Abstract
Background
Hepatic encephalopathy is a common complication of cirrhosis, with high related morbidity and mortality. Its presence is associated with a wide spectrum of change ranging from clinically obvious neuropsychiatric features, known as 'overt' hepatic encephalopathy, to abnormalities manifest only on psychometric or electrophysiological testing, 'minimal' hepatic encephalopathy. The exact pathogenesis of the syndrome is unknown but ammonia plays a key role. Drugs that specifically target ammonia include sodium benzoate, glycerol phenylbutyrate, ornithine phenylacetate, AST‐120 (spherical carbon adsorbent), and polyethylene glycol.
Objectives
To evaluate the beneficial and harmful effects of pharmacotherapies that specifically target ammonia versus placebo, no intervention, or other active interventions, for the prevention and treatment of hepatic encephalopathy in people with cirrhosis.
Search methods
We searched the Cochrane Hepato‐Biliary Controlled Trials Register, CENTRAL, MEDLINE, Embase, and three other databases to March 2019. We also searched online trials registries such as ClinicalTrials.gov, European Medicines Agency, WHO International Clinical Trial Registry Platform, and the Food and Drug Administration for ongoing or unpublished trials. In addition, we searched conference proceedings, checked bibliographies, and corresponded with investigators.
Selection criteria
We included randomised clinical trials comparing sodium benzoate, glycerol phenylbutyrate, ornithine phenylacetate, AST‐120, and polyethylene glycol versus placebo or non‐absorbable disaccharides, irrespective of blinding, language, or publication status. We included participants with minimal or overt hepatic encephalopathy or participants who were at risk of developing hepatic encephalopathy.
Data collection and analysis
Two review authors independently extracted data from the included reports. The primary outcomes were mortality, hepatic encephalopathy, and serious adverse events. We undertook meta‐analyses and presented results using risk ratios (RR) or mean differences (MD), both with 95% confidence intervals (CIs), and I2 statistic values as a marker of heterogeneity. We assessed bias control using the Cochrane Hepato‐Biliary domains and the certainty of the evidence using GRADE.
Main results
Eleven randomised clinical trials fulfilled our inclusion criteria. Two trials evaluated the prevention of hepatic encephalopathy while nine evaluated the treatment of hepatic encephalopathy. The trials assessed sodium benzoate (three trials), glycerol phenylbutyrate (one trial), ornithine phenylacetate (two trials), AST‐120 (two trials), and polyethylene glycol (three trials). Overall, 499 participants received these pharmacotherapies while 444 participants received a placebo preparation or a non‐absorbable disaccharide. We classified eight of the 11 trials as at 'high risk of bias' and downgraded the certainty of the evidence to very low for all outcomes.
Eleven trials, involving 943 participants, reported mortality data, although there were no events in five trials. Our analyses found no beneficial or harmful effects of sodium benzoate versus non‐absorbable disaccharides (RR 1.26, 95% CI 0.49 to 3.28; 101 participants; 2 trials; I2 = 0%), glycerol phenylbutyrate versus placebo (RR 0.65, 95% CI 0.11 to 3.81; 178 participants; 1 trial), ornithine phenylacetate versus placebo (RR 0.73, 95% CI 0.35 to 1.51; 269 participants; 2 trials; I2 = 0%), AST‐120 versus lactulose (RR 1.05, 95% CI 0.59 to 1.85; 41 participants; 1 trial), or polyethylene glycol versus lactulose (RR 0.50, 95% CI 0.09 to 2.64; 190 participants; 3 trials; I2 = 0%).
Seven trials involving 521 participants reported data on hepatic encephalopathy. Our analyses showed a beneficial effect of glycerol phenylbutyrate versus placebo (RR 0.57, 95% CI 0.36 to 0.90; 178 participants; 1 trial; number needed to treat for an additional beneficial outcome (NNTB) 6), and of polyethylene glycol versus lactulose (RR 0.19, 95% CI 0.08 to 0.44; 190 participants; 3 trials; NNTB 4). We did not observe beneficial effects in the remaining three trials with extractable data: sodium benzoate versus non‐absorbable disaccharides (RR 1.22, 95% CI 0.51 to 2.93; 74 participants; 1 trial); ornithine phenylacetate versus placebo (RR 2.71, 95% CI 0.12 to 62.70; 38 participants; 1 trial); or AST‐120 versus lactulose (RR 1.05, 95% CI 0.59 to 1.85; 41 participants; 1 trial).
Ten trials, involving 790 participants, reported a total of 130 serious adverse events. Our analyses found no evidence of beneficial or harmful effects of sodium benzoate versus non‐absorbable disaccharides (RR 1.08, 95% CI 0.44 to 2.68; 101 participants; 2 trials), glycerol phenylbutyrate versus placebo (RR 1.63, 95% CI 0.85 to 3.13; 178 participants; 1 trial), ornithine phenylacetate versus placebo (RR 0.92, 95% CI 0.62 to 1.36; 264 participants; 2 trials; I2 = 0%), or polyethylene glycol versus lactulose (RR 0.57, 95% CI 0.18 to 1.82; 190 participants; 3 trials; I2 = 0%). Likewise, eight trials, involving 782 participants, reported a total of 374 non‐serious adverse events and again our analyses found no beneficial or harmful effects of the pharmacotherapies under review when compared to placebo or to lactulose/lactitol.
Nine trials, involving 733 participants, reported data on blood ammonia. We observed significant reductions in blood ammonia in placebo‐controlled trials evaluating sodium benzoate (MD −32.00 µg/dL, 95% CI −46.85 to −17.15; 16 participants; 1 trial), glycerol phenylbutyrate (MD −12.00 µmol/L*week, 95% CI −23.37 to −0.63; 178 participants; 1 trial), ornithine phenylacetate (MD −27.10 µmol/L, 95% CI −48.55 to −5.65; 231 participants; 1 trial), and AST‐120 (MD −22.00 µg/dL, 95% CI −26.75 to −17.25; 98 participants; 1 trial). However, there were no significant differences in blood ammonia concentrations in comparison with lactulose/lactitol with sodium benzoate (MD 9.00, 95% CI −1.10 to 19.11; 85 participants; 2 trials; I2 = 0% ), AST‐120 (MD 5.20 units not specified, 95% CI −2.75 to 13.15; 35 participants; 1 trial), and polyethylene glycol (MD −29.28 µmol/L, 95% CI −95.96 to 37.39; 90 participants; 2 trials; I2 = 88%).
Five trials received support from pharmaceutical companies while four did not; two did not provide this information.
Authors' conclusions
There is insufficient evidence to determine the effects of these pharmacotherapies on the prevention and treatment of hepatic encephalopathy in adults with cirrhosis. They have the potential to reduce blood ammonia concentrations when compared to placebo, but their overall effects on clinical outcomes of interest and the potential harms associated with their use remain uncertain. Further evidence is needed to evaluate the potential beneficial and harmful effects of these pharmacotherapies in this clinical setting.
Drug treatments that specifically target ammonia for adults with cirrhosis and hepatic encephalopathy
Background
Cirrhosis is a chronic disorder of the liver. People with cirrhosis may develop hepatic encephalopathy, a condition that results in poor brain functioning. Some people with hepatic encephalopathy show clear evidence of brain dysfunction and are said to have 'overt' hepatic encephalopathy. They may have a poor memory, difficulty concentrating, speech problems, a tremor, particularly of their hands, or stiffness of their limbs. These changes may occur in bouts or may be persistent. Other people with cirrhosis may not show any obvious signs of brain dysfunction, but some aspects of their brain function, such as attention and the ability to perform complex tasks are found to be impaired when tested. They are said to have 'minimal' hepatic encephalopathy. The reason why people develop hepatic encephalopathy is complex, but the build up in the blood of toxins from the gut, particularly of a compound called ammonia, plays a key role. Certain drugs have been developed specifically to lower blood ammonia levels and may help prevent people from developing hepatic encephalopathy and have beneficial effects in those already suffering from this disorder. However, the evidence that they are beneficial is unclear. The five drugs (pharmacotherapies) considered in this review are sodium benzoate, glycerol phenylbutyrate, ornithine phenylacetate, AST‐120 (spherical carbon adsorbent), and polyethylene glycol.
Review question
We investigated the use of five pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. We did this by reviewing clinical trials in which people with cirrhosis were randomly allocated to treatment with one of these drugs or to an inactive dummy (placebo), to no treatment or to other drugs that are also used to manage this condition, such as, lactulose and lactitol (these are non‐absorbable disaccharides). We included people with cirrhosis who had minimal or overt hepatic encephalopathy and people who were at risk of developing this complication.
Search date
5 March 2019
Study funding sources
Five of the 11 randomised clinical trials we included in the review received support from pharmaceutical companies. Two trials did not provide information on potential financial support or links to pharmaceutical companies. Four trials did not receive funding or other support from this source.
Study characteristics
We identified 11 randomised clinical trials comparing drugs that specifically target ammonia with inactive placebo or a non‐absorbable disaccharide; two trials evaluated prevention of hepatic encephalopathy while nine trials evaluated treatment of hepatic encephalopathy. The trials assessed sodium benzoate (three trials), glycerol phenylbutyrate (one trial), ornithine phenylacetate (two trials), AST‐120 (two trials) and polyethylene glycol (three trials). Participants were treated for varying periods ranging from five days to 16 weeks.
Key results
Sodium benzoate, glycerol phenylbutyrate, ornithine phenylacetate, and AST‐120 lowered blood ammonia levels when compared to placebo, but none of the drugs lowered the blood ammonia levels when compared to a non‐absorbable disaccharide. Glycerol phenylbutyrate seemed to have a beneficial effect on hepatic encephalopathy when compared to placebo, as did polyethylene glycol when compared to lactulose. None of the drugs appeared to affect the risk of death and did not have any notable adverse effects.
Quality of the evidence
The evidence we found was very uncertain, and so we are not confident that these drugs are useful for preventing or treating hepatic encephalopathy in people with cirrhosis. There were very few trials available, and not all of them provided sufficient data for us to include in our analyses. In addition, many of the published trials received support from the pharmaceutical industry which introduces an element of bias. Thus, we need more information to obtain a better idea if these drugs are useful and safe for use in this context.
Summary of findings
Summary of findings for the main comparison.
