Abstract
Establishing the validity of appropriate use criteria (AUC) for percutaneous coronary intervention (PCI) in the setting of stable ischemic heart disease can support their adoption for quality improvement. We conducted a post hoc analysis of 2,287 Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation trial patients with stable ischemic heart disease randomized to PCI with optimal medical therapy (OMT) or OMT alone. Within appropriateness categories, we compared rates of death, myocardial infarction, revascularization subsequent to initial therapy, and angina-specific health status as determined by the Seattle Angina Questionnaire in patients randomized to PCI D OMT to those randomized to OMT alone. A total of 1,987 patients (87.9%) were mapped to the 2012 publication of the AUC, with 1,334 (67.1%) classified as appropriate, 551 (27.7%) uncertain, and 102 (5.1%) as inappropriate. There were no significant differences between PCI and OMT alone in the rate of mortality and myocardial infarction by appropriateness classification. Rates of revascularization were significantly lower in patients initially receiving PCI D OMT who were classified as appropriate (hazard ratio 0.65; 95% confidence interval 0.53 to 0.80; p <0.001) or uncertain (hazard ratio 0.49; 95% confidence interval 0.32 to 0.76; p = 0.001). Furthermore, among patients classified as appropriate by the AUC, Seattle Angina Questionnaire scores at 1 month were better in the PCI-treated group compared with the medical therapy group (80 ± 23 vs 75 ± 24 for angina frequency, 73 ± 24 vs 68 ± 24 for physical limitations, and 68 ± 23 vs 60 ± 24 for quality of life; all p <0.01), with differences generally persisting through 12 months. In contrast, health status scores were similar throughout the first year of follow-up in PCI D OMT patients compared with OMT alone in patients classified as uncertain or inappropriate. In conclusion, these findings support the validity of the AUC in efforts to improve health care quality through optimal use of PCI.
The appropriate use criteria (AUC) were developed through the collaborative efforts of multiple cardiovascular professional organizations to quantify the anticipated benefits of percutaneous coronary intervention (PCI), in terms of survival or health status outcomes, relative to the procedural risks for a given clinical scenario.1 Some have criticized the AUC for lacking evidence to support their validity, particularly as applied to patients with stable ischemic heart disease (SIHD).2,3 Providing empirical evidence to support the AUC ratings could enhance their use in supporting safer and potentially more cost-effective care. We leveraged the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) study, which randomized patients with SIHD to PCI and optimal medical therapy (OMT) or OMT alone, to assess the incremental benefits of PCI across different categories of appropriateness.4 Finding heterogeneity of health status treatment benefit with PCI that was greater in the more appropriate patients would validate the AUC as a tool for improving evidence-based and patientcentered care for patients with SIHD.
Methods
Details of the COURAGE trial have been described previously.4,5 Briefly, from 1999 to 2004, 2,287 of 3,071 eligible patients with stable coronary artery disease (CAD) were randomized at 50 US and Canadian centers to PCI with OMT or OMT alone. The study enrolled patients with at least a 70% diameter stenosis in 1 or more major epicardial coronary arteries and evidence of myocardial ischemia or a stenosis of at least 80% in 1 or more coronary arteries and classic angina pectoris without provocative testing. Patients were excluded if they had persistent Canadian Cardiovascular Society (CCS) class IV angina symptoms, a markedly positive stress test (substantial ST-segment depression or hypotensive response during stage 1 of the Bruce protocol), refractory heart failure or cardiogenic shock, the ejection fraction of <30%, revascularization in the previous 6 months, and coronary anatomy that was not amenable to PCI. Randomized patients were followed for a median 4.6 years (range 2.5 to 7.0 years).
Details regarding the methodology of AUC development have been described previously.1 In the AUC, PCI was considered “appropriate” for a clinical scenario when the expected benefits, in terms of survival or quality of life, exceeded the expected negative consequences of the procedure and “inappropriate” when the perceived risks outweighed potential benefits. Classification as “uncertain” in the AUC implies inadequate data to classify the balance of anticipated risk and benefit definitively. In patients with SIHD being considered for PCI, the principal determinants of procedural appropriateness were (1) extent of CAD, (2) ischemic risk, as determined by noninvasive testing, (3) intensity of antianginal therapy, and (4) symptom burden, as determined by CCS class.1 COURAGE was designed before to the first publication of AUC for PCI. We used baseline clinical and angiographic data to map COURAGE trial patients to an AUC scenario, which was then categorized as “appropriate,” “uncertain,” or “inappropriate” based on the 2012 publication of the AUC.1
Ascertainment of clinical outcomes in the COURAGE trial has been previously described.4 The primary outcome measure was a composite of death from any cause and nonfatal myocardial infarction (MI). Clinical outcomes were adjudicated by an independent committee whose members were blinded to patients’ treatment assignments. COURAGE also collected information on patient-reported health status, which was assessed using the Seattle Angina Questionnaire (SAQ) at baseline and at the 1-, 3-, 6-, 12-, 24-, and 36-month follow-up visits. The SAQ is a 19-item questionnaire that quantifies the frequency of angina, any recent change in the severity of angina, physical limitations because of angina, satisfaction with treatment, and quality of life.6–8 Scores range from 0 to 100, with higher scores indicating better health status. For this study, we focused our analyses on the most relevant SAQ domains: angina frequency, physical limitation, and quality of life. A clinically significant change is defined as a difference of ≥20 on the angina frequency scale, ≥8 points on the physical limitation scale, and ≥16 on the quality of life scale.9
To ensure balance between treatment strategies within each appropriateness category, we stratified COURAGE participants by their appropriateness class and compared baseline characteristics between PCI and OMT-only patients, including demographics, coronary anatomy, ischemic risk by noninvasive testing, medical therapy, symptom burden by CCS, and patient-reported health status of patients, within each AUC classification. Categorical variables were compared using frequencies and the chi-square tests or Fisher’s exact test, and means ± SD and t tests were used for continuous variables.
