Abstract
Spore reduction can be used as a surrogate measure of Cryptosporidium natural filtration efficiency. Estimates of log10 (log) reduction were derived from spore measurements in paired surface and well water samples in Casper Wyoming and Kearney Nebraska. We found that these data were suitable for testing the hypothesis (H0) that the average reduction at each site was 2 log or less, using a one-sided Student’s t-test. After establishing data quality objectives for the test (expressed as tolerable Type I and Type II error rates), we evaluated the test’s performance as a function of the (a) true log reduction, (b) number of paired samples assayed and (c) variance of observed log reductions. We found that 36 paired spore samples are sufficient to achieve the objectives over a wide range of variance, including the variances observed in the two data sets. We also explored the feasibility of using smaller numbers of paired spore samples to supplement bioparticle counts for screening purposes in alluvial aquifers, to differentiate wells with large volume surface water induced recharge from wells with negligible surface water induced recharge. With key assumptions, we propose a normal statistical test of the same hypothesis (H0), but with different performance objectives. As few as six paired spore samples appear adequate as a screening metric to supplement bioparticle counts to differentiate wells in alluvial aquifers with large volume surface water induced recharge. For the case when all available information (including failure to reject H0 based on the limited paired spore data) leads to the conclusion that wells have large surface water induced recharge, we recommend further evaluation using additional paired biweekly spore samples.
Keywords: total aerobic spores, alluvial aquifers, inadequately treated groundwater, surface water induced recharge, Cryptosporidium
1. Introduction
Drinking water providers are often charged with making treatment decisions based on limited information about the microbial pathogen hazard at a well or wellfield (e.g. Hancock et al. 1998). Often, these decisions are primarily based on indicator bacteria rather than pathogen occurrence. In the United States, public water systems (PWSs) are routinely tested for total coliform (TC) and E. coli (EC) under the total coliform and revised total coliform rules (TCR) (USEPA, 2013). In an evaluation of TC and EC data collected by undisinfected PWS wells for the year 2011 (TCR time period), Messner et al. (2017) estimated that about 5% of all undisinfected PWS wells (about several thousand across the United States) have TC detection rates of 20% or greater. They suggested that higher TC detection rates may indicate recent 1) vertical infiltration from the surface or 2) pumping-induced lateral recharge from adjacent surface water.
In the United States, public health authorities have established practices or regulations that govern the public health response when a well water sample is EC positive. Messner et al. (2017) suggest that, for undisinfected PWS wells with high TC detection rates but no EC occurrence, total aerobic spore bacterial samples collected together with TC samples could be used to gain additional information about well vulnerability to pathogens.
Total aerobic spores, like the total coliform, are ubiquitous bacteria, primarily found in soil (Headd and Bradford, 2016; Bradford et al. 2016). Like the total coliform, the total aerobic spores are transported from the soil into surface water by overland flow. Spores may also be entrained within infiltrating ground water and transported to the saturated ground water zone where they may enter wells directly due to pumping. Additionally, spores may enter ground water from surface water by normal surface water/groundwater exchange or be induced into ground water by well pumping. Unlike the total coliforms, spores are resistant to inactivation because the vegetative cell is enclosed by an environmentally protective coat (Headd and Bradford, 2016). Spores may survive for years in the subsurface (Meschke, 2001; Setlow, 2007), as compared with total coliforms, which may survive only weeks (Sidhu et al. 2015). Spores are found at high concentrations in surface water (Weiss et al. 2005).
Given pathogen sources at close proximity to surface locations of wells, water recharge, infiltrating vertically from the ground surface could entrain viral, bacterial and parasitic protozoan pathogens (e.g., Cryptosporidium). Messner et al. (2017) suggest three metrics for using spores to evaluate well vulnerability to pathogens: 1) spore presence/absence in well water, 2) spore concentration in well water or 3) spore log10 (log) reduction, based on paired surface and well water samples in sandy alluvial aquifers. Wells believed to be receiving high volumes of recent surface water would be evaluated using paired samples. Wells likely receiving only infiltrating recharge or a mixture of infiltration and induced surface water could be evaluated by any of the three metrics. However, paired spore samples probably are most informative for wells located in alluvial aquifers (stream deposits).
In this paper, we focus only on the relative vulnerability of wells in sandy alluvial aquifers inducing large volumes of recent surface water as shown by spore log reduction values. Wells with paired spore samples showing large reductions are less likely to be producing well water with a large volume of recent surface water. Measured spore reduction in sandy alluvial aquifers by subsurface passage (natural filtration) is recommended in USEPA guidance (USEPA, 2010) as an alternative treatment.
