Abstract
The functional diversity and molecular adaptations of reactive microglia in the chronically inflamed central nervous system (CNS) are poorly understood. We previously showed that mice lacking multifunctional protein 2 (MFP2), a pivotal enzyme in peroxisomal β-oxidation, persistently accumulate reactive myeloid cells in the gray matter of the CNS. Here we show that the increased numbers of myeloid cells solely derive from proliferation of resident microglia and not from infiltrating monocytes. We defined the signature of Mfp2−/− microglia by gene expression profiling after acute isolation, which was validated by quantitative PCR, immunohistochemical and flow cytometric analysis. The features of Mfp2−/− microglia were compared with those from SOD1G93A mice, an amyotrophic lateral sclerosis model. In contrast to the neurodegenerative milieu of SOD1G93A spinal cord, neurons were intact in Mfp2−/− brain and Mfp2−/− microglia lacked signs of phagocytic and neurotoxic activity. The chronically reactive state of Mfp2−/− microglia was accompanied by downregulation of markers that specify the unique microglial signature in homeostatic conditions. In contrast, mammalian target of rapamycin (mTOR) and downstream glycolytic and protein translation pathways were induced, indicative of metabolic adaptations. Mfp2−/− microglia were immunologically activated but not polarized to a pro- or anti-inflammatory phenotype. A peripheral lipopolysaccharide challenge provoked an exaggerated inflammatory response in Mfp2−/− brain, consistent with a primed state. Taken together, we demonstrate that chronic activation of resident microglia does not necessarily lead to phagocytosis nor overt neurotoxicity.
Keywords: Neuroinflammation, microglia, peroxisomes, phagocytosis
Introduction
Microglia, the resident immune cells of the CNS, are actively engaged in both homeostasis and pathology in the adult brain. Under physiological conditions, microglia constantly survey the microenvironment and communicate in a bidirectional way with neural cells (Ransohoff and Perry, 2009, Hughes, 2012, Gomez-Nicola et al., 2013, Prinz and Priller, 2014). It has become clear that microglia play a critical role in neuronal plasticity by controlling synaptic function and synaptogenesis (Tremblay et al., 2010, Salter and Beggs, 2014). Recently, important progress was made in the molecular characterization of homeostatic microglia, by differentiating their transcriptome signature from that of neural cells and other myeloid cells (Butovsky et al., 2012, Gautier et al., 2012, Chiu et al., 2013, Hickman et al., 2013, Butovsky et al., 2014).
In addition to their role in CNS homeostasis, microglia sense CNS damage and can act as versatile effector cells during neuropathological conditions. To this end, microglia adopt a range of activation states which remain ill-defined at the molecular level. In particular, the diverse phenotypes of microglia in conditions of chronic stress in the brain are poorly understood (Gomez-Nicola et al., 2013, Cherry et al., 2014). The morphological transition from ramified to amoeboid is accompanied by new effector functions that can vary widely and encompass proliferation, migration, production of cytokines and chemokines, and phagocytic activity. Whether chronically activated microglia always attain neurotoxic properties is still a matter of debate (Biber et al., 2014). An undervalued aspect in chronic neuroinflammation is that concomitant with acquiring novel shapes and functions, microglia might also lose features related to their homeostatic role. To better understand the spectrum of phenotypes of reactive microglia, information on gene expression and insights in signaling pathways are necessary. Recently, the microglia transcriptome of SOD1G93A mice, an amyotrophic lateral sclerosis (ALS) model was defined (Butovsky et al., 2012, Chiu et al., 2013). These microglia concurrently upregulate both neurotoxic and neuroprotective factors as well as genes associated with lysosomal activity and phagocytosis (Chiu et al., 2013). Their molecular profile was different from that of lipopolysaccharide (LPS)-activated microglia and of M1 or M2 macrophages, and was denoted as a neurodegeneration-specific signature (Chiu et al., 2013).
Chronic microglia activation is an important hallmark of many neurodegenerative diseases and contributes significantly to the progression of these disorders (Glass et al., 2010). Moreover, microglial dysfunction might underlie some neurological disorders, such as Rett’s syndrome (Derecki et al., 2013) and Nasu-Hakola disease (Satoh et al., 2011). Also in X-linked adrenoleukodystrophy, a neurometabolic disorder caused by peroxisomal β-oxidation deficiency (Kemp et al., 2012), microglia seem to play a pivotal role in disease pathogenesis. This is inferred from the therapeutic efficacy of bone marrow transplantation or hematopoietic stem cell gene therapy to halt the inflammatory demyelination seen in the cerebral childhood form of the disease (ccALD) (Cartier et al., 2009, Cartier and Aubourg, 2010). Due to the absence of neuroinflammation in the corresponding mouse model (Abcd1−/−), the precise molecular abnormalities leading to cerebral inflammation and/or microglial dysfunction cannot be investigated (Kemp et al., 2012). Interestingly, we recently showed that mice lacking multifunctional protein-2 (MFP2; also known as D-bifunctional protein), a peroxisomal β-oxidation enzyme downstream of the ABCD1 transporter, develop robust neuroinflammation (Huyghe et al., 2006c, Verheijden et al., 2013). This initiates before the age of 8 weeks and is confined to the gray matter throughout the brain and spinal cord. The expanding inflammation parallels the aggravating neurological phenotype of the mice, characterized by motor and cognitive impairments, lethargy and death before the age of 6 months ((Huyghe et al., 2006c) and unpublished observations). With the exception of testicular degeneration, no peripheral organ failure was observed (Jia et al., 2003, Huyghe et al., 2006a, Huyghe et al., 2006b). Interestingly, mice with selective elimination of MFP2 from neural cells develop minor neuroinflammation and have an extended lifespan compared to general knockouts (Verheijden et al., 2013). This suggests that loss of peroxisomal β-oxidation from non-neural cells (e.g. microglia and/or infiltrating monocytes) worsens the inflammatory state of the brain.
For unknown reasons, the phenotype of Mfp2−/− mice deviates from the severe neurodevelopmental pathology of patients with total ablation of MFP2 (Ferdinandusse et al., 2006b). Milder mutations give rise to degenerative neurological anomalies including ataxia, leukodystrophy and vision/hearing problems (Ferdinandusse et al., 2006a, Khan et al., 2010, van der Knaap et al., 2012) but it has not been assessed whether neuroinflammation develops. The precise metabolic role of MFP2 in the formation and maintenance of the CNS remains unresolved. Apart from increased levels of very long chain fatty acids, no other metabolic anomalies have been identified (Huyghe et al., 2006a).
In this study, we used Mfp2−/− mice as a unique tool to study the cellular and molecular aspects of neuroinflammation in peroxisomal β-oxidation deficiency. Specifically, we aimed to molecularly define the activation state of microglia and to uncover the cellular mechanisms driving the neuroinflammatory response. Our findings show that Mfp2−/− microglia adopt a strongly proliferative and immunologically activated phenotype that is neuron-sparing. As this contrasts with microglia in the neurodegenerative milieu of the SOD1G93A spinal cord (Chiu et al., 2013), we directly compared gene expression and histological features of microglia in both mouse models.
Materials and Methods
Mouse breeding
The generation of Mfp2−/− mice has been described (Baes et al., 2000). Mfp2−/− mice were bred on a Swiss/Webster background in the specific pathogen free animal housing facility of the KU Leuven, had ad libitum access to water and standard rodent food, and were kept on a 12-hour light and dark cycle. As we did not detect differences between wild type and heterozygous mice in our previous investigations, they were both used as controls. Unless stated otherwise, all experiments were performed on 17–20 week old mice with mixed gender and were in accordance with the “Guidelines for Care and Use of Experimental Animals” and fully approved by the Research Ethical committee of the KU Leuven (#190/2012).
