Abstract
In a recent study, repeated cold application induced beiging in subcutaneous white adipose tissue (SC WAT) of humans independent of body mass index. To identify factors that promote or inhibit beiging, we performed multiplex analysis of gene expression with the Nanostring nCounter system (the probe set contained genes for specific immune cell markers, cytokines, and chemokines) on the SC WAT from lean subjects. Multiple correlations analysis identified mast cell tryptase and CCL26, a chemokine for mast cells, as genes whose change correlated positively with the change in UCP1 in SC WAT, leading to the hypothesis that mast cells promote SC WAT beiging in response to cold. We quantified mast cell recruitment into SC WAT and degranulation. Mast cells increased in number in SC WAT in lean subjects, and there was an increase in the number of degranulated mast cells in both lean subjects and subjects with obesity. We determined that norepinephrine stimulated mast cell degranulation and histamine release in vitro. In conclusion, cold stimulated adipose tissue mast cell recruitment in lean subjects and mast cell degranulation in SC WAT of all research participants independent of baseline body mass index, suggesting that mast cells promote adipose beiging through the release of histamine or other products.
Subject terms: Diseases, Endocrine system and metabolic diseases
Introduction
Subcutaneous white adipose tissue (SC WAT) of adult humans is usually composed of adipocytes that function to store lipid in a single large lipid droplet. In response to cold or β-adrenergic receptor agonists, SC WAT undergoes a process called beiging1. Beige adipose has increased abundance of uncoupling protein 1 (UCP1), which promotes thermogenesis by uncoupling the electron transport chain in mitochondria. In addition to thermogenesis, beige adipocytes are associated with improved glucose and lipid metabolism in rodents2,3. Thus, understanding mechanisms that regulate beiging is an important goal towards identifying strategies that can be therapeutically exploited to improve glucose and lipid homeostasis. Beiging has been extensively studied in rodents, and more recently, beiging of SC WAT has been demonstrated in humans4–12. Our recent study indicates that beige adipose can be induced in lean humans or humans with obesity by cold or by treatment of subjects with obesity with the β3 agonist mirabegron6.
In addition to β-adrenergic signaling and the sympathetic nervous system, the immune system has been shown to modify adipose beiging (recently reviewed13). Studies in mice have implicated macrophages, eosinophils, type 2 innate lymphoid cells, and iNKT cells in SC WAT beiging13. In addition, our recent study in humans implicated mast cells in the seasonal regulation of UCP17. We found that mast cells function as cold sensors that release histamine, which stimulates lipolysis and UCP1 induction in adipocytes7. These immune cells interact in complicated ways with each other, with adipocytes, and with nerves to influence catecholamine levels, innervation, and the production of numerous factors that promote adipose beiging13–22. Alternatively, macrophage retention in adipose tissue has been shown in mice to inhibit beiging23, and in vitro studies have shown that macrophage conditioned medium and inflammatory cytokines inhibit UCP1 expression in adipocytes5,23–26.
We have recently shown that an acute localized cold exposure on one leg induced SC WAT beiging in humans6. Cold increased UCP1 and TMEM26 to the same extent in SC WAT from both legs, suggesting that SNS activation induces beiging rather than a localized decrease in SC WAT temperature6. Here, in that same cohort of lean subjects, we measured SC WAT gene expression of immune cell markers, cytokines, chemokines, and proteins involved in adipocyte function and dysfunction to gain further mechanistic insight into the beiging response in humans. We performed multiple correlations analysis to identify genes whose change in expression correlated with the change in UCP1 protein expression. In the cold treated leg, this analysis identified tryptase, an enzyme specifically expressed by mast cells, and CCL26, a chemokine for CCR3 receptor expressing cells, including mast cells, basophils, and eosinophils27–29, implicating mast cells in the induction of UCP1 expression by cold. We hypothesized that mast cell degranulation is involved in SC WAT beiging in response to cold, and therefore characterized mast cell recruitment and degranulation in SC WAT in response to cold and performed in vitro studies on mast cell degranulation in response to cold and norepinephrine.
Research Design and Methods
Human subjects and study design
The baseline characteristics and additional details about the research participants have been described elsewhere6. In brief, subjects were recruited from the Lexington, KY area in the summer (June 1 and September 15; mean temperature 20–24 °C). Baseline biopsies of thigh adipose were performed, the subjects then applied an icepack to one leg for 30 minutes each day for 10 consecutive days, and thigh biopsies were performed on the cold treated leg and the contralateral leg6. All subjects gave informed consent, and the protocols were approved by the Institutional Review Board at the University of Kentucky. All experiments were performed in accordance with relevant guidelines and regulations. The Clinicaltrials.gov registration identifier is NCT02596776 (date of registration: 04/11/2015).
mRNA quantification
We used the Nanostring ncounter multiplex system to measure the expression of 130 genes and six housekeeping genes in purified RNA from SC WAT. The genes in the code set are described in Table S1 and reference30. βAR receptor expression was determined by real-time RT PCR as described7. The primer sequences are in Table S2.