We use statistical analysis to evaluate some of the available total aerobic spore log reduction data. We evaluate biweekly spore data from two sites: 1) Casper Wyoming (data available from 2002–2017, e.g. Gollnitz et al. 2005), and 2) Kearney Nebraska (data available for 2011–2012, Miller and Miller and Associates, 2013), (referred to as “Casper” and “Kearney,” respectively). At each site, “demonstration of performance” studies were conducted using methods consistent with those recommended in EPA’s Long Term 2 Enhanced Surface Water Treatment Rule Toolbox Guidance Manual (USEPA, 2010). One site (Kearney) has the minimum number of spore samples recommended by the document (36 paired samples collected over 18 months); the other site (Casper) has a large number of supplemental spore samples.
We establish quantitation objectives for demonstrating log reduction, then use Casper and Kearney data to: 1) assess statistical variability for the two data sets, 2) evaluate the statistical power associated with different numbers of paired samples, 3) assess the impact of differing levels of variability on the statistical power of a fixed number of paired samples, and 4) design and test spore reduction performance goals. Finally, we consider the use of paired spore samples as part of a screening decision, i.e. to decide whether a well is receiving large volumes of recent surface water, based on a much smaller number of samples.
2. Total Aerobic Spores as Surrogates for Cryptosporidium
Due to their environmental resistance, physical properties, and ubiquity in ambient waters, total aerobic spores are used to indicate the vulnerability of wells inducing large volumes of recent surface water into wells located in sandy alluvial aquifers. Paired surface water and ground water spore measurements are recommended by USEPA guidance (USEPA, 2010) as surrogate measures of Cryptosporidium removal (reduction) via subsurface passage (natural filtration). Headd and Bradford (2016) and Bradford et al. (2016) reviewed and analyzed the suitability of total aerobic spores as Cryptosporidium surrogates in ground water. They conclude; “spores and oocysts share many commonalities with regard to biology and survivability, and the environmental prevalence and ease of detection make aerobic spores a promising surrogate for Cryptosporidium oocysts in surface and groundwater.” However, the authors suggest that more research is needed. For example, as reported by Bradford et al. (2016), enhanced Cryptosporidium mobility in sand aquifers occurs at a range of physicochemical conditions.
Wells located near surface water can receive substantial recent recharge from that surface water, either ponded water or from flowing streams, enhancing the likelihood of Cryptosporidium occurrence in well water (Gallas-Lindemann et al. 2013). In the United States and elsewhere, water from many wells located on lake or river banks receive chlorination plus full conventional treatment, including coagulation, settling and rapid sand filtration (Hunt et al. 2002). As was demonstrated in Davenport, Iowa (Colford et al. 2005), optimized conventional surface water treatment plants using engineered filtration can provide adequate treatment of poor quality surface water thereby minimizing the Cryptosporidium hazard. However, wells located adjacent to surface water and producing water from sand and gravel (alluvial) aquifers rely on bioparticle reduction by subsurface passage and disinfection (Ray et al. 2002; Abbaszadegan et al, 2011). If the disinfection process includes treatment by ultraviolet (UV) light, then the Cryptosporidium hazard is minimized (USEPA, 2006). However, if the PWS relies only on subsurface passage and chlorination, then an unknown magnitude Cryptosporidium hazard remains because Cryptosporidium is resistant to inactivation by chlorination and may be incompletely removed by subsurface passage (e.g. Hancock et al. 1994).
Gallas-Lindemann et al. (2013) collected samples from “radial and vertical wells” from 2009 to 2010 and report 5 (of 66) well samples positive for Cryptosporidium. “Radial wells” are horizontal collector wells and are always installed in alluvial or other sand aquifer types. Cryptosporidium measurements from the adjacent Rhine river were 66–250 oocysts/1000 liter (L) while Cryptosporidium measurements from the wells were 4–66 oocysts/1000 L, when detected. Based on these data, Gallas-Lindemann et al. (2013) suggest that the Rhine bank-filtered wells exhibited a Cryptosporidium reduction of “1–2 orders of magnitude.” Cryptosporidium occurrence in well water is also reported in bedrock aquifers such as karst limestone (Fuchslin et al. 2012; Khaldi et al. 2011; Schijven et al. 2003).
Total aerobic spore removal to monitor drinking water treatment plant bioparticle removal performance is well established (Brown and Cornwell, 2007; Nieminski et al. 2000; Mazoua and Chauveheid, 2005; Huertas et al. 2003). Others have used spores of sulfite-reducing clostridia (anaerobic bacteria) to monitor treatment plant performance (e.g. Hijnen et al. 1997) or to evaluate log reduction by subsurface passage (e.g. Schijven et al. 1998).
Although there is no US EPA-approved standard method for total aerobic spore assay in drinking water samples, the laboratories performing the sample assays for Casper and Kearney used a protocol similar to that described in Rice et al (2012).