Microglia isolation by FACS sorting
Microglia were acutely isolated from Mfp2−/− and control mice (n=4 for each genotype). After transcardiac perfusion with ice-cold PBS, brains were quickly dissected and mechanically homogenized with a tissue homogenizer in ice-cold HBSS containing 15mM HEPES and 0.5 % glucose (De Haas et al., 2007). Cells were filtered over a 70 μm strainer and pelleted at 220 g for 10 min at 4 °C. Contaminating myelin was removed by resuspending the pellet in 25 ml ice-cold 22% Percoll buffer, overlaying with 5 mL ice-cold PBS followed by centrifugation in a swinging bucket rotor at 950 g for 25 min at 4 °C (modified from (Olah et al., 2012)). The cells were incubated with mouse anti-CD45-FITC (eBiosciences 11–0451; 1:40) and mouse anti-CD11b-APC (eBiosciences 17–0112; 1:100) for 30 minutes at 4 °C in the dark. CD11bhigh/CD45mid cells were isolated using an Aria I fluorescence-activated cell sorter (FACS) (BD Biosciences) equipped with a 488 nm and 633 nm laser. Cells with low forward/sideward scatter profile were excluded from the isolation. Microglia yield ranged between 1 × 105 to 3 × 105 cells/mouse brain. Cells were collected in RNA lysis buffer provided with the RNeasy microkit (Qiagen, 74004). Data from FACS sorting of CD11bhigh/CD45mid myeloid cells were analyzed using BD FACsuite software (BD Biosciences).
Microarray
Total RNA was isolated from FACS sorted microglia by using the RNeasy Micro kit (Qiagen). The quality of the RNA samples was determined with an Agilent 2100 Bioanalyzer (Agilent Technologies, California, USA). Only samples with RIN values (RNA Integrity Number) higher or equal to 8 were used. The RNA was amplified with the NuGEN amplification kit (Agilent technologies). For microarray analysis, the whole genome GeneChip Mouse Gene 1.0 ST Array was used as described previously. Quality control, amplification, labeling of the samples, hybridization, washing and scanning of the chips and first-line bioinformatics was carried out at the MicroArray Facility (MAF, VIB, Leuven, Belgium). The complete dataset is available under GEO record GSE66420.
Flow cytometry
Brain cells were isolated by Percoll gradient centrifugation as described above. For antibody labeling, 1 × 105 cells were incubated in 200 μl for 20 min at 4 °C with combinations of mouse anti-Cd11b-APC (1:200) and either rat anti-LY6C-FITC (1:200), rat anti-5E12 (CD39) (1:300) rat anti-FCRLS (1:300) antibodies (Butovsky et al., 2012, Butovsky et al., 2014). The latter two were detected with goat anti-rat IgG conjugated to FITC (1:300; BioLegend). To demonstrate cell proliferation, bromodeoxyuridine (BrdU) (Sigma B5002) was dissolved in sterile Dulbecco’s phosphate buffered saline at 37°C and injected intraperitoneally (50 mg/kg body weight) daily for 3 consecutive days in 12-week-old or 16-week-old Mfp2 knockout and wild type littermates. Twelve hours after the last BrdU injection, proliferating cells were detected using flow cytometry with anti-BrDU antibodies (BrDU Flow Kit; BD Biosciences). Alternatively, animals were sacrificed for immunohistochemical detection of BrdU. Flow cytometry was performed on a FACSVerse (BD Biosciences), and data analyzed with BD FACsuite software (BD Biosciences). For immunological profiling, CD11b-Pe Cy7 (clone M1/70, Biolegend) was used in combination with CD11c-APC (clone HL3, BD Pharmingen), F4/80-APC (clone BM8, eBioscience), CX3CR1-FITC (Goat polyclonal, R&D Systems), CD204-Alexa 647 (clone MR5D3, AbD Serotec), CD206-Alexa 647 (clone 2F8, AbD Serotec) or IL10R (Imtec Diagnostics) all at dilutions of 1:200.
Real time PCR
Real time PCR was performed as previously described (Bottelbergs et al., 2010) using an ABI PRISM 7500 Real Time PCR instrument (Applied Biosystems, Lennik, Belgium). Primers and probes were either designed using Primer Express Software (Applied Biosystems) (β-actin, iNOS, Tnf sequences available on request) or ordered from Applied Biosystems as premade Taqman Gene Expression assays (Il1b, Mm011336189_m1; Il6, Mm01210733_m1; Cx3cr1, Mm0262011_s1; Tgfbr1, Mm00436964_m1; arginase, Mm00475991_m1; Mrc1, Mm00485148_m1). Assays were performed in duplicate or triplicate in 10 μL TaqMan Fast Universal PCR Master Mix (Applied Biosystems). Relative expression levels of the target genes were calculated taking into account the amplification efficiency as described (Giulietti et al., 2001). The relative expression levels of the target genes were calculated as a ratio to the housekeeping gene β-actin.
Bioinformatics
Differential expression of genes between the two conditions is based on the robust multi-array analysis (RMA) expression values (Irizarry et al., 2003) as obtained with the xps package (version 1.18.1) of BioConductor (www.bioconductor.org). We tested whether it significantly deviates from 0 with a moderated t-statistic (implemented in limma). The resulting p-values are corrected for multiple testing with Benjamini-Hochberg (Hochberg and Benjamini, 1990) to control the false discovery rate. In general, differential expression is based on the p-values, corrected for multiple testing (e.g. all probes with a corrected p-value less than 0.01) together with a cut-off on the fold-change of 1.5. Pathway analysis is based on information provided by The Ingenuity Knowledge Base. For data interpretation and visualization Ingenuity pathway analysis (IPA) and the functional annotation tool KEGG (Kyoto Encyclopedia for Genes and Genomes database) from DAVID (Database for Annotation, Visualization and Integrated Discovery v6.7) was used. In addition, the dataset from Mfp2−/− microglia was compared to transcriptomics datasets based on either RNA-sequencing of microglia in homeostatic conditions and from SOD1G93A mice (Chiu et al., 2013) (GEO dataset GSE43366) or gene expression profiling of microglia in homeostatic conditions using a NanoString nCounter platform (Butovsky et al., 2014). The RPKM gene levels from the RNA-seq experiment were converted to fold change as compared to control values. Differentially expressed genes (fold > 1.5, p-value < 0.01) were used for all analyses.
Histology
Anesthesia of the mice and tissue processing and immunohistochemical (IHC) staining were performed as described (Hulshagen et al., 2008). Routinely, paraffin sections (7 μm) were used for immunofluorescent stainings. The following primary antibodies were used: polyclonal rabbit anti-Iba1 (1:500; Wako D19–19741), rabbit anti-phospho-S6 ribosomal protein mAb (Ser235/236) (1:400; Cell signaling D57.2.2E), rabbit Cathepsin D (1:200 Santa Cruz), Lamp2/Mac3 (1:100, BD Pharmingen), rat Anti-BrDU mAb (1:100; AbD Serotec OBT0030), rabbit 3-nitrotyrosine (1:100; Millipore 06–284), and rabbit 4-hydroxynonenal (1:500; Millipore 393204). Cryo sections were used for staining with polyclonal rabbit anti-P2RY12 (1:500; provided by Prof. O. Butovsky, Boston, USA). Frozen sections of spinal cord of endstage SOD1G93A mice were kindly provided by Prof. L. Van Den Bosch (VIB and KU Leuven, Belgium). After incubation with primary antibodies overnight at room temperature (at 4°C for anti-P2RY12 staining) HRP-labeled secondary antibodies (1:200) were applied for 1 hour, followed by fluorescent labeling with a cyanine 2 (FITC) TSA kit (Perkin Elmer Life sciences, Boston, USA). P2ry12 antibodies were detected with goat anti-rabbit IgG conjugated to Alexa647 (1:300; Life technologies A21244). When double immunolabeling was performed, sets of primary and secondary antibodies were applied sequentially. As second fluorescent labels, cyanine 3 TSA kits (Perkin-Elmer) were used. Images were acquired with a motorized inverted IX-81 microscope connected to a CCD-FV2T digital camera (Olympus, Aartselaar, Belgium) and processed with LSM Image browser software (Zeiss, Germany). Microglia cell numbers were quantified on Iba-1 immunofluorescent stained paraffin sections (7 μm) around the sagittal midline (n=4). Within one plane (20 × magnification), only Iba-1-positive cells that fully colocalized with DAPI-positive nuclei were counted in the different regions of the brain, namely cornu ammonis (CA) 1–3 and dentate gyrus (DG) of hippocampus, somatosensory cortex, motor cortex and dorsal pons. Microglia number/frame was corrected for surface area. Quantification of neuronal numbers in CA3 of hippocampus was performed on cresyl violet stained sections (n=5) in a similar way as described for microglia. Only cells in which the Nissl substance was clearly visible were quantified.
