Abstract
African Americans remain significantly underrepresented in clinical research. Mistrust in medical researchers has been named a key barrier to the successful enrollment of minority study participants. However, trust is a social-interactional construct, and its effects on behavior are is complex. This study hypothesized that intention to participate in clinical research is mediated by trust in medical researchers, eHealth literacy, and information seeking behavior. The data were collected through an online survey (N=340) and analyzed to identify serial mediation. The model showed insignificant direct effect of race identity on behavioral intention, c`=−.19, t(335)=−1.22, p=.22, but a significant total effect, c=−.44, t(335)=−2.59, p<.01. The indirect effect of race identity on behavioral intention was also significant. The positive effect of trust in medical researchers on decisions to participate in clinical research can be amplified by stronger eHealth literacy and active information seeking, which can be supported through focused strategic health education and communication interventions. A focus on the development of information literacy could provide prospective minority research volunteers with skills for informed decision-making should be explored as an option for increasing mindful, informed participation in clinical research among currently underrepresented racial and ethnic groups.
Keywords: information seeking, participation in clinical research, serial mediation, trust in medical researchers
Introduction
Racial and ethnic diversity in the U.S. is growing, and about 40% of the U.S. population is currently comprised of ethnic and racial minorities (United States Census Bureau, 2016). The growing rate of racial and ethnic multicultural populations contributes to the variability of health conditions and care needs among them as well as possible disparities and unaddressed disease burdens, which, consequently, lead to the increasing necessity of the participation of minorities in clinical research. Greater rate of enrollment of minorities in clinical research is essential for the development and assessment of medical treatments that are tailored to be effective for these population groups. Furthermore, under-representation of minorities in clinical research has been noted previously as a serious shortcoming for the progress of and a barrier to the generalizability of clinical research findings (Lara et al., 2001; Mouton, Harris, Rovi, Solorzano, & Johnson, 1997). Although African Americans are as likely as white participants to express intentions to participate in clinical research (Wendler et al., 2005), they still remain significantly underrepresented in most studies (George, Duran, & Norris, 2014). Therefore, several national-level policy initiatives have been undertaken to address this issue (Centers for Medicare and Medicaid Services, 2006; National Institutes of Health, 2001; U.S. Food and Drug Administration, 2014).
For minority participants, study enrollment concerns have ranged from structural barriers, like the lack of insurance coverage for studies that involve a standard-of-care treatment comparison (Comis, Miller, Aldigé, Krebs, & Stoval, 2003a), to the constraints of time commitments and travel costs associated with study participation (George et al., 2014). A number of information dissemination and cognitive processing barriers can also contribute to low clinical study participation. Ineffective dissemination of information leads to a lack of awareness about available clinical studies and subsequent challenges in clinical research enrollment (Advani et al., 2003). Despite efforts to make information about clinical research available to prospective participants (Friedman et al., 2014), patient-centered recruitment messages that communicate about clinical research, its importance, and study-related specifics are still inadequate in providing sufficient knowledge (Kim, Tanner, Friedman, Foster, & Bergeron, 2015). Consequently, cognitive and psychological barriers that stem from the lack of understanding of clinical research further contribute to low participation in clinical research. These factors include the lack of knowledge about diseases and conditions, poor understanding of the process of clinical research, misunderstanding of randomization, and general distrust in medical researchers (Biedrzycki, 2010; Comis, Miller, Aldigé, Krebs, & Stoval, 2003b). Low levels of knowledge about the process of clinical research (e.g., randomization, subject protection) can impede participation (Jones et al., 2007). Specifically, as earlier research showed, if prospective participants perceive themselves to be more knowledgeable about clinical research, they are more likely to express an intention to participate in a study (Jones et al., 2007).
Another barrier to participation in clinical research is the lack of public trust in medical research in general and medical researchers in particular. Mistrust in medical researchers has been previously named a key barrier to enrollment of minority study participants (George et al., 2014; Hall et al., 2006). However, its effect is complex. The skepticism and distrust in medical research originates from the historical abuse of vulnerable study participants (Fisher & Kalbaugh, 2011; Rothman, 1982) and is fueled by news reports about the unethical behavior of some clinical researchers (Cohen, 2003).