Assuming spores are Cryptosporidium surrogates, we evaluate the reduction of total aerobic spores by subsurface passage from river to well water in sandy alluvial aquifers. We assume that high spore reduction is equivalent to high Cryptosporidium reduction and therefore lower Cryptosporidium risk. We focus on spore reduction and its uncertainty because 1) spore reduction is adequately measured in multiple locations (Weiss et al. 2005; Ray et al. 2003; Gollnitz et al. 2005, Miller and Miller and Associates, 2013) and 2) Cryptosporidium hazard decision-making may benefit from more insight into the uncertainty associated with average spore reduction values. When evaluating reduction data, it is difficult to directly compare spore reduction by subsurface passage with Cryptosporidium reduction by engineered conventional filtration. Conventional filtration includes adding a coagulant which together with the settling step enhances Cryptosporidium reduction during engineered rapid sand filtration.
3. Total aerobic spore reduction
Total aerobic spores were successfully used to demonstrate at least 2.0 log Cryptosporidium removal at the Casper alluvial aquifer adjacent to the North Platte river (Gollnitz et al. 2005). As part of the Cryptosporidium “demonstration of performance” decision, total aerobic spore removal is periodically monitored at select wells (Gollnitz et al. 2005) (Wyoming is the only US state that does not have state authority to implement the Safe Drinking Water Act). The monitoring is intended as a wellfield management tool to minimize Cryptosporidium well breakthrough risk during, for example, periods of high well water demand or high river stage. However, the spore data from Casper were not statistically analyzed to evaluate uncertainty. The log reduction data from Casper, continuously collected since 2003, represents the longest known periodic spore data set. Gollnitz et al. (2005) provide more details about the Casper wellfield. Berger (2002) and Schijven et al. (2003) provide additional data from the Kearney wellfield.
Most recently, Kearney staff collected 36 biweekly paired total aerobic spore samples in an attempt to demonstrate 3.0 log Cryptosporidium removal at their Platte river Kilgore island alluvial wellfield (Miller and Miller and Associates, 2013) using larger (one-liter) samples. Although high spore log removals were demonstrated, the spore removals were determined to be lower than the stated goal of 3.0 log Cryptosporidium removal (Miller and Miller and Associates, 2013). We use statistical analysis to evaluate the 2.0 log Cryptosporidium reduction capability at Kearney rather than the 3.0 log reduction evaluated by the Nebraska Department of Health and Human Services. We test the hypothesis that spore log reduction is at most 2.0 at both Casper and Kearney.
USEPA (2010) suggests that measured total aerobic spore values less than 10 spore forming units (SFU) per 100 mL be evaluated as equal to 10 SFU/100 mL (or 100 SFU/L). As described in the guidance, limited available total aerobic spore measurements from ground water suggested that sample sites had low background spore concentrations of about 100 SFU/L or less. Because it was assumed that these spore concentrations did not originate in recent surface water, they were not counted to determine recent surface water proportions. Given the capability of spores to survive (dormancy) in the subsurface for periods measured in years, it is not surprising to find background spores in ground water samples. However, there are many unknowns associated with the background spore measurements (e.g. spore presence/absence, spore concentration and spore log reduction). Thus, in this paper, we choose to evaluate all data as measured rather than assume that values less than 100 SFU/L are background spore detections.
4. Measured data from Casper and Kearney
4.1. Casper
Beginning in September, 2002 and continuing until the present, total aerobic spore concentrations were measured (100 mL samples) from a total of ten alluvial wells and the adjacent North Platte river. The study used designated “worst case” wells to represent the wellfield. Although Caisson 3 (horizontal collector well) and Caspar 6 (vertical well) were the primary sampling sites, at times other wells were sampled due to maintenance and other activities that rendered the designated wells unavailable for sampling. Only Caisson 3 and Caspar 6 had sufficient spore samples to explore individual well sampling performance. The initial 18-month suite of 36 paired biweekly aerobic spore samples were used to make the Cryptosporidium removal decision (Gollnitz et al. 2005).
Fig. 1 shows the available total aerobic spore data from Casper. A total of 607 samples were taken from the North Platte river at Casper. Spore counts in the river ranged from 400 to 500,000 with a median of 7,800/L. Well spore count data were mostly from Caisson 3 and Caspar 6. There were 587 spore count samples from Caisson 3 and 593 samples from Caspar 6. Spore counts in Caisson 3 ranged from 0 to 30,000 with a median of 20 SFU per liter. Twenty-seven percent of the Caisson 3 results were non-detects (zero spores counted) (Fig. 1). Caspar 6 spore counts also ranged from 0 to 30,000, but with a median of 5 SFU/L. Nearly half of the Caspar 6 results were non-detects. In Fig. 1, the non-detects are shown as “rugs” along the horizontal axis.
Fig. 1.
River and well total aerobic spore (TAS) data from Casper.