In vivo LPS challenge and cytokine measurements
Mfp2−/− and control mice 14 weeks of age received an intraperitoneal injection of LPS (1mg/kg, Sigma, L4391) or sterile saline vehicle in a total volume of 100μl. Four hours later, mice were sacrificed, plasma and brain regions were collected and flash frozen in liquid nitrogen. Brainstem was homogenized 1:10 (w/v) in ice-cold PBS containing a cocktail of protease inhibitors (Roche) and lysates were cleared by centrifugation at 14000g for 20 min at 4°C. Immunoreactive levels of TNF, IL1β and IL6 were measured in plasma and brainstem lysates by using a cytometric bead array mouse soluble protein flex set system (BD Biosciences). The samples were prepared according to the manufacturer’s instructions and analyzed on a FACSCanto HTS (BD Biosciences) and analyzed by FCAP array software (BD Biosciences).
Statistical analysis
One-way ANOVA was carried out using Graphpad Prism 3.0 software (San Diego, CA). For microarray data, p-values are corrected for multiple testing with Benjamini-Hochberg (Hochberg and Benjamini, 1990).
Results
No invasion of Ly6C+ monocytes but proliferation of resident microglia in Mfp2−/− brain
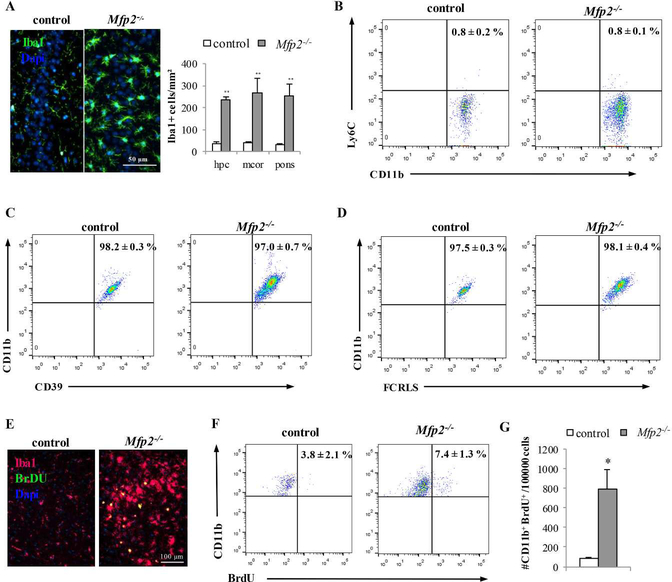
One of the most striking neuropathological hallmarks of Mfp2−/− mice is the development of extensive neuroinflammation involving astro- and microgliosis (Huyghe et al., 2006c, Verheijden et al., 2013). During the disease course, Iba1+ brain macrophages acquire an activated phenotype and display enlarged cell bodies and a deramified morphology together with increased F4/80 expression (Verheijden et al., 2013). In order to estimate whether these cells increase in number, Iba1+ cells were quantified in different regions of the CNS of mice at end stage (5 months old). In hippocampus, motor cortex and pons, microglia/macrophage numbers were 4–6 fold increased in Mfp2−/− compared to control brain (Fig. 1A). To investigate whether this increase arises from influx of peripheral monocytes or accumulation of resident microglia, we evaluated the presence of markers that are respectively expressed by blood monocytes (Ly6C) and resident microglia (CD39, FCRLS). Indeed, Butovsky et al recently showed that the expression of Ly6C and CD39/FCRLS effectively distinguishes non-overlapping populations of peripheral inflammatory monocytes and CNS-resident microglia in mice (Butovsky et al, 2012; Butovsky et al, 2014). The efficacy of these discriminating markers has been convincingly proven in a mouse model of ALS (SOD1G93A)(Butovsky et al., 2012, Butovsky et al., 2014). FACS analysis on brain homogenates of endstage Mfp2−/− mice showed that almost all CD11b+ cells are negative for the monocyte marker Ly6C (> 99%) (Fig.1B). In contrast, the vast majority of CD11b+ cells (> 97%) is positive for CD39 and FCRLS, two specific markers for resident microglia (Fig. 1C–D). Moreover, all CD11b+ cells are CD45dim, which is another characteristic of resident microglia (data not shown). Together, these data show that the expanded myeloid population is of local origin allowing to further designate these cells as microglia.
Figure 1. Increased numbers of myeloid cells in Mfp2−/− brain do not derive from influx of peripheral monocytes but from proliferation of resident microglia.
(A) Immunofluorescent staining for the microglia/macrophage marker Iba-1 reveals an increased number and altered shape of brain myeloid cells in the CA3 region of hippocampus in Mfp2−/− mice at 5 months compared to an age-matched control. Quantification of Iba-1+ cells in different brain regions shows increased microglia cell numbers in hippocampus (hpc), motor cortex (mcor) and pons of Mfp2−/− mice (** p < 0.01). Nuclei are stained blue with DAPI. (B) FACS analysis of Ly6C expression in CD11b-gated cells (n = 6 per group). Quantification shows that nearly all CD11b+ cells in brain of control and Mfp2−/− mice are Ly6c negative. (C) Nearly all Cd11b myeloid cells from control and Mfp2−/− brain express the microglial specific surface marker CD39 (n = 6 per group). (D) Nearly all Cd11b+ myeloid cells from control and Mfp2−/− brain express the microglial specific surface marker FCRLS (n = 4 per group). All FACS data are presented as mean ± SEM (n = 4– 6 per group) **p < 0.01. (E) BrdU incorporation (green) in Iba-1+ cells (red) in Mfp2−/− but not in control brain using immunofluorescent staining. Nuclei are stained blue with DAPI. (F,G) BrDU incorporation in CD11b-gated cells is higher in Mfp2−/− compared to control mice (n = 3–4 per group, mean ± SEM) *p < 0.05.
Having excluded influx of inflammatory monocytes in the Mfp2−/− brain, we next wanted to verify whether the accumulation of brain myeloid cells originates from proliferation of CNS resident microglia. We analyzed BrdU incorporation in microglial cells and indeed found significantly increased numbers of proliferating Iba1+/BrdU+ cells by IHC analysis (Fig. 1E) and Cd11b+/BrdU+ cells by FACS analysis in Mfp2−/− compared to control mice (Fig. 1F,G). Together, these data demonstrate that the inflammatory response in Mfp2−/− brain is governed by proliferation of resident microglia and not by infiltration of peripheral cells.