African Americans express less support for medical institutions in general and are less likely than white participants to trust that medical researchers will explain and disclose the details of study protocols (Wendler et al., 2005). Some institutions have been successful in recruiting minority participants in Phase I studies at rates over-representing the general population (Fisher & Kalbaugh, 2011), which questions the argument of the exclusive role of mistrust in explaining the enrollment of minorities in research. Early-stage clinical trials that tests safety of a particular drug frequently carry greater health risks for volunteers, and it is unclear if such over-enrollment came at a price of uninformed decision-making about participation in clinical research. Therefore, calls for future research urged to assess the process of recruitment and provision of research information systematically as it might explain the complexity of interrelationships among trust, informed consent, and decisions to participate in research (Fisher & Kalbaugh, 2011).
Conceptually, trust could be viewed as a social construct that is reflective of the relationships among people rather than their individual psychological states (Lewis & Weigert, 1985). Furthre elevated from the assessment of individual interactions, the social conceptualization of trust suggests that it is a characteristic of a system in which symbolically represented participants act in a secure and expected manner (Simmel, 2011). It could be expected that in the context of clinical studies attitudes of prospective study participants toward research are defined by situational individual experiences (Cohn, 2015), by what medical researchers represent as a social group, and by the interpersonal communication among participants and researchers (Hamel et al., 2016). Trust also creates a foundation for cognitive familiarity with the object of trust by enhancing the efficacy to find information about the object and activating subsequent information behavior (Lewis & Weigert, 1985).
Trust as a social reality is not only the prerequisite for participation in research, but it is an essential component for the development of health literacy (Ratzan, 2001) and information seeking skills (Yang et al., 2010). While online access to health information is equal among racial groups and no longer contributes to the digital divide (Kontos, Blake, Chou, & Prestin, 2014), information seeking and eHealth literacy skills remain a challenge across all racial and ethnic groups (Norman & Skinner, 2006). Yet, the ultimate manifestation of trust on the cognitive level is reached when social actors no longer need or want any further evidence or rational reasons for their confidence in the objects of trust (Lewis & Weigert, 1985), which may lead to withdrawal from information seeking.
In addition to the possibility of lower trust in medical researchers and healthcare institutions, recent reports on the use of online information sources report that African Americans are less likely to look for information—including health information—online, yet the race-based digital divide no longer explains this difference (Kontos et al., 2014). One study showed that in the context of clinical interactions, African American patients were less likely to ask questions than white patients (Eggly et al., 2011). This evidence suggests that information behavior and active information seeking could play a role in the low participation of minorities in medical research. Together, trust, health literacy, and information seeking are necessary antecedents of an informed decision to participate in a clinical study, but their interrelations have not been examined empirically. This study hypothesized that although racial identity has an overall effect on intention to participate in clinical research, this relationship is mediated by trust in medical researchers, eHealth literacy, and information seeking behavior.
H1: Race identity has an overall effect on intention to participate in clinical research.
H2: Trust in medical researchers is predictive of (a) eHealth information efficacy, (b) information seeking, and (c) behavioral intention to participate in clinical research.
H3: The race identity-behavioral intention relationship is mediated by trust in medical researchers, eHealth information efficacy, and information seeking behavior.
Methods
The research protocol was approved by an IRB, and informed consent was obtained from those who volunteered to participate in the study.
Design
The data were collected through an online survey in June 2016. Participants responded to questions about their demographics, trust in medical researchers, and eHealth literacy and were asked to read a message about a clinical research study for healthy volunteers. Then, participants were offered to explore questions and answers related to the prospective study and clinical research in general. Finally, they were asked to report on their information seeking behavior and intention to participate in the advertised study. Mediating (trust, eHealth literacy, and information seeking) and dependent (behavioral intention) variables were measured on a 7-point scale with anchors “strongly disagree” and “strongly agree.”