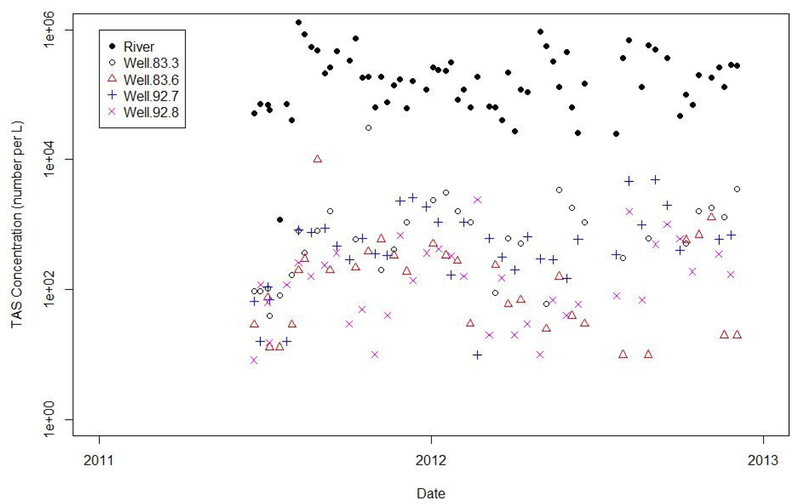
4.2. Kearney
At Kearney (Fig. 2) there were 65 Platte river samples collected approximately weekly over an 18-month period beginning in June 2011 and concluding in December 2012. A total of 139 one-liter samples were taken from the four wells. One well (92.7) was deemed most at risk because of its location on the narrow end of the Kilgore Island wellfield about 100 feet from the nearest surface water. Because the island is small and the wellfield yield is large, the well is likely inducing direct surface water withdrawal from both sides of the island with little supplemental flow from natural ground water.
Fig. 2.
River and well total aerobic spore (TAS) data from Kearney.
One sample (collected from well 92.7 on October 15, 2012) had a spore measurement of 1.4 million SFU/L. On that same day, the river spore measurement was 70,000 SFU/L and a sample from the adjacent well (well 92.8) was reported as 190 SFU/L. We removed the high well 92.7 spore measurement from the data set because it appears to be in error. There were no non-detection results among the 139 ground water samples.
5. Methods, analysis and results
The previous sections describe data collected by others. The following sections report our method, analyses and results.
5.1. Calculation of total aerobic spore reduction using paired samples
Total aerobic spore log reduction by use of paired surface water and ground water spore samples is calculated using Eq. (1) (Brown and Cornwell, 2007) for both treatment plants and, in this study, for wellfields.
For paired surface water (swi) and ground water (gwi) measurements of sampling event i:
| (1) |
5.2. Calculation of total aerobic spore reduction over time
Some Casper well samples have reported values of zero total aerobic spore counts. Because the log reduction calculation is indeterminate when the spore count is zero, we replace values of zero counts in wells with an assumed value of one SFU. An alternative (replacing zero values with 0.5 SFU) was also evaluated to assess the impact of this assumption. It is likely that the replacement will result in an increased calculated log reduction because samples demonstrating high log reduction (low spore counts) will be included rather than excluded in the final assessment.
Table 1 reports the summary statistics for total aerobic spore sample counts, calculated log reduction values and log reduction variability. Standard deviations are calculated for each wellfield rather than for each well because there are insufficient samples from each Kearney well for a well-specific standard deviation to be meaningful. There is a larger number of samples from Casper as compared with Kearney so it is noteworthy that the variabilities in the log reduction calculations are similar. Casper well Caisson 3 exhibits, on average, poorer log reduction performance as compared with Casper well Caspar 6. Caisson 3 is a horizontal collector well, designed to collect large volumes of recent surface water so it is expected to have shorter and more direct flow paths than the vertical well Caspar 6, resulting in poorer log reduction performance. Mean log reduction performance differed among all the wells at the two locations, with all wells showing a mean log reduction between 2.38 and 3.12. Casper wells are recharged by both vertical recharge from infiltration basins and by pumping-induced lateral infiltration from the North Platte River. Kearney wells are recharged by induced bi-lateral infiltration from each adjacent branch of the Platte River.
Table 1.
Summary statistics for spore log reduction values at Casper and Kearney wells.
| Number of Paired Total Aerobic Spore Samples | Median Log10 Reduction* | Mean Log10 Reduction* | Wellfield Log10 Reduction Standard Deviation* | |
|---|---|---|---|---|
| Casper Caisson 3 | 562 | 2.67 (2.74) |
2.58 (2.67) |
0.59 |
| Casper Caspar 6 | 576 | 2.76 (2.96) |
2.68 (2.83) |
|
| Kearney 83.3 | 33 | 2.37 | 2.38 | 0.74 |
| Kearney 83.6 | 32 | 3.13 | 3.12 | |
| Kearney 92.7 | 37 | 2.36 | 2.58 | |
| Kearney 92.8 | 37 | 3.03 | 3.10 |
Numbers in parentheses are based on substituting 0.5 SFU rather than 1.0 SFU for non-detects (zeros)
Fig. 3 shows calculated log reduction over the sampling interval for Casper and Fig. 4 shows the same for Kearney. To calculate values shown in Fig. 3, sample non-detection values were replaced with values of 1.0 SFU. We provide summary statistics but do not analyze how log reduction change with season or year. We suggest that future work might show that both wellfield pumping volume and river stage might reasonably be shown to have some relationship with spore log reduction.