Phamacotherapies that specifically target ammonia versus placebo or non‐absorbable disaccharides for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis
| Phamacotherapies that specifically target ammonia versus placebo or non‐absorbable disaccharides for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis | ||||||
| 1. Sodium benzoate compared to placebo for the treatment of hepatic encephalopathy in adults with cirrhosis | ||||||
| Patient or population: adults with cirrhosis and hepatic encephalopathy Setting: hospital Intervention: sodium benzoate Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE)a | Comments | |
| Risk with placebo | Risk with sodium benzoate | |||||
| All‐cause mortality | Study population | not estimable | 16 (1 RCT) |
⊕⊝⊝⊝ Very low | ||
| 0 per 1000 | 0 per 1000 (0 to 0) |
|||||
| Hepatic encephalopathy | No data reported | |||||
| Serious adverse events | Study population | not estimable | 16 (1 RCT) |
⊕⊝⊝⊝ Very low | ||
| 0 per 1000 | 0 per 1000 (0 to 0) |
|||||
| Non‐serious adverse events | Study population | not estimable | 16 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Health‐related quality of life | No data reported | |||||
| Blood ammonia concentrations (µg/dL) | ‐ | MD 32 lower (46.85 lower to 17.15 lower) | ‐ | 16 (1 RCT) | ⊕⊝⊝⊝ Very low | |
| 2. Sodium benzoate compared to non‐absorbable disaccharides for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis | ||||||
| Participants: people with cirrhosis and hepatic encephalopathy Setting: hospital Intervention: sodium benzoate Comparison: non‐absorbable disaccharides (lactulose or lactitol) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE)b | Comments | |
| Risk with lactulose/lactitol | Risk with sodium benzoate | |||||
| All‐cause mortality | Study population | RR 1.26 (0.49 to 3.28) | 101 (2 RCTs) | ⊕⊝⊝⊝ Very low | ||
| 118 per 1000 | 148 per 1000 (58 to 386) | |||||
| Hepatic encephalopathy | Study population | RR 1.22 (0.51 to 2.93) | 74 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 194 per 1000 | 237 per 1000 (99 to 570) | |||||
| Serious adverse events | Study population | RR 1.08 (0.44 to 2.68) | 101 (2 RCTs) | ⊕⊝⊝⊝ Very low | ||
| 137 per 1000 | 148 per 1000 (60 to 368) | |||||
| Non‐serious adverse events | Study population | RR 1.13 (0.96 to 1.32) | 182 (2 RCTs) | ⊕⊝⊝⊝ Very low | ||
| 469 per 1000 | 530 per 1000 (450 to 619) | |||||
| Health‐related quality of life | No data reported | |||||
| Blood ammonia concentrations | ‐ | MD 9 higher (1.10 lower to 19.11 higher) | ‐ | 85 (2 RCTs) | ⊕⊝⊝⊝ Very low | |
| 3. Glycerol phenylbutyrate compared to placebo for the prevention of hepatic encephalopathy in adults with cirrhosis | ||||||
| Participants: people with cirrhosis and at least two previous episodes of hepatic encephalopathy in the previous 6 months Setting: outpatients Intervention: glycerol phenylbutyrate Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE)c | Comments | |
| Risk with placebo | Risk with glycerol phenylbutyrate | |||||
| All‐cause mortality | Study population | RR 0.65 (0.11 to 3.81) | 178 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 34 per 1000 | 22 per 1000 (4 to 130) | |||||
| Hepatic encephalopathy | Study population | RR 0.57 (0.36 to 0.90) | 178 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 409 per 1000 | 233 per 1000 (147 to 368) | |||||
| Serious adverse events | Study population | RR 1.63 (0.85 to 3.13) | 178 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 136 per 1000 | 222 per 1000 (116 to 427) | |||||
| Non‐serious adverse events | Study population | RR 1.04 (0.88 to 1.21) | 178 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 761 per 1000 | 792 per 1000 (670 to 921) | |||||
| Health‐related quality of life | No data reported | |||||
| Blood ammonia concentrations (µmol/L*week) | ‐ | MD 12 lower (23.37 lower to 0.63 lower) | ‐ | 178 (1 RCT) | ⊕⊝⊝⊝ Very low | |
| 4. Ornithine phenylacetate compared to placebo for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis and hepatic encephalopathy | ||||||
| Participants: adults with cirrhosis and an acute episode of hepatic encephalopathy or adults with cirrhosis presenting with an episode of acute upper gastrointestinal bleeding Setting: hospital Intervention: ornithine phenylacetate Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE)d | Comments | |
| Risk with placebo | Risk with ornithine phenylacetate | |||||
| All‐cause mortality | Study population | RR 0.73 (0.35 to 1.51) | 269 (2 RCTs) | ⊕⊝⊝⊝ Very low | ||
| 113 per 1000 | 82 per 1000 (39 to 170) | |||||
| Hepatic encephalopathy | Study population | RR 2.71 (0.12 to 62.70) | 38 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Serious adverse events | Study population | RR 0.92 (0.62 to 1.36) | 264 (2 RCTs) | ⊕⊝⊝⊝ Very low | ||
| 285 per 1000 | 262 per 1000 (176 to 387) | |||||
| Non‐serious adverse events | Study population | RR 1.08 (0.78 to 1.51) | 269 (2 RCTs) | ⊕⊝⊝⊝ Very low | ||
| 368 per 1000 | 398 per 1000 (287 to 556) | |||||
| Health‐related quality of life | No data reported | |||||
| Blood ammonia concentrations (µmol/L) | ‐ | MD 27.1 lower (48.55 lower to 5.65 lower) | ‐ | 231 (1 RCT) | ⊕⊝⊝⊝ Very low | |
| 5. AST‐120 compared to placebo for the treatment of hepatic encephalopathy in adults with cirrhosis | ||||||
| Participants: adults with cirrhosis and hepatic encephalopathy Setting: hospital Intervention: AST‐120 Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE)e | Comments | |
| Risk with placebo | Risk with AST‐120 | |||||
| All‐cause mortality | Study population | not estimable | 148 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Hepatic encephalopathy | No data reported | |||||
| Serious adverse events | No data reported | |||||
| Non‐serious adverse events | No data reported | |||||
| Health‐related quality of life | No data reported | |||||
| Blood ammonia concentrations (µg/dL) | ‐ | MD 22 lower (26.75 lower to 17.25 lower) | ‐ | 98 (1 RCT) | ⊕⊝⊝⊝ Very low | We were not able to gather data on blood ammonia concentration separately for the two treatment groups |
| 6. AST‐120 compared to non‐absorbable disaccharide (lactulose) for the treatment of hepatic encephalopathy in adults with cirrhosis | ||||||
| Patient or population: adults with cirrhosis and hepatic encephalopathy Setting: hospital Intervention: AST‐120 Comparison: lactulose | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE)f | Comments | |
| Risk with lactulose | Risk with AST‐120 | |||||
| All‐cause mortality | Study population | RR 1.05 (0.59 to 1.85) | 41 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 524 per 1000 | 550 per 1000 (0 to 0) | |||||
| Hepatic encephalopathy | Study population | RR 1.05 (0.59 to 1.85) | 41 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 524 per 1000 | 550 per 1000 (309 to 969) | |||||
| Serious adverse events | Study population | not estimable | 41 (1 RCT) | ⊕⊝⊝⊝ Very low | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Non‐serious adverse events | No data reported | |||||
| Health‐related quality of life | No data reported | |||||
| Blood ammonia concentrations (units unspecified) | ‐ | MD 5.2 higher (2.75 lower to 13.15 higher) | ‐ | 35 (1 RCT) | ⊕⊝⊝⊝ Very low | We were only able to gather data on blood ammonia concentrations on a subgroup of participants. |
| 7. Polyethylene glycol compared non‐absorbable disaccharide (lactulose) for the treatment of hepatic encephalopathy in adults with cirrhosis | ||||||
| Participants: adults with cirrhosis and hepatic encephalopathy Setting: hospital Intervention: polyethylene glycol Comparison: lactulose | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE)g | Comments | |
| Risk with lactulose | Risk with polyethylene glycol | |||||
| All‐cause mortality | Study population | RR 0.50 (0.09 to 2.64) | 190 (3 RCTs) | ⊕⊝⊝⊝ Very low | ||
| 43 per 1000 | 21 per 1000 (4 to 112) | |||||
| Hepatic encephalopathy | Study population | RR 0.19 (0.08 to 0.44) | 190 (3 RCTs) | ⊕⊝⊝⊝ Very low | ||
| 330 per 1000 | 63 per 1000 (26 to 145) | |||||
| Serious adverse events | Study population | RR 0.57 (0.18 to 1.82) | 190 (3 RCTs) | ⊕⊝⊝⊝ Very low | ||
| 74 per 1000 | 42 per 1000 (13 to 136) | |||||
| Non‐serious adverse events | Study population | RR 0.71 (0.40 to 1.27) |
117 (2 RCTs) | ⊕⊝⊝⊝ Very low | ||
| 191 per 1000 | 136 per 1000 (77 to 243) | |||||
| Health‐related quality of life | No data reported | |||||
| Blood ammonia levels (µmol/L) | ‐ | MD 29.28 lower (95.96 lower to 37.39 higher) | ‐ | 90 (2 RCTs) | ⊕⊝⊝⊝ Very low | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; RCT: randomised clinical trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded the evidence by three levels because of the serious risk of bias (two levels) and uncertainty; only one small trial is included in the analysis and there were no reported events (one level).
bWe downgraded the evidence by three levels because of the serious risk of bias; only one of the two trials had a low risk of bias (two levels) and uncertainty; only two small trials are included in the analysis (one level).
cWe downgraded the evidence by three levels because of the serious risk of bias (two levels) and uncertainty; only one small trial is included in the analysis (one level).
dWe downgraded the evidence by three levels because of the serious risk of bias (two levels) and uncertainty; only two small trials are included in the analysis (one level).
eWe downgraded the evidence by three levels because of the serious risk of bias (two levels) and uncertainty; only one small trial is included in the analyses, there were no reported deaths and the blood ammonia data were incomplete (one level).
fWe downgraded the evidence by three levels because of the serious risk of bias (two levels) and uncertainty (only one small trial is included in the analyses; there were no reported deaths and the blood ammonia data were incomplete) (one level).
gWe downgraded the evidence by three levels because of the serious risk of bias (two levels) and uncertainty; only two small trials are included in the analyses (one level).
Background
Description of the condition
The term 'hepatic encephalopathy' is used to describe the spectrum of neuropsychiatric change that can arise in people with cirrhosis. The joint guideline from the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) defines hepatic encephalopathy as, "brain dysfunction associated with liver insufficiency or portal‐systemic shunting" (AASLD/EASL 2014; Vilstrup 2014).
Clinically apparent or 'overt' hepatic encephalopathy manifests as a neuropsychiatric syndrome encompassing a wide spectrum of mental and motor disorders (Ferenci 2002; Weissenborn 1998). It may develop over a period of hours or days, apparently spontaneously, or else, in 50% to 70% of instances, follow an identifiable precipitating event such as gastrointestinal bleeding, infection, dehydration, or constipation (Pantham 2017). Episodes may recur. Between episodes, people may return to their baseline neuropsychiatric status or retain a degree of impairment (Bajaj 2010). Less frequently, people present with persistent neuropsychiatric abnormalities, which are always present to some degree, but which may fluctuate in severity (Ferenci 2002). The changes in mental state in people with overt hepatic encephalopathy range from subtle alterations in personality, intellectual capacity and cognitive function to deep coma. The changes in motor function may include asterixis (flapping tremor), rigidity, speech disorders, tremor, and delayed diadochocinetic movements (Cadranel 2001; Victor 1965; Weissenborn 1998). People with overt hepatic encephalopathy may show other associated abnormalities, including: impaired psychomotor performance (Schomerus 1998); disturbed neurophysiological function (Chu 1997; Parsons‐Smith 1957); altered cerebral neurochemical/neurotransmitter homeostasis (Taylor‐Robinson 1994); reductions in global and regional cerebral blood flow and metabolism (O'Carroll 1991); and changes in cerebral fluid homeostasis (Haussinger 2000). In general, the degree of impairment in these variables increases with the severity of the underlying liver disease (Bajaj 2009). 'Minimal' hepatic encephalopathy, in the older literature referred to as ’subclinical’ or ’latent’ hepatic encephalopathy, is the term applied to people with cirrhosis with no clinical neuropsychiatric abnormalities who, nevertheless, show abnormalities in neuropsychometric or neurophysiological performance, when tested (Ferenci 2002; Guérit 2009). Recently, the term covert hepatic encephalopathy has been introduced to encompass patients with minimal hepatic encephalopathy and those with low‐grade overt hepatic encephalopathy. While this approach may be pragmatic, it is not clear how informative or valuable it is in clinical and particularly research settings. Indeed, it has already been shown that patients classified as having covert hepatic encephalopathy behave, when tested, as two relatively independent groups (Montagnese 2014; Zacharias 2017).
There is no gold standard test for the diagnosis of hepatic encephalopathy (AASLD/EASL 2014; Montagnese 2004; Vilstrup 2014), but rather a range of diagnostic tests that can be used singly or in combination. A detailed neuropsychiatric history and examination (Montagnese 2004), should be undertaken with particular attention paid to changes in memory, concentration, cognition, and consciousness. Clinicians and researchers often use the West Haven Criteria to grade mental status (Conn 1977), and the Glasgow Coma Score to grade the level of consciousness (Teasdale 1974). The history and examination will identify the clinical features suggestive of hepatic encephalopathy, which are often subtle, or confirm their absence. Further, they will allow exclusion of other causes of neuropsychiatric abnormalities, such as certain neurological disorders and other metabolic encephalopathies, including those associated with diabetes, renal failure, and chronic pulmonary insufficiency. People with hepatic encephalopathy also show impaired performance on a range of psychometric tests (Montagnese 2004; Randolph 2009). Those with minimal hepatic encephalopathy show deficits in attention, visuo‐spatial abilities, fine motor skills, and memory, while other cognitive functions are relatively well preserved. People with overt hepatic encephalopathy show additional disturbances in psychomotor speed, executive function, and concentration. Several paper and pencil psychometric tests are used in the evaluation of cognitive performance in people suspected of having hepatic encephalopathy. These tests are either used individually or are grouped together into test batteries or systems. Of the tests used singly, Number Connection Tests A and B are the best known (Ferenci 2002). The Psychometric Hepatic Encephalopathy Score (PHES), which comprises five paper and pencil tests covering the domains of attention, visual perception, and visuo‐constructive abilities, is the most widely used psychometric test battery and has high diagnostic specificity (Schomerus 1998; Weissenborn 2001). PHES test scores have to be normalised to take account of factors such as age, sex, and educational level. At present, normative databases are available in several countries, including Germany, Italy, Spain, Mexico, Korea, Romania, India, and the UK. In countries where levels of illiteracy are high, Figure Connection Tests A and B are often used either alone or as part of the PHES battery (Dhiman 1995). People with hepatic encephalopathy may also show several neurophysiological abnormalities (Guérit 2009). The electroencephalogram (EEG), which primarily reflects cortical neuronal activity, may show progressive slowing of the background activity and abnormal wave morphology (Parsons‐Smith 1957). Recent advances in electroencephalogram analysis provide better quantifiable and more informative data (Jackson 2016; Olesen 2016). The brain responses, or evoked potentials, to stimuli such as light and sound may show abnormal slowing or wave forms, or both (Chu 1997; Guérit 2009). Other potential diagnostic techniques, such as the Critical Flicker Fusion Frequency (Kircheis 2002), the Inhibitory Control Test (Bajaj 2008), and the Stroop test (Allampati 2016), need further validation. Blood ammonia concentrations are not routinely measured to diagnose hepatic encephalopathy (Blanco Vela 2011; Lockwood 2004), but are often monitored in clinical trials.