Trust in medical researchers was measured using the 12-item scale developed by Hall and colleagues (Hall et al., 2006). The scale uses items that focus on trust in doctors doing medical research (e.g., I completely trust doctors who do medical research) and trust in medical research or researchers (e.g., Medical researchers have no selfish reasons for doing research studies). Scale reliability measured at alpha = .87. eHealth literacy, or the perceived ability to look for and identify helpful information on the Internet, was measured by the eHealth Literacy Scale (eHEALS) (Norman & Skinner, 2006), alpha = .92. The 8-item scale assesses consumers’ combined knowledge, confidence, and perceived skills in finding, evaluating, and applying electronic health information to health problems (Norman & Skinner, 2006) (e.g., I know what health resources are available on the Internet; I know how to use the health information I find on the Internet to help me). Participants reported their information seeking behavior using a 2-item scale that asked if they looked for information about clinical studies and relevant health topics, alpha = .89.
Behavioral intention to participate in a study was the dependent variable. A comprehensive meta-analysis looked at the measurement of behavioral intention and identified that it can be measured as desire (‘I want to perform behavior x’), self-prediction (‘I will perform behavior x’) or intention (‘I intend to perform behavior x’) (Armitage & Conner, 2001). Among these measures, intention was identified as the strongest. Additional methodological research has labeled these measures as goal intention, which is different from implementation intention (Gollwitzer & Brandstätter, 1997; Milne, Orbell, & Sheeran, 2002). The latter may include specifics in terms of how, when, and what behavioral actions might take place. Behavioral intention for the present study was measured using a 2-item scale to indicate a combination of a goal and implementation intention items: I plan to leave my contact information to be contacted about the advertised research study and I intend to participate in the advertised research study.
SPSS v.23 and the PROCESS macro model 6, which allows assessing mediation models with up to four mediators operating in series (Hayes, 2013), were used for data analyses.
Participants
Participants were invited through a nationally representative panel provided by a consumer research organization, Survey Sample International (SSI). The sample was stratified by gender (50% male and 50% female) and race (50% white and 50% African American), and a random sample of participants within each stratum was invited to participate. This sampling method allowed addressing some of the limitations noted by previous online experiments that reported lower participation of males and racial minorities (Yang et al., 2010). Participants (N = 340) were English-speaking adults over 18 years old (M = 41.57, SD = 15.28).
Materials
One of the screens in the survey that was shown to all participants included a page with recruitment information for a hypothetical study for healthy volunteers. The study focused on assessing the dosage of aspirin as a potential disease prevention drug and was based on the existing evidence that daily use of aspirin can prevent many diseases including heart disease, cancer, and rheumatoid arthritis (Hoffmeister, Chang-Claude, & Brenner, 2007; Shadick et al., 2010; Thun, Jacobs, & Patrono, 2012). Participants were asked to consider joining a study assessing the long-term effects of aspirin on health.
Results
The study sample consisted of 340 participants (170 male, 170 female; 170 white, 170 African American). The age of participants ranged from 18 to 83 (M = 41.57, SD = 15.82). Twenty-two participants (6.5%) indicated their ethnicity as “Hispanic or Latino.” Participants’ levels of education included less than high school (n = 4, 1.2%), high school/GED (n = 55, 16.2%), some college (n = 100, 29.4%), college graduate (n = 130, 38.2%), and post-graduate (n = 51, 15.0%); levels of income included less than $20,000 (n = 55, 16.2%), $20,000-$49,999 (n = 114, 33.5%), $50,000–99,999 (n = 113, 33.2%), and $100,000 or more (n = 49, 14.4%). Nine participants (2.6%) chose not to disclose their level of income. Reporting about their health status, eight (2.4%) participants self-assessed it as “poor,” 65 (19.1%) self-assessed it as “fair,” 132 (38.8%) self-assessed it as “good,” 101 (29.7%) self-assessed it as “very good,” and 34 (10.0%) self-assessed it as “excellent.”