Fig. 3.
Time series plot of calculated spore log reduction for two Casper wells.
Fig. 4.
Time series plot of calculated spore log reduction for four Kearney wells.
5.3. Hypothesis testing with large numbers of paired samples (“demonstration of performance test”)
We wish to test the hypothesis that average log reduction in any wellfield is 2 or less. In the absence of data – or if the results are inconclusive – we fail to reject this “null hypothesis” (H0) and the wellfield should not be given 2-log credit for Cryptosporidium reduction. The 2-log credit is given only if data lead us to reject H0 and thereby conclude that log reduction is significantly greater than 2. We wish to control the probability of giving credit when the true average log reduction is less than or equal to 2. We also wish to limit the probability of erring in the other direction by failing to give credit when the true log reduction is above 2.5 and above 3.0. Table 2 assigns tolerable limits to these hypothesis test errors. Fig. 5 displays these constraints graphically.
Table 2.
Students t-test hypothesis performance goals.
| True Log Reduction | Error | Desired Probability of Error |
|---|---|---|
| 2 or less | Concluding log reduction exceeds 2 and giving 2 log credit | 0.05 = 5% or less |
| 2.5 | Concluding log reduction is at most 2 and not giving 2 log credit | 0.5 = 50% or less |
| 3 | Concluding log reduction is at most 2 and not giving 2 log credit | 0.25 = 25% or less |
Fig. 5.
Spore log removal hypotheses testing diagram.
Fig. 5 shows that our greatest concern is concluding >2.0 log spore reduction (at most 5% of the time) when the true log spore reduction is ≤2.0 (Type I error). Establishing this constraint, we are limiting and controlling the likelihood that an unjustified 2.0 log reduction is determined. We also allow that, fifty percent of the time, we could incorrectly fail to reject H0 when the true average log spore reduction is 2.5 log or greater (Type II error). We tolerate a higher probability for Type II error because installation of supplemental treatment adds to public health protection, albeit at extra cost to the water provider. However, if true average log spore reduction is greater than 3 we allow only a 25% probability of failing to reject H0 (Type II error). This is because if the true average log spore reduction is 3, the likelihood that public health could be endangered becomes smaller. The shaded gray region in Fig. 5 outlines the domain that satisfies these constraints.
We assessed the spore count data deviation from log-normality for each well separately and then combine data from all wells in each wellfield to separately and in combination test for normality. We find that, for the Casper wellfield, the horizontal collector well (Caisson 3) had the greatest deviation from log-normality. Of the Casper wells evaluated, Caisson 3 likely pumps surface water having the shortest subsurface residence time, the shortest and most direct flow path from river to well and the largest yield in the wellfield and so is most likely to be affected by fluctuating surface water spore inputs. Thus, it is not surprising to see increased divergence from normality for this well. We conclude that the divergence from normality for each well (including Caisson 3) is relatively small and that a log-normal data distribution is still appropriate.
Given normally distributed concentration data and normally distributed log-reduction ratios, we can use the one-sided, Students t-test to evaluate spore log reduction, testing the null hypothesis at the 5% significance level.
The standard deviations of spore log reduction observed for the Casper (0.59) and Kearney (0.74) wellfields are presented in Table 1. Using Students t-test, we calculate the probability for rejecting H0, based on 36 randomly-selected paired samples over a range of true mean log reductions. Fig. 6 shows two calculated curves, one each for the Casper and Kearney calculated variability levels (standard deviations shown in Table 1). Both calculated curves are fixed to pass through the point representing the 0.05 probability of rejecting H0 when the true mean log reduction is 2.0. The two curves plotted in Fig. 6 show very little difference between the two. Both curves fall within the shaded gray region of the display (the region where our chosen performance goals are met). Fig. 6 shows that 36 samples are sufficient to satisfy our hypothesis test if variability (as expressed by the standard deviation) in log reduction is as observed for Casper and Kearney.
Fig. 6.
Spore log reduction hypothesis testing diagram for 36 random samples.