Within each appropriateness category (appropriate, uncertain, and inappropriate for PCI), we compared rates of death, nonfatal MI, and revascularization procedures subsequent to initial therapy in patients randomized to PCI + OMT to those randomized to OMT alone with Kaplan-Meier survival curves and log-rank tests. Similarly, within each appropriateness category, we evaluated whether there were differences in SAQ scores over time between patients randomized to PCI versus OMT alone. We compared SAQ scores for the angina frequency, physical limitations, and quality of life domains at baseline, 1, 3, 6, 12, 24, and 36 months using t tests. We then determined if SAQ scores were different for PCI versus OMT alone for different levels of appropriateness by test of interaction (appropriateness category by treatment) at these time points.
We also conducted repeated-measures analyses of the scores and the change from baseline of scores over time using maximum likelihood methods to estimate the average benefit of PCI over time. For these analyses, intermittent missing data (e.g., a missing observation at 6 but not at 12 months) were imputed using multivariate imputation by chained equations.10 We included in the models the squared and cubed effect of time and the interactions with treatment and appropriateness because of the rapid improvement in patients’ health status after randomization. A level of significance of p <0.01 was used for all analyses, consistent with all COURAGE post hoc analyses.
Results
Of the 2,287 patients randomized in the COURAGE trial, 1,987 (88%) could be mapped to the AUC at baseline, with most unmapped patients because of a lack of stress testing. Among patients mapped to the AUC, 986 (50%) were randomized to PCI + OMT as initial treatment and 1,001 (50%) to OMT alone. Of the patients who received PCI, 654 (66%) were classified as appropriate, 279 (28%) as uncertain, and 53 (5%) as inappropriate. Of the patients who received OMT alone, 680 (68%) were classified as appropriate, 272 (27%) as uncertain, and 49 (5%) as inappropriate.
As would be expected by randomization, patient characteristics were similar by treatment strategy within each appropriateness category (Table 1). Although patients were similar across treatment strategies, within categories of appropriateness, patient characteristics differed across categories of appropriateness (Supplementary Table 1). Compared with patients classified as inappropriate, those patients classified as appropriate were more likely to have multivessel disease, use ≥2 antianginal medications, and have at least CCS I angina. Furthermore, baseline SAQ scores for angina frequency, physical limitation, and quality of life were much lower, reflecting worse baseline health status in patients classified as appropriate.
Table 1.