Defining the molecular signature of a non-neurodegenerative microglia phenotype
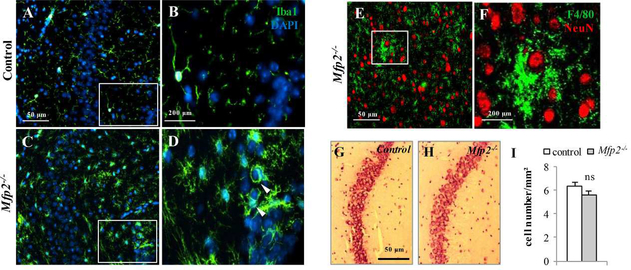
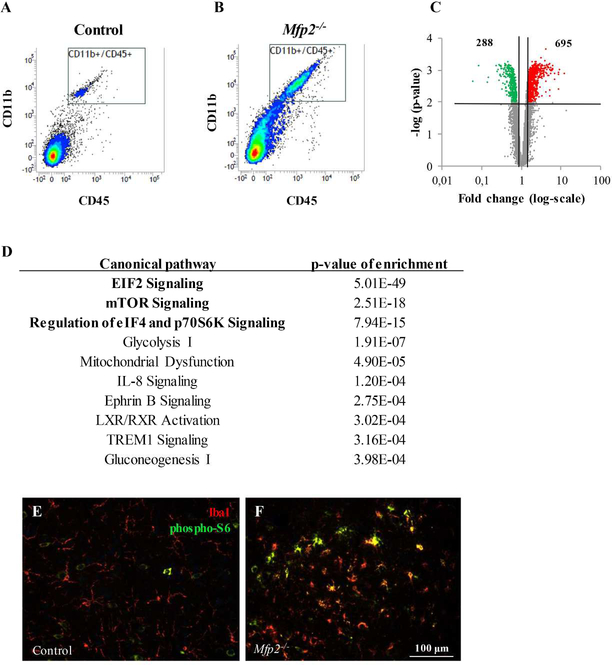
As microgliosis in Mfp2−/− mice is confined to gray matter areas such as cortex, hippocampus and brainstem (Verheijden et al., 2013), we performed double immunofluorescence stainings with microglial and neuronal markers. We observed that microglia enwrap neuronal cell bodies (Fig. 2A–F), suggesting that microglia in Mfp2−/− mice become activated in response to neuronal demise, analogous to several disease models of neurodegenerative disorders. However, previous FluoJade and caspase/TUNEL stainings did not show evidence for neuronal damage nor death in the CNS of Mfp2−/− mice (Huyghe et al., 2006c). To further confirm the conservation of neurons, we counted neuron numbers in the CA3 region of the hippocampus of endstage Mfp2−/− mice (Fig. 2G–I). We did not find significant differences between Mfp2−/− and control mice. Also in other brain regions, we could not detect an overt reduction of neuron numbers (not shown). The microglia microenvironment in Mfp2−/− brain thus deviates from the milieu in neurodegenerative disorders. We therefore characterized the molecular signature of this non-neurodegenerative microglia phenotype and performed microarray analysis on acutely isolated microglia (CD11b+/CD45dim) from whole brain of 5 months old control and Mfp2−/− mice (Fig. 3A,B). We found that 983 genes were differentially expressed in Mfp2−/− compared to control microglia (288 downregulated, 695 upregulated; fold ≥ 1.5, p-value < 0.01) (Fig. 3C). The top 50 up- and downregulated genes, the unsupervised hierarchical clustering of RMA values and the principle component analysis of gene expression data are shown in Suppl. Fig.1. Pathway enrichment by Ingenuity and DAVID analysis demonstrated pronounced induction of pathways related to protein translation, cellular growth and proliferation, such as Eif2 signaling, mTOR signaling and regulation of eIF4 and p70S6K signaling (Fig. 3D and Suppl. Table 1). Activation of mTOR was validated by IHC, showing increased phosphorylation of the downstream target ribosomal S6 protein (pS6) in Mfp2−/− compared to wild type microglia (Fig. 3E,F). The transcription factor Hypoxia-inducible factor 1-alpha (HIF1α), another downstream target of mTOR (Duvel et al., 2010), was significantly induced according to the microarray data, which was further supported by elevated transcripts of several enzymes of the glycolytic pathway and VEGF (Suppl. Table 1). Interestingly, the regulatory subunit of 5’ AMP-activated protein kinase (AMPK) that inhibits mTOR in conditions of cellular energy depletion, was downregulated by 50%. The enrichment of mTOR and eIF2 signaling is in accordance with the strong proliferative response and cellular growth observed in Mfp2−/− microglia.
Figure 2. Abundant microglia-neuron contacts but no neuronal loss in Mfp2−/− mice.
(A-D) Activated microglia (Iba1+, green) enwrap cell bodies (DAPI, blue) in the CA3 region of the hippocampus in Mfp2−/− mice (C,D) but not in control (A,B). B and D are magnifications of the highlighted area in A and C, respectively. Nuclei are stained blue with DAPI. (E) F4/80+ cells contact NeuN neurons in the brainstem of Mfp2−/− mice. (F) Magnification of E. (G – I) H&E staining and quantification in the CA3 region of hippocampus reveals preservation of neurons in Mfp2−/− mice.
Figure 3. Transcriptomics analysis on acutely isolated microglia from control and Mfp2−/− mouse brain.
(A,B) Cd11b/Cd45 FACS sorting of microglia isolated from control (A) and Mfp2−/− (B) mouse brain. (C) Volcano plot of differentially expressed genes of Mfp2−/− versus control microglia (cut-off: 0.75 ≥ fold change ≥ 1.5; p < 0.01). (D) The 10 most upregulated pathways after applying the canonical pathway enrichment tool of Ingenuity software on the microarray dataset of Mfp2−/− microglia (differentially expressed genes: 0.75 ≤ fold ≤1.5; p-value 0.01 ). (E,F) Increased IHC staining for phosphorylated ribosomal S6 (pS6, green), a downstream target of mTOR, in Iba1+ microglia (red) in Mfp2−/− mice (F) as compared to control (E), shown for thalamus region.
Microglia in Mfp2−/− brain are not phagocytic and do not produce reactive oxygen/nitrogen species
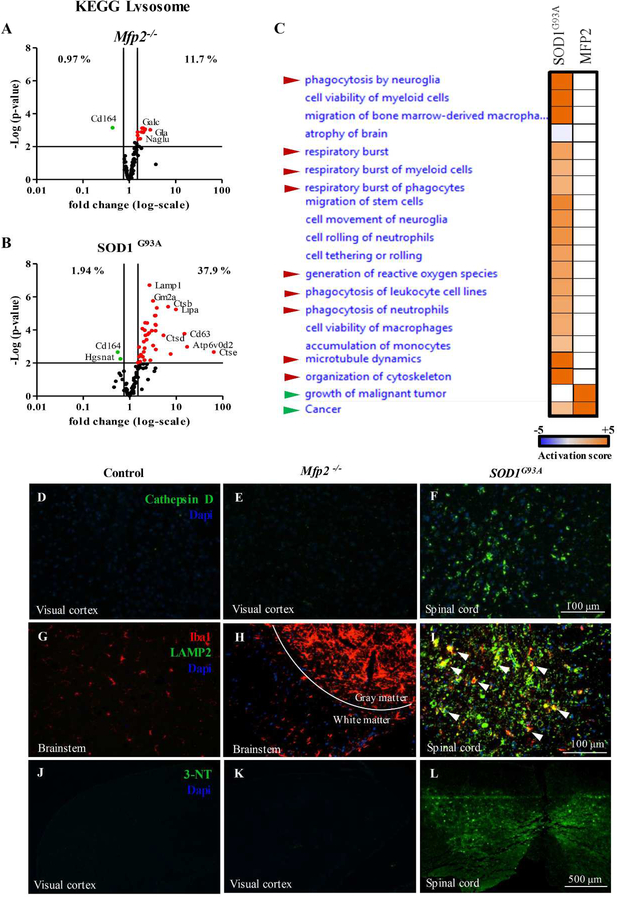
We compared the transcriptional signature of Mfp2−/− microglia residing in a neuron-sparing milieu, with the previously published neurodegeneration-specific microglial signature in SOD1G93A mice, a model for ALS (GEO dataset GSE43366)(Chiu et al., 2013). The most pronounced difference between the Mfp2−/− and SOD1G93A model was the strong enrichment of the KEGG pathway Lysosome in SOD1G93A microglia (37.9% of genes related to Lysosome upregulated) (Fig. 4B) whereas this was less obvious for Mfp2−/− microglia (11.7% of genes upregulated) (Fig. 4A). Comparison for enriched functions between the two models using Ingenuity analysis demonstrated marked enrichment for ‘phagocytosis’, ‘cytoskeletal organization’ and ‘respiratory burst’ in the SOD1G93A as compared to Mfp2−/− microglia (Fig. 4C). As these data suggest that microglia in Mfp2−/− mice do not acquire phagocytic properties, in contrast to microglia in a neurodegenerative disease model (SOD1G93A), we performed IHC for markers associated with lysosomal activation and phagocytosis. There was no increased immunoreactivity of Cathepsin D (Fig. 4D,E), LAMP2/MAC3 (Fig. 4G,H) and CD68 (not shown) in brain of Mfp2−/− versus control mice (shown for visual cortex and brainstem). In contrast, we detected clear positive staining for these markers in the lumbal spinal cord of endstage SOD1G93A mice (Fig. 4F,I).
Figure 4. Differences in expression of lysosomal, phagocytic and nitrosative stress markers in Mfp2−/− versus SOD1G93A microglia.