Immunohistochemistry
Mast cells were identified in SC WAT using mouse anti-tryptase (#sc-33676, Santa Cruz Biotechnology Inc, Dallas, TX). Sections were deparaffinized, subjected to antigen retrieval, blocked with 5% normal goat serum followed with a Streptavidin/Biotin block (# SP-2002, Vector Labs, Burlingame, CA), and then incubated consecutively with anti-tryptase primary antibody overnight. Samples were rinsed and incubated with biotinylated goat anti-mouse antibody (# 1-065-003, Jackson ImmunoResearch, West Grove, PA), strepavidin-HRP (#S911, Life Technologies, Carlsbad, CA), and then AlexaFluor 594 tyramide reagent (#B40957, Invitrogen, Carlsbad, CA). The slides were cover slipped using vectashield with DAPI (Vector Labs). Mast cells were counted in the non-fibrotic areas of the adipose tissue using images captured with a Zeiss AxioImager MI upright fluorescent microscope (Zeiss, Gottingen, Germany), and analysis was performed using Zen software (Zeiss). Degranulated mast cells were defined as having irregular shape with jagged edges and visible tryptase-filled vacuoles surrounding the cell. Capillary and vessel density was determined by staining with lectin-TRITC (#L4889, Sigma-Aldrich). Sections were prepared as above and incubated with lectin-TRITC for 2 hours followed by 4%PFA post fixation, and then cover slipped with vectashield with DAPI. Capillaries were counted as structures between 5 and 10 microns and vessels were above 10 microns.
Histamine release
TIB64 cells (P815, ATCC, Manassas, VA) were grown at 37 °C in DMEM (#11885-092; Thermo Fisher Scientific, Grand Island, NY) with10% fetal bovine serum (FBS; #101; Tissue Culture Biologicals, Tulare, CA). One mL of TIB64 cells at a concentration of 1 × 106 cells/mL was transferred to a centrifuge tube, spun down to remove growth medium, and resuspended in 2% FBS-DMEM medium warmed to the indicated temperature; the medium contained 100 nM norepinephrine as indicated. The cells were incubated at 37 °C or 32 °C for the indicated times and medium was harvested by centrifugation. Histamine was detected with a histamine EIA kit (Cayman Chemical, Ann Arbor, MI) following the manufacturer’s protocol.
Statistics
Paired student’s t tests were conducted in Graphpad Prism version 7.0. The change in UCP1 protein determined by immunohistochemistry (post-pre) was determined from our previous study6. Histamine release was analyzed by one-way analysis of variance to determine group differences at both 120 and 240 minutes. Furthermore, pairwise comparisons were made based on Fisher’s least significant difference approach. Repeated measures multivariate analysis of variance (RM MANOVA) was performed as described6 to analyze mast cell recruitment and degranulation. Normality was assessed via the use of Q-Q plots. SAS version 9.4 was utilized for these analyses.
Results
Repeated cold exposure changes immune cell and angiogenic gene expression in SC WAT
We have recently characterized beiging of human SC WAT in response to cold6. The experimental design of that study was to biopsy SC WAT of one leg (baseline), apply an ice pack to that leg for 30 minutes a day for 10 days, and then perform SC WAT biopsies on both legs6. This design allowed us to address direct effects of cold and effects on sympathetic nervous system activation by cold (contralateral leg) on beiging. We observed equivalent beiging after cold exposure in SC WAT from both legs in 26 subjects with a wide range of body mass index (BMI), and notably, neither baseline body mass index nor insulin sensitivity affected the beiging response6. To gain a better understanding of human adipose beiging, we analyzed the mRNA expression of genes involved in adipocyte function, lipid metabolism, immune cell markers, chemokines, and inflammation (Table S1) in SC WAT obtained from lean subjects. As shown in Table 1, there was a significant decrease in CD68, a pan macrophage marker, and a significant increase in MRC2, a marker of alternative macrophage activation, in the cold treated leg. Cold decreased the gene expression of MMP9 and increased TIMP2 and thrombospondin 2. These gene changes suggest that macrophages and tissue remodeling are potentially involved in adipose tissue beiging, and this was further evaluated by multiple correlation analysis as described below. Finally, cold increased PPAR delta expression, which is involved in lipid catabolism.