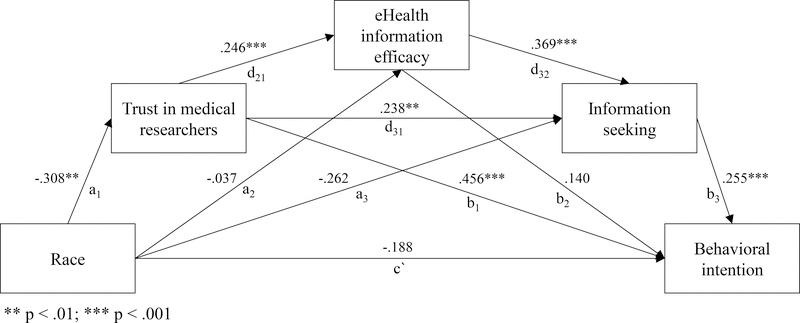
H1 proposed that race identity affects the intention to participate in clinical research. The hypothesis was supported. Total effect was significant, c = −.44, t(335) = −2.59, p < .01. However, direct effect of race identity on behavioral intention was not statistically significant, c` = −.19, t(335) = −1.22, p = .22, which suggests mediation through other constructs.
H2 stated that trust in medical researchers is predictive of (a) eHealth information efficacy, (b) information seeking, and (c) behavioral intention to participate in clinical research. H2a-c were supported. All three relationships were significant, including those between trust and eHealth information efficacy, b = .25, SE = .07, p < .01, trust and information seeking, b = .24, SE = .10, p < .01, and trust and behavioral intention to participate in clinical research b = .46, SE = .07, p < .10.
Finally, H3 proposed that race identity, trust in medical researchers, eHealth literacy, and information seeking form a serial mediation model that predicts behavioral intention to participate in clinical research. Subsequently, a model tested the relationship between race as the independent variable, trust in medical researchers, information-seeking self-efficacy, and information seeking as moderators, and behavioral intention as the dependent variable. The hypothesis was supported. The model showed four pathways that explain the relationship between race identity and behavioral intention to participate in clinical research (Figure 1). The indirect effect of race identity on behavioral intention was significant and showed a negative effect, effect = −.25, 95% bias-corrected bootstrap CI (based on 5,000 samples) [−.434, −.100]. Although all pathways resulted in the overall negative relationship between participants who self-identified as African Americans and their intention to participate in clinical research, trust in medical researchers, t(335) = 4.51, b = .45, p < .01, and information seeking behavior, t(335) = 4.35, b = .26, p<.01, minimized the negative effect and had direct positive effects on behavioral intention. Furthermore, higher eHealth literacy was identified as a positive predictor of information seeking, which further attenuated the negative relationship between race identity and behavioral intention, t(335) = 1.71, effect = −.007, 95% bias-corrected bootstrap CI (based on 5,000 samples) [−.021, −.002].
Figure 1.
Path coefficients for the serial mediation model of race and behavioral intention to participate in clinical research.
Discussion and Conclusion
Guided by the previously noted inconsistencies in the participation of African Americans in clinical studies (Fisher & Kalbaugh, 2011), this study assessed direct and mediated effects of race identity on intention to participate in clinical research. This study connected several lines of research and provided a comprehensive understanding of the factors that affect participation decisions. The model presented in this study showed that trust, eHealth literacy, and information seeking play mediating roles. Before discussing theoretical and practical implications of the present study, two limitations should be noted. First, this study used a realistic but hypothetical scenario of a clinical research study. One earlier paper discussed that when faced with a decision to participate in an actual study, only a fifth of participants who expressed an earlier intention proceeded with enrollment (Buchbinder et al., 2004). Therefore, differences in expressed intentions and actual behavior should be expected, which is likely to affect effect sizes observed in the present study. Also, patients’ health status has been long noted to influence the intentions to participate in clinical research (Bevan, Chee, McGhee, & McInnes, 1993). Therefore, the generalization of the evidence reported in this study should be limited to healthy participants and used with caution when extrapolated onto patients with diagnosed conditions.