(A-B) After transcriptomics analyses of Mfp2−/− microglia (A) and SOD1G93A microglia (B) (data from GEO GSE43366) the expression of genes associated with the Lysosome (mmu04142) KEGG pathway were plotted. The percentage of genes of this pathway differentially expressed in both mouse models is shown. (C) Heatmap for enriched functions of SOD1G93A microglia as compared to Mfp2−/− microglia (Ingenuity analysis). Enrichment is depicted as activated (orange), not enriched (white) or deactivated (blue). Red arrows point to functions related to phagocytosis, green arrows to growth and proliferation. (D-F) Staining of the lysosomal marker Cathepsin D (green) in visual cortex of control (D) and Mfp2−/− mice (E) and in spinal cord of endstage SOD1G93A mice (F). (G-I) Co-staining of the microglial marker Iba1 (red) and the lysosomal marker LAMP2/MAC3 (green) in brainstem of control (G) and Mfp2−/− mice (H) and in spinal cord of endstage SOD1G93A mice (I). White arrowheads point to LAMP2+ microglia. (J-L) Staining for nitrosylated proteins (green) in visual cortex of control (J) and Mfp2−/− mice (K) and in spinal cord of endstage SOD1G93A mice (L). Nuclei are stained blue with DAPI.
The low expression of ‘respiratory burst’ genes in Mfp2−/− as compared to SOD1G93A microglia comprised normal levels of Nos2, Nox1 and Nox2 (Cybb) versus controls. In contrast, SOD1G93A microglia showed a very strong upregulation of Cybb compared to control microglia (> 40 fold) (Chiu et al., 2013). As reactive oxygen and nitrogen species are known to be important mediators of neurotoxicity, and upregulation might take place at the posttranscriptional level, we further evaluated oxidative stress markers. IHC stainings for nitrated proteins (Fig. 4J–L) and lipid peroxidation (4HNE, not shown) were not detectable in Mfp2−/− brain (Fig. 4K), whereas both were positive in SOD1G93A spinal cord (Fig. 4L and data not shown).
In summary, despite their chronic active state, Mfp2−/− microglia do not upregulate pathways associated with phagocytic clearance, lysosomal activity and reactive oxygen/nitrogen species production. This is in striking contrast to the neurodegeneration-specific microglial signature of SOD1G93A mice.
Mfp2−/− microglia are immunologically activated but not polarized
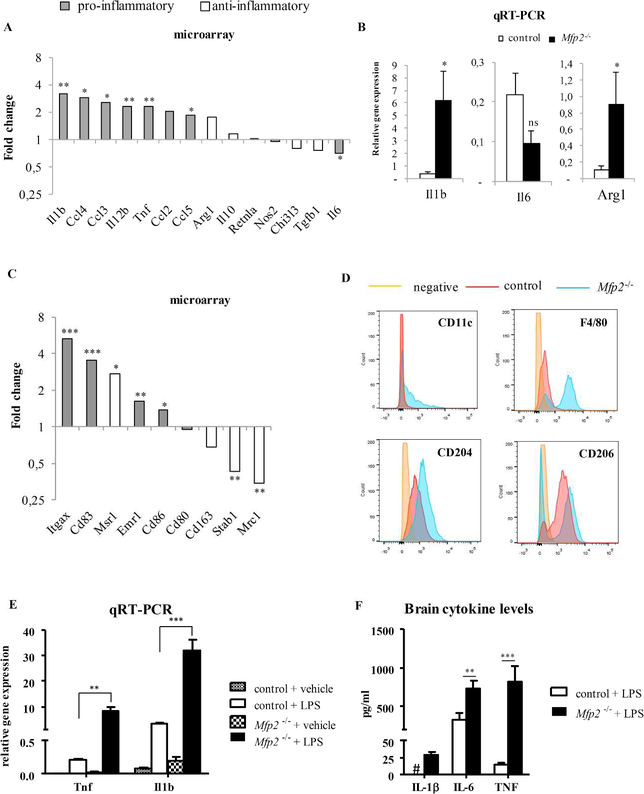
In response to disruption of CNS homeostasis by infectious or sterile conditions, reactive microglia can produce pro-inflammatory cytokines such as TNF. Chronic release of these substances by microglia can affect neuronal viability (Block et al., 2007). In contrast, the induction of an anti-inflammatory microglia phenotype is generally linked to neuroprotection (Kigerl et al., 2009, Liao et al., 2012). In view of preserved neuron numbers in Mfp2−/− mice and the absence of a neurodegeneration-specific microglial signature, we explored transcript levels of genes associated with pro- and anti-inflammatory activation in the micro-array dataset. Microglia in Mfp2−/− mice are clearly immunologically activated as several genes encoding cytokines (Il1b, Tnf, Il12b), chemokines (Ccl5, Ccl2) and surface markers (Itgax, Emr1, Cd83, Cd86) associated with inflammatory activation were strongly induced (Fig. 5A,C). Remarkably, the expression of several key pro-inflammatory genes was unchanged (Nos2, Il12a, Cxcl9, Ifnb1, Icam1) or even downregulated (Il6), suggesting that microglia in Mfp2−/− mice do not acquire a typical pro-inflammatory activation state. However, Mfp2−/− microglia could also not be categorized as anti-inflammatory, because typical anti-inflammatory genes were either induced (Arg1, Msr1) or repressed (Mrc1, Stab1) (Fig. 5A–C). qRT-PCR and FACS analysis of some key inflammatory markers further confirmed that the microglia activation state in Mfp2−/− mice cannot be categorized as pro- or anti-inflammatory (Fig. 5B,D). Analysis of cytokine levels in brain homogenates revealed that despite strongly increased transcript levels of Tnf and Il1b in microglia, the respective cytokine levels were not detectable (TNF) or only 1.5 fold increased (IL1β) (not shown) in Mfp2−/− brain, further supporting the absence of a typical pro-inflammatory effector state. Interestingly, after intraperitoneal injection of LPS, both transcript (Tnf, Il1b) and protein levels (IL1β, IL6 and TNFα) were more strongly induced in Mfp2−/− compared to control mice, consistent with a primed state (Perry and Holmes, 2014) (Fig. 5E,F). In order to exclude that this particular immunological state of microglia is induced by systemic inflammation, we analyzed cytokine levels in plasma (Il1β, Il6, TNFα) and the leucocyte profile in blood. There were however no signs of peripheral inflammatory anomalies in Mfp2−/− mice and after LPS administration cytokine levels increased similarly in control and mutant mice (data not shown).
Figure 5. The immunological profile of Mfp2−/− microglia is not polarized to a pro- or antiinflammatory state.
(A) Relative transcript levels of pro-inflammatory (dark gray bars) and anti-inflammatory (white bars) activation markers as determined by microarray analysis in Mfp2−/− microglia compared to wild type (n=4). (B) qRT-PCR analyses of the pro-inflammatory markers IL1β and IL6 and the anti-inflammatory marker Arg-1 confirm micro-array data and indicate that microglia are not unequivocally polarized. mRNA expression levels were normalized to β-actin. Data are presented as mean ± SEM (n = 3–4). * p < 0.05; ** p < 0.01. (C) Surface markers expression associated with classical (gray) and alternative (white) macrophage activation as determined by microarray analysis. (D) FACS analysis of CD11b+ microglia shows increased expression of CD11c and F4/80 surface markers associated with classical activation. There is also increased expression of the anti-inflammatory marker CD204pos (Msr1) as well as an increase in the proportion of microglia that are CD206neg (Mrc1) (representative experiment out of two). (E-F) Mfp2−/− and control mice (n=3–5) were challenged with intraperitoneal LPS and brainstem tissue was analyzed after 4 hrs. Transcripts (E) and cytokine levels (F) of Il1b, Il6 and Tnf were more elevated in Mfp2−/− as compared to control mice. # = not detectable, * p<0.05, ** p < 0.01, ***p<0.001.
It thus seems that Mfp2−/− microglia exhibit an immunologically activated and primed state, but they are not polarized and only acquire a typical pro-inflammatory effector state after a secondary stimulus.
