Abstract
Measurable residual disease (MRD) that persists after initial therapy is a powerful predictor of relapse and survival in acute lymphoblastic leukemia (ALL). However, the optimal use of this information to influence therapeutic decisions is controversial. Herein, we comprehensively review the role of MRD assessment in adults with ALL, including methods to quantify residual leukemia cells during remission, prognostic impact of MRD across ALL subtypes, and available therapeutic approaches to eradicate MRD. This review presents consensus statements and provides an evidence-based framework for practicing hematologists and oncologists to use MRD information to make rational treatment decisions in adult patients with ALL.
Keywords: Acute lymphoblastic leukemia, measurable residual disease, minimal residual disease, Philadelphia chromosome, prognosis, risk stratification
Introduction
The vast majority of adults with acute lymphoblastic leukemia (ALL) achieve remission with standard chemotherapy regimens, but many of these patients ultimately relapse and die from leukemia.1,2 In these patients, relapse occurs despite achievement of morphologic remission (i.e., bone marrow blasts <5%), suggesting that low levels of measurable residual disease (MRD), also called “minimal residual disease,” persist in the remission bone marrow (Figure 13). Compared with morphologic assessment alone, sensitive methods of MRD quantification can better estimate the reduction in posttreatment disease burden and provide information about the leukemia biology and treatment response of individual patients. Posttreatment MRD status is a powerful prognostic factor in all subtypes of ALL and, in many studies, supersedes historically relevant prognostic factors, including age, white blood cell count, and cytogenetics.4–7 Given the significant impact of MRD on survival outcomes in adults with ALL, many authorities suggest that MRD status can be used to inform postremission strategies, such as allogeneic hematopoietic stem cell transplantation (HSCT) in first remission. The development of novel approaches (e.g., blinatumomab, inotuzumab ozogamicin, and chimeric antigen receptor [CAR] T cells) that are highly effective in eradicating residual disease has further increased the complexity of decision-making regarding MRD.
Figure 1. Patterns of response and relapse in ALL according to quantitative clearance of leukemic burden.
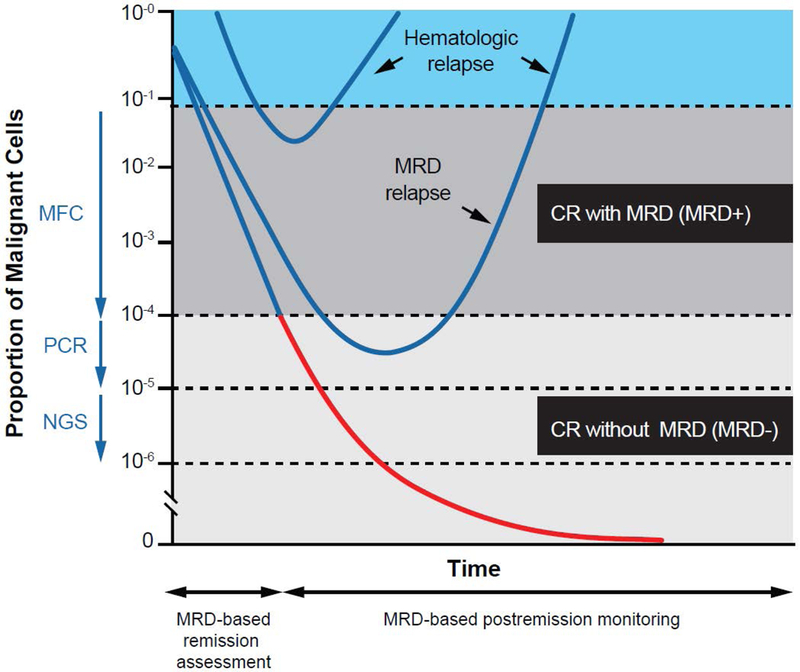
Arrows represent sensitivity of assay. CR, complete remission; MFC, multiparameter flow cytometry; NGS, next-generation sequencing; PCR, polymerase chain reaction. Republished with permission of the American Society of Hematology, from “Has MRD monitoring superseded other prognostic factors in adult ALL?” Brüggemann M, Raff T, Kneba M, 120(23), 2012; permission conveyed through Copyright Clearance Center, Inc.3
Herein we review the published data on MRD in ALL, with the goal of developing evidence-based consensus recommendations for the detection and management of MRD in adult patients with ALL. Specifically, we review various MRD detection methodologies and discuss how detection and quantification of MRD in various ALL subtypes correlate with outcomes. We also discuss evidence supporting various therapeutic options for patients with MRD-positive remission, including both HSCT and non-HSCT approaches, and provide consensus recommendations to help guide clinician decision-making in this setting.
Methods of MRD Assessment
Various methods to quantify posttreatment MRD in patients with ALL are available in clinical practice and in the research setting, including multiparameter flow cytometry (MFC), quantitative polymerase chain reaction (PCR), and next-generation sequencing (NGS). The advantages and disadvantages of these various methods of MRD assessment are summarized in Table 1. Regardless of the methodology used, for optimal sensitivity MRD assessment should generally be performed on a bone marrow specimen, as levels of detectable MRD may be 1 to 3 logs lower in the peripheral blood than in the bone marrow.8–10 This difference between peripheral blood and bone marrow MRD assessment appears to be most pronounced in patients with B-cell ALL.10
Table 1.
Methods of Measurable Residual Disease Assessment in ALL
| Method | Specimen | Sensitivity | Advantages | Disadvantages |
|---|---|---|---|---|
| Flow cytometry for “difference from normal” | Fresh viable cells | ~10−4 | • Fast • Relatively inexpensive • Potential to detect phenotypic shifts • Does not require access to pretreatment specimens |
• Confounders: increased benign B-cell precursors during marrow recovery; potential phenotypic shifts • Requires significant technical expertise • Limited standardization (though attempts in progress) |
| RQ-PCR for IGH/TCR gene rearrangements | DNA | ~10−4 to 10−5 | • Sensitive • Well standardized with consensus guidelines • Requires access to pretreatment specimens |
• Time consuming and labor intensive • Requires significant technical expertise • May not detect small subclones at diagnosis, thus may miss clonal evolution • Expensive |
| RQ-PCR for recurrent gene fusions | RNA | ~10−4 to 10−5 | • Sensitive • Uses standard primers utilized for diagnostic purposes |
• Applicable to <50% of ALL cases • Limited standardization |
| NGS | DNA | ~10−6 | • Very sensitive • Fast (uses consensus primers) • Requires access to pretreatment specimens • Potential to track small subclones and clonal evolution |
• Not standardized • Requires complex bioinformatics • Minimal clinical validation • Expensive |
RQ-PCR, real-time quantitative polymerase chain reaction; TCR, T-cell receptor.