Table 1.
Gene Expression Changed in SC WAT of the Cold Treated Leg in lean research participants.
| Gene Symbola | Name | Pre | Post | Fold Change Post/Pre | P |
|---|---|---|---|---|---|
| ANGPT2 | Angiopoietin 2 | 128 ± 9 | 181 ± 21 | 1.42 | 0.003 |
| F3 | Tissue Factor | 737 ± 45 | 810 ± 49 | 1.10 | 0.011 |
| MMP9 | Matrix metallopeptidase 9 | 503 ± 134 | 262 ± 69 | 0.52 | 0.017 |
| CD68 | Cluster of Differentiation 68 | 1251 ± 185 | 843 ± 99 | 0.67 | 0.017 |
| UCP2 | uncoupling protein 2 | 816 ± 64 | 687 ± 69 | 0.84 | 0.017 |
| PPARD | Peroxisome proliferator-activated receptor delta | 44 ± 2 | 54 ± 3 | 1.25 | 0.017 |
| MRC2 | mannose receptor C type 2 | 513 ± 53 | 655 ± 78 | 1.28 | 0.031 |
| FBXO31 | F-box only protein 31 | 87 ± 5 | 105 ± 6 | 1.22 | 0.037 |
| TIMP2 | Tissue inhibitor of metalloproteinases 2 | 3054 ± 191 | 3514 ± 259 | 1.15 | 0.037 |
| THBS2 | Thrombospondin 2 | 505 ± 45 | 652 ± 76 | 1.29 | 0.038 |
| NFE2L2 | Nuclear factor (erythroid-derived 2)-like 2 | 958 ± 24 | 1055 ± 37 | 1.10 | 0.048 |
aSC WAT was isolated before and after cold treatment and gene expression determined with the Nanostring nCounter system as described in research design and methods. The data are presented as means (nCounter counts) ± the standard error of the mean (n = 12; two-tailed, paired student’s t-test).
We also analyzed gene expression in the contralateral leg since there was an increase in UCP1 in SC WAT that was similar to the cold-treated leg6. As shown in Table 2, different genes were changed in SC WAT of the contralateral leg in comparison to the cold-treated leg. Strikingly, the five genes with the lowest P values were all related to the regulation of angiogenesis including angiopoietin-2, which was also upregulated in the cold treated leg (Table 1). The expression of VEGFA was increased, and VEGFA has been found to stimulate adipose tissue beiging31,32. Because of these changes in angiogenic genes, we examined the vascularity (capillary density and larger blood vessels) the SC WAT from the cold-treated and contralateral legs of five random subjects, but did not find significant changes (Fig. S1). Other genes changed included tryptase, a marker of mast cells, and CCL26, which is chemotactic for CCR3 expressing cells such as basophils, mast cells, and eosinophils27–29. This finding was of interest since we recently defined a role for mast cells in seasonal induction of UCP1 mRNA7.
Table 2.
Gene Expression Changed in SC WAT of the Contralateral Leg in lean research participants.
| Gene Symbola | Name | Pre | Post | Fold Change Post/Pre | P |
|---|---|---|---|---|---|
| VEGFA | Vascular endothelial growth factor A | 1312 ± 78 | 1548 ± 82 | 1.18 | 0.006 |
| ANGPT2 | Angiopoietin 2 | 128 ± 9 | 163 ± 15 | 1.27 | 0.007 |
| ANGPT4 | Angiopoietin 4 | 14 ± 1 | 11 ± 1 | 0.75 | 0.009 |
| ANGPT1 | Angiopoietin 1 | 932 ± 84 | 826 ± 99 | 0.89 | 0.014 |
| ANGPTL4 | Angiopoietin Like 4 | 2063 ± 157 | 1585 ± 177 | 0.77 | 0.024 |
| FNDC5 | Fibronectin type III domain-containing protein 5 | 26 ± 3 | 21 ± 2 | 0.80 | 0.034 |
| TPSAB1 | Tryptase | 2562 ± 318 | 3172 ± 398 | 1.24 | 0.034 |
| IL18 | Interleukin-18 | 80 ± 9 | 98 ± 10 | 1.23 | 0.047 |
| CCL26 | Chemokine (C-C motif) ligand 26 | 13 ± 2 | 17 ± 2 | 1.31 | 0.048 |
| TNFRSF12A | TWEAK receptor | 17 ± 2 | 14 ± 2 | 0.84 | 0.050 |
aSC WAT was isolated before and after Cold treatment and gene expression determined with the Nanostring nCounter system as described in research design and methods. The data are presented as means (nCounter Counts) ± the standard error of the mean (n = 12; two-tailed, paired student’s t-test).