Baseline patient characteristics stratified by appropriateness classification and initial treatment
| Characteristic | Appropriate | Uncertain | Inappropriate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PCI (n=654) |
OMT (n=680) |
P value | PCI (n=279) |
OMT (n=272) |
P value | PCI (n=53) |
OMT (n=49) |
P value | |
| Age, mean (SD) (years) | 62±10 | 63±10 | 0.33 | 61±10 | 60±10 | 0.71 | 61 ±11 | 60±10 | 0.32 |
| Men | 86% | 85% | 0.58 | 85% | 86% | 0.63 | 77% | 78% | 0.98 |
| White | 85% | 87% | 0.24 | 89% | 86% | 0.28 | 91% | 83% | 0.28 |
| Body mass index, mean (SD) (kg/m2) | 30±5 | 30±5 | 0.65 | 29±5 | 30±5 | 0.46 | 29±4 | 29±5 | 0.48 |
| Diabetes mellitus | 35% | 36% | 0.68 | 28% | 34% | 0.14 | 22% | 41% | 0.04 |
| Heart failure | 5% | 5% | 0.65 | 6% | 2% | 0.02 | 6% | 4% | 0.71 |
| Chronic lung disease | 10% | 13% | 0.12 | 7% | 12% | 0.04 | 6% | 10% | 0.48 |
| Current smoker (<30 days) | 27% | 26% | 0.51 | 31% | 32% | 0.84 | 34% | 43% | 0.42 |
| Cancer (other than skin) | 5% | 4% | 0.58 | 4% | 6% | 0.33 | 15% | 0% | <0.01 |
| Family history of CAD | 55% | 51% | 0.16 | 54% | 52% | 0.65 | 58% | 55% | 0.83 |
| Hypertension | 70% | 69% | 0.66 | 63% | 65% | 0.52 | 45% | 55% | 0.32 |
| Hypercholesterolemia | 47% | 52% | 0.08 | 53% | 52% | 0.74 | 49% | 59% | 0.31 |
| Prior myocardial infarction | 36% | 36% | 0.77 | 37% | 42% | 0.23 | 37% | 44% | 0.46 |
| Prior percutaneous coronary intervention | 16% | 15% | 0.64 | 13% | 14% | 0.71 | 8% | 12% | 0.51 |
| Prior coronary artery bypass grafting | 10% | 11% | 0.71 | 9% | 7% | 0.48 | 2% | 4% | 0.61 |
| Prior Stroke or TIA | 9% | 9% | 0.71 | 7% | 6% | 0.60 | 2% | 4% | 0.61 |
| Peripheral Vascular Disease | 8% | 9% | 0.49 | 4% | 7% | 0.16 | 11% | 10% | 0.86 |
| Systolic blood pressure, mean (SD) (mmHg) | 134±21 | 133±20 | 0.28 | 131±19 | 131±18 | 0.79 | 132±18 | 130±17 | 0.57 |
| Diastolic blood pressure, mean (SD) (mmHg) | 74±12 | 74±11 | 0.69 | 74±11 | 75±10 | 0.27 | 74±11 | 74±10 | 0.92 |
| Estimated GFR, mean (SD) (mL/min/1.73 m2) | 80±20 | 77±20 | <0.01 | 82±19 | 81 ±19 | 0.68 | 84±23 | 80±20 | 0.34 |
| Ejection fraction, mean (SD) (%) | 62±11 | 61±10 | 0.98 | 62±11 | 62±10 | 0.94 | 63±10 | 60±10 | 0.08 |
| No. of Coronary Arteries Narrowed | |||||||||
| 1 | 22% | 24% | 57% | 51% | 45% | 37% | |||
| 2 | 41% | 37% | 0.21 | 38% | 44% | 0.37 | 53% | 59% | 0.59 |
| 3 | 36% | 40% | 5% | 6% | 2% | 4% | |||
| Proximal Left Anterior Descending | 58% | 66% | 0.02 | 20% | 26% | 0.14 | 0% | 2% | 0.48 |
| Baseline medications | |||||||||
| β-blockers | 77% | 75% | 0.54 | 60% | 61% | 0.81 | 67% | 74% | 0.52 |
| Nitrates | 65% | 67% | 0.61 | 41% | 47% | 0.23 | 29% | 29% | 0.97 |
| ACE-I/ARBs | 52% | 52% | 0.84 | 53% | 51% | 0.61 | 43% | 66% | 0.05 |
| Statins | 68% | 68% | 0.92 | 63% | 66% | 0.44 | 53% | 58% | 0.67 |
| Calcium-channel blockers | 38% | 34% | 0.22 | 23% | 21.9% | 0.83 | 6.1% | 7.9% | 1.00 |
| ≥ 2 antianginal medications | 69% | 67% | 0.53 | 34% | 39.3% | 0.23 | 26.5% | 34.2% | 0.48 |
| Risk from stress test results | |||||||||
| Low | 2% | 1% | 4% | 4.0% | 36.6% | 51.3% | |||
| Intermediate | 54% | 50% | 0.35 | 86% | 88.6% | 0.64 | 63.4% | 48.7% | ND |
| High | 45% | 49% | 10% | 7.5% | 0% | 0% | |||
| Baseline health status | |||||||||
| CCS class | |||||||||
| None | 7% | 8% | 12% | 17% | 77% | 69% | |||
| I | 29% | 30% | 0.12 | 36% | 37% | 0.15 | 4% | 16% | ND |
| II | 35% | 39% | 41% | 39% | 19% | 14% | |||
| III | 29% | 24% | 11% | 7% | 0% | 0% | |||
| SAQ angina frequency score, mean ± SD (IQR) | 66±27 (40,90) |
66±26 (50,90) |
0.58 | 73±24 (60,100) |
76±24 (60,100) |
0.26 | 83±24 (75,100) |
85±23 (75,100) |
0.72 |
| SAQ physical limitation score, mean ± SD (IQR) | 64±25 (44,83) |
64±25 (44,83) |
0.88 | 70±24 (50,92) |
73±24 (53,94) |
0.29 | 78±20 (64,96) |
79±25 (69,100) |
0.88 |
| SAQ quality of life score, mean ± SD (IQR) | 50±25 (33,67) |
49 ±24 (33,67) |
0.36 | 52±26 (33,75) |
57±26 (42,83) |
0.05 | 64±25 (46,83) |
63±27 (42,83) |
0.86 |
Hypertension was defined by clinical history, systolic blood pressure >140 mm Hg, or diastolic blood pressure >90 mm Hg at the time of enrollment. Hyperlipidemia was defined by the patient’s clinical history at the time of enrollment.
ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CCS = Canadian Cardiovascular Society; GFR = glomerular filtration rate; IQR = interquartile range; OMT = optimal medical therapy; PCI = percutaneous coronary intervention; SAQ = Seattle Angina Questionnaire; SD = standard deviation; TIA = transient ischemic attack.
Outcomes of death, nonfatal MI, and revascularization stratified by appropriateness classification and initial treatment are listed in Table 2. There were no significant differences in the rates of death, MI, or the composite outcome of both comparing PCI versus OMT alone within each appropriateness category, and there was no significant interaction between the appropriateness categories and treatment. Of patients classified as appropriate or uncertain, those randomized to PCI + OMT were significantly less likely to undergo subsequent revascularization procedures. Rates of subsequent revascularization were not statistically different between treatment arms for patients classified as inappropriate for PCI, and the interaction between appropriateness category and treatment was not significant for subsequent revascularization.
Table 2.
Patient outcomes of death and non-fatal myocardial infarction stratified by appropriateness classification and initial treatment
| Outcome | Appropriateness Classification |
OMT | PCI | Hazard Ratio (95% Cl) | P Value | P Value for Interaction | ||
|---|---|---|---|---|---|---|---|---|
| Events | Rate | Events | Rate | |||||
| Death | Inappropriate | 4 (8%) | 7.2 | 1 (2%) | 2.0 | 0.22 (0.02, 1.94) | 0.17 | |
| Uncertain | 17 (6%) | 6.6 | 11 (4%) | 4.0 | 0.59 (0.28, 1.25) | 0.17 | 0.25 | |
| Appropriate | 61 (9%) | 8.2 | 56 (9%) | 8.8 | 0.96 (0.67, 1.37) | 0.81 | ||
| MI | Inappropriate | 7 (14%) | 15.7 | 5 (9%) | 9.7 | 0.63 (0.20, 2.01) | 0.43 | |
| Uncertain | 13 (5%) | 5.3 | 25 (9%) | 9.9 | 1.85 (0.95, 3.61) | 0.07 | 0.27 | |
| Appropriate | 88 (13%) | 13.6 | 91 (14%) | 14.6 | 1.11 (0.83, 1.49) | 0.48 | ||
| Death or MI | Inappropriate | 11 (22%) | 22.6 | 6 (11%) | 11.6 | 0.48 (0.18, 1.30) | 0.15 | |
| Uncertain | 28 (10%) | 11.3 | 34 (12%) | 13.1 | 1.15 (0.70, 1.90) | 0.58 | 0.22 | |
| Appropriate | 133 (20%) | 19.6 | 135 (21%) | 21.1 | 1.09 (0.86, 1.39) | 0.48 | ||
| Coronary revascularization procedure | Inappropriate | 10 (20%) | 26.3 | 5 (9%) | 9.8 | 0.42 (0.14, 1.22) | 0.11 | |
| Uncertain | 57 (21%) | 22.5 | 32 (11%) | 12.3 | 0.49 (0.32, 0.76) | 0.001 | 0.35 | |
| Appropriate | 232 (34%) | 36.1 | 154 (24%) | 25.0 | 0.65 (0.53, 0.80) | <0.001 | ||
Rates represent the estimated 4.6-year event rate.
MI = myocardial infarction; OMT = optimal medical therapy; PCI = percutaneous coronary intervention.