Flow Cytometry
MFC is performed by identifying and tracking aberrant leukemia-associated immunophenotypes (LAIPs) on leukemic blasts. This can be done either by searching for the diagnostic aberrant LAIP in the remission sample (e.g., aberrant expression of myeloid antigens or increased or decreased density of antigens normally expressed on benign B-cell precursors), or by measuring any difference in immunophenotypes from the highly stereotypical normal immunophenotype distribution in the remission sample.11 In most experienced centers in the U.S., the latter “difference from normal” (DfN) approach is now preferred, in part because it does not require knowledge of the original immunophenotype. Challenges to interpretation include increased normal benign B-cell precursors during bone marrow recovery, which can potentially mask a small residual ALL population (false negative) or be misinterpreted as residual ALL (false positive). The DfN strategy is advantageous for detecting residual ALL populations even after a phenotypic shift, which may arise as a direct result of therapy, or due to a shift in clonal architecture.12 Regardless of the method used, MRD assessment with MFC requires substantial expertise on the part of the interpreting pathologist, who appears to be the primary source of inconsistent interlaboratory interpretations. Although MFC MRD measurement has been standardized in Europe13, there is currently no formal standardization of MFC MRD measurement across institutions and laboratories in the United States. Fresh, viable cells are also required for analyses. Despite these disadvantages, MFC-based MRD assessment is significantly faster, less expensive, and less labor-intensive than PCR-based methods. Standard flow-based methodologies are capable of detecting MRD with a sensitivity of approximately 1 leukemic cell per 10,000 nucleated cells. Techniques using ≥8-color flow cytometry may achieve better sensitivity (theoretically as low as 10−6), although such high levels of sensitivity require input of 2–5 × 107 nucleated cells, which is rarely obtainable from remission marrows.9,13
PCR
Quantitative PCR may be used to identify 1) clonal immunoglobulin heavy chain (IGH) gene rearrangements, 2) T-cell receptor (TCR) gene rearrangements, or 3) recurrent leukemia-associated translocations. While recombination of the V, D, and J segments of IGH and the genes encoding the TCR receptor complex are random events in normal B and T cells early in the maturation process, leukemia transformation subsequent to VDJ recombination results in identical IGH and TCR rearrangements being present in malignant lymphoblast clones in an individual patient. Notably, the PCR assay is directed at the junctional regions of these rearrangement events, which are the most diverse in sequence. Compared with North America, where MFC is more commonly used to detect MRD, PCR-based MRD assessment is used frequently in European countries, where a substantial effort by the EuroMRD consortium has been undertaken to standardize this assay.14 Furthermore, quantitative PCR is approximately 1 log more sensitive than can typically be achieved with MFC.15–17 However, quantitative PCR assays require construction of patient-tailored allele-specific oligonucleotide (ASO) primers, which is time-consuming and laborious. Additionally, because minor malignant subclones present at diagnosis may not be appreciated when developing patient-specific primers, these may be subsequently overlooked in remission samples, leading to false-negative results. Unlike flow cytometry, PCR-based MRD assessment of IGH or TCR rearrangements cannot be performed in the absence of a reference sample with high leukemic load in which leukemia-associated rearrangements can be clearly identified.
PCR can also be used to detect MRD in patients with recurrent leukemia-associated translocations, such as BCR-ABL1. Because PCR for these gene translocations is performed using the same universal primer probes used for diagnostic purposes, this method is generally simpler than using either MFC or patient-specific PCR. A sensitivity similar to patient-specific PCR assays can be reached with this approach (ie, up to 10−5), although commercially available assays may not always provide this level of sensitivity. The primary drawback is that only a minority of adults with ALL carry a recurrent gene fusion that can be used as an MRD marker of their disease. These PCR assays are not fully standardized, which complicates the interpretation of results. Even though there has been careful standardization of molecular response using BCR-ABL1 in chronic myeloid leukemia (CML)18, these molecular response milestones cannot necessarily be extrapolated to the majority of cases of Philadelphia chromosome (Ph)-positive ALL in which the p190 transcript is present. Furthermore, although most studies have evaluated the prognostic impact of BCR-ABL1-based MRD in patients with Ph-positive ALL, PCR for patient-specific IGH and TCR rearrangements may be more specific than BCR-ABL1 monitoring, as BCR-ABL1 can rarely be detected in non-ALL hematopoietic cells.19 This latter scenario may represent a “CML-like” Ph-positive ALL in which the BCR-ABL1 residual disease status does not necessarily affect prognosis.20 Despite these potential disadvantages, PCR for BCR-ABL1 is the most common method used for MRD assessment of Ph-positive ALL in U.S. academic centers and appears to be superior to MFC in predicting outcomes in this ALL subtype.21
NGS
The development of NGS as a tool to identify MRD may overcome some of the limitations of the current MFC and PCR methodologies described above. In ALL, the targets of NGS-based MRD assays have thus far been the same leukemic clone-specific IGH and TCR gene rearrangements as with PCR assays.22–26 NGS utilizes rapid, parallel sequencing using consensus primers. Therefore, NGS does not require the construction of patient-specific reagents. This feature also makes standardization and performance characterization possible. The sensitivity achieved with NGS is up to 1 to 2 logs deeper than with other currently available methods of MRD detection in the U.S., and there is evidence that NGS can identify clinically significant MRD in patients who are MRD negative by other MRD methodologies (ie, MFC or PCR).22–26 It is important to note, however, that MFC and PCR can at least theoretically achieve similar levels of sensitivity as NGS if an adequate number of cells or DNA are analyzed, although this is not done in standard practice. In addition to a high degree of sensitivity, NGS is also highly specific for residual disease and may outperform other methods of MRD, particularly PCR.27 NGS also offers the advantage of being able to track minor subclones, which may be missed with other methodologies; the importance of these subclones in driving relapse is becoming increasingly appreciated in ALL.28,29 However, despite the theoretically excellent sensitivity of NGS-based MRD assays (ie, up to 1 leukemia cell per 106 nucleated cells), large amounts of cells/DNA from the remission bone marrow are required to achieve this level of sensitivity, which may limit their utility in some scenarios. For example, NGS may not be optimal for quantification of MRD in post-treatment aplastic samples, as this may lead to overamplification of rare non-malignant rearrangements and overestimation MRD. Like with ASO PCR, pretreatment samples are also required. Notably, on September 28, 2018, the U.S. Food and Drug Administration approved the clonoSEQ NGS assay for the detection of MRD in ALL and multiple myeloma; this represents the first approval of an NGS-based test for MRD in any hematologic malignancy and is likely to lead to wider clinical adoption of this assay in the coming years.