Fig. 7 illustrates the power of using only 36 random samples for any well or wellfield that exhibits spore log reduction variability differing from the variability observed for Casper or Kearney. We calculate and display performance curves for log reductions with standard deviations of 0.5, 1.0, 1.5 and 2.0 using 36 random, normally distributed, samples. Fig. 7 shows that 36 samples appear to be sufficient to meet our hypothesis test performance goals as long as the standard deviation of log reduction is less than about 2. For that case, performance at 2.5 log reduction just misses our goal. Conclusions based on Fig. 7 apply to any wellfield with characteristics similar to Casper or Kearney (bank filtered wells in alluvial aquifers as described in USEPA, 2010 guidance) but with greater variability in calculated spore log reduction.
Fig. 7.
Spore log reduction hypothesis testing diagram for 36 samples in wells or wellfields with known reduction variability.
5.4. Hypothesis testing with a small number of paired samples (screening test)
Using the data collected at Casper and Kearney, we use a similar type of hypothesis testing framework, as a screening test rather than as a “demonstration of performance” test. Our goal is to test the information value of small numbers of paired spore samples (e.g. 2 paired samples) from alluvial aquifers to determine 2 log spore reduction.
We evaluate the same null hypothesis (H0) described above but in this case, we wish to have a very low probability (0.01) that we reject H0 when it is false (Table 3).
Table 3.
Normal screening test hypothesis performance goals.
| True Log Reduction | Error | Desired Probability of Error |
|---|---|---|
| 2 or less | Concluding log reduction exceeds 2 and giving 2 log credit | 0.01 = 1% or less |
| 3 | Concluding log reduction is at most 2 and not giving 2 log credit | 0.5 = 50% or less |
| 4 | Concluding log reduction is at most 2 and not giving 2 log credit | 0.25 = 25% or less |
We assume normal error and that the standard deviation is known to be 1.0. This is based on the normal error structure observed following log transform of the Kearney and Casper data. We believe the standard deviation of 1.0 is conservative, because it is somewhat greater than observed in Kearney and Casper, but also because the river and well water were only weakly correlated in those water systems. If there had been a more direct connection between those rivers and wells (for example, due to passage through gravel rather than sand), then the observed log reductions would vary less across paired samples.
This standard deviation is a little greater than was observed in the Casper and Kearney data (Table 1). For this hypothesis, we use the normal screening test rather than Students t-test. We determine that the mean from two paired samples is 0.71 and the critical value for the sample mean is 3.65.
Fig. 8 shows the spore log reduction performance diagram for a screening test of 2 to 12 samples with very high probability that there will be few false positives (Type I errors). This figure shows that, for a screening test to estimate log reduction in alluvial aquifers as a component of a decision process for surface water induced recharge, six samples will be most appropriate (plotted within the region outlining our performance goals).
Fig. 8.
Spore log reduction hypothesis testing diagram for screening tests with few samples.
6. Discussion
In this paper, we determine that 36 paired spore samples are an appropriate number of samples to estimate a bioparticle log removal “demonstration of performance” at Casper and Kearney based on a set of constraints for uncertainty. We can use the same hypothesis testing procedure to test other sample numbers for their information value. We have evaluated the null hypothesis H0 when the mean log reduction is at most 2.0 (not presented herein) for 36, 28, 20, 12 and 4 paired samples. As would be expected, with fewer samples there is greater probability of not rejecting Ho when log reduction is truly satisfactory (2.5 or greater).
We also assess the utility of small numbers of paired samples to evaluate the need for further spore log reduction testing. Two bioparticle well (only) samples are currently used by many states, not to evaluate Cryptosporidium risk, but to help identify wells or well fields (in all aquifer types) that may need further evaluation to determine Cryptosporidium risk (USEPA, 1992). Thus, two samples are currently recommended by USEPA guidance, in conjunction with other site data, as a screening test. We suggest, for sandy alluvial aquifers only, as a supplement to the current two (well) sample screening test, two or more total aerobic spore paired samples to evaluate spore log reduction. Addition of the supplemental spore log reduction measurement could enhance the information value of the current two sample bioparticle-count method, at relatively low cost. Utilities could increase the number of spore log reduction paired samples in the screening test to increase their success probability that paired spore samples (together with bioparticle counts) indicate a relatively low contribution of recent surface water in their pumped source ground water.
7. Conclusions
Total aerobic spore data from two locations, Casper and Kearney, were statistically analyzed. Based on that analysis, we conclude:
Average total aerobic spore log reduction differed in the two locations but the uncertainty in the log reduction was similar, despite a large difference in the number of samples.
36 random paired surface water/ground water total aerobic spore samples (collected biweekly over 18 months, as recommended in the 2010 USEPA guidance) were sufficient to reject the null hypothesis that spore log reduction was 2 or less 95% of the time. Our analysis supports its continued use in a “demonstration of performance.”
Expected log reduction variability (0.5 – 2.0 standard deviations) will not affect the conclusion that 36 samples are sufficient.