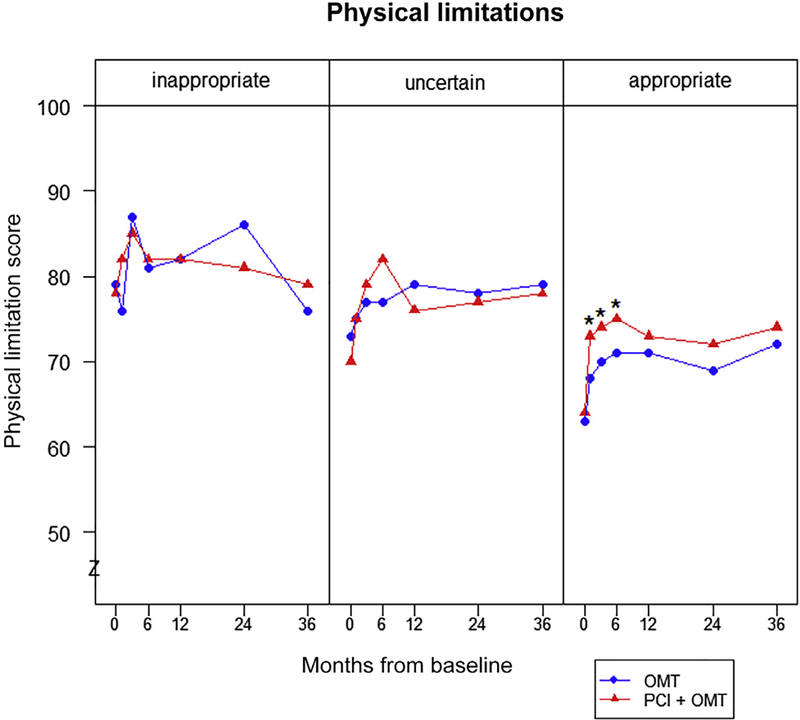
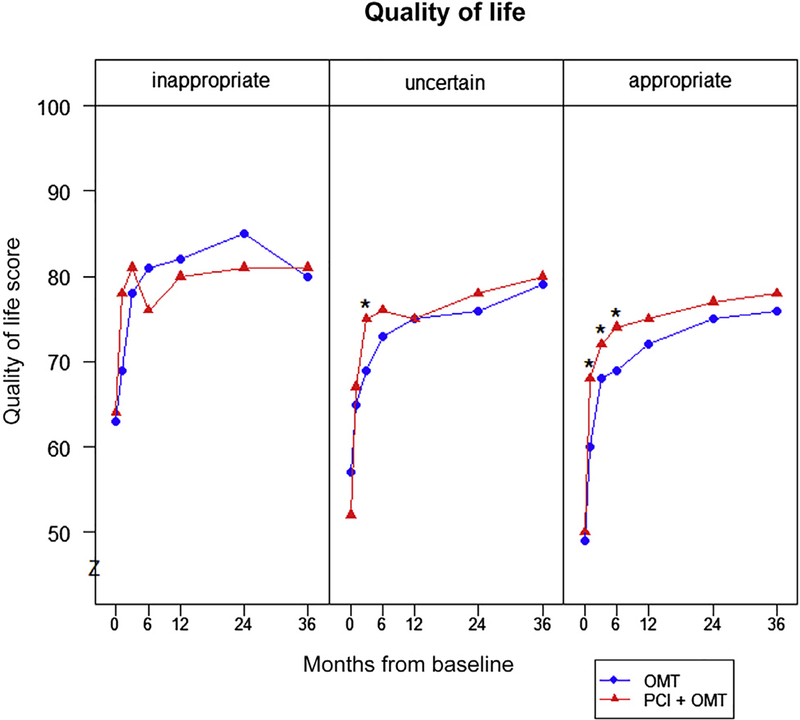
There were significant differences in the benefits of PCI for health status outcomes by appropriateness category. Among patients classified as appropriate, scores for angina frequency were significantly better in patients treated with PCI than OMT alone at 1 month and throughout the first 2 years of follow-up (Figure 1). Similarly, physical limitation and quality of life scores for patients with appropriate indications for revascularization were significantly higher in patients treated with PCI, compared with OMT alone, throughout the first 6 months of follow-up. In patients classified as uncertain for PCI, health status scores for all 3 SAQ domains were similar throughout follow-up between patients treated with PCI and OMT alone (Figure 2). Finally, there were no differences in health status scores between the 2 treatment groups for patients classified as inappropriate for PCI (Figure 3). Despite these differences by treatment within appropriateness categories, the interactions of appropriateness category by treatment were not significant for any of the SAQ domains (p >0.05 for all domains at all time points), likely because of the small sample of inappropriate patients randomized in COURAGE. The comparisons between PCI and OMT alone for each category of appropriateness are provided in the Supplementary Table 2.
Figure 1.
Mean angina frequency scores over time by appropriateness classification. *p <0.01.
Figure 2.
Mean physical limitation scores over time by appropriateness classification. *p <0.01.
Figure 3.
Mean quality of life scores over time by appropriateness classification. *p <0.01.
Repeated-measures analyses agreed with the primary findings (Supplementary Table 3). The repeated-measures analysis suggested that the average benefit of PCI + OMT relative to OMT alone was 8.7 points higher in patients classified as appropriate versus uncertain and 5.3 points higher for patients classified as uncertain versus inappropriate. The differences were 6.1 and 5.5 points for the SAQ quality of life and 7.4 and 5.1 points for the SAQ physical limitation scales, respectively (p <0.001 for all).
Discussion
Using the randomized allocation of revascularization in the COURAGE trial, we found that patients with SIHD rated appropriate for revascularization had better health status in the year after treatment with PCI + OMT than those treated with OMT alone. In contrast, there were no significant differences in patients’ health status after treatment with PCI in patients rated uncertain or inappropriate for PCI. PCI was also associated with lower rates of revascularization subsequent to initial therapy in patients rated as appropriate or uncertain for the procedure. In contrast, PCI was not associated with lower rates of mortality or nonfatal MI for any AUC category. Collectively, our findings provide empirical evidence to validate the AUC, by affirming the benefits of revascularization for reducing angina in patients with SIHD and an appropriate indication for PCI.