The homeostatic microglial signature is downregulated in Mfp2−/− microglia
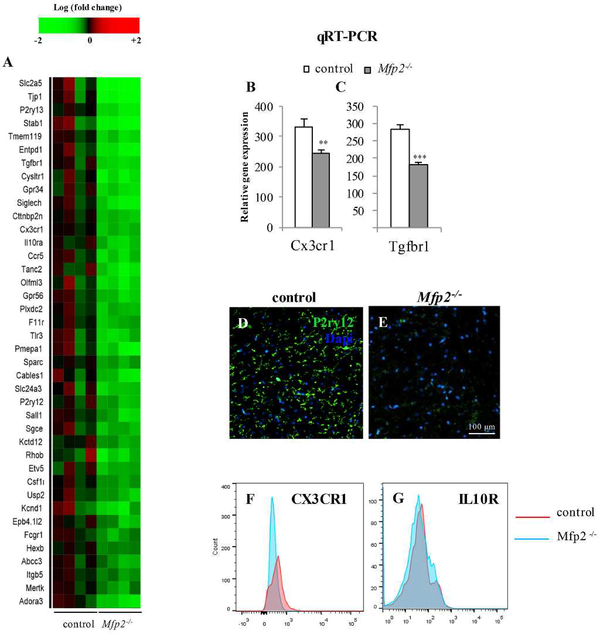
Several research groups recently reported the specific transcript signature of homeostatic microglia (Gautier et al., 2012, Chiu et al., 2013, Hickman et al., 2013, Butovsky et al., 2014) by comparing the transcriptomes of microglia with those of other CNS cell types and/or peripheral myeloid cells. This resulted in the identification of specific markers of microglia in their surveillance mode. When comparing our data set with the homeostatic microglial signature, it was conspicuous that the bulk of microglia specific genes was significantly suppressed in Mfp2−/− microglia (46% when compared with Butovsky et al.(Butovsky et al., 2014) (Fig. 6A) and 53 % compared with Chiu et al. (Chiu et al., 2013)), whereas only a few were upregulated (6–9%). These data demonstrate that in the inflamed Mfp2−/− brain, microglia loose typical features related to their homeostatic function. In order to validate the microarray data, we performed qRT-PCR of Cx3cr1 and Tgfbr1 on isolated microglia (Fig. 6B,C) and IHC analysis of P2RY12 (Fig. 6D,E) which confirmed the downregulation of these surface markers in Mfp2−/− microglia. FACS analysis for CX3CR1 and IL10R showed a clear reduction in CX3CR1 expression and a slight reduction in IL10R expression (Fig. 6F,G). Taken together, a hallmark of the microglia phenotype in Mfp2−/− brain is the loss of surface and other markers that were shown to be highly and exclusively expressed in these cells in homeostatic conditions.
Figure 6. Repression of homeostatic gene signature in Mfp2−/− microglia.
(A) Heatmap showing expression values of signature genes of microglia (according to Butovsky et al, 2013) in control versus Mfp2−/− microglia. In Mfp2−/− microglia, 40 out of 86 genes are significantly downregulated (47%). Expression values are expressed as robust multi-array analysis (RMA). (B,C) qRT-PCR confirms the downregulation of the microglia-enriched genes Cx3cr1 (A) and Tgfbr1 (B) in Mfp2−/− microglia. Expression levels were normalized to β-actin. Data are presented as mean ± SEM (n = 3–4, ** p < 0.01; *** p < 0.001). (D,E) Immunohistochemical staining confirms downregulation of the purinergic receptor P2RY12 on microglia of Mfp2−/− as compared to wild type mice. (F,G) FACS analysis of CD11b+ microglia shows reduced expression of CX3CR1 and to a lesser extent of IL10R in Mfp2−/− microglia (1 experiment out of 2 with similar data).
Discussion
This study contributes to a better understanding of the wide spectrum of phenotypes that microglia can adopt in the diseased brain. We determined the microglial signature in a murine model of peroxisomal β-oxidation deficiency in which robust and chronic inflammation in the gray matter occurs in the absence of neuronal loss. We show that the resident microglia in Mfp2−/− mice proliferate, are permanently in an activated non-phagocytic state and lose their typical homeostatic markers. Our data prove that microglia can remain in a reactive state without acquiring overt neurotoxic and phagocytic properties.
As infiltrating bone marrow-derived monocytes may have a major impact on the pathological course of neurological conditions (Prinz and Mildner, 2011, Yamasaki et al., 2014), it was of prime importance to establish the source of the expanded microglial compartment in Mfp2−/− mice. Analysis of specific markers corresponding to peripheral monocytes and resident microglia clearly showed that the myeloid cells in Mfp2−/− brain, even at late stages of disease, solely derive from local microglia. This differs from the neuroinflammatory demyelination condition in the peroxisomal β-oxidation disorder, ccALD in which the fast progressive phase coincides with opening of the blood brain barrier (Kemp et al., 2012).
Although the immunological profile of Mfp2−/− microglia is clearly distinct from naive microglia, the affected cytokines and inflammatory mediators could not be clustered in currently known pro- or anti-inflammatory states. In contrast to acute insults to the brain, where microglia switch from an initial proinflammatory to a resolving anti-inflammatory state, the microglial phenotype in chronic sterile stress conditions can be very heterogeneous. In the SOD1G93A mouse model of ALS, microglia were shown to convert during the course of disease from an alternatively activated to a classically activated state (Liao et al., 2012). The latter were shown to be neurotoxic to motor neurons in vitro. It is now recognized that microglia induce inflammatory markers in the aged rodent brain and it is hypothesized that this modest chronic neuroinflammation impairs neuronal functioning (Norden and Godbout, 2013). Age is not contributing to the inflammatory response in our model as mice only survive up to 5–6 months of age. It remains however unsolved whether long-term activation of microglia is necessarily deleterious for neural cells (Biber et al., 2014). Interestingly, crucial players in the neurotoxic properties are reactive oxygen and nitrogen species (Block et al., 2007, Brown and Neher, 2014). Although a set of pro-inflammatory surface and cytokine markers are induced in Mfp2−/− microglia, the genes responsible for generation of reactive oxygen and nitrogen species are not upregulated, even at endstage of disease. The induction of Arg1, a well characterized anti-inflammatory marker gene, also indicates that Mfp2−/− microglia are rather protective for their environment than toxic. Indeed, the enzyme arginase 1 utilizes, similar to iNOS, arginine as a substrate, but converts it to products that serve as precursors in repair processes and not to destructive nitrogen species (Lange et al., 2004, Estevez et al., 2006).
Gene expression screening and histological analyses further indicated absence of lysosomal activity and cytoskeletal remodeling that normally accompany phagocytosis. This is in sharp contrast with the gene expression signature of SOD1G93A microglia in which these pathways were strongly induced (Chiu et al., 2013). In the latter model, microglia respond to early changes in motor neurons and develop phagocytic and neurotoxic properties even in a presymptomatic stage (Sanagi et al., 2010). On the contrary, reactive microglia in Mfp2−/− brain seem to get stuck in their graded response from a ramified to a fully activated phagocytic state. This raises the question whether Mfp2−/− microglia have the ability to develop lysosomal activity and execute phagocytosis. In this respect it is important to note that cathepsin D positive microglia were present in cerebellar white matter (data not shown), where we previously showed degeneration of Purkinje cell axons (Verheijden et al., 2013), indicative of a proper phagocytic function. Microglia in MFP2 deficient mice thus transform for a prolonged period in a reactive state that lacks neurodestructive behavior within their lifetime. It needs to be elucidated whether this state is triggered by cell autonomous processes in microglia resulting from peroxisomal β-oxidation inactivity or by microglial responses to MFP2 deficiency in neighbouring cells or a combination of these mechanisms. The fact that the microglial reactivity is confined to the gray matter strongly suggests that metabolic distress caused by peroxisomal MFP2 deficiency in neurons contributes to pathology.
