may have other potential pharmacological effects as well, such as antiviral and restoration of glucocorticoid sensitivity. In animal experiments, macrolides have been observed to have a therapeutic effect on rhinoviruses and influenza viruses. Furthermore, macrolide antibiotics can reduce allergic inflammation or lipopolysaccharide-induced airway goblet cell hypertrophy and hyperplasia, promotes mucociliary transporting, and inhibits the inflammation and remodeling of airway and pulmonary vessels.23 Since macrolides in the airway mucosa and lung tissue can be 20–30 times the plasma concentration and have good tissue penetration, they occupy an important position in clinical anti-infective drugs.
Based on pharmacology, macrolides could be effective anti-inflammatory agents.24 Many clinical studies have confirmed the role of macrolides in the prevention and treatment of COPD.25 Of the papers we selected, one study reported that clarithromycin did not reduce COPD exacerbations, but improved symptom score in St George’s respiratory questionnaire, body function score in SF-3 questionnaire, neutrophils in sputum, and the chemotaxis of neutrophils.10 We consider this negative result was limited by the length of the study and the small number of patients enrolled. In Suzuki et al’s9 study, the main outcome was cough, not exacerbations. We still enrolled it because it was the first study that confirmed the positive effect in preventing exacerbations after the application of macrolides in a stable period, and, what is more, cough was one of the indicators of exacerbation. The COLUMBUS trial showed that, in patients experiencing acute exacerbations more than three times annually, oral administration of 500 mg azithromycin three times a week significantly reduced the frequency of the acute exacerbation of COPD.15 Our meta-analysis also confirmed that prophylactic macrolides were statistically significant in reducing the COPD exacerbations.
To data, no studies have confirmed which macrolides are most effective. Pharmacological studies showed that anti-inflammatory and immunomodulatory effects were mainly found in 14-membered ring macrolides, such as erythromycin, clarithromycin, and roxithromycin, and 15-membered ring macrolides, such as azithromycin, while the 16-membered josamycin and midecamycin had no immunomodulatory effect.26 Azithromycin is considered to be an effective option, because it has stronger antibacterial activity, better gastrointestinal tolerance, less hepatotoxicity, and will not lead to a prolonged QT interval with better safety in long-term medication.27 This may be because its metabolism will not interfere with cytochrome P450, thus avoiding metabolic interference with other medication for COPD, such as glucocorticoids and theophylline. Our sub-group analysis showed erythromycin had several advantages over other macrolides, which was consistent with He et al’s12 study. The reason was unclear. We may not have a large number of RCTs collected or the lung function was still in early stage of COPD. Which macrolide has better efficacy, and whether there is a racial difference in the effect of macrolides still needs further discussion.
There has been no detailed description about “low dose”. By carefully analyzing the literature we have included, the so-called low dose is a dose lower than the effective antibacterial concentration of macrolides, generally 40–60% of the normal dose. As reported, H. influenzae, S. pneumoniae, and Pseudomonas aeruginosa were the usual pathogens in COPD, and they can form a bacteria biofilm (BBF), and bacteria in biofilm can be released as a potential source result in recurrent infection and exacerbation. Gillis and Iglewski28 showed that azithromycin delayed initial biofilm formation of Pseudomonas aeruginosa at subMIC (2 µg/mL), but did not affect mature biofilms at subMIC (8 µg/mL). Starner et al29 observed that azithromycin at subMIC (0.125 µg/mL) could not only disrupt the formation of biofilms, but also damage the extracellular matrix of mature biofilms of H. influenzae. This may be why it is “low dose”. It is expected that more research will be carried out in the future to set a dose ranging and a cut-off value for MICs of the macrolide antibiotic to reduce the long-term oral risk-benefit ratio.
Previous meta-analyses showed that only long-term oral administration could reduce the frequency of acute exacerbations, while the short-term therapy could not benefit from it.30 According to the 2019 GOLD Report, the 12-month course had better tolerability. However, in our study, there was no difference in the effects between 3 and 12 months. Recently, a study conducted by Spanish scholar enrolled 505 patients in GOLD grading D group, the results of which showed that, after long-term continuous cycles of azithromycin treatment for more than 24 months to 36 months, the rate of exacerbations and hospitalizations continuously reduced by more than 50% with very few adverse events. The findings will recently be published in CHEST. Herath et al31 reported that continuous and intermittent (three times per week) prophylactic usage of macrolides resulted in significant reduction of exacerbations, but not pulsed treatment (eg, 5 days every 8 weeks). The best course of prophylactic antibiotic therapy remains to be determined.
Han et al32 reported that azithromycin was more effective in elderly patients and patients with low GOLD grade, but less effective in smoking patients. In our study, the average age of patients was over 65, and the result showed that the treatment effect was less effective in older patients. We consider that the elderly were more likely to have multiple underlying diseases, and the BMI index is low. They might had more comorbidities, such as pneumonia, respiratory failure, heart failure, etc. These factors could affect curative effect.