Consensus recommendations:
MRD assessment should be performed on bone marrow specimens for optimal sensitivity (particularly for B-cell ALL). PCR for BCR-ABL1 is preferable to MFC for patients with Ph-positive ALL. Depending on access to different assays, either MFC, ASO PCR, or NGS are reasonable MRD quantification techniques for patients with Ph-negative B-cell ALL or T-cell ALL. MFC should be performed in a high-volume clinical laboratory with a pathologist experienced in MFC-based MRD interpretation. ASO PCR should only be performed in well-standardized laboratories. Regardless of the method used, a sensitivity of at least 10−4 should be reached for adequate assessment of MRD.
Prognostic and Predictive Impact of MRD in ALL
Pediatric ALL
The impact of MRD in determining outcomes in ALL was first appreciated in pediatric studies. Although a full discussion of the prognostic and therapeutic role of MRD assessment in childhood ALL is outside the scope of the present review, it is important to acknowledge the vital role that MRD assessment has played in informing prognosis and therapy allocation in this population. Achievement of MRD negativity after induction chemotherapy has been shown to be highly associated with long-term outcomes in numerous studies, including a meta-analysis of 20 pediatric studies incorporating data from over 10,000 patients.30 Given the strong impact of MRD on survival in pediatric ALL, MRD status is now systematically used by all cooperative groups worldwide to risk-stratify patients and guide treatment decisions, including treatment intensification or deintensification.31–38 For children with ALL who receive HSCT in first remission, levels of MRD before transplant are predictive for posttransplant relapse.39–41 Similarly, higher levels of MRD after HSCT have been shown to predict impending relapse, particularly when detected after day +60 posttransplantation.42 Despite the very clear prognostic role of MRD in pediatric ALL, recent studies have suggested that other genetic factors (e.g., IKZF1 deletions co-occurring with other copy number alterations, also known as “IKZF1 plus”) may influence MRD kinetics, the optimal cutoff for MRD assessment, and the predictive impact of MRD response.43,44 These findings all serve as a framework for future studies of MRD in adult ALL, including how MRD information can be used to rationally inform postremission therapies such as HSCT.
T-Cell ALL and Ph-Negative B-Cell ALL in Adults
In adults with T-cell ALL or Ph-negative B-cell ALL, the achievement of MRD negativity is predictive for long-term outcomes regardless of whether MFC- or PCR-based assays are used.4–7,45–47 When comparing MRD studies, several important considerations influence the predictive impact of MRD, including the treatment regimen used, the sensitivity of the MRD assay and timing of assessment, and the rate of HSCT in the cohort, among others. Despite differences across studies, posttreatment MRD status has consistently emerged as one of the most powerful predictors of outcomes, and is frequently identified as the only independently prognostic factor for relapse and survival.4–6 For example, in a meta-analysis of 16 studies comprising 2076 adult patients with ALL (including T-cell ALL and both Ph-negative and Ph-positive B-cell ALL), achievement of MRD negativity was associated with significant improvement in event-free survival (EFS) and overall survival (OS), with hazard ratios of 0.28 (95% Bayesian credible interval, 0.24–0.33) and 0.28 (95% Bayesian credible interval, 0.20–0.39), respectively.30 The 10-year disease-free survival rate was 64% for patients who achieved MRD negativity compared with 21% for those who were MRD positive. The studies included in this meta-analysis evaluated MRD at the end of induction or early consolidation, using either MFC or PCR. Notably, the predictive impact of MRD clearance was consistent across therapies, methods and timing of MRD assessment, level of MRD cutoff, and ALL subtypes.
Recent reports have suggested that MRD information may be combined with cytogenetics or genomic profiling in order to further improve risk-stratification in adults with ALL.7,48,49 In particular, adult patients with Ph-like ALL, MLL gene rearrangement, and early T-cell precursor ALL appear to have relatively poor outcomes regardless of MRD status (when quantified at conventional levels of 10−4).7,50–52 These disease subtypes are also more likely to have persistent MRD despite intensive therapy. Other cytogenetic abnormalities including low hypodiploidy and complex cytogenetics (ie, ≥5 chromosomal abnormalities) have also been reported to be associated with poor outcomes, independent of MRD, in some but not all studies.7,53
Ph-Positive ALL in Adults
In individual studies of chemotherapy plus a BCR-ABL1 tyrosine kinase inhibitor (TKI), achievement of a deeper molecular response (using reverse transcription [RT]-PCR of BCR-ABL1 transcripts) has been associated with superior outcomes.19,54–56 In pooled analyses from MD Anderson Cancer Center of patients with Ph-positive ALL who received intensive chemotherapy plus a TKI but did not undergo HSCT in first remission, patients who achieved deeper molecular responses had better long-term survival than those with less-deep responses.21,57 In one analysis, patients who achieved a complete molecular response (CMR; ie, the absence of a quantifiable BCR-ABL1 transcript by RT-PCR with a threshold of detection in the 10−4 to 10−5 range) after approximately 3 months of treatment had a 4-year OS rate of 66% despite not undergoing HSCT in first remission. The median OS for patients who achieved CMR by 3 months was 127 months vs 38 months for patients who did not achieve CMR (P = .009). Achievement of CMR was the only variable independently predictive for OS by multivariate analysis.57
MRD has also been suggested to predict outcomes in patients with Ph-positive ALL treated with lower-intensity regimens. In a GIMEMA study of younger patients with newly diagnosed Ph-positive ALL treated with dasatinib plus corticosteroids, patients who did not achieve CMR by day 85 were assigned to receive chemotherapy (clofarabine-cyclophosphamide) and/or allogeneic HSCT, depending on patient fitness and donor availability. Patients who achieved CMR received only TKI maintenance.58 In an interim analysis of this prospective study, the outcomes of patients who achieved CMR were superior to those who did not (30-month disease-free survival rate 75% vs 44%, respectively; P = .06), despite the CMR patients receiving less intensive therapy.