Although criteria to assess the appropriateness of coronary procedures were first developed nearly 30 years ago,11 their validity in assessing the quality of care have remained in question. This concern relates to the consensus method by which AUC are developed and the lack of prospective validation of the criteria.12–15 The present study adds significantly to previous observational studies that have examined the validity of the AUC for invasive coronary procedures. Many of these previous studies are not contemporary,16–19 were limited to the evaluation of coronary angiography rather than coronary revascularization,16,17 or did not assess health status outcomes in addition to mortality and acute coronary syndrome events—a notable limitation as the primary potential benefit of PCI in SIHD is to improve patients’ health status.16,17,20 Furthermore, as these previous studies evaluated the relation between procedural appropriateness and outcomes in routine clinical practice, there was the potential for selection bias as to who received revascularization under a given appropriateness rating. Our study was able to assess the validity of the AUC in the context of the COURAGE trial, which is less susceptible to selection bias given that the decision to treat patients with SIHD using PCI or medical therapy alone was randomized.
We found that, compared with OMT alone, patients with SIHD treated with PCI with appropriate indications for intervention had notable and sustained 1-year gains in angina frequency, physical limitations, and CAD-related quality of life, whereas there were no significant or sustained improvements with PCI in any of these health status domains in patients with uncertain or inappropriate AUC indications. It is important to note that COURAGE patients classified as inappropriate for PCI had generally good baseline health status, as reflected by their SAQ scores. In comparison, patients classified as appropriate had much lower baseline health status. These baseline comparisons offer additional face validity to the AUC given that the opportunity for health status improvement is much more limited in those with high SAQ scores and favorable health status before treatment. In fact, nearly 75% of patients classified as inappropriate for revascularization were asymptomatic and did not have the potential to have improved angina control as they had no angina to begin with.
Recent studies applying contemporary AUC to PCI registries in the United States suggest that at least 12% to 17% of PCIs performed for patients with SIHD are categorized as inappropriate.21–23 Application of the AUC represents an opportunity to improve PCI quality by reducing unnecessary complications and resource utilization associated with inappropriate procedures. Accordingly, ascertainment of procedural indication to inform PCI appropriateness has been incorporated in recently published PCI performance measures.24 Furthermore, open discussion with the patient about procedural appropriateness as a measure of anticipated procedural benefits relative to risks may lead to more patient-centered decisions in the use of revascularization therapy. From our findings, clinicians could inform patients rated as “appropriate” for PCI that they are, on average, more likely to have a better health status over time with PCI compared with OMT. Conversely, among those rated as “inappropriate,” there is unlikely to be any difference in their health status with the more invasive PCI strategy. The findings of the present study support the validity of the AUC in guiding future quality improvement efforts and patient-centered care.
The present study should be considered in light of the following potential limitations. Although we have complete outcomes for death and nonfatal MI, there were missing health status outcomes in COURAGE. Although we did not observe differences in the completeness of follow-up across treatment groups,10 we cannot exclude the possibility of some bias in our estimates. However, the results of our repeated-measures analyses that accounted for missing data were consistent with our primary findings. A second potential limitation relates to the number of inclusion and exclusion criteria in COURAGE (e.g., left main disease, depressed ejection fraction) that preclude a complete assessment of all the AUC scenarios; therefore, our findings are not generalizable to these patient populations. Similar concerns about the generalizability of the COURAGE trial in relation to women and nonwhites also pertain to the present analysis. A third concern is that the entry criteria for COURAGE led to the vast majority of patients being appropriate for revascularization, and it is possible we were underpowered in our ability to precisely define the benefits or harms of PCI in patients considered inappropriate for PCI. This limitation in power is further suggested by the lack of statistical significance for our tests of interaction of appropriateness category by treatment for any of the SAQ domains and the lack of clinical significance in SAQ score differences. A fourth concern relates to the control arm of medical therapy being administered to a very high standard in the trial, which may be challenging to achieve in clinical practice.25–27 This may affect the relative difference in health status between PCI and OMT alone for SIHD when applied to routine clinical practice. Although randomization was preserved in the 2 treatment arms in the AUC subgroups, this study was a post hoc analysis, and thus, the results should be considered exploratory and hypothesis generating. Furthermore, the results from COURAGE do not reflect recent advances in PCI, particularly with drugeluting stents.4,10 Additionally, COURAGE had a minority of patients with moderate or severe ischemia.28 Patients with a greater degree of ischemia are currently being studied in the International Study of Comparative Health Effectiveness With Medical and Invasive Approaches trial.29 It is also important to note that the AUC reflect a large number of clinical scenarios that are categorized into 3 broad categories of appropriateness. We are unable to assess for heterogeneity of effect within categories of appropriateness in the present study given our sample size, and this is an important area for future research. Finally, the results of the COURAGE trial reflect an initial strategy of PCI + OMT versus OMT alone. Just under one third of patients in COURAGE crossed over from OMT to PCI during followup.4 As the use of subsequent revascularization is a process of care that does not reflect the initial decision to perform PCI, we did not separately analyze the outcomes of the subgroup of crossover patients.