The analysis of gene expression above revealed numerous changes that may be related to the induction of UCP1 protein. We performed a multiple correlations analysis to identify changes in gene expression that predict the increase in UCP1 protein expression in SC WAT of the cold treated leg (Table 3) using the data on UCP1 protein expression that we previously determined6. This analysis identified the change in tryptase and CCL26 gene expression as positively correlated to the change in UCP1 protein expression (Table 3), suggesting mast cells are involved in the induction of UCP1 by cold. Figure 1A shows the positive correlation between the change in tryptase and the change in UCP1 expression in SC WAT of the cold treated leg, and Fig. 1B shows the result for CCL26. Tryptase was not found as a significantly increased gene in Table 1. Closer examination of tryptase gene expression in the cold treated leg revealed that the gene expression is not normally distributed at baseline and that tryptase gene expression significantly increases in the cold treated leg if non parametric analysis is used (Fig. 1C). We also investigated the relationship between tryptase and UCP1 in SC WAT of the contralateral leg and found a trend between the change in tryptase and the change in UCP1 protein expression (Fig. S2). Together, these findings suggest that cold stimulates adipose tissue mast cells, and this contributes to UCP1 induction. We also performed the multiplex analysis of gene expression in research participants with obesity and found that tryptase expression did not change significantly in either the cold treated or contralateral leg. Overall, the response of subjects with obesity was different and will be reported in the future. Notably, tryptase mRNA expression was not significantly altered by cold in the research participants with obesity.
Table 3.
Multiple Correlations Analysis of Change in Gene Expression with Change in SC WAT UCP1 protein of the Cold Treated Leg.
| Gene Symbola | Name | r | P |
|---|---|---|---|
| Positive correlation | |||
| TPSAB1 | Tryptase | 0.77 | 0.0033 |
| CCL26 | Chemokine (C-C motif) ligand 26 | 0.71 | 0.0092 |
| Negative correlation | |||
| ANGPTL4 | Angiopoietin Like 4 | −0.80 | 0.0016 |
| TNFRSF12A | TWEAK receptor | −0.63 | 0.0324 |
| CIDEA | cell death-inducing DNA fragmentation factor alpha-like effector A | −0.58 | 0.0464 |
| ADIPOR2 | Adiponectin receptor 2 | −0.58 | 0.0467 |
aSC WAT was isolated before and after cold treatment6 and gene expression determined with the Nanostring nCounter system as described in research design and methods. We performed multiple correlations analysis to identify changes in gene expression that predicted the change in UCP1 protein expression as described in research design and methods. The data used to calculate the change in UCP1 were reported in6. Pearson correlation coefficients (r) and P values are given.
Figure 1.

Correlations of changes in gene expression with changes in UCP1. The change in UCP1 protein expression in SC WAT by acute cold treatment (post – pre) was calculated for the cold treated and contralateral legs using our previously published results6. The change in UCP1 protein is plotted versus the change in gene expression of genes identified by multiple correlations analysis. (A,B) The change of tryptase or CCL26 versus the change in UCP1 protein the cold leg are shown. The data were analyzed by linear regression analysis, and Pearson correlation coefficients (r) and P values are shown (n = 12). (C) The quantification of tryptase gene expression at baseline and after cold in SC WAT of the cold treated leg is shown. Data represent means ± SEM (n = 12). *P < 0.05 (Wilcoxon matched-pairs signed rank test).
SC WAT mast cells increase in number in lean subjects and degranulate in response to cold
Cold could affect mast cell recruitment, stimulate mast cell degranulation, or affect both. We therefore investigated whether cold affects SC WAT mast cells in both the cold treated and contralateral leg of all subjects of the study6. Mast cells are dispersed throughout adipose tissue and tend to be enriched in fibrotic areas of fat (Fig. S3); however, not all adipose tissue samples contain fibrotic areas, and hence we only counted mast cells in the non-fibrotic areas. Representative images of mast cell staining at baseline and after cold (cold treated leg) are shown in Fig. 2A,B, and the quantification of mast cell density is shown in Fig. 2C. Cold significantly increased the mast cell per adipocyte ratio by approximately 1.6-fold in the cold-treated and contralateral legs of lean subjects (Fig. 2C; cold: P < 0.01; contralateral: P < 0.01). As expected33, subjects with obesity had a higher baseline level of mast cells than lean subjects (Fig. 2C; P < 0.01), and mast cells did not increase in number in SC WAT of the cold treated or contralateral legs of subjects with obesity (Fig. 2C), consistent with the lack of change in mast cell tryptase gene expression noted above.