In view of the seemingly harmless microglial activation, the question arises whether this contributes to disease pathogenesis in Mfp2−/− mice as increasing microgliosis coincides with the neurological symptoms. In this respect, it should be emphasized that the most prominent coordinated change in Mfp2−/− microglia is rather a loss-of-function than a toxic-gain-of-function. Several research groups recently defined the unique transcriptome signature of homeostatic microglia by comparison with their neural cell neighbors and myeloid cell family members (Gautier et al., 2012, Chiu et al., 2013, Hickman et al., 2013, Butovsky et al., 2014). These typical microglial markers were generally downregulated in Mfp2−/− microglia pointing to a loss of their surveillance state. Butovsky et al recently reported that the microglia specific gene signature is mainly driven by transforming growth factor β (TGFβ) and that loss of TGFβ signaling leads to downregulation of the homeostatic gene signature (Butovsky et al., 2014). In Mfp2−/− microglia the expression of Tgbr1 was downregulated, probably contributing to their loss-of-homeostatic-function phenotype. A large fraction of the downregulated genes encode cell surface proteins such as the purinergic receptors P2RY12 and P2RY13. It should however be noted that this loss of microglial membrane proteins is selective as several others are either unchanged (e.g. P2RY6) or increased (e.g. P2RX4) in expression. It is plausible that these alterations will perturb the microglial communication with the microenvironment or that chronic deprivation of homeostatic microglial properties impairs neurological function. It was indeed shown that lack of CX3CR1 signaling in microglia perturbs synaptic function in the postnatal period resulting in persistently disturbed brain connectivity and behavioral disorders (Zhan et al., 2014). Furthermore, loss of microglia in adulthood was proven to impact on memory and motor learning through impaired synapse formation, emphasizing the importance of proper neuron-microglia crosstalk in normal brain functioning (Parkhurst et al., 2013).
Whereas the primary trigger for microglia activation in Mfp2−/− brain remains unknown, we found that mTOR and other pathways driving protein synthesis were induced in the reactive microglia. Activation of these pathways leads to cell proliferation and growth, in line with the observed morphological and mitogenic response of Mfp2−/− microglia. The pathways downstream of mTOR are known to be involved in ribosomal activity, protein synthesis and energy generation through induction of HIF1α, glycolysis, and enhanced mitochondrial functioning (Hay and Sonenberg, 2004, Duvel et al., 2010). In fact, little is known on the role of mTOR in the diverse microglial phenotypes. In vitro experiments with primary microglia and BV2 cells showed increased mTOR signaling after exposure to hypoxia, LPS or a mixture of cytokines (Lu et al., 2006). Mouse models with various neurological conditions such as spinal cord injury and epilepsy, were shown to improve when treated with mTOR inhibitors but the impact on the inflammatory reaction remains poorly documented (Sekiguchi et al., 2012, van Vliet et al., 2012, Lu et al., 2014). Interestingly, mTOR was recently identified as a novel factor involved in macrophage polarization although contradictory findings were reported whether mTOR activation biases the cytokine response towards a pro- or an anti-inflammatory state (Byles et al., 2013, Luo et al., 2014). In addition, it was recently shown that the AKT-mTOR-HIF1α pathway is instrumental for ‘training’ of macrophages whereby cytokine expression is enhanced following a prior exposure to β-glycan (Cheng et al., 2014, Saeed et al., 2014). This signaling orchestrates a switch in macrophages from oxidative metabolism to aerobic glycolysis. In this respect, the upregulation of the mTOR pathway in Mfp2−/− microglia, may play a broader role than only sustain growth and proliferation. The activation of the transcription factor HIF1α and its downstream glycolytic enzymes might play a pivotal role in the primed state of Mfp2−/− brain and possibly in other chronic neuroinflammatory states. In view of the premise that primed microglia in neurodegenerative diseases become detrimental after a systemic insult such as infection or illness, it is important to further investigate whether targeting mTOR can modify the microglia expression pattern in Mfp2−/− mice and in other neuroinflammatory conditions.
In conclusion, our data indicate that chronically reactive microglia in Mfp2−/− brain adopt an immunologically activated but non-polarized phenotype that is marked by morphological and metabolic adaptations. These microglia act in a pro-survival environment without overt neuronal demise, although an adverse effect on neuronal functioning through loss of microglial homeostatic functions cannot be excluded.
Supplementary Material
Supplemental figure 1 Transcriptomics analysis of isolated microglia from Mfp2−/− and control brain
(A) Heatmap and unsupervised hierarchical clustering of RMA values. (B) Principle component analysis (PCA) of gene expression data. (C) Heatmaps of top 50 genes upregulated and downregulated in Mfp2−/− as compared to wild type microglia, ranked by fold change.
Main points.
Resident microglia in MFP2 deficient mice exhibit a proliferative and immunologically activated state but they lack signs of phagocytic and neurotoxic activity
The chronically reactive microglia lose their typical homeostatic markers but upregulate pathways associated with growth and proliferation
The molecular signature of microglia in Mfp2−/− brain strongly diverges from microglia in the neurodegenerative milieu of an ALS mouse model
Acknowledgments
The authors wish to thank Benno Das and Lies Pauwels for excellent technical assistance, Nieske Brouwer and Prof. dr. H.W.G.M. Boddeke (Rijksuniversiteit Groningen, The Netherlands) for the guidance with the microglia isolation and Prof L. Van Den Bosch (VIB and KU Leuven, Belgium) for providing sections of spinal cord of SOD1G93A mice. This work was funded by grants from Fonds Wetenschappelijk Onderzoek Vlaanderen (G.0675.12N and G.0A15.13), and KU Leuven (OT12/79).
References
- Baes M, Huyghe S, Carmeliet P, Declercq PE, Collen D, Mannaerts GP, Van Veldhoven PP. 2000. Inactivation of the peroxisomal multifunctional protein-2 in mice impedes the degradation of not only 2-methyl branched fatty acids and bile acid intermediates but also of very long chain fatty acids. J Biol Chem 275:16329–16336. [DOI] [PubMed] [Google Scholar]
- Biber K, Owens T, Boddeke E. 2014. What is microglia neurotoxicity (Not)? Glia 62:841–854. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69. [DOI] [PubMed] [Google Scholar]
- Bottelbergs A, Verheijden S, Hulshagen L, Gutmann DH, Goebbels S, Nave KA, Kassmann C, Baes M. 2010. Axonal integrity in the absence of functional peroxisomes from projection neurons and astrocytes. Glia 58:1532–1543. [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. 2014. Microglial phagocytosis of live neurons. Nat Rev Neurosci 15:209–216. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. 2014. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 17:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, Lawson R, Berry J, Krichevsky AM, Cudkowicz ME, Weiner HL. 2012. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest 122:3063–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, Horng T. 2013. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun 4:2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Aubourg P. 2010. Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleukodystrophy. Brain Pathology 20:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrere F, Blanche S, Audit M, Payen E, Leboulch P, l’Homme B, Bougneres P, Von KC, Fischer A, Cavazzana-Calvo M, Aubourg P. 2009. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326:818–823. [DOI] [PubMed] [Google Scholar]
- Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. 2014. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345:1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK. 2014. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP, Muratet M, Carroll MC, Levy S, Tavazoie S, Myers RM, Maniatis T. 2013. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 4:385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haas AH, Boddeke HW, Brouwer N, Biber K. 2007. Optimized isolation enables ex vivo analysis of microglia from various central nervous system regions. Glia 55:1374–1384. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Kipnis J. 2013. The role of microglia in brain maintenance: implications for Rett syndrome. Trends Immunol 34:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. 2010. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39:171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez AG, Sahawneh MA, Lange PS, Bae N, Egea M, Ratan RR. 2006. Arginase 1 regulation of nitric oxide production is key to survival of trophic factor-deprived motor neurons. J Neurosci 26:8512–8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandusse S, Denis S, Mooyer PA, Dekker C, Duran M, Soorani-Lunsing RJ, Boltshauser E, Macaya A, Gartner J, Majoie CB, Barth PG, Wanders RJ, Poll-The BT. 2006a. Clinical and biochemical spectrum of D-bifunctional protein deficiency. Ann Neurol 59:92–104. [DOI] [PubMed] [Google Scholar]