Relapsed or Refractory ALL in Adults
In adults, information about the prognostic and predictive impact of MRD in the salvage setting comes predominantly from studies of novel monoclonal antibodies such as inotuzumab ozogamicin and blinatumomab, or CAR T cells. Patients with relapsed or refractory ALL treated with blinatumomab who achieved MRD negativity using an allele-specific PCR assay (with sensitivity of at least 10−4) had longer survival than patients who remained MRD positive, with a 67% reduction in the risk of death in patients who exhibited MRD response.59,60 In patients treated with inotuzumab ozogamicin in the salvage setting, achievement of MRD negativity by MFC (assessed at the end of treatment) was associated with longer remission durations and better survival (median OS 14.1 months if MRD negative vs 7.2 months if MRD positive).61–63 In single-institution retrospective analyses, MRD status after salvage therapy has been reported to be an important predictive factor in adults with relapsed or refractory ALL.64,65 The impact of MRD negativity in the salvage setting may differ according to the number of prior lines of therapy received. In one retrospective analysis of adults with B-cell ALL in first or second salvage who received inotuzumab ozogamicin- or blinatumomab-containing regimens, MRD negativity at the time of morphologic remission was associated with superior survival only when achieved in first salvage. In contrast, patients in second salvage had poor outcomes regardless of MRD response when quantified at the level of 10-4.65
Consensus Recommendations
In adults with ALL undergoing frontline treatment, MRD from the bone marrow should be assessed at a minimum: after the end of induction, in early consolidation (approximately after 3 months of therapy), and then approximately every 3 months for at least 3 years (5 years for patients with Ph-positive ALL who do not undergo HSCT in first remission). Patients who undergo HSCT should have MRD assessment performed immediately prior to HSCT, and serial MRD measurements after HSCT (approximately every 3 months).
In patients with relapsed or refractory ALL receiving salvage therapy, MRD should be assessed at least at the time of morphologic remission and at the end of treatment, particularly for patients in first salvage in whom this information has greater predictive importance.
Therapeutic Approaches for Patients With Persistent or Recurrent MRD
The detection of MRD in patients with ALL serves not only to predict outcomes; it can also inform risk-adapted strategies. For patients with high-risk disease, the goal is to increase the cure rate by intensifying therapies, such as HSCT in first CR, intensification of chemotherapy, or the introduction of novel agents. Conversely, it is also important to identify patients at relatively low risk for relapse, as the goal is to spare these patients from the potential morbidity of these high-intensity treatment approaches. For instance, the treatment-related mortality of HSCT for adults with ALL may be as high as 30%, with rates of acute and chronic graft-vs-host disease approaching 50%.66 Utilization of MRD assessment to help avoid allogeneic HSCT in appropriate patients represents a critically important treatment decision.
Impact of MRD on Decision to Perform HSCT
T-Cell ALL and Ph-Negative B-Cell ALL
Several prospective studies have suggested that HSCT improves survival in adults with Ph-negative ALL who have persistently positive MRD after initial chemotherapy.5,6,48,67 In one study of 522 adults with Ph-negative ALL, HSCT benefited patients with poor MRD response (hazard ratio for OS 0.41; P = .005), but not those with adequate MRD response (defined as MRD level <10−3 by PCR at the end of induction).40 Similarly, the PETHEMA ALL-AR-03 trial evaluated adolescent and adult patients with Ph-negative ALL with high-risk baseline prognostic factors (e.g., age 30 to 60 years, white blood cells >30 × 106/L, or MLL gene rearrangement).6 Patients with poor day 14 bone marrow morphologic response (ie, ≥10% blasts) and/or suboptimal MRD response after induction and at the end of early consolidation (ie, MRD ≥5 × 10−4 by MFC) were assigned to undergo myeloablative HSCT (n = 71), whereas patients with favorable early morphologic and MRD response were assigned to receive chemotherapy alone (n = 108). Patients with favorable response had 5-year relapse-free survival (RFS) and OS rates of 55% and 59%, respectively, despite the presence of historically adverse pretreatment prognostic characteristics and not undergoing HSCT in first remission.
Overall, HSCT has generally been shown to improve outcomes among patients with persistently positive MRD. HSCT seems to have less relative benefit in patients who achieve an optimal MRD response. However, when comparing patients who underwent HSCT, those who had achieved MRD negativity prior to HSCT have superior outcomes compared with those who continued to have detectable MRD. The differential outcomes by pre-HSCT MRD status in adults with ALL who undergo HSCT has been shown both when MRD is measured at the end of induction48 and immediately prior to HSCT.8,25,41,68 Serial MRD assessment post-HSCT can also identify patients with impending relapse.8,25,69–71
Ph-Positive ALL
In contrast to Ph-negative ALL, there are minimal prospective data in Ph-positive ALL regarding the relationship between MRD status and the role of HSCT in first remission. In a GRAALL study of imatinib-based regimens as frontline treatment for adults with Ph-positive ALL, HSCT was associated with a significant benefit in RFS for the whole cohort.72 However, when patients were stratified according to molecular response, those who achieved a major molecular response after the second cycle of therapy did not seem to benefit from HSCT. A retrospective analysis of patients treated with intensive chemotherapy plus various TKIs who did not undergo HSCT in first remission showed that patients who achieved CMR by 3 months had a 4-year OS rate of 66%. These results were suggestive that a majority of patients with Ph-positive ALL who achieve CMR can be cured with chemotherapy and TKI alone, without need for HSCT in first remission. Similar to the data in Ph-negative ALL, pre-HSCT MRD status appears to influence post-HSCT outcomes in Ph-positive ALL.55,73 Post-HSCT MRD may also predict for increased likelihood of relapse.55
Non-HSCT Options for MRD Positivity
Historically, further cycles of cytotoxic chemotherapy or HSCT were the only treatment modalities available to salvage patients with MRD-positive disease. The development of novel monoclonal antibodies and cellular therapies has increased the options for these patients. In phase III studies of the anti-CD22 antibody-drug conjugate inotuzumab ozogamicin and the CD3-CD19 bispecific T-cell engager blinatumomab in adults with relapsed or refractory ALL, the MRD negativity rates in responders were 78% and 76%, respectively.62,74 Blinatumomab was also evaluated as an MRD-directed treatment in patients with B-cell ALL and persistent or recurrent MRD after intensive chemotherapy. In a study of 116 patients with MRD-positive ALL (65% of whom were in first remission), MRD negativity was achieved in 78% of patients after 1 cycle of blinatumomab, which translated to superior median RFS (23.6 months vs 5.7 months; P = .002) and OS (38.9 months vs 12.5 months; P = .002) compared with MRD nonresponders.75 MRD responders in first remission had superior EFS compared with those in second or later remission who responded to blinatumomab. In a post-hoc analysis, there was no difference in RFS between patients who did or did not undergo post-blinatumomab HSCT in first remission (P = .24), whereas there was a significant benefit to HSCT for patients in second or later remission who underwent post-blinatumomab HSCT (P = .02). These phase II data contributed to the approval of blinatumomab by the US Food and Drug Administration in March 2018 for the treatment of patients with B-cell ALL in first or second remission with MRD ≥0.1%. There are no randomized data comparing immediate HSCT versus blinatumomab followed by HSCT for patients with persistent or recurrent MRD, and therefore treatment decisions for this scenario must be extrapolated from and guided by phase II data.