Theoretical implications
Race identity does not directly explain intentions to participate in clinical research but does so through mediators of trust, eHealth information efficacy, and information seeking. Consistent with prior research, there was no direct effect of race on intention to join a clinical study (Fisher & Kalbaugh, 2011). However, the overall negative relationship between race and trust in medical researchers explained lower intentions to join a clinical study among African Americans compared to white participants. Previously reported differences in information behaviors among white and African American participants were observed in this study as well but only through mediation by trust in medical researchers. Also in line with previous research (Kontos et al., 2014), race identity was not shown to be a direct predictor of eHealth literacy or information seeking. However, supporting the hypothesis put forth by this study, trust was identified as a direct positive antecedent for both. Furthermore, the model presented in this study showed that race identity was indirectly associated with information seeking through trust and eHealth literacy. Conceptually, these findings signal that race should be considered as a theoretical construct (i.e., race identity) and not as just a demographic variable. Identity has been long recognized as a social (Hogg, Terry, & White, 1995) and communication (Hecht, Warren, Jung, & Krieger, 2004) construct. This study showed that mediating factors, like trust, could play a key role in explaining the relationship between race identity and behavior.
Earlier research has suggested that perceived racial discordance can have a negative effect on communication between healthcare professionals and African Americans who are invited to participate in clinical research (George et al., 2014). Trust toward medical research has been identified as one of the main—but not exclusive—constructs explaining participation in clinical research. This finding supports conceptualization of trust as a social construct (Lewis & Weigert, 1985). As this study showed, trust is predictive of prospective participants’ self-assessed ability use online health information and intentions to search for additional information, which are essential for voluntary and informed decisions about participation in clinical research. This finding adds to the original conceptualization of trust showing that it manifests behaviorally. Future research should also consider the interaction of identity and trust and the mediating role of the latter in explaining specific behaviors.
Practical implications
This study showed that the positive effect of trust in medical researchers on decisions to participate in clinical studies can be amplified by higher levels of eHealth literacy and active information seeking, both of which can be supported through focused strategic health education and communication interventions. Most current recruitment methods focus on overcoming the barriers for individual enrollment. The results reported in this study also signal that high level of trust could result in uninformed decisions, when prospective participants express intentions to participate in clinical research while neglecting information seeking. These effects could be further amplified by the focus on prospective participants’ behaviors (i.e., participation in clinical research) rather than the attention to skill development necessary to informed decision making. Therefore, an alternative approach could aim at the development of information literacy and self-efficacy skills among minority research volunteers by providing them with skills for informed decision-making. This approach should be explored as an option for increasing mindful, informed participation in clinical research among currently underrepresented racial and ethnic groups.
Conclusion
Trust remains a key factor explaining low levels of participation of African Americans in medical research. As an interactional, social experience it serves as a necessary antecedent to the development of information literacy skills and engagement with information about clinical studies. Trust can also serve as a heuristic shortcut for uninformed intentions to become a research participant. Therefore, while measures to achieve trusting relationships among medical researchers and minority communities are essential, they should be strategically planned in combination with activities that promote eHealth literacy skills and information seeking. Together, such multipronged efforts stand a better chance in countering the effects of mistrust and promoting greater participation of racial minorities in clinical studies.
Acknowledgement
Research reported in this publication was partially supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The author declares that she has no conflict of interest.