The most common side-effect of macrolide antibiotics is gastrointestinal dysfunction. Liver function damage and allergic reactions are rare. Arrhythmia and ototoxicity are even rarer. Fourteen-membered macrolide antibiotics can enhance drug interactions and cause adverse events. Erythromycin, spiramycin, and clarithromycin can cause QT interval prolongation and torsades de pointes ventricular tachycardia. As Moj et al33 reported, clarithromycin is a cytochrome P450 (CYP) 3A4 substrate, as well as a P-glycoprotein (P-gp) substrate and organic anion-transporting polypeptides (OATP) 1B1 and 1B3. Of all the macrolides, clarithromycin has the highest risk of digoxin toxicity by inhibiting the action against P-gp–mediated effluxthe, while erythromycin and azithromycin have much lower risk.34,35 Albert et al13 observed another important side-effect, which was hearing impairment.Herath et al31 also reported that development of long QTc or tinnitus were not significantly more frequent in the treatment group than the placebo. Ray et al36 found the death from cardiovascular. But it was not clear whether the macrolides were the direct cause of death or the cardiovascular disease. In our study, the adverse reaction ratio of long-term use of macrolides was not high, and the main side-effect was gastrointestinal reaction. The incidence rate of hearing impairment was higher in the treatment group than in the control group (P<0.05), and the rate of cardiovascular events did not differ significantly between the two groups.
Another non-negligible problem is antibiotic resistance in the long-term use of macrolides. Albert et al13 collected nasopharyngeal swabs for bacterial culture to detect the resistance of respiratory pathogens to macrolides. Although the respiratory colonized bacteria were significantly reduced after the treatment of azithromycin, the resistant strains isolated from the azithromycin group were significantly increased (P<0.05). But, in another study about CF, chronic azithromycin usage did not alter community resistance patterns.3 We speculate that the combination with other drugs, or different courses and doses may reduce the bacterial resistance to macrolides. CSY0073, a new type of macrocyclolactone with no antibacterial activity, but anti-inflammatory activity,37 has been clinically proven to be useful in the treatment of inflammatory bowel disease and arthritis. The application of this new drug may reduce bacterial resistance.
Conclusion
Our meta-analysis has analyzed and confirmed that long-term low dose usage of macrolides could significantly reduce the frequency of the acute exacerbation of COPD. The treatment was well tolerated with few adverse reactions, but it was not suitable for the elderly. We recommend that this treatment regimen could be used in patients with GOLD grading C or D because they have a higher risk of acute exacerbation and mortality. There is a need to further discuss whether this treatment must last for 12 months or longer. Clinically, it should combine with individual factors in COPD patients and we need to weigh the pros and cons before giving preventive use of macrolides. In the literature included, the sample size of some is small, which reduces the efficiency of statistical analysis. In order to obtain more comprehensive and objective conclusions on the efficacy of macrolide antibiotics in the treatment of stable COPD, more randomized, double-blind controlled trials with a dedicated design, reliable methods, and high quality are needed.
Acknowledgment
The authors received no specific funding for this work.
Abbreviation list
COPD, chronic obstructive pulmonary disease; AECOPD, acute exacerbations of chronic obstructive pulmonary disease; RCT, randomized controlled trial; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; CI, confidence interval; IL-8, interleukin-8; TNF-α, tumor necrosis factor-α.
Ethics approval
All analyses were based on previous published studies, thus no ethical approval or patient consent are required.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–963. doi: 10.1136/thoraxjnl-2011-201518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferkol T, Schrau fnagel D. The global burden of respiratory disease. Ann Am Thorac Soc 2014;11:404–406. doi: 10.1513/AnnalsATS-201311-405PS. [DOI] [PubMed] [Google Scholar]
- 3.Marchetti N, Criner GJ, Albert RK. Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013;143:1444–1454. doi: 10.1378/chest.12-1801 [DOI] [PubMed] [Google Scholar]
- 4.Criner GJ, Bourbeau J, Diekemper RL, et al. Executive summary: prevention of acute exacerbation of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:883–893. doi: 10.1378/chest.14-1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donath E, Chaudhry A, Hernandez-Aya LF, et al. A meta-analysis on the prophylactic use of macrolide antibiotics for the prevention of disease exacerbations in patients with chronic obstructive pulmonary disease. Respir Med. 2013;107:1385–1392. doi: 10.1016/j.rmed.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMoa0801936 [DOI] [PubMed] [Google Scholar]
- 7.Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflmmation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC [DOI] [PubMed] [Google Scholar]