Description of the intervention
We assessed five separate pharmacotherapies that specifically target ammonia. These differed in their formulation, routes of administration, and modes of action (Table 3).
Table 1.
Pharmacotherapeutic agents that specifically target ammonia
| Agent | Appearance | Doses used | Mechanisms of action |
| Sodium benzoatea | Crystalline powder | Given orally in solution: standard dose 10 g daily in divided doses | Conjugates with glycine to form hippurate, which is then excreted via the kidneys. |
| Sodium phenylbutyratea | Crystalline powder | Administered orally or via nasogastric tube; 200 mg/kg/day | Conjugates with glutamine to form phenylacetylglutamine (PAG) in the liver and kidneys, which is then eliminated in the urine. As glutamine is incorporated into PAG, more is synthesized by amidation of glutamic acid by ammonia through glutamine synthetase. |
| Glycerol phenylbutyrate | Liquid | Administered orally, 6 mL twice daily for 16 weeks | Prodrug of sodium phenylbutyrate. Conjugates with glutamine to form phenylacetylglutamine (PAG) in the liver and kidneys, which is then eliminated in the urine. As glutamine is incorporated into PAG, more is synthesized by amidation of glutamic acid by ammonia through glutamine synthetase. |
| Sodium phenylacetate | Crystalline powder | Sodium phenylacetate is usually used in combination with sodium benzoate as adjunctive therapy for the treatment of acute hyperammonaemia and associated encephalopathy in people with urea cycle enzyme deficiencies. It is supplied as a solution containing 100 mg/mL of sodium phenylacetate and 100 mg/mL of sodium benzoate (Ammonul 10%/10%). It is administered as an IV infusion 5.5 g/m2/day | Conjugates with glutamine to form phenylacetylglutamine (PAG) in the liver and kidneys, which is then eliminated in the urine. As glutamine is incorporated into PAG, more is synthesized by amidation of glutamic acid by ammonia through glutamine synthetase. |
| Ornithine phenylacetate (OCR‐002) | Crystalline salt | Administered as IV infusion 10 g/24 h (0.42 g/h) | Reduces ammonia through 2 pathways:
|
| AST‐120 (spherical carbon microsphere adsorbent) | Powder | Orally administered powder (sachets), 2 g three times daily | Differs structurally from activated charcoal and exhibits superior adsorptive capacity for certain organic compounds typically those with a low molecular weight < 10 kDa. It binds ammonia in the lumen of the lower gastrointestinal tract and facilitates its excretion. |
| Polyethylene glycol (PEG) | Solution of 280 g of PEG in 4 L of water | Administered orally or via a nasogastric tube in a single 4 L dose over 20‐30 min | A cathartic which causes rapid clearance of ammonia‐synthesising gut bacteria from the gut lumen. |
| IV: intravenous | |||
aIncludes relatively high amounts of sodium.
How the intervention might work
The exact pathogenesis of hepatic encephalopathy is unknown but ammonia is known to play a key role (Butterworth 2013; Morgan 2018). Ammonia is produced in the intestine from dietary protein, deamination of glutamine via glutaminase and bacterial action in the colon. It is absorbed by non–ionic diffusion but specific ammonia transporters may also be involved; ammonia concentrations in the portal vein are ten‐fold higher than in arterial blood. The hepatic extraction rate is high. The ammonia in portal blood, together with the ammonia derived from hepatic amino acid metabolism, is taken up primarily by periportal hepatocytes and metabolised to urea via the urea cycle. The kidneys and muscles also play a role in ammonia homeostasis (Wright 2011). In skeletal muscle, ammonia is transformed into glutamine through the action of glutamine synthetase. In the kidneys, ammonia is generated from the deamination of glutamine. In people with cirrhosis, blood ammonia levels increase primarily because of a reduction in first‐pass metabolism of ammonia as a result of portal systemic shunting and a loss of hepatic metabolic capacity. As a result, gut‐derived ammonia is not effectively cleared from the blood by the liver; it consequently enters the systemic circulation and impinges on the brain where it has both direct and indirect effects on cerebral function. Treatment is aimed, primarily, at reducing the production and absorption of ammonia from the gastrointestinal tract and this is usually affected by use of non‐absorbable disaccharides and non‐absorbable antibiotics.
Hyperammonaemia is also a major consequence of genetic disorders of the urea cycle enzymes. In these conditions the increase in blood ammonia concentrations results directly from failure of hepatic ammonia metabolism. Treatment is based on providing alternative pathways for the removal of nitrogen waste (Berry 2014). A small number of drugs, so‐called 'ammonia scavengers', have been developed for use in urea cycle disorders. The best known of these agents are sodium benzoate, and sodium/glycerol phenylbutyrate. Both decrease ammonia concentrations by serving as alternatives to urea for the excretion of waste nitrogen. Benzoate conjugates with glycine to form hippuric acid while phenylacetate conjugates with glutamine in the liver and kidneys to form phenylacetylglutamine. Hippuric acid and phenylacetylglutamine are subsequently excreted in the urine (Table 3). These ‘ammonia scavenging agents’ have also been used to treat hepatic encephalopathy in people with cirrhosis (Campollo 1992; Efrati 2000; Gonzalez 1994; Mendenhall 1986; Misel 2013; Rockey 2014; Sushma 1992; Uribe 1990; Weiss 2018). Ornithine phenylacetate was specifically developed for the treatment of hepatic encephalopathy in people with cirrhosis; the L‐ornithine moiety acts as a substrate for the synthesis of glutamine from ammonia in skeletal muscle, while the phenylacetate moiety combines with glutamine to form phenylacetylglutamine, which is excreted in the urine (Jalan 2007; Rahimi 2016; Rose 2012; STOP‐HE 2017; Ventura‐Cots 2016). This agent is not intended for use in the treatment of urea cycle enzyme disorders (Rahimi 2016). AST‐120 is a carbon microsphere adsorbent that differs structurally from activated charcoal in that it has a selective binding surface. It exhibits superior adsorption of low molecular weight organic compounds such as ammonia from the lumen of the lower gastrointestinal tract, which are then excreted in the faeces (Bajaj 2013; Bosoi 2011; Pockros 2009). Polyethylene glycol (PEG) is a cathartic; it causes rapid clearance of the gut bacteria that synthesize ammonia, thereby reducing its production (Naderian 2017; Rahimi 2014; Rahimi 2016). The adverse events associated with the use of these drugs are mainly gastrointestinal and include diarrhoea, constipation, dry mouth, and changes in appetite (Lee 2010; Rahimi 2016).
Why it is important to do this review
Hepatic encephalopathy is a common and debilitating complication of cirrhosis. Approximately 10% to 14% of people with cirrhosis have overt hepatic encephalopathy when they are first diagnosed with liver disease (Saunders 1981). In people with decompensated cirrhosis, the prevalence of overt hepatic encephalopathy at presentation is about 20% (D’Amico 1986; De Jongh 1992; Zipprich 2012). In people with cirrhosis who have no evidence of neuropsychiatric impairment, the risk of developing an episode of overt hepatic encephalopathy within five years of presentation varies from 5% to 25%, depending on the presence or absence of other risk factors; the cumulative incidence of overt hepatic encephalopathy is as high as 40% (Bajaj 2011a; Randolph 2009). The prevalence of minimal hepatic encephalopathy may be more than 50% in people with previous overt hepatic encephalopathy (Lauridsen 2011; Sharma 2010).
The presence of hepatic encephalopathy, whether minimal or overt, is associated with significant impairment in the performance of complex tasks, such as driving (Kircheis 2009; Schomerus 1981), and a detrimental effect on quality of life (Groeneweg 1998), and safety (Roman 2011). In addition, the presence of overt hepatic encephalopathy pre‐transplantation has a detrimental effect on neurocognitive function post‐transplantation (Sotil 2009), and on survival (Bustamante 1999; D’Amico 2006; Jepsen 2010; Stewart 2007). The one‐year survival rate in people who have hepatic encephalopathy at presentation is 36%, with a five‐year survival rate of 15% (Jepsen 2010), while the survival probability after a first episode of hepatic encephalopathy is 42% at one year but only 23% at three years (Bustamante 1999). Overt hepatic encephalopathy also poses a substantial burden for the affected families (Bajaj 2011b), and a significant financial burden on healthcare systems (Poodad 2007; Stepanova 2012).
Strategies to prevent and treat hepatic encephalopathy in people with cirrhosis are clearly needed (Morgan 2018). At present, treatment is directed primarily at reducing the production and absorption of gut‐derived neurotoxins, particularly ammonia, mainly through dietary manipulation, bowel cleansing, non‐absorbable disaccharides and non‐absorbable antibiotics (AASLD/EASL 2014; Vilstrup 2014). Interventions that specifically target the metabolism and elimination of ammonia may provide new treatment options (Jover‐Cobos 2013; McGuire 2010; Rahimi 2016; Rose 2012). There are several potential candidates (Table 3). Some, for example, sodium benzoate and glycerol phenylbutyrate, are used to treat the hyperammonaemia associated with urea cycle enzyme deficiencies; they serve as 'ammonia scavengers', providing alternative, non‐urea cycle pathways for removal of ammonia. Ornithine phenylacetate is also an ammonia scavenger; it was developed specifically for the treatment of hepatic encephalopathy in people with cirrhosis. AST‐120 and polyethylene glycol speed elimination of ammonia or ammonia‐generating bacteria via the large intestine (Table 3). None of these pharmacotherapies are currently licensed for the indication of hepatic encephalopathy, although several are undergoing phase IIB and III clinical trials. Thus, presently these agents do not have a place in routine clinical practice.
Very little is known about the potential beneficial and harmful effects of these pharmacotherapies. We have, therefore, conducted a systematic review with meta‐analyses of the available randomised clinical trials of five pharmacotherapeutic agents that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in people with cirrhosis, following recommendations for best practice.
Objectives
To evaluate the beneficial and harmful effects of pharmacotherapies that specifically target ammonia versus placebo, no intervention, or other active interventions, for the prevention and treatment of hepatic encephalopathy in people with cirrhosis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials, irrespective of blinding, language, or publication status in our primary analyses. If, during the selection of trials, we identified observational studies, for example, quasi‐randomised studies, cohort studies or patient reports that described adverse events caused by or associated with use of the interventions under review, then we included these data in our qualitative analyses. We did not specifically search for observational studies for inclusion in this review, which is a known limitation.
Types of participants
We included adults with cirrhosis and minimal or overt hepatic encephalopathy, or adults who were at risk of developing overt hepatic encephalopathy. We included participants in our primary analyses irrespective of age, sex, the aetiology and severity of the underlying liver disease, or the presence or absence of precipitating factors. We excluded trials involving people with hepatic encephalopathy associated with acute liver failure. We included trials involving people with hepatic encephalopathy associated with either cirrhosis or non‐cirrhotic portal hypertension provided that subgroup analyses were available or else the proportion of participants with non‐cirrhotic portal hypertension was very small.
Types of interventions
We evaluated drugs that specifically target ammonia, including sodium benzoate, glycerol phenylbutyrate, ornithine phenylacetate, spherical carbon adsorbents (AST‐120), and polyethylene glycol versus placebo, no treatment or interventions that have a potentially beneficial effect on hepatic encephalopathy, such as the non‐absorbable disaccharides (Gluud 2016). We included trials irrespective of the dose, treatment duration, or mode of administration of the drugs under review. We allowed co‐interventions if they were administered equally to all comparison groups.