Supplementary Material
Disclosures
This study was supported by the Cooperative Studies Program of the US Department of Veterans Affairs Office of Research and Development (Washington, DC), in collaboration with the Canadian Institutes of Health Research (Ottawa, Ontario, Canada), and by unrestricted research grants from Merck, Pfizer, Bristol-Myers Squibb, Fujisawa, Kos Pharmaceuticals, Datascope, AstraZeneca, Key Pharmaceutical, Sanofi-Aventis, First Horizon, and GE Healthcare, including in-kind support with Food and Drug Administration-approved drugs used by study participants. All industrial funding in support of the trial was directed through the US Department of Veterans Affairs. Dr. Bradley is supported by VA Health Services Research and Development Career Development Award (HSR&D-CDA2 10199). Dr. Weintraub has received consulting fees from Sanofi-Aventis and Bristol-Myers Squibb and grant support from Sanofi-Aventis. Dr. Berman reports receiving grant support, consulting fees, and lecture fees from Floura Pharma, Bracco Diagnostics, GE Healthcare, Siemens, Lantheus Medical Imaging, and Astellas Healthcare and software royalties from Cedars-Sinai Medical Center. Dr. Teo reports receiving lecture and consulting fees and grant support from Boehringer Ingelheim. Dr. Boden reports receiving honoraria and lecture fees from Gilead and Sanofi-Aventis. Dr. Mancini reports receiving consulting fees from Pfizer, AstraZeneca, and GlaxoSmithKline and lecture fees from Merck, AstraZeneca, GlaxoSmithKline, and Sanofi-Aventis. Dr. Spertus discloses that he holds the copyright to the SAQ and has received grant support from Lilly and Genentech and as a consultant to United Healthcare, St. Jude Medical, and the American Heart Association. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The other authors report no conflicts to disclose.
Footnotes
Disclaimer: The views expressed in this report represent those of the authors and do not necessarily represent the official views, position, or policy of the Department of Veterans Affairs or the US government.
Supplementary Data
Supplementary data related with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.amjcard.2015.03.057.
References
- 1.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2012;59:857–881. [DOI] [PubMed] [Google Scholar]
- 2.Marso SP, Teirstein PS, Kereiakes DJ, Moses J, Lasala J, Grantham JA. Percutaneous coronary intervention use in the United States defining measures of appropriateness. JACC Cardiovasc Interv 2012;5:229–235. [DOI] [PubMed] [Google Scholar]
- 3.Kereiakes DJ, Stone GW. Appropriate use criteria to reduce underuse and overuse of revascularization. J Am Coll Cardiol 2013;61:2024. [DOI] [PubMed] [Google Scholar]
- 4.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 5.Boden WE, O’rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk W, Knudtson M, Dada M, Casperson P, Harris CL, Spertus JA, Shaw L, Chaitman BR, Mancini GBJ, Berman DS, Weintraub WS; COURAGE trial coprincipal investigators and study coordinators. Design and rationale of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) Trial Veterans Affairs cooperative studies program no. 424. Am Heart J 2006;151:1173–1179. [DOI] [PubMed] [Google Scholar]
- 6.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 7.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol 1994;74:1240–1244. [DOI] [PubMed] [Google Scholar]
- 8.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation 2002;106:43–49. [DOI] [PubMed] [Google Scholar]
- 9.Wyrwich KW, Spertus JA, Kroenke K, Tierney WM, Babu AN, Wolinsky FD; Heart Disease Expert Panel. Clinically important differences in health status for patients with heart disease: an expert consensus panel report. Am Heart J 2004;147:615–622. [DOI] [PubMed] [Google Scholar]
- 10.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O’Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008;359:677–687. [DOI] [PubMed] [Google Scholar]
- 11.Chassin MR, Park RE, Fink A, Rauchman S, Keesey J, Brook RH. Indications for Selected Medical and Surgical Procedures: A Literature Review and Ratings of Appropriateness: Coronary Artery Bypass Surgery. Santa Monica: RAND, 1986; Publication R-3204/2-CWF-HF-HCFA-PMT-RWJ. [Google Scholar]
- 12.Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health 1984;74: 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassirer JP. The quality of care and the quality of measuring it. N Engl J Med 1993;329:1263–1265. [DOI] [PubMed] [Google Scholar]