- Ferdinandusse S, Denis S, Mooyer PAW, Dekker C, Duran M, Soorani-Lunsing RJ, Boltshauser E, Macaya A, G,,rtner J, Majoie CBLM, Barth PG, Wanders RJA, Poll-The BT. 2006b. Clinical and biochemical spectrum of D-bifunctional protein deficiency. Ann Neurol 59:92–104. [DOI] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. 2012. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13:1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. 2001. An overview of realtime quantitative PCR: applications to quantify cytokine gene expression. Methods 25:386–401. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. 2010. Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nicola D, Fransen NL, Suzzi S, Perry VH. 2013. Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci 33:2481–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. 2004. Upstream and downstream of mTOR. Genes Dev 18:1926–1945. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. 2013. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 16:1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. 1990. More powerful procedures for multiple significance testing. Stat Med 9:811–818. [DOI] [PubMed] [Google Scholar]
- Hughes V 2012. Microglia: The constant gardeners. Nature 485:570–572. [DOI] [PubMed] [Google Scholar]
- Hulshagen L, Krysko O, Bottelbergs A, Huyghe S, Klein R, Van Veldhoven PP, De Deyn PP, D’Hooge R, Hartmann D, Baes M. 2008. Absence of functional peroxisomes from mouse CNS causes dysmyelination and axon degeneration. J Neurosci 28:4015–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghe S, Mannaerts GP, Baes M, Van Veldhoven PP. 2006a. Peroxisomal multifunctional protein-2: the enzyme, the patients and the knockout mouse model. BBA Mol Cell Biol L 1761:973–994. [DOI] [PubMed] [Google Scholar]
- Huyghe S, Schmalbruch H, De Gendt K, Verhoeven G, Guillou F, Van Veldhoven PP, Baes M. 2006b. Peroxisomal multifunctional protein 2 is essential for lipid homeostasis in Sertoli cells and for male fertility in mice. Endocrinology 147:2228–2236. [DOI] [PubMed] [Google Scholar]
- Huyghe S, Schmalbruch H, Hulshagen L, Van Veldhoven PP, Baes M, Hartmann D. 2006c. Peroxisomal multifunctional protein-2 deficiency causes motor deficits and glial lesions in the adult CNS. Am J Pathology 168:1321–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264. [DOI] [PubMed] [Google Scholar]
- Jia Y, Qi C, Zhang Z, Hashimoto T, Rao MS, Huyghe S, Suzuki Y, Van Veldhoven PP, Baes M, Reddy JK. 2003. Overexpression of peroxisome proliferator-activated receptor-alpha (PPARalpha)-regulated genes in liver in the absence of peroxisome proliferation in mice deficient in both L- and D- forms of enoyl-CoA hydratase/dehydrogenase enzymes of peroxisomal beta-oxidation system. J Biol Chem 278:47232–47239. [DOI] [PubMed] [Google Scholar]
- Kemp S, Berger J, Aubourg P. 2012. X-linked adrenoleukodystrophy: metabolic, genetic and clinical aspects. BBA-Mol Basis Dis 1822:1465–1474. [DOI] [PubMed] [Google Scholar]
- Khan A, Wei XC, Snyder FF, Mah JK, Waterham H, Wanders RJ. 2010. Neurodegeneration in D-bifunctional protein deficiency: diagnostic clues and natural history using serial magnetic resonance imaging. Neuroradiology 52:1163–1166. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. 2009. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29:13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange PS, Langley B, Lu P, Ratan RR. 2004. Novel roles for arginase in cell survival, regeneration, and translation in the central nervous system. J Nutr 134:2812S–2817S. [DOI] [PubMed] [Google Scholar]
- Liao B, Zhao W, Beers DR, Henkel JS, Appel SH. 2012. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol 237:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DY, Liou HC, Tang CH, Fu WM. 2006. Hypoxia-induced iNOS expression in microglia is regulated by the PI3-kinase/Akt/mTOR signaling pathway and activation of hypoxia inducible factor-1alpha. Biochem Pharmacol 72:992–1000. [DOI] [PubMed] [Google Scholar]
- Lu Q, Gao L, Huang L, Ruan L, Yang J, Huang W, Li Z, Zhang Y, Jin K, Zhuge Q. 2014. Inhibition of mammalian target of rapamycin improves neurobehavioral deficit and modulates immune response after intracerebral hemorrhage in rat. J Neuroinflammation 11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Wall AA, Yeo JC, Condon ND, Norwood SJ, Schoenwaelder S, Chen KW, Jackson S, Jenkins BJ, Hartland EL, Schroder K, Collins BM, Sweet MJ, Stow JL. 2014. Rab8a interacts directly with PI3Kgamma to modulate TLR4-driven PI3K and mTOR signalling. Nat Commun 5:4407. [DOI] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. 2013. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol 39:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah M, Raj D, Brouwer N, De Haas AH, Eggen BJ, Den Dunnen WF, Biber KP, Boddeke HW. 2012. An optimized protocol for the acute isolation of human microglia from autopsy brain samples. Glia 60:96–111. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. 2013. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155:1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Holmes C. 2014. Microglial priming in neurodegenerative disease. Nat Rev Neurol 10:217–224. [DOI] [PubMed] [Google Scholar]
- Prinz M, Mildner A. 2011. Microglia in the CNS: immigrants from another world. Glia 59:177–187. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. 2014. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15:300–312. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. 2009. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27:119–145. [DOI] [PubMed] [Google Scholar]
- Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, Cheng SC, Ratter J, Berentsen K, van der Ent MA, Sharifi N, Janssen-Megens EM, Ter Huurne M, Mandoli A, van Schaik T, Ng A, Burden F, Downes K, Frontini M, Kumar V, Giamarellos-Bourboulis EJ, Ouwehand WH, van der Meer JW, Joosten LA, Wijmenga C, Martens JH, Xavier RJ, Logie C, Netea MG, Stunnenberg HG. 2014. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345:1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Beggs S. 2014. Sublime microglia: expanding roles for the guardians of the CNS. Cell 158:15–24. [DOI] [PubMed] [Google Scholar]
- Sanagi T, Yuasa S, Nakamura Y, Suzuki E, Aoki M, Warita H, Itoyama Y, Uchino S, Kohsaka S, Ohsawa K. 2010. Appearance of phagocytic microglia adjacent to motoneurons in spinal cord tissue from a presymptomatic transgenic rat model of amyotrophic lateral sclerosis. J Neurosci Res 88:2736–2746. [DOI] [PubMed] [Google Scholar]
- Satoh J, Tabunoki H, Ishida T, Yagishita S, Jinnai K, Futamura N, Kobayashi M, Toyoshima I, Yoshioka T, Enomoto K, Arai N, Arima K. 2011. Immunohistochemical characterization of microglia in Nasu-Hakola disease brains. Neuropathology 31:363–375. [DOI] [PubMed] [Google Scholar]
- Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E. 2012. Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma 29:946–956. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. 2010. Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8:e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, Wassmer E, Wolf NI, Ferreira P, Topcu M, Wanders RJ, Waterham HR, Ferdinandusse S. 2012. MRI as diagnostic tool in early-onset peroxisomal disorders. Neurology 78:1304–1308. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Forte G, Holtman L, den Burger JC, Sinjewel A, de Vries HE, Aronica E, Gorter JA. 2012. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia 53:1254–1263. [DOI] [PubMed] [Google Scholar]
- Verheijden S, Bottelbergs A, Krysko O, Krysko DV, Beckers L, De Munter S, Van Veldhoven PP, Wyns S, Kulik W, Nave KA, Ramer MS, Carmeliet P, Kassmann CM, Baes M. 2013. Peroxisomal multifunctional protein-2 deficiency causes neuroinflammation and degeneration of Purkinje cells independent of very long chain fatty acid accumulation. Neurobiol Dis 58:258–269. [DOI] [PubMed] [Google Scholar]
- Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, Kidd G, Zorlu MM, Sun N, Hu W, Liu L, Lee JC, Taylor SE, Uehlein L, Dixon D, Gu J, Floruta CM, Zhu M, Charo IF, Weiner HL, Ransohoff RM. 2014. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 211:1533–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT. 2014. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17:400–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1 Transcriptomics analysis of isolated microglia from Mfp2−/− and control brain
(A) Heatmap and unsupervised hierarchical clustering of RMA values. (B) Principle component analysis (PCA) of gene expression data. (C) Heatmaps of top 50 genes upregulated and downregulated in Mfp2−/− as compared to wild type microglia, ranked by fold change.