We did not include trials involving use of L‐ornithine L‐aspartate as these are the subject of a separate Cochrane Review (Goh 2018).
Types of outcome measures
We assessed all outcomes at the maximum duration of follow‐up.
Primary outcomes
All‐cause mortality.
Hepatic encephalopathy. We assessed this outcome using the primary investigators' overall assessment of: i) the number of participants who developed hepatic encephalopathy, and ii) the number of participants without a clinically‐relevant improvement in hepatic encephalopathy.
Serious adverse events. We defined adverse events as any untoward medical occurrence (ICH‐GCP 1997) and considered adverse events as serious if they resulted in death; were life‐threatening; required inpatient hospitalisation or prolongation of existing hospitalisation; or resulted in persistent or significant disability or incapacity. In this review, serious adverse events included mortality and hepatic encephalopathy, and they were analysed as a composite outcome.
Secondary outcomes
Non‐serious adverse events. We considered as non‐serious all adverse events that did not fulfil the criteria for serious adverse events, as described above (ICH‐GCP 1997).
Health‐related quality of life.
Blood ammonia.
Search methods for identification of studies
We combined the electronic and manual searches.
Electronic searches
We searched the Cochrane Hepato‐Biliary Specialised Register (March 2019: hbg.cochrane.org/specialised‐register), the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 3) in the Cochrane Library; MEDLINE Ovid (1946 to March 2019); Embase Ovid (1974 to March 2019); LILACs (Bireme; 1982 to March 2019); Science Citation Index Expanded (Web of Science; 1900 to March 2019); and Conference Prodeedings Citation Index ‐ Science (Web of Science; 1990 to March 2019; Royle 2003) using the strategies outlined in Appendix 1.
We did not have access to Chinese, Russian, or Japanese databases, but we plan to search these in future updates should they become available to us via the Cochrane Hepato‐Biliary Group.
Searching other resources
We searched the reference lists of papers identified in the electronic searches and wrote to authors of the identified clinical trials and relevant pharmaceutical companies for additional data, if required. We searched the conference proceedings of the annual meetings of the British Society of Gastroenterology (BSG), the European Association for the Study of the Liver (EASL), the United European Gastroenterology Week (UEGW), the American Gastroenterological Association (AGA), and the American Association for the Study of Liver Diseases (AASLD) from 2000 to 2018/9. We searched online trials registries such as ClinicalTrials.gov (clinicaltrials.gov/); European Medicines Agency (EMA; www.ema.europa.eu/ema/); the World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp), and the Food and Drug Administration (FDA; www.fda.gov) in March 2019. We also searched Google Scholar using the search terms cirrhosis AND ammonia scavenging agents; and pharmaceutical company sources for ongoing or unpublished trials with no date restriction.
Data collection and analysis
We performed the review following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), Cochrane Hepato‐Biliary information for authors (hbg.cochrane.org/), and the Methodological Expectations of Cochrane Intervention Reviews (MECIR) guidelines (MECIR 2018).
Selection of studies
Three review authors (HDZ, APZ, and MYM), working independently, read the electronic search output, performed additional manual searches, and listed potentially eligible trials. All review authors read the potentially eligible trials and participated in the final selection of trials for inclusion. If trial data were reported in more than one publication, we selected the report with the largest number of participants and the longest duration of follow‐up as our primary reference. We listed details of all the included trials in the Characteristics of included studies table, and listed all the excluded trials with the reasons for their exclusion in the Characteristics of excluded studies table. A fourth review author (LLG) acted as ombudsman in case of disagreements on trial suitability for inclusion or exclusion. We resolved contrary opinions through discussion.
Data extraction and management
All review authors independently extracted data and evaluated bias. We requested missing data and other information from the published trial reports from the corresponding authors of the included trials. We sought information and data from identified but unpublished trials or ongoing trials from the principal investigators and sponsors. We gathered the following data from the included trials.
Trials: design (cross‐over or parallel); settings (number of clinical sites; outpatient or inpatient; inclusion period); country of origin; publication status; funding sources;
Participants: mean age; proportion of men; aetiology and severity of the liver disease; type of hepatic encephalopathy (diagnostic criteria and definitions/terminology); previous history of hepatic encephalopathy;
Interventions: type, dose, duration of therapy, mode of administration; co‐interventions;
Outcomes: including definitions used in the assessment and duration of follow‐up; number of participants included in the assessment of outcomes (number of losses to follow‐up/withdrawals); outcomes included in the meta‐analyses.
Assessment of risk of bias in included studies
We followed Cochrane Hepato‐Biliary recommendations for assessing the risk of bias in the included trials, based on the definitions described below (hbg.cochrane.org/information‐authors). We assessed each domain separately as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017) and combined the domains to provide an overall assessment of bias control for both mortality and non‐mortality outcomes. We classified trials as low risk of bias only if none of the domains was designated as being at unclear or high risk of bias.
Allocation sequence generation
Low risk of bias: sequence generation achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, or throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Unclear risk of bias: the method of sequence generation was not specified.
High risk of bias: sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment; allocation was controlled by a central and independent randomisation unit or similar adequate method (e.g. serially numbered opaque sealed envelopes) to ensure that the allocation sequence was unknown to the investigators (Savović 2012a; Savović 2012b).
Unclear risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel
Low risk of bias: blinding of participants and personnel performed adequately using a placebo. We defined lack of blinding as not likely to affect the evaluation of mortality (Savović 2012a; Savović 2012b).
Unclear risk of bias: insufficient information to assess blinding.
High risk of bias: no blinding or incomplete blinding.
Blinding of outcome assessors
Low risk of bias: blinding of outcome assessors performed adequately using a placebo. We defined lack of blinding as not likely to affect the evaluation of mortality (Savović 2012a; Savović 2012b).
Unclear risk of bias: there was insufficient information to blinding.
High risk of bias: no blinding or incomplete blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The investigators used sufficient methods, such as intention‐to‐treat analyses with multiple imputations or carry‐forward analyses to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported clinically relevant outcomes (all‐cause mortality, hepatic encephalopathy, and serious adverse events). If we had access to the original trial protocol, the outcomes selected should be those called for in the protocol. If we obtained information from a trial registry (such as www.clinicaltrials.gov), we only used that information if the investigators registered the trial before inclusion of the first participant.
Unclear risk of bias: predefined relevant outcomes were not reported fully or the reporting was unclear.
High risk of bias: one or more predefined outcomes were not reported.
Other bias
Low risk of bias: the trial appeared free of other biases including: medicinal dosing, medicinal problems, or follow‐up (as defined below).
Unclear risk of bias: the trial may or may not have been free of other domains that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias such as inappropriate treatments being given to the controls (e.g. an inappropriate dose) or follow‐up (e.g. the trial included different follow‐up schedules for participants in the allocation groups).
Overall bias assessment
Low risk of bias: all domains were low risk of bias using the definitions above.
High risk of bias: one or more of the bias domains was of unclear or high risk of bias.
Measures of treatment effect
We analysed dichotomous outcomes using risk ratios (RR) and continuous outcomes using mean differences (MD), both with 95% confidence intervals (CI). For primary outcomes, we calculated the number needed to treat for an additional beneficial outcome (NNTB) as 1 / risk difference (RD) based on the highest‐quality evidence (randomised clinical trials with a low risk of bias where available).
Unit of analysis issues
We included randomised clinical trials using a parallel‐group design. We did not identify any multi‐armed trials, however, if we identify any such trials in future updates, then we will undertake separate pair‐wise comparisons of the treatments of interest. We did not identify any cross‐over trials, however, if we identify any such trials in future updates, we will only use data from the first treatment period (Deeks 2017).
Dealing with missing data
We collected data on all participants randomised and included all participants irrespective of compliance or follow‐up. We planned to evaluate the influence of missing data (Higgins 2008), by undertaking best‐case scenario, worst‐case scenario, and extreme worst‐case scenario analyses (hbg.cochrane.org/information‐authors). However, we did not identify any randomised clinical trials with missing outcome data.
Assessment of heterogeneity
We expressed heterogeneity as I2 statistic values using the following thresholds: 0% to 40% (unimportant), 40% to 60% (moderate), 60% to 80% (substantial), and more than 80% (considerable). We used this information when describing and interpreting our analyses and included the information in the 'Summary of findings' table.
Assessment of reporting biases
We evaluated reporting bias based on the definition and reporting of key outcomes (the most clinically relevant) and by comparing protocols, online trial registrations, and trial publications if available. We planned to use visual inspection of funnel plots and regression analyses to evaluate reporting biases if our analysis included at least 10 trials with reported events for an individual pharmacotherapy (Egger 1997; Harbord 2006), however, our review did not reach this number threshold.
Data synthesis
We performed the analysis in Review Manager 5 (Review Manager 2014) and STATA version 14 (Stata 2015).
Meta‐analyses
We analysed trials for each of the drugs that specifically target ammonia separately, using fixed‐effect and random‐effects meta‐analyses (Deeks 2017). The individual meta‐analyses included a small number of trials, and we did not identify differences between the two models. We chose to report random‐effects meta‐analyses based on an expected clinical difference between trials.
In the case that estimates of the random‐effects and fixed‐effect meta‐analyses are similar in future updates, then we will assume that any small‐study effect had little effect on the intervention effect estimate. If the random‐effects estimate is more beneficial, we will re‐evaluate whether it is reasonable to conclude that the intervention was more effective in the smaller trials. If the larger trials tend to be those conducted with greater methodological rigour, or conducted in circumstances more typical of the use of the intervention in practice, then we will report the results of meta‐analyses restricted to the larger, more rigorous trials. Based on the expected clinical heterogeneity, we anticipated that a number of analyses would display statistical between‐trial heterogeneity (I2 > 0%).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses to determine the influence of:
risk of bias;
type of encephalopathy;
aetiology of the liver disease (alcohol or hepatitis).
We were not able to undertake subgroup analyses because the number of trials was too small.
Sensitivity analysis
We planned to undertake worst‐case scenario analyses, as described in Dealing with missing data. However, outcome data sets were complete in the intervention and control groups in all of the included trials.
Trial Sequential Analysis
We planned to perform Trial Sequential Analyses of our primary outcomes to evaluate the risk of random error associated with sparse data and cumulative testing, and to evaluate futility (Higgins 2008; Wetterslev 2008). However, the number of events, participants, and trials were clearly insufficient, so we did not undertake these analyses.
In future updates of our review, if the data allow, we plan to undertake Trial Sequential Analyses with alpha 3%, power 90%, and the results of the random‐effects meta‐analyses (upper 95% CI) in order to determine the relative risk reduction and the control group event.
Certainty of evidence, GRADE
We used the GRADE system to evaluate the certainty of the evidence for all outcomes reported in the review, considering the within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimate, and risk of publication bias (Schünemann 2013).
'Summary of findings' tables
We used GradePro 2015 to generate a 'Summary of findings' table with information about outcomes, risk of bias, and the results of the meta‐analyses (Table 1).
Results
Description of studies
We included 11 randomised clinical trials (Characteristics of included studies), and excluded three observational trials, one randomised clinical trial comparing two agents that specifically target ammonia, and one randomised clinical trial in which the drug of interest was used as adjuvant therapy to another active agent (Characteristics of excluded studies). In addition, we identified two ongoing trials that may be eligible for inclusion in future updates (NCT00558038; NCT03448770). We did not have access to data from these ongoing trials. Four of the 11 trials were published in abstract form only (Bajaj 2013; Gonzalez 1994; Pockros 2009; STOP‐HE 2017), while the remaining seven trials were published as full papers (Naderian 2017; Rahimi 2014; Rockey 2014; Shehata 2018; Sushma 1992; Uribe 1990; Ventura‐Cots 2016).
We included 11 trials in our quantitative and qualitative analyses (Bajaj 2013; Gonzalez 1994; Naderian 2017; Pockros 2009; Rahimi 2014; Rockey 2014; Shehata 2018; STOP‐HE 2017; Sushma 1992; Uribe 1990; Ventura‐Cots 2016).