Figure 2.
Mast cell density and degranulation in SC WAT of research participants in response to acute cold treatment. (A,B) Tryptase staining of SC WAT at baseline and after cold (scale bar for C: 50 μm; scale bar for D: 100 μm). White arrows point to intact mast cells and yellow arrows point to mast cells with diffuse, punctate staining. (C) Quantification of mast cell density in lean subjects and subjects with obesity at baseline and in SC WAT from the cold and contralateral legs after 10 days of acute cold exposure. (D,E) Higher magnification images demonstrating degranulated and intact mast cells (scale bar: 20 μm). (F) Quantification of degranulated mast cells in lean subjects and subjects with obesity at baseline and in SC WAT from the cold and contralateral legs after 10 days of acute cold exposure. Data represent means ± SEM. Data were analyzed by RM MANOVA as described in research design and methods. **P < 0.01; ****P < 0.0001 (lean n = 17; obese n = 8).
We have previously shown that cold stimulates mast cell degranulation and histamine release in vitro and that histamine stimulates adipocyte UCP1 expression7. Here, we noted a diffuse pattern of tryptase staining in the adipose tissue after cold, indicating mast cell degranulation (Fig. 2B). Therefore, we determined whether cold stimulates mast cell degranulation in vivo in response to cold. When mast cells degranulate the pattern of tryptase staining changes from cellular staining, usually in a circular pattern, to diffuse staining that is punctate and larger than unstimulated mast cells. Closer examination of the pattern of tryptase staining in SC WAT of the cold treated leg revealed tryptase staining of intact and degranulated mast cells. Images of tryptase staining taken at higher magnification are shown in Fig. 2D,E. We quantified the number of degranulated mast cells and observed that cold treatment stimulated mast cell degranulation in SC WAT from both the cold-treated and contralateral legs in lean subjects (Fig. 2F; cold: P < 0.0001; contralateral: P < 0.01). Cold also stimulated mast cell degranulation in SC WAT from both legs in subjects with obesity (Fig. 2F; cold: P < 0.0001; contralateral: P < 0.01), even though there was no increase in total mast cells (Fig. 2C).
In vitro studies on mast cell degranulation
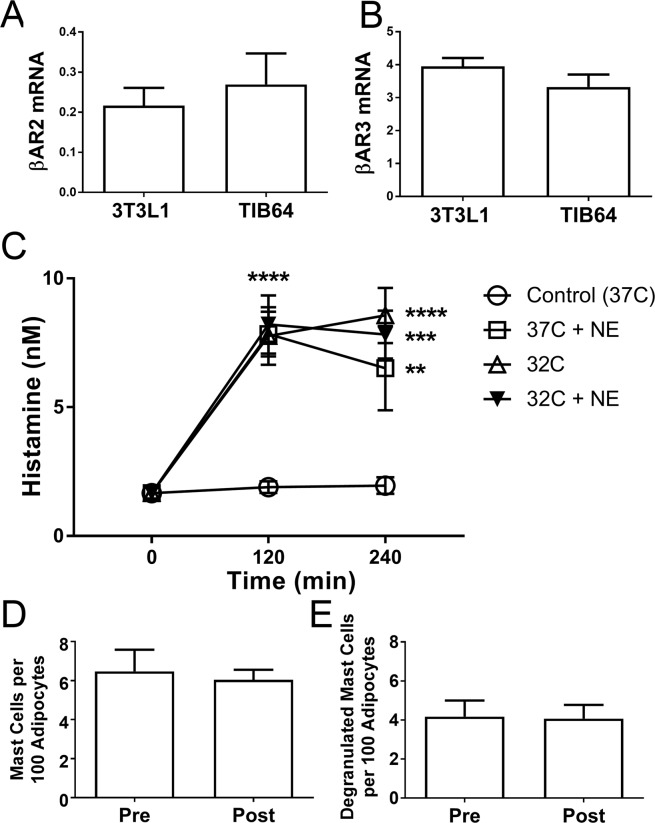
We previously found that cold causes mast cell degranulation in vitro, which possibly explains mast cell degranulation in the cold treated leg in vivo. However, the contralateral leg demonstrated equivalent mast cell degranulation (Fig. 2F), suggesting a different mechanism, and not solely a temperature effect. We previously found that the level of UCP1 and TMEM26 induction is the same in SC WAT of the contralateral leg as the cold treated leg, suggesting that cold stimulated the SNS, which then stimulated WAT beiging6. To determine whether mast cells could be responsive to SNS stimulation, we evaluated βAR expression on mast cells and determined whether NE stimulates mast cell degranulation. As shown in Fig. 3A,B, TIB64 mast cells express β2AR and β3ARs at similar levels as 3T3L1 adipocytes; we did not detect βAR1 expression in TIB64 cells. We treated TIB64 mast cells with norepinephrine (100 nM) at 32 °C and at 37 °C and measured histamine levels in the media 120 and 240 min after treatment. Norepinephine at 37 °C stimulated histamine release to the same extent as cold (32 °C) treatment; however, treatment with NE at 32 °C did not result in more histamine release than cold (32 °C) (Fig. 3C).