CAR T cells have also been evaluated in the treatment of MRD-positive disease. In a study of 19–28z CAR T cells in patients with relapsed B-cell ALL, 21 patients in morphologic remission were treated (15 with MRD-positive disease and 6 who were MRD negative).76 The median EFS and OS of these 21 patients with low-burden disease (ie, bone marrow blasts <5%) were 10.6 months and 20.1 months, respectively. These patients with low-burden disease had significantly lower rates of severe cytokine release syndrome and neurotoxic effects compared with patients not in morphologic remission at the time of CAR T-cell infusion.
Consensus recommendations:
A recommended MRD-guided treatment algorithm for fit adult patients is shown in Figure 2. For patients with Ph-negative B-cell ALL or T-cell ALL who achieve MRD negativity within 3 months from the start of treatment (early consolidation), HSCT is recommended only in those with high-risk cytogenetic or genetic features (e.g., Ph-like ALL, MLL gene rearrangement, or early T-cell precursor ALL). Given the poor outcomes of patients who undergo HSCT with detectable MRD, patients who remain MRD positive after initial therapy should be considered for MRD-directed therapies prior to HSCT. Blinatumomab for 2–4 cycles is recommended for patients with B-cell ALL and any level of detectable MRD prior to HSCT. Patients who achieve MRD negativity should undergo HSCT, whereas those who remain MRD positive should be considered for clinical trial (if available) or proceed to HSCT.
Figure 2. Consensus algorithm for MRD-based management of adults with ALL.
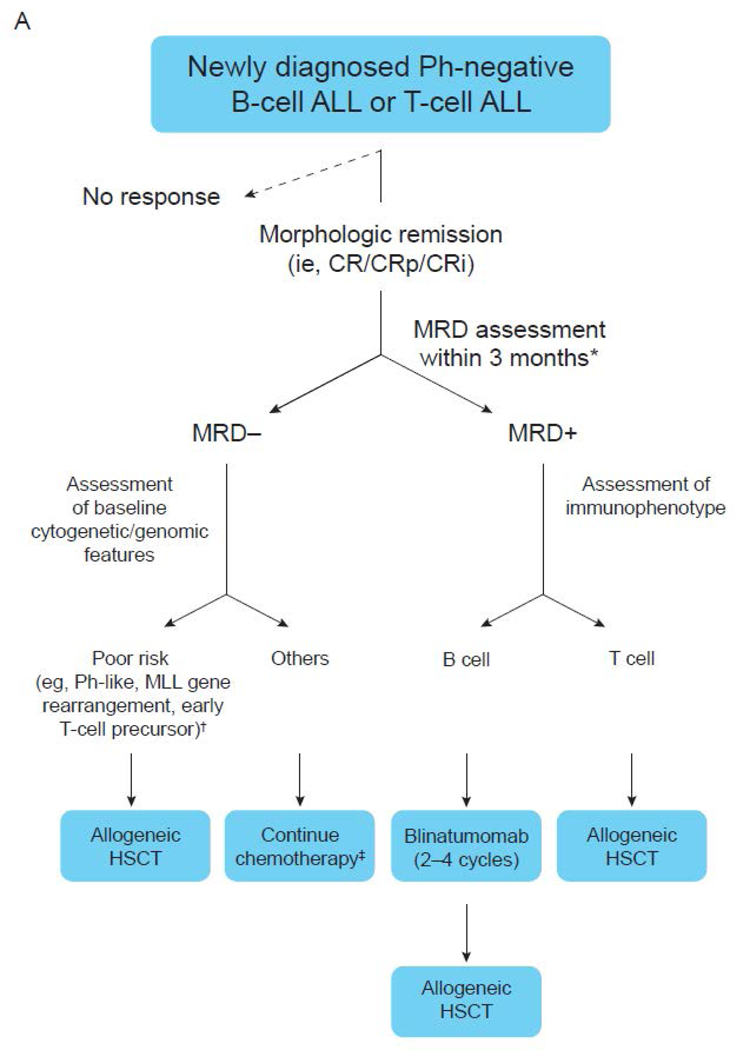
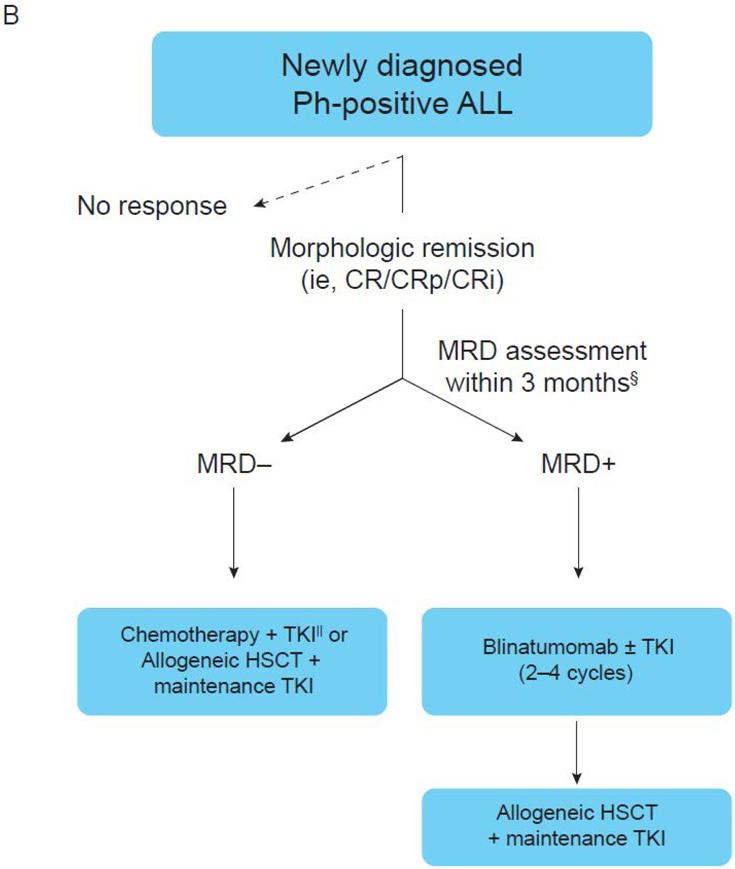
(A) Newly diagnosed Ph-negative B-cell ALL or T-cell ALL. (B) Newly diagnosed Ph-positive ALL. *MRD should be assessed on a bone marrow specimen using an assay with a sensitivity of at least 10-4. MRD negativity should be achieved within 3 months from the start of therapy. †Allogeneic HSCT may also be considered in patients with low hypodiploidy or complex karyotype. ‡In patients who do not undergo HSCT, MRD should be assessed approximately every 3 months for at least 3 years. MRD should be assessed immediately prior to HSCT in all patients undergoing transplant. §MRD should be assessed with PCR for BCR-ABL on a bone marrow specimen. MRD negativity should be achieved within 3 months from the start of therapy. ||In patients who do not undergo HSCT, MRD should be assessed approximately every 3 months for at least 5 years. MRD should also be assessed immediately prior to HSCT in all patients undergoing transplant. CRi, CR with incomplete blood count recovery; CRp, CR with incomplete platelet recovery; MLL, mixed lineage leukemia.
Patients with Ph-positive ALL who achieve CMR within 3 months of therapy may continue to be treated with chemotherapy plus a BCR-ABL1 TKI or undergo HSCT followed by maintenance TKI. Patients who remain MRD positive after 3 months of therapy should undergo HSCT followed by maintenance TKI. Blinatumomab for 2–4 cycles, with or without concomitant TKI, should be given prior to HSCT, in an attempt to convert to CMR prior to HSCT.
Future Directions and Areas of Research