Results of the search
We identified 513 potentially relevant records from electronic databases, and 10 additional records through manual searches and enquires (Figure 1). After removing duplicate references and references that were irrelevant to this review, we identified 17 records reporting 11 randomised clinical trials that fulfilled our inclusion criteria (Bajaj 2013; Gonzalez 1994; Naderian 2017; Pockros 2009; Rahimi 2014; Rockey 2014; Shehata 2018; STOP‐HE 2017; Sushma 1992; Uribe 1990; Ventura‐Cots 2016). In five of the 11 trials the control group received a placebo preparation (Bajaj 2013; Gonzalez 1994; Rockey 2014; STOP‐HE 2017; Ventura‐Cots 2016); in the remaining six trials they received a non‐absorbable disaccharide (Naderian 2017; Pockros 2009; Rahimi 2014; Shehata 2018; Sushma 1992; Uribe 1990). We did not identify any trials that compared these drugs with no treatment or with interventions that might potentially benefit hepatic encephalopathy, other than the non‐absorbable disaccharides.
Figure 1.
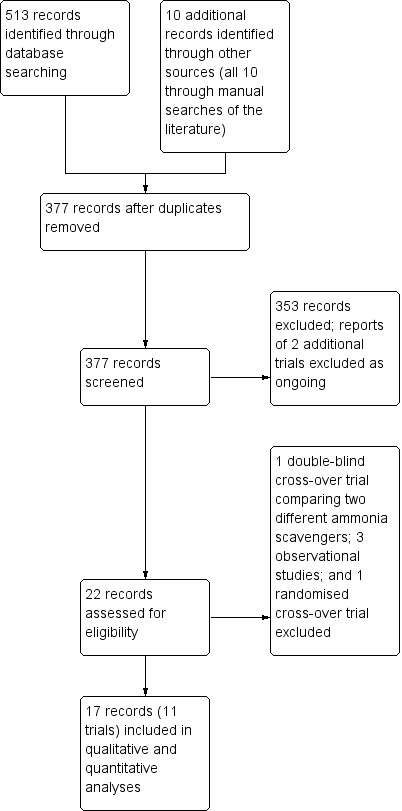
Study flow diagram
The countries of origin were Egypt (Shehata 2018), India (Sushma 1992), Iran (Naderian 2017), Mexico (Gonzalez 1994; Uribe 1990), Spain (Ventura‐Cots 2016), and the USA (Bajaj 2013; Pockros 2009; Rahimi 2014). One study was undertaken in centres in the USA, Ukraine, and Russia (Rockey 2014); one study was undertaken in centres in Australia, Austria, Belgium, Bulgaria, Czech Republic, Denmark, Estonia, France, Germany, Hungary, Israel, Italy, Netherlands, New Zealand, Russia, Spain, and the USA (STOP‐HE 2017).
Included studies
Participants
In total, 499 participants received pharmacotherapies that specifically target ammonia and 444 participants received placebo or a non‐absorbable disaccharide. The mean age of participants in the included trials ranged from 35.6 to 59.6 years and the proportion of men from 41.7% to 79.0%. The proportion of participants with cirrhosis secondary to hepatitis B or C infection ranged from 4% to 100%; the proportion with alcohol‐related cirrhosis ranged from 4.8% to 70%.
One trial, involving 38 participants with cirrhosis, evaluated prevention of hepatic encephalopathy following an upper gastrointestinal bleed (Ventura‐Cots 2016); six participants had overt hepatic encephalopathy at the time of inclusion while the remaining 32 participants did not. One trial evaluated the secondary prevention of hepatic encephalopathy in participants who had had at least two previous episodes of hepatic encephalopathy Grade 2 or greater in the previous six months (Rockey 2014).
The remaining nine trials included participants with current hepatic encephalopathy which was classified as covert (minimal and Grade 1) in one trial (Bajaj 2013), and as overt (Grades 1 to 4) in eight (Gonzalez 1994; Naderian 2017; Pockros 2009; Rahimi 2014; Shehata 2018; STOP‐HE 2017; Sushma 1992; Uribe 1990).
Interventions
Three trials evaluated sodium benzoate in doses of 5.6 to 10 g a day given orally or via a nasogastric tube (Gonzalez 1994; Sushma 1992; Uribe 1990); one trial evaluated oral glycerol phenylbutyrate 12 mL a day (Rockey 2014); two trials evaluated intravenous ornithine phenylacetate in doses ranging from 5 g to 20 g a day (STOP‐HE 2017; Ventura‐Cots 2016); two trials evaluated oral AST‐120 in doses ranging from 6 g to 12 g a day (Bajaj 2013; Pockros 2009); and three trials evaluated polyethylene glycol given orally or via a nasogastric tube at a dose of 280 grams in 4 litres of water daily (Naderian 2017; Rahimi 2014), or three to four sachets at 64 grams per sachet dissolved in one litre of water, given orally over three to four hours or via nasogastric tube at a rate of 20 to 30 millilitres per minute (Shehata 2018).
Comparisons
The control groups received either a placebo preparation (Bajaj 2013; Gonzalez 1994; Rockey 2014; STOP‐HE 2017; Ventura‐Cots 2016), or a non‐absorbable disaccharide in a dose adjusted to produce two to three semi‐soft stools per day (Naderian 2017; Pockros 2009; Rahimi 2014; Sushma 1992; Uribe 1990), or a fixed dose of 20‐30 millilitres orally or via nasogastric tube, given as 3 doses over 24 hours with 200 millilitres as a retention enema every four hours (Shehata 2018).
Co‐interventions
The majority of trials used co‐interventions. It is unclear if one study used additional active agents, which included rifaximin, L‐ornithine L‐aspartate and lactulose, in similar proportions of participants in the treatment and placebo groups (STOP‐HE 2017). Three trials did not report the use of co‐interventions (Bajaj 2013; Naderian 2017; Shehata 2018). The remaining seven trials used co‐interventions in similar proportions of participants in the treatment and control groups.
Outcomes
All 11 trials reported on mortality although there were no events in one trial (Gonzalez 1994). Ten trials reported the other primary outcomes (Bajaj 2013; Naderian 2017; Pockros 2009; Rahimi 2014; Rockey 2014; Shehata 2018; STOP‐HE 2017; Sushma 1992; Uribe 1990; Ventura‐Cots 2016). Investigators assessed hepatic encephalopathy using several different methods. Seven of 10 trials assessed mental status using West Haven Criteria (Bajaj 2013; Gonzalez 1994; Pockros 2009; Rockey 2014; Sushma 1992; Uribe 1990; Ventura‐Cots 2016). They also used several composite assessment techniques (Table 4) including the Portal‐Systemic Encephalopathy Sum and Index (Gonzalez 1994; Sushma 1992; Uribe 1990); the Hepatic Encephalopathy Scoring Algorithm (HESA) (Naderian 2017; Pockros 2009; Rahimi 2014; Shehata 2018; STOP‐HE 2017); the Clinical Hepatic Encephalopathy Staging Scale (CHESS) (Ventura‐Cots 2016); and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Bajaj 2013). One trial used visual, auditory, and somatosensory‐evoked potentials as an additional test moiety (Sushma 1992), while the only trial that included participants with minimal hepatic encephalopathy used the Psychometric Hepatic Encephalopathy Score (PHES) test battery (Bajaj 2013).
Table 2.
Composite neurocognitive assessment tools
| Assessment tool | Description | Advantages | Disadvantages |
|
Portal‐Systemic Encephalopathy Sum and Index (PSE Sum/PSE Index) (Conn 1977) |
Provides an index of the severity of hepatic encephalopathy derived by adding scores for the degree of abnormality, expressed on a 0 to 4+ scale, for:
Each component is arbitrarily weighted in proportion to its importance; mental state is weighted by a factor of 3, while the other variables are assigned a factor of 1. The PSE Sum is the total of the weighted scores; its maximum possible value is 28. The PSE Index is the ratio of the estimated PSE Sum to the maximum possible Approximate time required: dependent on the time taken to obtain the results of the blood ammonia and the EEG. |
|
|
|
Psychometric Hepatic Encephalopathy Score (PHES) (Weissenborn 2001) |
Format: a battery of five pencil and paper tests Aproximate time required: 20 minutes Domains tested:
|
|
|
|
Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph 1998) |
A battery of tests that are also used to assess dementia, traumatic brain injury, stroke, multiple sclerosis and bipolar disorder. Recommended for use in the USA, where normative data is more widely available. Format: a battery of pencil and paper tests Approximate time required: 25 minutes Domains tested:
|
|
|
|
Hepatic Encephalopathy Scoring Algorithm (HESA) (Hassanein 2008) |
An algorithm originally developed to assess the utility of extracorporeal albumin dialysis in the treatment of people with severe hepatic encephalopathy. Format: a combination of clinical indicators and results from neurophysiological tests Approximate time required: 15 minutes Domains tested:
|
|
|
|
Clinical Hepatic Encephalopathy Staging Scale (CHESS) (Ortiz 2007) |
A scale from 0‐9, designed to reduce interobserver variability. Format: a set of nine questions that the observer must answer Approximate time required: 10 minutes Domains tested:
|
|
|
|
Cognitive Drug Research (CDR) (Mardini 2008) |
Developed in the UK specifically for people with minimal hepatic encephalopathy. Format: computerized test consisting of a set of increasingly complex tasks based on yes/no responses. Seven tests with 50 parallel forms of each task. Approximate time required: up to 30 minutes Domains tested: attention power and continuity, speed and quality of working and episodic memory |
|
|
| EEG: electroencephalogram | |||
Ten trials measured blood ammonia concentrations (Bajaj 2013; Gonzalez 1994; Naderian 2017; Pockros 2009; Rahimi 2014; Rockey 2014; STOP‐HE 2017; Sushma 1992; Uribe 1990; Ventura‐Cots 2016). However, we could not extract the data from one trial as they were displayed in graph form in the published paper and the corresponding data were not included separately in the text (Ventura‐Cots 2016). Blood ammonia concentrations were measured variously in venous and arterial blood; the timing of the blood sampling varied between trials as did the laboratory measurement techniques and the expression of the results (Table 5)
Table 3.
Measurement of ammonia concentrations in the included trials
| Trial | Type of blood sample | Unit measure | Time period for measurement differences |
| Gonzalez 1994 | Venous | µg/dL | Baseline, 72 hours and 7 days |
| Sushma 1992 | Arterial | µg/dL | Baseline, 72 hours and at recovery |
| Uribe 1990 | Not specified | µg/dL | Baseline and 2 weeks |
| Rockey 2014 | Venous | µmol/L*week | Measure based on the time normalised area under the time‐concentration curve (TN‐AUCweek) |
| STOP‐HE 2017 | Venous | µmol/L | Mean reduction in ammonia concentrations |
| Ventura‐Cots 2016 | Venous | µmol/L*120 hr | Measurement based on the time‐normalized area under the curve time concentration curve (TN‐AUC0–120h) |
| Bajaj 2013 | Venous | µg/dL | Baseline and 8 weeks |
| Pockros 2009 | Venous | Not specified | Baseline and 4 weeks |
| Naderian 2017 | Not specified | µmol/L | Reported as the mean difference from baseline to 24 hours |
| Rahimi 2014 | Not specified | µmol/L | Reported as the mean difference from 6 to 24 hours |
hr: hour
Excluded studies
We excluded five trials (Campollo 1992; Ghabril 2013; Mendenhall 1986; Panella 1993; Weiss 2018). See Characteristics of excluded studies.
We excluded one trial because it did not meet our inclusion criteria in that it compared two oral interventions that specifically target ammonia: sodium benzoate or sodium phenylacetate (Mendenhall 1986); both agents were effective at improving or maintaining participants' mental status. We excluded one further trial because it did not meet our inclusion criteria in that it assessed sodium benzoate as an adjuvant to branched chain amino acids for the treatment of chronic stable hepatic encephalopathy versus placebo (Panella 1993); participants receiving adjuvant sodium benzoate showed a significantly greater reduction in blood ammonia concentrations compared to the group receiving placebo and a trend to a greater reduction in Number Connection Test times and the Portal‐Systemic Encephalopathy Index.