Figure 3.
Mast cells express βARs and degranulate in response to norepinephrine. (A,B) βAR2 and 3 mRNA expression was determined in 3T3L1 and TIB64 mast cells by real-time RT PCR as described in methods. (C) TIB64 cells were changed into media at 32 C or 37 C with 0 or 100 nM norepinephrine (NE) for 0, 120 or 240 minutes as indicated. The media was removed from the cells and histamine determined as described in research design and methods. Data are presented as means ± SEM. The data were analyzed by ANOVA as described in research design and methods. **P < 0.01; ***P < 0.001; ****P < 0.0001. (D) Mast cell density and degranulated mast cells were quantified in SC WAT from human research participants with obesity treated with 50 mg mirabegron per day for 10 weeks. Data are presented as means ± SEM and were analyzed by a paired, two-tailed student’s t test (n = 6).
We previously showed that mirabegron, a specific β3AR agonist, stimulated pHSL phosphorylation and induced UCP1 and TMEM26 protein expression in SC WAT, suggesting βAR3 receptor stimulation in vivo6. Here, we determined whether mirabegron treatment stimulated mast cell degranulation in the same cohort of insulin resistant research participants with obesity (see6 for baseline characteristics). As shown in Fig. 3D,E, there was no change in mast cell number or degranulation in response to mirabegron, suggesting that cold-mediated SNS activation and not just β3-adrenergic receptor activation is necessary to cause mast cell degranulation in vivo.
Conclusions
The results presented in this study suggest that mast cell degranulation contributes to the induction of UCP1 in SC WAT by cold. Mast cells express βARs, and degranulate and release histamine in response to norepinephrine, suggesting that they are responsive to SNS activation.
Discussion
Results from gene expression analysis suggested that mast cells are positive regulators of SC WAT beiging, which occurs in response to acute cold6. We found that cold induced mast cell recruitment and degranulation in SC WAT of lean research participants and mast cell degranulation in research participants with obesity. These effects on mast cells occurred equally in SC WAT from both the cold treated and contralateral legs, and we furthermore demonstrated that norepinephrine stimulates mast cell degranulation in vitro. Together, these observations and our previous work implicating mast cells as positive regulators of seasonal beiging7, strongly support a role for mast cells as positive regulators of SC WAT beiging, adding them to the list of immune cells involved in this process13.
An important finding from our previous study was that SC WAT beiging and increased UCP1 protein occurred to the same extent in the contralateral leg as the cold treated leg, suggesting that cold activated the SNS6. Here, we found that mast cell degranulation is also stimulated to the same extent in SC WAT of the contralateral leg as the cold treated leg, suggesting that mast cell degranulation may be stimulated by SNS activation. In vitro studies demonstrated that mast cells express β-adrenergic receptors and that norepinephrine induces mast cell degranulation, providing a mechanistic link between cold and mast cell degranulation. Indeed, mast cells are found in in anatomic association with nerves in other tissues34, indicating that mast cells may be an important link between the nervous system and immune cells. The findings of this study, in addition to our previous study on seasonal SC WAT beiging in humans7, suggest that β-adrenergic stimulation and subsequent degranulation and histamine release are the mechanisms by which mast cells are involved in stimulation of adipose beiging.
Previously, we demonstrated that mast cells are cold sensors; mast cells exposed to a physiologic cold exposure (32 °C) degranulate, releasing histamine in vitro7. Adipocytes express histamine receptors, and histamine receptor activation increases cAMP, stimulating lipolysis and UCP1 expression in adipocytes7. Previous studies have shown that histamine promotes thermogenic responses by additional mechanisms besides these direct effects on adipocytes. Histamine increases blood flow in BAT, which is an important physiologic response for thermogenesis35,36, and it also renders cold sensitive nerves more sensitive to stimuli37. Overall, results from these studies35–37 and the current study demonstrating mast cell degranulation in vivo in response to cold and the responsiveness of mast cells to catecholamine suggest a positive role for histamine in promoting thermogenic responses to defend against cold. It is of interest to note that stimulation of histamine receptors in the brain positively affects energy expenditure and UCP1 induction in BAT in rodents38–40. Thus, several studies indicate that histamine acts at multiple sites to promote thermogenesis. However, one study in rodents indicates that mast cells have several negative effects in the context of obesity, including the suppression of UCP1 in BAT33. It is therefore possible that mast cells have different effects on beiging in SC WAT and BAT in humans and rodents.