References
- Advani AS, Atkeson B, Brown CL, Peterson BL, Fish L, Johnson JL, … Gautier M (2003). Barriers to the participation of African-American patients with cancer in clinical trials: A pilot study. Cancer, 97(6), 1499–1506. 10.1002/cncr.11213 [DOI] [PubMed] [Google Scholar]
- Armitage CJ, & Conner M (2001). Efficacy of the theory of planned behaviour: A meta-analytic review. British Journal of Social Psychology, 40(4), 471–499. 10.1348/014466601164939 [DOI] [PubMed] [Google Scholar]
- Bevan E, Chee L, McGhee S, & McInnes G (1993). Patients’ attitudes to participation in clinical trials. British Journal of Clinical Pharmacology, 35(2), 204–207. 10.1111/j.1365-2125.1993.tb05687.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedrzycki B (2010). Decision making for cancer clinical trial participation: A systematic review. Oncology Nursing Forum, 37(6), E387–E399. 10.1188/10.ONF.E387-E399 [DOI] [PubMed] [Google Scholar]
- Buchbinder SP, Metch B, Holte SE, Scheer S, Coletti A, & Vittinghoff E (2004). Determinants of Enrollment in a Preventive HIV Vaccine Trial: Hypothetical Versus Actual Willingness and Barriers to Participation. JAIDS Journal of Acquired Immune Deficiency Syndromes, 36(1), 604. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. (2006). Medicare clinical trial policies. Retrieved from https://www.cms.gov/Medicare/Coverage/ClinicalTrialPolicies/index.html?redirect=/ClinicalTrialPolicies
- Cohen GI (2003). Clinical research by community oncologists. CA: A Cancer Journal for Clinicians, 53(2), 73–81. 10.3322/canjclin.53.2.73 [DOI] [PubMed] [Google Scholar]
- Cohn S (2015). “Trust my doctor, trust my pancreas”: Trust as an emergent quality of social practice. Philosophy, Ethics, and Humanities in Medicine, 10, 9 10.1186/s13010-015-0029-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comis RL, Miller JD, Aldigé CR, Krebs L, & Stoval E (2003a). Public attitudes toward participation in cancer clinical trials. Journal of Clinical Oncology, 21(5), 830–835. 10.1200/JCO.2003.02.105 [DOI] [PubMed] [Google Scholar]
- Comis RL, Miller JD, Aldigé CR, Krebs L, & Stoval E (2003b). Public Attitudes Toward Participation in Cancer Clinical Trials. Journal of Clinical Oncology, 21(5), 830–835. 10.1200/JCO.2003.02.105 [DOI] [PubMed] [Google Scholar]
- Eggly S, Harper FWK, Penner LA, Gleason MJ, Foster T, & Albrecht TL (2011). Variation in question asking during cancer clinical interactions: A potential source of disparities in access to information. Patient Education and Counseling, 82(1), 63–68. 10.1016/j.pec.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JA, & Kalbaugh CA (2011). Challenging assumptions about minority participation in US clinical research. American Journal of Public Health, 101(12), 2217–2222. 10.2105/AJPH.2011.300279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, Kim S-H, Tanner A, Bergeron CD, Foster C, & General K (2014). How are we communicating about clinical trials?: an assessment of the content and readability of recruitment resources. Contemporary Clinical Trials, 38(2), 275–283. 10.1016/j.cct.2014.05.004 [DOI] [PubMed] [Google Scholar]
- George S, Duran N, & Norris K (2014). A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. American Journal of Public Health, 104(2), e16–e31. 10.2105/AJPH.2013.301706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer PM, & Brandstätter V (1997). Implementation intentions and effective goal pursuit. Journal of Personality and Social Psychology, 73(1), 186–199. 10.1037/0022-3514.73.1.186 [DOI] [PubMed] [Google Scholar]
- Hall MA, Camacho F, Lawlor JS, DePuy V, Sugarman J, & Weinfurt K (2006). Measuring trust in medical researchers. Medical Care, 44(11), 1048–1053. 10.1097/01.mlr.0000228023.37087.cb [DOI] [PubMed] [Google Scholar]
- Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, & Eggly S (2016). Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients With Cancer., Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients With Cancer. Cancer Control : Journal of the Moffitt Cancer Center, Cancer Control : Journal of the Moffitt Cancer Center, 23, 23(4, 4), 327, 327–337. 10.1177/107327481602300404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press. [Google Scholar]
- Hecht ML, Warren JR, Jung E, & Krieger JL (2004). The communication theory of identity. In W. B. Gudykunst, Theorizing About Intercultural Communication (pp. 257–278). [Google Scholar]