We excluded three trials because they were observational. The first included 18 participants with cirrhosis and chronic persistent hepatic encephalopathy, given sodium benzoate in a mean dose of 6.4 grams daily for six months (Campollo 1992). Three participants were withdrawn within the first month of treatment with nausea and abdominal pain. The remaining 15 participants showed improvement in their Portal‐Systemic Encephalopathy Sum and Index (Table 4). The second included 18 participants with overt hepatic encephalopathy and hyperammonaemia admitted to an intensive care unit, given sodium phenylbutyrate 200 mg/kg per day orally or via a nasogastric tube (Weiss 2018). They compared outcomes with those in an historical control group (matched for age, sex, MELD (Model for End‐stage Liver Disease) score, and severity of hepatic encephalopathy using West Haven Criteria), managed in the same unit, using the same guidelines. Blood ammonia concentrations were lower at 12 and 48 hours in those receiving sodium phenylbutyrate, while survival on discharge from the intensive care unit was significantly higher. Several side effects were recorded in the participants who received sodium phenylbutyrate including ascites (two), leucopenia (one), pancreatitis (one), herpes simplex infection (one), and renal tubulopathy (one). In the control group, one participant developed ascites and one developed a maculopapular eruption. The third observational study included 15 participants with cirrhosis and a history of at least two previous episodes of overt hepatic encephalopathy within the previous six months, given 6 mL of glycerol phenylbutyrate twice daily for one week followed by 9 mL twice daily for three weeks to assess tolerability and the effect on blood ammonia concentrations (Ghabril 2013). The lower of the two doses effectively reduced blood ammonia concentrations compared with baseline and was better tolerated.
Risk of bias in included studies
We based our 'Risk of bias' assessment on the published descriptions combined with additional information from the investigators and from ClinicalTrials.gov (Figure 2; Figure 3).
Figure 2.
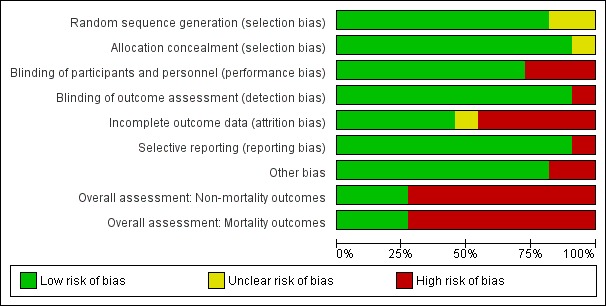
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Figure 3.
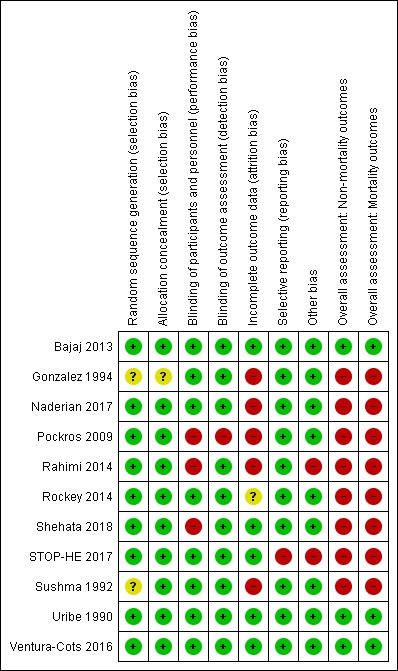
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
In nine of the 11 trials investigators generated the allocation sequence based on computer‐generated random numbers (Bajaj 2013; Naderian 2017; Pockros 2009; Rahimi 2014; Rockey 2014; Shehata 2018; STOP‐HE 2017; Uribe 1990; Ventura‐Cots 2016). Two trials did not specify the method of sequence generation (Gonzalez 1994; Sushma 1992).
Four trials concealed the allocation of participants using sealed envelopes (Rahimi 2014; Shehata 2018; Sushma 1992; Ventura‐Cots 2016). Four trials used central allocation (Bajaj 2013; Pockros 2009; Rockey 2014; Uribe 1990). One trial confirmed concealment of allocation, but not the specific method used (STOP‐HE 2017). One trial utilized different personnel to ensure allocation was concealed (Naderian 2017). One study lacked information regarding the blinding of allocation (Gonzalez 1994).
We graded nine trials as having a low risk of selection bias (Bajaj 2013; Naderian 2017; Pockros 2009; Rahimi 2014; Rockey 2014; Shehata 2018; STOP‐HE 2017; Uribe 1990; Ventura‐Cots 2016) and two as having an unclear risk of selection bias (Gonzalez 1994; Sushma 1992).
Blinding
We graded eight trials as having a low risk of performance and detection bias (Bajaj 2013; Gonzalez 1994; Naderian 2017; Rockey 2014; STOP‐HE 2017; Sushma 1992; Uribe 1990; Ventura‐Cots 2016). We graded three trials as having a high risk of bias, as they were open‐label trials without blinding (Pockros 2009; Rahimi 2014; Shehata 2018).
Incomplete outcome data
We graded five trials as having a low risk of attrition bias, as they used an intention‐to‐treat analysis, or included all participants in the analyses (Bajaj 2013; Shehata 2018; STOP‐HE 2017; Uribe 1990; Ventura‐Cots 2016). Five trials did not evaluate all randomised participants, and so we graded these as at high risk of attrition bias (Gonzalez 1994; Naderian 2017; Pockros 2009; Rahimi 2014; Sushma 1992). In one study the risk of attrition bias was unclear (Rockey 2014).
Selective reporting
Ten trials reported predefined, clinically relevant outcome measures, suggesting a low risk of selective reporting (Bajaj 2013; Gonzalez 1994; Naderian 2017; Pockros 2009; Rahimi 2014; Rockey 2014; Shehata 2018; Sushma 1992; Uribe 1990; Ventura‐Cots 2016). We classified the remaining trial as having a high risk of reporting bias as it did not report the primary outcome of a change from baseline in hepatic encephalopathy stage (STOP‐HE 2017).
Other potential sources of bias
We found no other potential sources of bias in nine trials (Bajaj 2013; Gonzalez 1994; Naderian 2017; Pockros 2009; Rockey 2014; Shehata 2018; Sushma 1992; Uribe 1990; Ventura‐Cots 2016). We classified two trials as at high risk in respect of other potential bias: in one study, baseline blood urea nitrogen concentration was significantly higher in participants in the treatment group compared to the control group (Rahimi 2014), while in another trial we observed differences, some of them significant, in baseline characteristics, the severity of the underlying liver disease, the precipitant factors, and use of additional anti‐encephalopathy treatments between participants recruited in the USA and elsewhere in the world (STOP‐HE 2017).
Overall risk of bias
We classified eight trials at high risk of bias for all outcomes (Gonzalez 1994; Naderian 2017; Pockros 2009; Rahimi 2014; Rockey 2014; Shehata 2018; STOP‐HE 2017; Sushma 1992), and three trials as at low risk of bias for all outcomes (Bajaj 2013; Uribe 1990; Ventura‐Cots 2016).
Effects of interventions
See: Table 1
Primary outcomes
All‐cause mortality
We were able to gather mortality data from 11 trials involving 943 participants (Analysis 1.1). Our analyses found no beneficial or harmful effects of sodium benzoate versus non‐absorbable disaccharides (RR 1.26, 95% CI 0.49 to 3.28; 101 participants; 2 trials; I2 = 0%), glycerol phenylbutyrate versus placebo (RR 0.65, 95% CI 0.11 to 3.81; 178 participants; 1 trial), ornithine phenylacetate versus placebo (RR 0.73, 95% CI 0.35 to 1.51; 269 participants; 2 trials; I2 = 0%), AST‐120 versus lactulose (RR 1.05, 95% CI 0.59 to 1.85; 41 participants; 1 trial), or polyethylene glycol versus lactulose (RR 0.50, 95% CI 0.09 to 2.64; 190 participants; 3 trials; I2 = 0%). There were no events in the trial of sodium benzoate versus placebo.
Analysis 1.1.
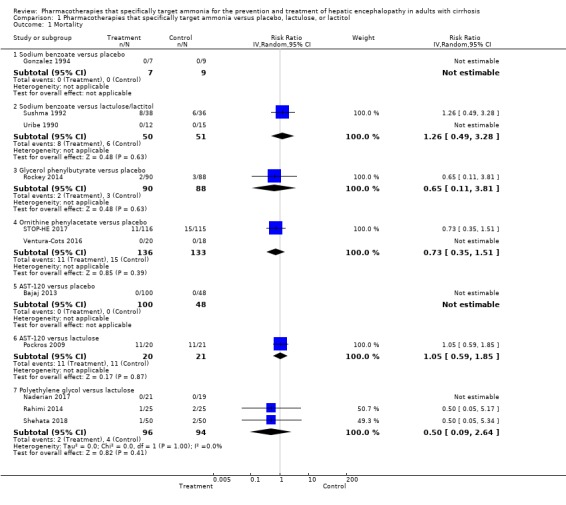
Comparison 1 Pharmacotherapies that specifically target ammonia versus placebo, lactulose, or lactitol, Outcome 1 Mortality.
Hepatic encephalopathy
Seven trials involving 521 participants reported data on hepatic encephalopathy (Analysis 1.2). Our analyses showed a beneficial effect of glycerol phenylbutyrate versus placebo (RR 0.57, 95% CI 0.36 to 0.90; 178 participants; 1 trial; NNTB 6), and of polyethylene glycol versus lactulose (RR 0.19, 95% CI 0.08 to 0.44; 190 participants; 3 trials; I2 = 0%; NNTB 4). We did not observe beneficial effects in the remaining three trials with extractable data viz. sodium benzoate versus non‐absorbable disaccharides (RR 1.22, 95% CI 0.51 to 2.93; 74 participants; 1 trial); ornithine phenylacetate versus placebo (RR 2.71, 95% CI 0.12 to 62.70; 38 participants; 1 trial); or AST‐120 versus lactulose (RR 1.05, 95% CI 0.59 to 1.85; 41 participants; 1 trial).
Analysis 1.2.
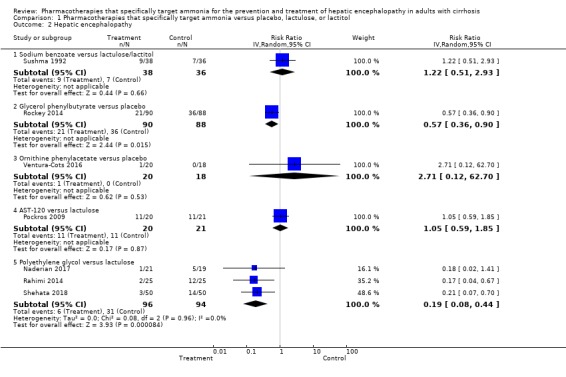
Comparison 1 Pharmacotherapies that specifically target ammonia versus placebo, lactulose, or lactitol, Outcome 2 Hepatic encephalopathy.
Serious adverse events
Ten trials, involving 790 participants, reported a total of 130 serious adverse events (Analysis 1.3). Our analyses found no beneficial or harmful effects of sodium benzoate versus non‐absorbable disaccharides (RR 1.08, 95% CI 0.44 to 2.68; 101 participants; 2 trials), glycerol phenylbutyrate versus placebo (RR 1.63, 95% CI 0.85 to 3.13; 178 participants; 1 trial), ornithine phenylacetate versus placebo (RR 0.92, 95% CI 0.62 to 1.36; 264 participants; 2 trials; I2 = 0%), or polyethylene glycol versus lactulose (RR 0.57, 95% CI 0.18 to 1.82; 190 participants; 3 trials; I2 = 0%). There were no events in the trial evaluating sodium benzoate versus placebo or in the trial evaluating AST‐120 versus lactulose.
Analysis 1.3.
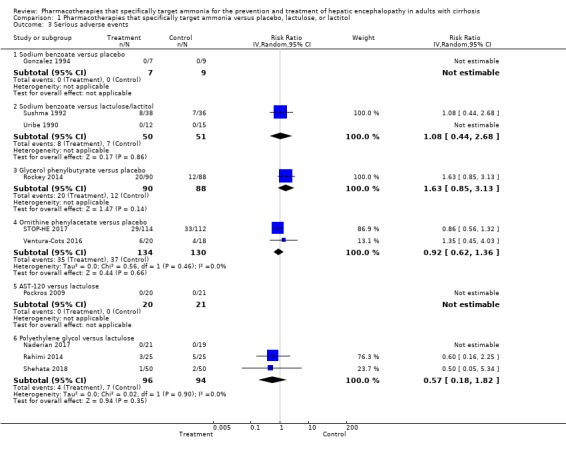
Comparison 1 Pharmacotherapies that specifically target ammonia versus placebo, lactulose, or lactitol, Outcome 3 Serious adverse events.