For alluvial aquifers, a screening test of as few as 6 paired surface water/ground water total aerobic spore samples may be used as a supplement to the existing USEPA guidance (USEPA, 1992). Our analysis shows that six paired samples are sufficient to meet our uncertainty constraints.
We suggest that paired (surface water/ground water) aerobic spore measurements could be used to supplement ground water bioparticle data. As few as two sample pairs may be adequate for some sites, e.g., those with low flux layers that restrict surface water contribution to ground water.
Time series log reduction data combined with river stage or well yield data can be used to identify periods with increased Cryptosporidium risk. We suggest further data analysis to explore this hypothesis using the Casper (and Kearney) log reduction time series data set.
Acknowledgements
We thank the public servants and private contractors associated with Kearney and Casper for many helpful discussions and permission to use their data. The views expressed in this article are solely those of the authors and do not represent those of the US Environmental Protection Agency or the federal government.
ABBREVIATIONS:
- EC
E. coli
- L
liter
- log
log base 10
- H0
null hypothesis
- mL
milliliter
- PWS
public water system(s)
- SFU
spore forming units
- TAS
total aerobic spore
- TC
total coliform
- TCR
total coliform rule
- USEPA
US Environmental Protection Agency
References
- Abbaszadegan M, Rauch-Williams T, Johnson WP and Hubbs SA, 2011. Methods to Assess GWUDI and Bank Filtration Performance Water Research Foundation, Denver. [Google Scholar]
- Berger P, 2002. Removal of Cryptosporidium using bank filtration, in: Ray C (Ed.), Riverbank Filtration: Understanding Contaminant Biogeochemistry and Pathogen Removal. Kluwer Academic Publishers, Dordrecht, pp. 85–121. [Google Scholar]
- Bradford SA, Kim H, Headd B and Torkzaban S, 2016. Evaluating the transport of Bacillus subtilis spores as a potential surrogate for Cryptosporidium parvum oocysts. Environmental Science & Technology. 50(3), 1295–1303. [DOI] [PubMed] [Google Scholar]
- Brown RA and Cornwell DA, 2007. Using spore removal to monitor plant performance for Cryptosporidium removal. Journal American Water Works Association. 99(3), 95–109. [Google Scholar]
- Colford JM, Wade TJ, Sandhu SK, Wright CC, Lee S, Shaw S, Fox K, Burns S, Benker A, Brookhart MA and Van der Laan M, 2005. A randomized, controlled trial of in-home drinking water intervention to reduce gastrointestinal illness. American Journal of Epidemiology. 161(5), 472–482. [DOI] [PubMed] [Google Scholar]
- Colford JM Jr, Hilton JF, Wright CC, Arnold BF, Saha S, Wade TJ, Scott J and Eisenberg JN, 2009. The Sonoma water evaluation trial: a randomized drinking water intervention trial to reduce gastrointestinal illness in older adults. American Journal of Public Health. 99(11), 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füchslin HP, Egli T and Kötzsch S, 2012. Cryptosporidium spp. in drinking water. Swiss Medical Weekly. 142, 13683– 13689. [DOI] [PubMed] [Google Scholar]
- Gallas-Lindemann C, Sotiriadou I, Plutzer J and Karanis P, 2013. Prevalence and distribution of Cryptosporidium and Giardia in wastewater and the surface, drinking and ground waters in the Lower Rhine, Germany. Epidemiology and Infection. 141(01), 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnitz WD, Clancy JL, McEwen JB and Garner SC, 2005. Riverbank filtration for IESWTR compliance. Journal American Water Works Association. 97(12), 64–76. [Google Scholar]
- Hancock CM, Rose JB and Callahan M, 1998. Crypto and Giardia in US groundwater. Journal American Water Works Association. 90(3), 58–61. [Google Scholar]
- Headd B and Bradford SA, 2016. Use of aerobic spores as a surrogate for Cryptosporidium oocysts in drinking water supplies. Water Research. 90, 185–202. [DOI] [PubMed] [Google Scholar]
- Hijnen WA, Willemsen-Zwaagstra J, Hiemstra P, Medema GJ and van der Kooij D, 2000. Removal of sulphite-reducing clostridia spores by full-scale water treatment processes as a surrogate for protozoan (oo)cysts removal. Water Science and Technology. 41(7), 165–171. [Google Scholar]
- Huertas A, Barbeau B, Desjardins C, Galarza A, Figueroa MA and Toranzos GA, 2003. Evaluation of Bacillus subtilis and coliphage MS2 as indicators of advanced water treatment efficiency. Water Science and Technology. 47(3), 255–259. [PubMed] [Google Scholar]