- 8.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Yanai M, Yamaya M, et al. Erythromycin and common cold in COPD. Chest. 2001;120:730–733. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee D, Khair OA, Honeybourne D. The effect of oral clarithromycin on health status and sputum bacteriology in stable COPD. Respir Med. 2005;99:208–215. [DOI] [PubMed] [Google Scholar]
- 11.Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178(11):1139–1147. doi: 10.1164/rccm.200801-145OC [DOI] [PubMed] [Google Scholar]
- 12.He ZY, Ou LM, Zhang JQ, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration. 2010;80:445–452. doi: 10.1159/000321374 [DOI] [PubMed] [Google Scholar]
- 13.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–698. doi: 10.1056/NEJMoa0910383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkhof FF, Doornewaard-Ten Hertog NE, Uil SM, et al. Azithromycin and cough-specific health status in patients with chronic obstructive pulmonary disease and chronic cough: a randomised controlled trial. Respir Res. 2013;14:125. doi: 10.1186/1465-9921-14-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361–368. doi: 10.1016/S2213-2600(14)70019-0 [DOI] [PubMed] [Google Scholar]
- 16.Simpson JL, Powell H, Baines KJ, et al. The effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trial. PLoS One. 2014;9:e105609. doi: 10.1371/journal.pone.0105609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shafuddin E, Mills GD, Holmes MD, et al. A double-blind, randomised, placebocontrolled study of roxithromycin and doxycycline combination, roxithromycin alone, or matching placebo for 12 weeks in adults with frequent exacerbations of chronic obstructive pulmonary disease. J Negat Results Biomed. 2015;14:15. doi: 10.1186/s12952-015-0034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ra SW, Sze MA, Lee EC, et al. Azithromycin and risk of COPD exacerbations in patients with and without Helicobacter pylori. Respir Res. 2017;18:109. doi: 10.1186/s12931-017-0594-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimoto K, Yasuo M, Urushibata K, et al. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir. 2005;25:640–646. doi: 10.1183/09031936.05.00047504 [DOI] [PubMed] [Google Scholar]
- 20.Drost EM, Skwarski KM, Sauleda J, et al. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang P, Yang J, Yang Y, et al. Effect of azithromycin in combination with simvastatin in the treatment of chronic obstructive pulmonary disease complicated by pulmonary arterial hypertension. Pak J Med Sci. 2017;33(2):260–264. doi: 10.12669/pjms.332.11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wouters E, Groenewegen K, Dentener M, et al. Systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(8):626–634. doi: 10.1513/pats.200706-071TH [DOI] [PubMed] [Google Scholar]
- 23.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H, Shen C. Research progress of immunomodulatory effects of macrolide drugs. Sect Respirat Syst Foreign Med Sci. 2005;25(11):818–821. [Google Scholar]
- 25.Peckham DG. Macrolide antibiotics and cystic fibrosis. Thorax. 2002;57:189–190. doi: 10.1136/thorax.57.3.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin BK. Immunomodulatory properties of macrolides: overview and historical perspective. Am J Med. 2004;117:Suppl 9A, 2–4. [DOI] [PubMed] [Google Scholar]
- 27.Pomares X, Monton C, Espasa M, Casabon J, Monsó E, Gallego M. Long-term azithromycin therapy in patients with severe COPD and repeated exacerbations. Int J Chron Obstruct Pulmon Dis. 2011;6:449–456. doi: 10.2147/COPD.S23655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillis RJ, Iglewski BH. Azithromycin retards Pseudomonas K.aeruginosa biofilm formation. J Clin Microbiol. 2004;42:5842–5845. doi: 10.1128/JCM.42.12.5842-5845.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starner TD, Shrout JD, Parsek MR, et al. Subinhibitory concentrations of azithromycin decrease nontypeable haemophilus influenzae biofilm formation and diminish established biofilms. Antimicrob Agents Chemother. 2008;1(52):137–145. doi: 10.1128/AAC.00607-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni W, Shao X, Cai X, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One. 2015;10(3):1–13. doi: 10.1371/journal.pone.0121257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herath SC, Normansell R, Maisey S, et al. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2018;10:CD009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189:1503–1508. doi: 10.1164/rccm.201306-1150OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moj D, Hanke N, Britz H, et al. Clarithromycin midazolam, and digoxin: application of PBPK modeling to gain new insights into drug-drug interactions and co-medication regimens. AAPS J. 2017;19(1):298–312. doi: 10.1208/s12248-016-0009-9 [DOI] [PubMed] [Google Scholar]
- 34.Hughes J, Crowe A. Inhibition of P-glycoprotein–mediated efflux of digoxin and its metabolites by macrolide antibiotics. J Pharmacol Sci. 2010;113:315–324. [DOI] [PubMed] [Google Scholar]
- 35.Gomes CM, van Paassen H, Romeo S, et al. Multidrug resistance mediated by ABC transporters in osteosarcoma cell lines: mRNA analysis and functional radiotracer studies. Nuclear Med Biol. 2006;33:831–840. doi: 10.1016/j.nucmedbio.2006.07.011 [DOI] [PubMed] [Google Scholar]
- 36.Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu S, Xiaoning Z. Macrolides: a promising pharmacologic therapy for chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2017;11(3):147–155. doi: 10.1177/1753465816682677 [DOI] [PMC free article] [PubMed] [Google Scholar]