Secondary outcomes
Non‐serious adverse events
Eight trials, involving 782 participants, reported a total of 374 non‐serious adverse events (Analysis 1.4). Our analyses found no beneficial or harmful effects of sodium benzoate versus non‐absorbable disaccharides (RR 1.13, 95% CI 0.96 to 1.32; 182 participants; 2 trials; I2 = 0%), glycerol phenylbutyrate versus placebo (RR 1.04, 95% CI 0.88 to 1.21; 178 participants; 1 trial), ornithine phenylacetate versus placebo (RR 1.08, 95% CI 0.78 to 1.51; 269 participants; 2 trials; I2 = 11%), or polyethylene glycol versus non‐absorbable disaccharides (RR 0.71, 95% CI 0.43 to 1.18; 137 participants; 2 trials). There were no events in the trial evaluating sodium benzoate versus placebo.
Analysis 1.4.
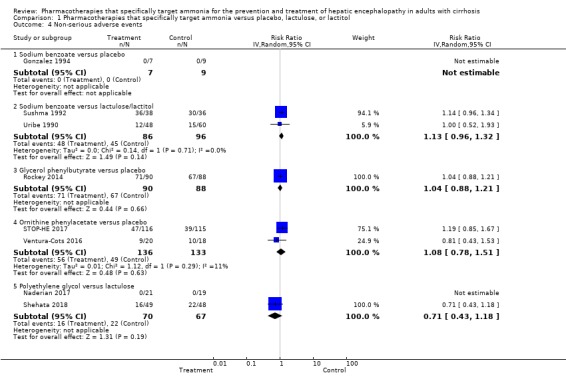
Comparison 1 Pharmacotherapies that specifically target ammonia versus placebo, lactulose, or lactitol, Outcome 4 Non‐serious adverse events.
Health‐related quality of life
One included trial with 48 participants (Pockros 2009), reported data on quality of life extracted from the sub‐scores of the Hepatic Encephalopathy Scoring Algorithm (HESA: Table 4). Use of both AST‐120 and lactulose resulted in improvement in anxiety and in memory for recent events; use of AST‐120 was associated, in addition, with improvements in complex computations.
No other trials reported quality‐of‐life outcomes.
Blood ammonia
Nine trials, involving 733 participants, reported data on blood ammonia concentrations measured variously in venous and arterial blood (Table 5), and at various time points in the trial (Analysis 1.5). Our analyses showed a beneficial effect on blood ammonia concentration in trials of sodium benzoate versus placebo (MD −32.00 µg/dL, 95% CI −46.85 to −17.15; 16 participants; 1 trial), glycerol phenylbutyrate versus placebo (MD −12.00 µmol/L*week, 95% CI −23.37 to −0.63; 178 participants; 1 trial), ornithine phenylacetate versus placebo (MD −27.10 µmol/L, 95% CI −48.55 to −5.65; 231 participants; 1 trial), and AST‐120 versus placebo (MD −22.00 µg/dL, 95% CI −26.75 to −17.25; 98 participants; 1 trial). However, there were no beneficial effects on blood ammonia concentration in the trials of sodium benzoate versus non‐absorbable disaccharides (MD 9.80 µg/dL, 95% CI −0.48 to 20.08; 58 participants; 1 trial; and MD ‐14.94 µg/dL, 95% CI ‐71.32 to 41.44; 27 participants; 1 trial), AST‐120 versus lactulose (MD 5.20 units not specified, 95% CI −2.75 to 13.15; 35 participants; 1 trial), or polyethylene glycol versus lactulose (MD −29.28 µmol/L, 95% CI −95.96 to 37.39; 90 participants; 2 trials).
Analysis 1.5.
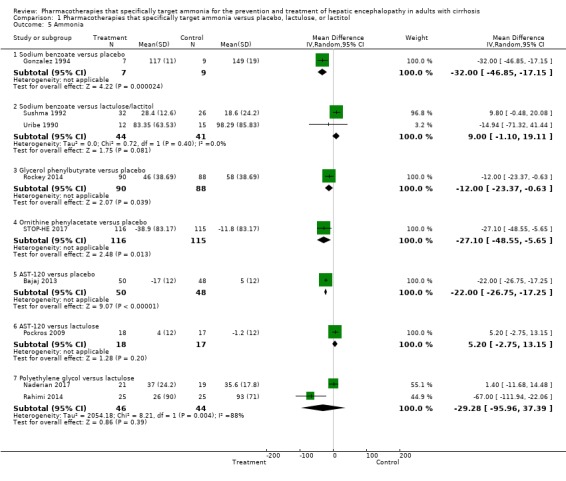
Comparison 1 Pharmacotherapies that specifically target ammonia versus placebo, lactulose, or lactitol, Outcome 5 Ammonia.
'Summary of findings' tables
For all outcomes, we downgraded the certainty of the evidence by three levels to 'very low' as eight trials were at high risk of bias (one level) and the number of trials, participants, and events in each meta‐analysis was small (two levels). See Table 1.
Discussion
Summary of main results
This systematic review included qualitative and quantitative data from 11 randomised clinical trials, involving 943 participants, treated with one of five drugs of interest. Although all five drugs specifically target ammonia, the ways in which they do so differ considerably. In consequence, we evaluated each separately and did not undertake any overall group analyses. In the primary analyses, we found evidence that glycerol phenylbutyrate had a beneficial effect on hepatic encephalopathy when compared to placebo (one trial) as did polyethylene glycol when compared to lactulose (three trials). We found no beneficial or harmful effects on hepatic encephalopathy in the other seven trials. None of these pharmacotherapies had a beneficial or harmful effect on the risk of mortality or the development of adverse events. The majority of these agents effectively lowered circulating ammonia concentrations when compared to placebo but not when compared to the non‐absorbable disaccharides.
The certainty of the evidence was very low. This was because there were only three trials at low risk of bias and the number of trials, participants, and events in each analysis was small. Hence, no conclusions can be made with certainty regarding the clinical benefits and harms of these five pharmacotherapies.
Overall completeness and applicability of evidence
We included 11 randomised clinical trials with 943 participants with cirrhosis and a history or current evidence of hepatic encephalopathy. The trials were generally small, involving an average of 86 (range 18 to 231) people. Only five of the 11 trials conducted a sample size calculation for assessment of statistical power (Naderian 2017; Rahimi 2014; Rockey 2014; Shehata 2018; Ventura‐Cots 2016). Of these, only two trials met the required sample size after withdrawal or loss of participants (Rahimi 2014; Shehata 2018). Thus, several of the trials are likely to be underpowered to detect a difference in the effectiveness and safety of the interventions. In addition, we classified eight of the 11 included trials at high risk of bias for all outcomes.
We evaluated five pharmacotherapies in this review. In most instances there was only one trial per comparison. Thus, three trials assessed sodium benzoate; one in a comparison with placebo (Gonzalez 1994), and two in comparison with non‐absorbable disaccharides but with different agents (Sushma 1992; Uribe 1990). One trial assessed glycerol phenylbutyrate versus placebo (Rockey 2014). Two trials assessed ornithine phenylacetate against placebo (STOP‐HE 2017; Ventura‐Cots 2016). Two trials assessed AST‐120, one in comparison with placebo (Bajaj 2013), and one in comparison with lactulose (Pockros 2009). Finally, three trials compared polyethylene glycol with lactulose (Naderian 2017; Rahimi 2014; Shehata 2018).
Overall, the included trials compared four of the five pharmacotherapies to placebo (Bajaj 2013; Gonzalez 1994; Rockey 2014; STOP‐HE 2017; Ventura‐Cots 2016); none of the trials undertook placebo‐controlled trials of polyethylene glycol; this would be extremely difficult to do except in an open, likely unblinded, fashion. The trials compared three of the five pharmacotherapies to non‐absorbable disaccharides (Naderian 2017; Pockros 2009; Rahimi 2014; Shehata 2018; Sushma 1992; Uribe 1990), the exceptions being glycerol phenylbutyrate and ornithine phenylacetate. None of the trials compared any of the five pharmacotherapies to other active agents, such as rifaximin or branched chain amino acids.
Four of the 11 trials were only published in abstract form and hence did not report all outcomes (Bajaj 2013; Gonzalez 1994; Pockros 2009; STOP‐HE 2017). Although further information was obtainable from some trials, many authors did not, or could not, provide us with the additional information we needed for our analyses. Data were also missing from some of the trials published as full papers; again some of these were retrievable from the trial authors but some were not. Thus, we could extract mortality data from all 11 trials but six of them reported no events. This limits the usefulness of these analyses, although we included three of the latter six trials in meta‐analyses (Naderian 2017; Uribe 1990; Ventura‐Cots 2016). We were able to retrieve data on hepatic encephalopathy from seven trials only, while data on serious adverse events were available from 10 trials, but with the same issue that we could not estimate the data in the four trials reporting no events.
Differences in the way in which the trials utilized the drugs could confound the results. For example, in two of the three trials comparing polyethylene glycol to lactulose, participants were allowed to receive a single dose of lactulose prior to randomisation and after 24 hours of treatment regardless of their group allocation (Rahimi 2014), or were not given lactulose at all (Shehata 2018). However, the third trial gave lactulose to the participants allocated to polyethylene glycol in the same amount enterally or rectally as in the control group (Naderian 2017). Thus, two trials assessed polyethylene glycol as an alternative to lactulose (Rahimi 2014; Shehata 2018), whereas the other assessed it as an adjuvant (Naderian 2017).
The majority of trials used co‐interventions alongside the study drug and, in the main, participants randomised to experimental or control groups had equal access to them. However, in the large, placebo‐controlled trial of ornithine phenylacetate (STOP‐HE 2017), 58% of participants were taking rifaximin, 5.2% were taking L‐ornithine L‐aspartate, and an unspecified proportion were taking lactulose, but no information was provided on the numbers of participants in the experimental and control groups involved. In addition, there were differences in the route of drug administration both between and within trials; for example, some trials administered study or control drugs or both by either enema or orally. Between trials, doses of the study or control substance or both also varied, which might have altered the effect size, particularly if the therapeutic effect is dose‐dependent.
All participants included in our review had cirrhosis and a history of or current clinical evidence of hepatic encephalopathy. The majority of the included trials involved participants with an acute episode of hepatic encephalopathy (Gonzalez 1994; Naderian 2017; Rahimi 2014; Shehata 2018; STOP‐HE 2017; Sushma 1992). Two trials involved participants with stable chronic hepatic encephalopathy (Pockros 2009; Uribe 1990), while one study involved participants with minimal and Grade 1 hepatic encephalopathy (Bajaj 2013). One study evaluated the primary prevention of hepatic encephalopathy following an upper gastrointestinal bleed (Ventura‐Cots 2016), while one further trial evaluated the secondary prevention of hepatic encephalopathy in participants who had had at least two previous episodes of hepatic encephalopathy Grade 2 or greater in the previous six months (Rockey 2014). Thus, the included trials evaluated both the prevention of hepatic encephalopathy and treatment of the various manifestations of the syndrome. However, no systematic evaluation of the use of the five pharmacotherapies across the spectrum of hepatic encephalopathy is possible based on the trials available to date. It would, therefore, be difficult to predict where these drugs might best fit into clinical practice.
Hepatic encephalopathy may also develop in people with non‐cirrhotic portal hypertension and in people with acute liver failure but these people are encountered much less frequently than people with cirrhosis in clinical practice; these populations were not represented in the included trials. However, there is no reason why our results could not be extrapolated to people with hepatic encephalopathy associated with non‐cirrhotic portal hypertension (e.g. portal vein block). The results may, on the other hand, not be directly applicable to people with fulminant hepatic failure.
Hepatic encephalopathy can often be precipitated by stressors such as infection, gastrointestinal bleeding, alcohol misuse or electrolyte disturbances. Therefore, it is important to be able to identify and treat these precipitating factors (AASLD/EASL 2014; Vilstrup 2014). The trials assessed in this review did not provide detailed information on possible precipitating events and the effects of the interventions designed to ameliorate them, nor did they evaluate the effects, if any, of the addition of pharmacotherapies that specifically target ammonia. It is, therefore, unclear whether these pharmacotherapies provide additional benefit in situations where hepatic encephalopathy is precipitated by a treatable event.
Hepatic encephalopathy imposes a significant burden on healthcare systems, and resource utilization associated with its management is increasing (Stepanova 2012). None of the randomised clinical trials included in this review conducted a detailed assessment of the costs associated with hospitalisation and treatment with pharmacotherapies that target ammonia. One study found that the cost of lactulose was 30 times the cost of sodium benzoate, although there is a possibility that this has changed, as the study was conducted over 15 years ago (Sushma 1992). Another study found that polyethylene glycol significantly reduced the length of hospital admission versus lactulose (Shehata 2018).