While the prognostic impact of MRD assessment is clear across ALL subtypes, many important questions remain about the optimal method of MRD assessment and how this information should be incorporated into risk-adapted therapies. In particular, the deep sensitivity achievable with NGS holds significant promise in the improvement of risk-assessment and treatment determination of ALL, although large prospective studies of this technology are needed. Studies are also needed to evaluate the utility of peripheral blood MRD monitoring using these more sensitive NGS-based techniques, including the potential application of circulating cell-free DNA. The complex interaction between molecular and genomic changes in ALL and the prognostic impact of MRD should continue to be explored. As our knowledge of the genomic landscape of ALL evolves, it is likely that optimal assessment of relapse risk, and consequently postremission therapies, will incorporate both pretreatment genomic alterations and posttreatment MRD status. Finally, prospective MRD-guided studies are needed in order to optimize ALL therapy. The development of novel strategies (e.g., monoclonal antibodies alone or in combinations with other antibodies or with chemotherapy; CAR T cells; Bcl-2 inhibitors) is needed to eradicate MRD that persists after initial therapy. Prospective studies should utilize the highest-sensitivity assays available (e.g., NGS) and should assess whether such eradication of MRD can obviate the need for HSCT in this setting.
Acknowledgments
The study was funded by the PLATO Foundation.
Editorial support was provided by Mary L. Smith, PhD, CMPP, from TRM Oncology, Atlanta, GA, and funded by the Plato Foundation.
Footnotes
Disclaimers
Conflict of interest disclosures
N. Short: Consultant for Takeda.
E. Jabbour: Research grants from Amgen, Pfizer, Adaptive Biotechnologies, AbbVie, Takeda, BMS, Novartis.
M. Albitar: Employed by a diagnostic company that offers testing for MRD.
M. de Lima: Nothing to disclose.
L. Gore: Consulting/Medical advisory board service for Amgen, Celgene, Novartis, Roche/Genentech. Travel expenses paid by Amgen, BMS, Novartis, Roche/Genentech (for advisory board service noted above), US Food and Drug Administration. Stock in Amgen, Celgene, Clovis, Sanofi Paris. Research funding from NCI, Alex’s Lemonade Stand Foundation, Children’s Hospital Colorado Foundation, Hyundai Hope on Wheels Foundation, St. Baldrick’s Foundation.
J. Jorgensen: Nothing to disclose.
A.C. Logan: Research funding from Astellas, Novartis, Pharmacyclics, Kite, Jazz. Consulting fees from Amgen, Incyte, Jazz, Pfizer, Shire.
J. Park: Consulting fees from Amgen, Shire, Novartis, Adaptive Biotechnologies, Kite Pharma.
F. Ravandi: Nothing to disclose.
B. Shah: Advisory board for Adaptive Biotechnologies. Honoraria, Pfizer. Speaker board, Amgen. Research grant from Incyte. Foundation support from Chotiner Research Foundation, Lymphoma Research Foundation, and NIH. CME advisory board from NCCN.
J. Radich: Scientific advisory board from Adaptive Biotechnologies. Laboratory research contracts with Novartis.
H. Kantarjian: Research grants from Amgen, Ariad, Astex, BMS, Novartis, Pfizer. Honoraria from AbbVie, Amgen, Ariad, BMS, ImmunoGen, Orsenix, Pfizer.