- 14.Mulley AG Jr, Eagle KA. What is inappropriate care? JAMA 1988;260: 540–541. [PubMed] [Google Scholar]
- 15.Hicks NR. Some observations on attempts to measure appropriateness of care. BMJ 1994;309:730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby JV, Fireman BH, Lundstrom RJ, Swain BE, Truman AF, Wong CC, Froelicher ES, Barron HV, Hlatky MA. Variation among hospitals in coronary-angiography practices and outcomes after myocardial infarction in a large health maintenance organization. N Engl J Med 1996;335:1888–1896. [DOI] [PubMed] [Google Scholar]
- 17.Hemingway H, Chen R, Junghans C, Timmis A, Eldridge S, Black N, Shekelle P, Feder G. Appropriateness criteria for coronary angiography in angina: reliability and validity. Ann Intern Med 2008;149:221–231. [DOI] [PubMed] [Google Scholar]
- 18.Kravitz RL, Laouri M, Kahan JP, Guzy P, Sherman T, Hilborne L, Brook RH. Validity of criteria used for detecting underuse of coronary revascularization. JAMA 1995;274:632–638. [PubMed] [Google Scholar]
- 19.Hemingway H, Crook AM, Feder G, Banerjee S, Dawson JR, Magee P, Philpott S, Sanders J, Wood A, Timmis AD. Underuse of coronary revascularization procedures in patients considered appropriate candidates for revascularization. N Engl J Med 2001;344:645–654. [DOI] [PubMed] [Google Scholar]
- 20.Ko DT, Guo H, Wijeysundera HC, Natarajan MK, Nagpal AD, Feindel CM, Kingsbury K, Cohen EA, Tu JV; Cardiac Care Network (CCN) of Ontario Variations in Revascularization Practice in Ontario (VRPO) Working Group. Assessing the association of appropriateness of coronary revascularization and clinical outcomes for patients with stable coronary artery disease. J Am Coll Cardiol 2012;60:1876–1884. [DOI] [PubMed] [Google Scholar]
- 21.Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, Nallamothu BK, Weaver WD, Masoudi FA, Rumsfeld JS, Brindis RG, Spertus JA. Appropriateness of percutaneous coronary intervention. JAMA 2011;306:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley SM, Maynard C, Bryson CL. Appropriateness of percutaneous coronary interventions in Washington state. Circ Cardiovasc Qual Outcomes 2012;5:445–453. [DOI] [PubMed] [Google Scholar]
- 23.Hannan EL, Cozzens K, Samadashvili Z, Walford G, Jacobs AK, Holmes DR, Stamato NJ, Sharma S, Venditti FJ, Fergus I, King SB. Appropriateness of coronary revascularization for patients without acute coronary syndromes. J Am Coll Cardiol 2012;59:1870–1876. [DOI] [PubMed] [Google Scholar]
- 24.Nallamothu BK, Tommaso CL, Anderson HV, Anderson JL, Cleveland JC, Dudley RA, Duffy PL, Faxon DP, Gurm HS, Hamilton LA, Jensen NC, Josephson RA, Malenka DJ, Maniu CV, McCabe KW, Mortimer JD, Patel MR, Persell SD, Rumsfeld JS, Shunk KA, Smith SC, Stanko SJ, Watts B. ACC/AHA/SCAI/AMA-convened PCPI/NCQA 2013 performance measures for adults undergoing percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on performance measures, the Society for Cardiovascular Angiography and Interventions, the American Medical Association-Convened Physician Consortium for performance improvement, and the National Committee for Quality Assurance. J Am Coll Cardiol 2014;63:722–745. [DOI] [PubMed] [Google Scholar]
- 25.Maron DJ, Boden WE. As REGARDS treatment goal attainment compared with COURAGE: the perfect should not be the enemy of the good. J Am Coll Cardiol 2014;63:1634–1635. [DOI] [PubMed] [Google Scholar]
- 26.Maron DJ, Boden WE, Weintraub WS, Calfas KJ, O’Rourke RA. Is optimal medical therapy as used in the COURAGE trial feasible for widespread use? Curr Treat Options Cardiovasc Med 2011;13:16–25. [DOI] [PubMed] [Google Scholar]
- 27.Kereiakes DJ, Teirstein PS, Sarembock IJ, Holmes DR Jr, Krucoff MW, O’Neill WW, Waksman R, Williams DO, Popma JJ, Buchbinder M, Mehran R, Meredith IT, Moses JW, Stone GW. The truth and consequences of the COURAGE trial. J Am Coll Cardiol 2007;50: 1598–1603. [DOI] [PubMed] [Google Scholar]
- 28.Shaw LJ, Berman DS, Maron DJ, Mancini GBJ, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117: 1283–1291. [DOI] [PubMed] [Google Scholar]
- 29.International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA)—Full Text View—ClinicalTrials.gov. Available at: http://clinicaltrials.gov/show/NCT01471522. Accessed July 24, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.