We gained additional insight into SC WAT beiging from the analysis of gene expression in this study. Importantly, the multiple correlations analysis identified CCL26 as a gene whose change in expression positively correlated with the change in UCP1 expression in the cold treated leg. CCL26 is a chemokine for CCR3 expressing cells such as mast cells, basophils, and eosinophils27–29, and induction of CCL26 by acute cold exposure could thus be the mechanism leading to mast cell recruitment to adipose tissue. CCL26 was also identified as a significantly induced gene in the contralateral leg; therefore, CCL26 may be increased by SNS activity. CCL26 may thus be an important link between the SNS and the recruitment of immune cells involved in type 2 immune responses in adipose tissue. CCL26 mRNA was not increased in the cold treated leg. It is possible that cold caused a transient increase in CCL26. Further studies on the kinetics of CCL26 mRNA and protein induction will be necessary to elucidate the role of CCL26 in mast cell recruitment into adipose. We also found that the change in CIDEA gene expression inversely correlated with the change in UCP1 protein expression (Table 3). CIDEA increases with adipose beiging but is known to inhibit UCP1 activity in mice41. It will be import and to investigate this possible regulatory mechanism on UCP1 function in humans in future studies.
We found that VEGF and other genes that regulate vascularity were changed in the contralateral leg; in addition to being pro-angiogenic, VEGF also induces beiging31,32. Similarly, angiopoiten-2 was induced in both the cold treated and contralateral leg. Angiopoiten-2 was thought to be antiangiogenic; however, a recent study found that it is pro-angiogenic in adipose tissue42. One feature of beige adipose is increased vascularity to deliver oxygen and nutrients and remove heat, and we therefore measured capillary and vessel density. We did not find changes in SC WAT capillary density by histochemistry after acute cold; however, it may require longer than the 10 day time period of this study to increase capillarity. Finally, multiple correlations analysis identified angiopoietin like-4 (angptl4) as a gene negatively associated with UCP1. Angptl4 inhibits lipoprotein lipase (LPL), and it would thus be important to determine whether cold stimulates LPL to deliver more lipid to beige adipose for oxidation in future studies.
Finally, we note that a limitation of this study is that it was not adequately powered to detect an interaction between sex and mast cell recruitment or degranulation, which is an important question. In our previous study we were not able to detect an interaction between sex and adipose beiging from this same set of research participants6.
In conclusion, this study shows that mast cells are recruited to SC WAT of lean subjects in response to cold. Cold stimulates mast cell degranulation in all subjects regardless of baseline BMI, consistent with stimulation of SC WAT beiging6. This study, in combination with our recent study on the role of mast cells in SC WAT beiging in the winter, suggests that mast cells promote SC WAT beiging. Future clinical studies will employ histamine receptor antagonists or mast cell stabilizers to determine the importance of mast cells to SC WAT beiging in vivo.
Supplementary information
Acknowledgements
We wish to thank the staff of the University of Kentucky Clinical Research Unit for the assistance with this study and Dorothy Ross for assistance with coordinating the recruitment of the participants. This work was supported by the following NIH grants: RO1 DK107646, RO1 DK112282, CTSA grant UL1TR001998, and P20 GM103527-06.
Author Contributions
P.K., E.D.V. and B.F. designed the experiments, analyzed data and wrote the manuscript. A.C., B.Z., M.B., H.M., K.T. and Z.J. performed the experiments. P.W. analyzed data.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Brian S. Finlin and Amy L. Confides contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-45136-9.