- Hunt H, Schubert J and Ray C, 2003. Operation and maintenance considerations, in: Ray C, Melin G and Linsky RB (Eds.), Riverbank Filtration, Improving Source-Water Quality, Kluwer Academic Publishers, Dordrecht, pp. 61–70. [Google Scholar]
- Khaldi S, Ratajczak M, Gargala G, Fournier M, Berthe T, Favennec L, and Dupont JP. 2011. Intensive exploitation of a karst aquifer leads to Cryptosporidium water supply contamination, Water Research. 45(9), 2906–2914. [DOI] [PubMed] [Google Scholar]
- Mazoua S and Chauveheid E, 2005. Aerobic spore-forming bacteria for assessing quality of drinking water produced from surface water. Water Research. 39(20), 5186–5198. [DOI] [PubMed] [Google Scholar]
- Meschke JS, 2001. Comparative Adsorption, Persistence, and Mobility of Norwalk virus, Poliovirus Type I and F+RNA Coliphages in Soil and Groundwater. Ph.D. thesis, School of Public Health, University of North Carolina, Chapel Hill, North Carolina. [Google Scholar]
- Messner MJ, Berger P and Javier J, 2017. Total coliform and E. coli in public water systems using undisinfected ground water in the United States. International Journal of Hygiene and Environmental Health. 220, 736–743. [DOI] [PubMed] [Google Scholar]
- Miller CA and Miller and Associates, 2013. Demonstration of Performance to Establish Natural Filtration Credits for the City of Kearney, Nebraska Platte River Well Field. Unpublished report to the Nebraska Department of Health and Human Services, March 21, 2013, Project No. 130-C1–054. [Google Scholar]
- Nieminski EC, Bellamy WD and Moss LR, 2000. Using surrogates to improve performance. Journal American Water Works Association. 92(3), 67–78. [Google Scholar]
- Ray C, Schubert J, Linsky RB and Melin G, 2003. Introduction in: Ray C, Melin G and Linsky RB (Eds.), Riverbank Filtration, Improving Source-Water Quality, Kluwer Academic Publishers, Dordrecht, pp. 1–15. [Google Scholar]
- Rice EW, Baird RB, Eaton AD and Lenore SC, 2012. Standard Methods for the Examination of Water and Wastewater, twenty-second ed. American Public Health Association, Washington. [Google Scholar]
- Schijven J, Berger P and Miettinen I, 2003. Removal of pathogens, surrogates, indicators, and toxins using riverbank filtration in: Ray C, Melin G and Linsky RB (Eds.), Riverbank Filtration, Improving Source-Water Quality, Kluwer Academic Publishers, Dordrecht, pp. 73–116. [Google Scholar]
- Schijven JF, Hoogenboezem W, Nobel PJ, Medema GJ and Stakelbeek A, 1998. Reduction of FRNA-bacteriophages and faecal indicator bacteria by dune infiltration and estimation of sticking efficiencies. Water Science and Technology. 38(12), 127–131. [Google Scholar]
- Setlow P, 2007. I will survive: DNA protection in bacterial spores. Trends in Microbiology. 15(4), 172–180. [DOI] [PubMed] [Google Scholar]
- Sidhu JPS, Toze S, Hodgers L, Barry K, Page D, Li Y and Dillon P, 2015. Pathogen decay during managed aquifer recharge at four sites with different geochemical characteristics and recharge water sources. Journal of Environmental Quality. 44(5), 1402–1412. [DOI] [PubMed] [Google Scholar]
- USEPA, 1991. Guidance Manual for Compliance with the Filtration and Disinfection Requirements for Public Water Systems using Surface Water Sources, March 1991. https://www.epa.gov/dwreginfo/surface-water-treatment-rule-documents (accessed 17.07.17).
- USEPA, 1992. Consensus Method for Determining Groundwaters Under the Direct Influence of Surface Water Using Microscopic Particulate Analysis (MPA), USEPA Manchester Environmental Laboratory, Port Orchard WA: https://nepis.epa.gov (accessed 17.07.17). [Google Scholar]
- USEPA, 2006. Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface Water Treatment Rule, EPA 815-R-06–007.
- USEPA, 2010. Long Term 2 Enhanced Surface Water Treatment Rule Toolbox Guidance Manual, EPA 815-R-0–16.
- USEPA, 2013. 40 CFR PARTs 141 and 142; national primary drinking water regulations; revisions to the Total Coliform Rule; final rule. Fed. Register 78 (February (30)). 10270–10365 EPA-HQ-OW-2008–0878.
- USEPA. 2016. Six-Year Review 3 Technical Support Document for Long-Term 2 Enhanced Surface Water Treatment Rule. EPA-810-R-16–011.
- Weiss WJ, Bouwer EJ, Aboytes R, LeChevallier MW, O’Melia CR, Le BT and Schwab KJ, 2005. Riverbank filtration for control of microorganisms: Results from field monitoring. Water Research. 39(10), 1990–2001. [DOI] [PubMed] [Google Scholar]