References
- 1.Sive JI, Buck G, Fielding A, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. British journal of haematology 2012; 157(4): 463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood 2014; 123(6): 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruggemann M, Raff T, Kneba M. Has MRD monitoring superseded other prognostic factors in adult ALL? Blood 2012; 120(23): 4470–81. [DOI] [PubMed] [Google Scholar]
- 4.Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood 2006; 107(3): 1116–23. [DOI] [PubMed] [Google Scholar]
- 5.Gokbuget N, Kneba M, Raff T, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood 2012; 120(9): 1868–76. [DOI] [PubMed] [Google Scholar]
- 6.Ribera JM, Oriol A, Morgades M, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014; 32(15): 1595–604. [DOI] [PubMed] [Google Scholar]
- 7.Beldjord K, Chevret S, Asnafi V, et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood 2014; 123(24): 3739–49. [DOI] [PubMed] [Google Scholar]
- 8.Logan AC, Vashi N, Faham M, et al. Immunoglobulin and T cell receptor gene high-throughput sequencing quantifies minimal residual disease in acute lymphoblastic leukemia and predicts post-transplantation relapse and survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2014; 20(9): 1307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dongen JJ, van der Velden VH, Bruggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood 2015; 125(26): 3996–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Velden VH, Jacobs DC, Wijkhuijs AJ, et al. Minimal residual disease levels in bone marrow and peripheral blood are comparable in children with T cell acute lymphoblastic leukemia (ALL), but not in precursor-B-ALL. Leukemia 2002; 16(8): 1432–6. [DOI] [PubMed] [Google Scholar]
- 11.Wood BL. Principles of minimal residual disease detection for hematopoietic neoplasms by flow cytometry. Cytometry Part B, Clinical cytometry 2016; 90(1): 47–53. [DOI] [PubMed] [Google Scholar]
- 12.Lee D, Grigoriadis G, Westerman D. The role of multiparametric flow cytometry in the detection of minimal residual disease in acute leukaemia. Pathology 2015; 47(7): 609–21. [DOI] [PubMed] [Google Scholar]
- 13.Theunissen P, Mejstrikova E, Sedek L, et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood 2017; 129(3): 347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Velden VH, Cazzaniga G, Schrauder A, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia 2007; 21(4): 604–11. [DOI] [PubMed] [Google Scholar]
- 15.Ryan J, Quinn F, Meunier A, et al. Minimal residual disease detection in childhood acute lymphoblastic leukaemia patients at multiple time-points reveals high levels of concordance between molecular and immunophenotypic approaches. British journal of haematology 2009; 144(1): 107–15. [DOI] [PubMed] [Google Scholar]
- 16.Thorn I, Forestier E, Botling J, et al. Minimal residual disease assessment in childhood acute lymphoblastic leukaemia: a Swedish multi-centre study comparing real-time polymerase chain reaction and multicolour flow cytometry. British journal of haematology 2011; 152(6): 743–53. [DOI] [PubMed] [Google Scholar]
- 17.Gaipa G, Cazzaniga G, Valsecchi MG, et al. Time point-dependent concordance of flow cytometry and real-time quantitative polymerase chain reaction for minimal residual disease detection in childhood acute lymphoblastic leukemia. Haematologica 2012; 97(10): 1582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013; 122(6): 872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cazzaniga G, De Lorenzo P, Alten J, et al. Predictive value of minimal residual disease in Philadelphia-chromosome-positive acute lymphoblastic leukemia treated with imatinib in the European intergroup study of post-induction treatment of Philadelphia-chromosome-positive acute lymphoblastic leukemia, based on immunoglobulin/T-cell receptor and BCR/ABL1 methodologies. Haematologica 2018; 103(1): 107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovorkova L, Zaliova M, Venn NC, et al. Monitoring of childhood ALL using BCR-ABL1 genomic breakpoints identifies a subgroup with CML-like biology. Blood 2017; 129(20): 2771–81. [DOI] [PubMed] [Google Scholar]
- 21.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood 2013; 122(7): 1214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladetto M, Bruggemann M, Monitillo L, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia 2014; 28(6): 1299–307. [DOI] [PubMed] [Google Scholar]
- 23.Faham M, Zheng J, Moorhead M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2012; 120(26): 5173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sala Torra O, Othus M, Williamson DW, et al. Next-Generation Sequencing in Adult B Cell Acute Lymphoblastic Leukemia Patients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2017; 23(4): 691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulsipher MA, Carlson C, Langholz B, et al. IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood 2015; 125(22): 3501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood B, Wu D, Crossley B, et al. Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood 2018; 131(12): 1350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotrova M, van der Velden VHJ, van Dongen JJM, et al. Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone marrow transplantation 2017; 52(7): 962–8. [DOI] [PubMed] [Google Scholar]
- 28.Ma X, Edmonson M, Yergeau D, et al. Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia. 2015; 6: 6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gawad C, Pepin F, Carlton VE, et al. Massive evolution of the immunoglobulin heavy chain locus in children with B precursor acute lymphoblastic leukemia. Blood 2012; 120(22): 4407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry DA, Zhou S, Higley H, et al. Association of Minimal Residual Disease With Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia: A Meta-analysis. JAMA oncology 2017; 3(7): e170580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pieters R, de Groot-Kruseman H, Van der Velden V, et al. Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016; 34(22): 2591–601. [DOI] [PubMed] [Google Scholar]
- 32.Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. The Lancet Oncology 2013; 14(3): 199–209. [DOI] [PubMed] [Google Scholar]
- 33.Basso G, Veltroni M, Valsecchi MG, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009; 27(31): 5168–74. [DOI] [PubMed] [Google Scholar]
- 34.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 2010; 115(16): 3206–14. [DOI] [PubMed] [Google Scholar]
- 35.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood 2015; 126(8): 964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. The Lancet Oncology 2014; 15(8): 809–18. [DOI] [PubMed] [Google Scholar]
- 37.Pui CH, Pei D, Coustan-Smith E, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. The Lancet Oncology 2015; 16(4): 465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pui CH, Pei D, Raimondi SC, et al. Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with Response-Adapted therapy. Leukemia 2017; 31(2): 333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bader P, Kreyenberg H, Henze GH, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009; 27(3): 377–84. [DOI] [PubMed] [Google Scholar]