- Hoffmeister M, Chang-Claude J, & Brenner H (2007). Individual and joint use of statins and low-dose aspirin and risk of colorectal cancer: A population-based case–control study. International Journal of Cancer, 121(6), 1325–1330. 10.1002/ijc.22796 [DOI] [PubMed] [Google Scholar]
- Hogg MA, Terry DJ, & White KM (1995). A tale of two theories: A critical comparison of identity theory with social identity theory. Social Psychology Quarterly, 255–269. [Google Scholar]
- Jones JM, Nyhof-Young J, Moric J, Friedman A, Wells W, & Catton P (2007). Identifying motivations and barriers to patient participation in clinical trials. Journal of Cancer Education, 21(4), 237–242. 10.1080/08858190701347838 [DOI] [PubMed] [Google Scholar]
- Kim S-H, Tanner A, Friedman DB, Foster C, & Bergeron C (2015). Barriers to clinical trial participation: Comparing perceptions and knowledge of African American and White South Carolinians. Journal of Health Communication, 20(7), 816–826. 10.1080/10810730.2015.1018599 [DOI] [PubMed] [Google Scholar]
- Kontos E, Blake KD, Chou W-YS, & Prestin A (2014). Predictors of eHealth usage: Insights on The Digital Divide from the Health Information National Trends Survey 2012. Journal of Medical Internet Research, 16(7). 10.2196/jmir.3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara PN, Higdon R, Lim N, Kwan K, Tanaka M, Lau DHM, … Lam KS (2001). Prospective Evaluation of Cancer Clinical Trial Accrual Patterns: Identifying Potential Barriers to Enrollment. Journal of Clinical Oncology, 19(6), 1728–1733. [DOI] [PubMed] [Google Scholar]
- Lewis JD, & Weigert A (1985). Trust as a Social Reality. Social Forces, 63(4), 967–985. 10.1093/sf/63.4.967 [DOI] [Google Scholar]
- Milne S, Orbell S, & Sheeran P (2002). Combining motivational and volitional interventions to promote exercise participation: Protection motivation theory and implementation intentions. British Journal of Health Psychology, 7(2), 163–184. 10.1348/135910702169420 [DOI] [PubMed] [Google Scholar]
- Mouton CP, Harris S, Rovi S, Solorzano P, & Johnson MS (1997). Barriers to black women’s participation in cancer clinical trials. Journal of the National Medical Association, 89(11), 721–727. [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. (2001). NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. Retrieved from http://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm
- Norman CD, & Skinner HA (2006). eHEALS: The eHealth Literacy Scale. Journal of Medical Internet Research, 8(4), e27 10.2196/jmir.8.4.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzan SC (2001). Health literacy: communication for the public good. Health Promotion International, 16(2), 207–214. 10.1093/heapro/16.2.207 [DOI] [PubMed] [Google Scholar]
- Rothman DJ (1982). Were Tuskegee & Willowbrook “studies in nature”? The Hastings Center Report, 12(2), 5–7. 10.2307/3561798 [DOI] [PubMed] [Google Scholar]
- Shadick NA, Karlson EW, Cook NR, Maher NE, Buring JE, & Lee I-M (2010). Low-dose aspirin in the primary prevention of rheumatoid arthritis: The women’s health study. Arthritis Care & Research, 62(4), 545–550. 10.1002/acr.20042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmel G (2011). Georg Simmel on individuality and social forms. University of Chicago Press. [Google Scholar]
- Thun MJ, Jacobs EJ, & Patrono C (2012). The role of aspirin in cancer prevention. Nature Reviews Clinical Oncology, 9(5), 259–267. 10.1038/nrclinonc.2011.199 [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. (2016). QuickFacts. Retrieved November 1, 2017, from https://www.census.gov/quickfacts/fact/table/US/PST045216
- U.S. Food and Drug Administration. (2014, June 25). Recruiting study subjects: Information sheet [WebContent]. Retrieved July 8, 2015, from http://www.fda.gov/RegulatoryInformation/Guidances/ucm126428.htm
- Wendler D, Kington R, Madans J, Wye GV, Christ-Schmidt H, Pratt LA, … Emanuel E (2005). Are racial and ethnic minorities less willing to participate in health research? PLoS Med, 3(2), e19 10.1371/journal.pmed.0030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, McComas K, Gay G, Leonard JP, Dannenberg AJ, & Dillon H (2010). From information processing to behavioral intentions: Exploring cancer patients’ motivations for clinical trial enrollment. Patient Education and Counseling, 79(2), 231–238. 10.1016/j.pec.2009.08.010 [DOI] [PubMed] [Google Scholar]