References
- 1.Kajimura S, Spiegelman BM, Seale P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen P, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min SY, et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nature medicine. 2016;22:312–318. doi: 10.1038/nm.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chondronikola M, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kern Philip A., Finlin Brian S., Zhu Beibei, Rasouli Neda, McGehee Robert E., Westgate Philip M., Dupont-Versteegden Esther E. The Effects of Temperature and Seasons on Subcutaneous White Adipose Tissue in Humans: Evidence for Thermogenic Gene Induction. The Journal of Clinical Endocrinology & Metabolism. 2014;99(12):E2772–E2779. doi: 10.1210/jc.2014-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlin, B. S. et al. Human adipose beiging in response to cold and mirabegron. JCI Insight3, 10.1172/jci.insight.121510 (2018). [DOI] [PMC free article] [PubMed]
- 7.Finlin BS, et al. Mast Cells Promote Seasonal White Adipose Beiging in Humans. Diabetes. 2017;66:1237–1246. doi: 10.2337/db16-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. The Journal of clinical investigation. 2015;125:478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidossis LS, et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015;22:219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petruzzelli M, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Frontini A, et al. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochimica et biophysica acta. 2013;1831:950–959. doi: 10.1016/j.bbalip.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Kir S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villarroya F, Cereijo R, Villarroya J, Gavalda-Navarro A, Giralt M. Toward an Understanding of How Immune Cells Control Brown and Beige Adipobiology. Cell Metab. 2018;27:954–961. doi: 10.1016/j.cmet.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Reitman ML. How Does Fat Transition from White to Beige? Cell Metab. 2017;26:14–16. doi: 10.1016/j.cmet.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen KD, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Y, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao RR, et al. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MW, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brestoff JR, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer K, et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nature medicine. 2017;23:623–630. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camell CD, et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature. 2017;550:119–123. doi: 10.1038/nature24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz de Azua I, et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. The Journal of clinical investigation. 2017;127:4148–4162. doi: 10.1172/JCI83626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung KJ, et al. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat Immunol. 2017;18:654–664. doi: 10.1038/ni.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goto T, et al. Proinflammatory cytokine interleukin-1beta suppresses cold-induced thermogenesis in adipocytes. Cytokine. 2016;77:107–114. doi: 10.1016/j.cyto.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto Tomoya, Nitta Takahiro, Maruno Koji, Yeh Yu-Sheng, Kuwata Hidetoshi, Tomita Koichi, Goto Tsuyoshi, Takahashi Nobuyuki, Kawada Teruo. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. American Journal of Physiology-Endocrinology and Metabolism. 2016;310(8):E676–E687. doi: 10.1152/ajpendo.00028.2015. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto T, et al. Inflammation induced by RAW macrophages suppresses UCP1 mRNA induction via ERK activation in 10T1/2 adipocytes. Am.J.Physiol Cell Physiol. 2013;304:C729–C738. doi: 10.1152/ajpcell.00312.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochi H, et al. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999;190:267–280. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romagnani P, et al. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. The American journal of pathology. 1999;155:1195–1204. doi: 10.1016/S0002-9440(10)65222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 30.Finlin BS, et al. The Influence of a KDT501, a Novel Isohumulone, on Adipocyte Function in Humans. Frontiers in endocrinology. 2017;8:255. doi: 10.3389/fendo.2017.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun K, et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J, et al. VEGF-A-Expressing Adipose Tissue Shows Rapid Beiging and Enhanced Survival After Transplantation and Confers IL-4-Independent Metabolic Improvements. Diabetes. 2017;66:1479–1490. doi: 10.2337/db16-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nature medicine. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleij HP, Bienenstock J. Significance of Conversation between Mast Cells and Nerves. Allergy Asthma Clin Immunol. 2005;1:65–80. doi: 10.1186/1710-1492-1-2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothwell NJ, Stock MJ, Wyllie MG. Effects of histamine antagonists on noradrenaline-stimulated blood flow and oxygen consumption of brown adipose tissue in the rat. Pflugers Archiv: European journal of physiology. 1984;402:325–329. doi: 10.1007/BF00585518. [DOI] [PubMed] [Google Scholar]
- 36.Desautels M, Wollin A, Halvorson I, Muralidhara DV, Thornhill J. Role of mast cell histamine in brown adipose tissue thermogenic response to VMH stimulation. The American journal of physiology. 1994;266:R831–837. doi: 10.1152/ajpregu.1994.266.3.R831. [DOI] [PubMed] [Google Scholar]
- 37.Shen J, Yao JF, Tanida M, Nagai K. Regulation of sympathetic nerve activity by L-carnosine in mammalian white adipose tissue. Neuroscience letters. 2008;441:100–104. doi: 10.1016/j.neulet.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 38.Masaki T, et al. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes. 2004;53:2250–2260. doi: 10.2337/diabetes.53.9.2250. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi K, Suwa H, Ishikawa T, Kotani H. Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. The Journal of clinical investigation. 2002;110:1791–1799. doi: 10.1172/JCI15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snitker S, et al. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am J Clin Nutr. 2009;89:45–50. doi: 10.3945/ajcn.2008.26561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Z, et al. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 42.An, Y. A. et al. Angiopoietin-2 in white adipose tissue improves metabolic homeostasis through enhanced angiogenesis. Elife6, 10.7554/eLife.24071 (2017). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.