- 40.Elorza I, Palacio C, Dapena JL, Gallur L, Sanchez de Toledo J, Diaz de Heredia C. Relationship between minimal residual disease measured by multiparametric flow cytometry prior to allogeneic hematopoietic stem cell transplantation and outcome in children with acute lymphoblastic leukemia. Haematologica 2010; 95(6): 936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bachanova V, Burke MJ, Yohe S, et al. Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: effect of minimal residual disease on relapse and survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2012; 18(6): 963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bader P, Kreyenberg H, von Stackelberg A, et al. Monitoring of minimal residual disease after allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015; 33(11): 1275–84. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor D, Enshaei A, Bartram J, et al. Genotype-Specific Minimal Residual Disease Interpretation Improves Stratification in Pediatric Acute Lymphoblastic Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018; 36(1): 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanulla M, Dagdan E, Zaliova M, et al. IKZF1(plus) Defines a New Minimal Residual Disease-Dependent Very-Poor Prognostic Profile in Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018; 36(12): 1240–9. [DOI] [PubMed] [Google Scholar]
- 45.Ravandi F, Jorgensen JL, O’Brien SM, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. British journal of haematology 2016; 172(3): 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel B, Rai L, Buck G, et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. British journal of haematology 2010; 148(1): 80–9. [DOI] [PubMed] [Google Scholar]
- 47.Holowiecki J, Krawczyk-Kulis M, Giebel S, et al. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The Polish Adult Leukemia Group ALL 4–2002 MRD Study. British journal of haematology 2008; 142(2): 227–37. [DOI] [PubMed] [Google Scholar]
- 48.Dhedin N, Huynh A, Maury S, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood 2015; 125(16): 2486–96; quiz 586. [DOI] [PubMed] [Google Scholar]
- 49.Gupta SK, Bakhshi S, Chopra A, Kamal VK. Molecular genetic profile in BCR-ABL1 negative pediatric B-cell acute lymphoblastic leukemia can further refine outcome prediction in addition to that by end-induction minimal residual disease detection. Leukemia & lymphoma 2017: 1–6. [DOI] [PubMed] [Google Scholar]
- 50.Jain N, Roberts KG, Jabbour E, et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood 2017; 129(5): 572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain N, Lamb AV, O’Brien S, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood 2016; 127(15): 1863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lafage-Pochitaloff M, Baranger L, Hunault M, et al. Impact of cytogenetic abnormalities in adults with Ph-negative B-cell precursor acute lymphoblastic leukemia. Blood 2017; 130(16): 1832–44. [DOI] [PubMed] [Google Scholar]
- 53.Issa GC, Kantarjian HM, Yin CC, et al. Prognostic impact of pretreatment cytogenetics in adult Philadelphia chromosome-negative acute lymphoblastic leukemia in the era of minimal residual disease. Cancer 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S, Kim DW, Cho BS, et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia 2012; 26(11): 2367–74. [DOI] [PubMed] [Google Scholar]
- 55.Kim DY, Joo YD, Lim SN, et al. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood 2015; 126(6): 746–56. [DOI] [PubMed] [Google Scholar]
- 56.Yoon JH, Yhim HY, Kwak JY, et al. Minimal residual disease-based effect and long-term outcome of first-line dasatinib combined with chemotherapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2016. [DOI] [PubMed] [Google Scholar]
- 57.Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2016; 128(4): 504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiaretti S, Vitale A, Elia L, et al. Multicenter Total Therapy Gimema LAL 1509 Protocol for De Novo Adult Ph+ Acute Lymphoblastic Leukemia (ALL) Patients. Updated Results and Refined Genetic-Based Prognostic Stratification. Blood 2015; 126(23): abstract 81. [Google Scholar]
- 59.Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. The Lancet Oncology 2015; 16(1): 57–66. [DOI] [PubMed] [Google Scholar]
- 60.Zugmaier G, Gokbuget N, Klinger M, et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood 2015; 126(24): 2578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kantarjian H, Thomas D, Jorgensen J, et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer 2013; 119(15): 2728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. The New England journal of medicine 2016; 375(8): 740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jabbour E, Gokbuget N, Advani A, et al. Impact of minimal residual disease (MRD) status in clinical outcomes of patients with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL) treated with inotuzumab ozogamicin (InO) in the phase 3 INO-VATE trial. American Society of Clinical Oncology; 2018: J Clin Oncol; 2018. [Google Scholar]
- 64.Saygin C, Papadantonakis N, Cassaday RD, et al. Prognostic impact of incomplete hematologic count recovery and minimal residual disease on outcome in adult acute lymphoblastic leukemia at the time of second complete response. Leukemia & lymphoma 2018; 59(2): 363–71. [DOI] [PubMed] [Google Scholar]
- 65.Jabbour E, Short NJ, Jorgensen JL, et al. Differential impact of minimal residual disease negativity according to the salvage status in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Cancer 2017; 123(2): 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Speziali C, Paulson K, Seftel M. Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia in Adults. Current hematologic malignancy reports 2016; 11(3): 175–84. [DOI] [PubMed] [Google Scholar]
- 67.Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood 2009; 113(18): 4153–62. [DOI] [PubMed] [Google Scholar]
- 68.Sanchez-Garcia J, Serrano J, Serrano-Lopez J, et al. Quantification of minimal residual disease levels by flow cytometry at time of transplant predicts outcome after myeloablative allogeneic transplantation in ALL. Bone marrow transplantation 2013; 48(3): 396–402. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez J, Serrano J, Gomez P, et al. Clinical value of immunological monitoring of minimal residual disease in acute lymphoblastic leukaemia after allogeneic transplantation. British journal of haematology 2002; 116(3): 686–94. [DOI] [PubMed] [Google Scholar]
- 70.Spinelli O, Peruta B, Tosi M, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica 2007; 92(5): 612–8. [DOI] [PubMed] [Google Scholar]
- 71.Zhao XS, Liu YR, Zhu HH, et al. Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Annals of hematology 2012; 91(2): 183–92. [DOI] [PubMed] [Google Scholar]
- 72.Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood 2015; 125(24): 3711–9. [DOI] [PubMed] [Google Scholar]
- 73.Cai WZ, Cen JN, Chen J, et al. Major molecular response prior to allogeneic hematopoietic stem cell transplantation predicts better outcome in adult Philadelphia-positive acute lymphoblastic leukemia in first remission. Bone marrow transplantation 2017; 52(3): 470–2. [DOI] [PubMed] [Google Scholar]
- 74.Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. The New England journal of medicine 2017; 376(9): 836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018; 131(14): 1522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park JH, Riviere I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. The New England journal of medicine 2018; 378(5): 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]