Whilst ammonia levels significantly decreased during use of most of the study drugs, the degree of hepatic encephalopathy, on the whole, did not significantly improve. This may reflect the fact that, while ammonia is known to play an important role in the pathogenesis of the syndrome, simply reducing circulating ammonia level may not be sufficient to reverse the complex cascade of events that contribute to the pathophysiology of this condition.
Quality of the evidence
The main reason for downgrading the certainty of the evidence in this review were incomplete outcome data (attrition bias), and the small number of trials giving rise to uncertainty.
We included randomised clinical trials published as full papers or abstracts and attempted to obtain additional information on essential aspects of bias control from the authors of these works. We combined the individual bias domains into an overall assessment (hbg.cochrane.org/information‐authors). We identified potential biases in all but three of the included trials (Bajaj 2013; Uribe 1990; Ventura‐Cots 2016). We defined mortality, but not serious adverse events, as an outcome that is robust to performance and detection biases (Savović 2012a). This decision can be questioned, as lack of blinding is not likely to influence the assessment of serious adverse events such as variceal bleeding, hepatorenal syndrome, and liver failure. We classified all of the included trials but three (Bajaj 2013; Uribe 1990; Ventura‐Cots 2016), as being at high risk in the overall assessments of mortality and non‐mortality outcomes.
Based on the assessment of incomplete outcome data (attrition bias), and due to the small number of trials giving rise to uncertainty, we classified the certainty of the evidence as very low for the assessment of all our outcomes.
Potential biases in the review process
We undertook the review based on current recommendations for bias control (hbg.cochrane.org/information‐authors;Higgins 2017). We attempted to minimize possible selection bias (Page 2014), by using a comprehensive search strategy. We combined searches in electronic databases with hand searches of the biographies of identified trials, and the conference proceedings and abstract books from relevant national and international society meetings. We consider it unlikely that we failed to identify any published trials.
Two of the included trials, involving 216 participants, evaluated the prevention of hepatic encephalopathy while nine trials, involving 727 participants evaluated its treatment. We combined these trials in the analyses of the primary outcomes because they involved different agents and this may have introduced an element of bias.
Agreements and disagreements with other studies or reviews
This review is the first and largest identified systematic review of pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. It includes 11 randomised clinical trials involving five different specific pharmacotherapies and 943 participants. There are no other systematic reviews and meta‐analyses evaluating these drugs with which to compare the findings of the present review. A number of general reviews of the management of hepatic encephalopathy have been published (Ahuja 2014; Bass 2007; Butterworth 2000; Jawaro 2016; Toris 2011), some of which look specifically at the drugs included in this review (Acharya 2018; De Las Heras 2017; Hadjihambi 2018; Jover‐Cobos 2013; Kornerup 2018; Matoori 2015; Misel 2013; Rahimi 2016; Sturgeon 2014). The EASL/AASLD Practice Guidelines state that further clinical reports are awaited on ornithine phenylacetate and that more trials are needed on glyceryl phenylbutyrate which, if they confirmed the original results, 'might lead to clinical recommendations' (AASLD/EASL 2014; Vilstrup 2014).
Authors' conclusions
This review includes 11 randomised clinical trials evaluating the use of five pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis. The analyses found that these drugs have the potential to reduce blood ammonia concentrations, when compared to placebo, but the evidence was of low certainty. However, there is insufficient evidence to determine the effects of these pharmacotherapies on clinical outcomes and serious adverse events. Thus, further research is needed before these drugs can be fully evaluated and their place in clinical practice defined.
We used the EPICOT format (Brown 2006a), to define the implications for research.
Evidence (what is the current state of the evidence?). We included 11 trials involving 943 participants. We found evidence that sodium benzoate, glycerol phenylbutyrate, ornithine phenylacetate, and AST‐120 effectively lower blood ammonia concentrations when compared to placebo and that polyethylene glycol lowered blood ammonia concentrations when compared to lactulose. However, there was little evidence that this ammonia‐lowering effect translated into clinical benefit except that glycerol phenylbutyrate had a beneficial effect on hepatic encephalopathy when compared to placebo, and polyethylene glycol had a beneficial effect on hepatic encephalopathy when compared to lactulose. However, the certainty of the evidence was very low, and hence, we are very uncertain about these findings. Further high‐quality randomised clinical trials are needed.
Participants (what is the population of interest?). We focused on people with cirrhosis and hepatic encephalopathy or people with cirrhosis who were at risk of developing hepatic encephalopathy. Because of the small number of randomised clinical trials available, we were not able to undertake subgroup analyses to determine differences in outcomes between trials looking at the prevention or treatment of hepatic encephalopathy, or determine if there were differences in outcomes by the degree of hepatic encephalopathy. Future trials should be designed to look for these potential differences. The effects of these drugs should also be assessed in people with hepatic encephalopathy associated with acute liver failure or with non‐cirrhotic portal hypertension.
Interventions (what are the interventions of interest?): the interventions assessed are sodium benzoate, glycerol phenylbutyrate, ornithine phenylacetate, AST‐120 (spherical carbon adsorbent), and polyethylene glycol. Although they all specifically target ammonia, they differ by mode of action, formulation, and route of administration. In some cases, the evidence for beneficial and harmful effects was based on only one trial and at most three. All the interventions considered in this review should be evaluated in further high‐quality, randomised clinical trials.
Comparisons (what are the comparisons of interest?). The comparisons assessed in the trials in this review were a variety of placebo preparations and the non‐absorbable disaccharides lactulose and lactitol. Future trials should include comparisons against placebo and no intervention, where considered feasible, and also against other agents thought to benefit people with hepatic encephalopathy, including non‐absorbable disaccharides and non‐absorbable antibiotics. Future trials should also evaluate the effect of co‐interventions.
Outcomes (what are the outcomes of interest?). The primary outcomes included in this review ‐ mortality, hepatic encephalopathy, and serious adverse events ‐ should be included in any future trials. Health‐related quality of life is also an important outcome, particularly in any future trials involving participants with minimal or chronic persistent hepatic encephalopathy. As these agents specifically target ammonia, blood ammonia concentrations will need to be monitored; the best outcome measure in relation to blood ammonia is the percentage change in concentration over trial baseline.
Time stamp (date of literature search): 5 March 2019
Acknowledgements
We thank Sarah Louise Klingenberg, Cochrane Hepato‐Biliary's Information Specialist, for her help with the strategies for the electronic searches.
This review did not receive funding support.
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of Cochrane Hepato‐Biliary through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Peer reviewers of the protocol: Bruce F Scharschmidt, USA; Fernando Gomes Romeiro, Brazil; Baresh S Sharma, India; Peter Yepsen, Denmark Contact editor: Goran Bjelacovic, Serbia Sign‐off editor: Christian Gluud, Denmark
Peer reviewers of the review: Piero Amodio, Italy Contact editor: Goran Poropat, Croatia Sign‐off editor: Agostino Colli, Italy
Appendices
Appendix 1. Search strategy
| Database | Time span | Search terms |
| Cochrane Hepato‐Biliary Controlled Trials Register | March 2019 | ((sodium and (benzoate or phenylacetate or phenylbutyrate)) or glycerol phenylbutyrate or ornithine phenylacetate or spherical carbon absorbant or activated charcoal or ammonul or buphenyl or ravicti or AST‐120 or polyethylene* glycol or PEG or (ammoni* and scaveng*)) AND ((encephalopath* or HE) and cirrho*) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | 2019, Issue 3 | #1 MeSH descriptor: [Phenylbutyrates] explode all trees #2 MeSH descriptor: [Phenylacetates] explode all trees #3 MeSH descriptor: [Sodium Benzoate] explode all trees #4 MeSH descriptor: [Charcoal] explode all trees #5 ((sodium and (benzoate or phenylacetate or phenylbutyrate)) or glycerol phenylbutyrate or ornithine phenylacetate or spherical carbon absorbant or activated charcoal or ammonul or buphenyl or ravicti or AST‐120 or polyethylene* glycol or PEG or (ammoni* and scaveng*)) #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Hepatic Encephalopathy] explode all trees #8 MeSH descriptor: [Fibrosis] explode all trees #9 #7 and #8 #10 ((encephalopath* or HE) and cirrho*) #11 #9 or #10 #12 #6 and #11 |
| MEDLINE Ovid | 1946 to March 2019 | 1. exp Phenylbutyrates/ or exp Phenylacetates/ or exp Sodium Benzoate/ 2. exp Charcoal/ 3. ((sodium and (benzoate or phenylacetate or phenylbutyrate)) or glycerol phenylbutyrate or ornithine phenylacetate or spherical carbon absorbant or activated charcoal or ammonul or buphenyl or ravicti or AST‐120 or polyethylene* glycol or PEG or (ammoni* and scaveng*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 4. 1 or 2 or 3 5. exp Hepatic Encephalopathy/ 6. exp Fibrosis/ 7. 5 and 6 8. ((encephalopath* or HE) and cirrho*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 9. 7 or 8 10. 4 and 9 |
| Embase Ovid | 1974 to March 2019 | 1. exp benzoic acid/ 2. exp phenylacetic acid/ 3. exp 4 phenylbutyric acid/ 4. exp glycerol phenylbutyrate/ 5. exp ornithine phenylacetate/ 6. exp activated carbon/ 7. ((sodium and (benzoate or phenylacetate or phenylbutyrate)) or glycerol phenylbutyrate or ornithine phenylacetate or spherical carbon absorbant or activated charcoal or ammonul or buphenyl or ravicti or AST‐120 or polyethylene* glycol or PEG or (ammoni* and scaveng*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. exp hepatic encephalopathy/ 10. exp liver cirrhosis/ 11. 9 and 10 12. ((encephalopath* or HE) and cirrho*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 13. 11 or 12 14. 8 and 13 |
| LILACS (Bireme) | 1982 to March 2019 | ((encephalopath$ or HE) and cirrho$) [Words] and ((sodium and (benzoate or phenylacetate or phenylbutyrate)) or glycerol phenylbutyrate or ornithine phenylacetate or spherical carbon absorbant or activated charcoal or ammonul or buphenyl or ravicti or AST‐120 or polyethylene$ glycol or PEG or (ammoni$ and scaveng$)) [Words] |
| Science Citation Index Expanded (Web of Science) | 1900 to March 2019 | #3 #2 AND #1 #2 TS=((encephalopath* or HE) and cirrho*) #1 TS=((sodium and (benzoate or phenylacetate or phenylbutyrate)) or glycerol phenylbutyrate or ornithine phenylacetate or spherical carbon absorbant or activated charcoal or ammonul or buphenyl or ravicti or AST‐120 or polyethylene* glycol or PEG or (ammoni* and scaveng*)) |
| Conference Proceedings Citation Index – Science (Web of Science) |
1990 to March 2019 | #3 #2 AND #1 #2 TS=((encephalopath* or HE) and cirrho*) #1 TS=((sodium and (benzoate or phenylacetate or phenylbutyrate)) or glycerol phenylbutyrate or ornithine phenylacetate or spherical carbon absorbant or activated charcoal or ammonul or buphenyl or ravicti or AST‐120 or polyethylene* glycol or PEG or (ammoni* and scaveng*)) |
Data and analyses
Comparison 1.
Pharmacotherapies that specifically target ammonia versus placebo, lactulose, or lactitol
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 11 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Sodium benzoate versus placebo | 1 | 16 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Sodium benzoate versus lactulose/lactitol | 2 | 101 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.49, 3.28] |
| 1.3 Glycerol phenylbutyrate versus placebo | 1 | 178 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.11, 3.81] |
| 1.4 Ornithine phenylacetate versus placebo | 2 | 269 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.35, 1.51] |
| 1.5 AST‐120 versus placebo | 1 | 148 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 AST‐120 versus lactulose | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.59, 1.85] |
| 1.7 Polyethylene glycol versus lactulose | 3 | 190 | Risk Ratio (IV, Random, 95% CI) | 0.5 [0.09, 2.64] |
| 2 Hepatic encephalopathy | 7 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Sodium benzoate versus lactulose/lactitol | 1 | 74 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.51, 2.93] |
| 2.2 Glycerol phenylbutyrate versus placebo | 1 | 178 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.36, 0.90] |
| 2.3 Ornithine phenylacetate versus placebo | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 2.71 [0.12, 62.70] |
| 2.4 AST‐120 versus lactulose | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.59, 1.85] |
| 2.5 Polyethylene glycol versus lactulose | 3 | 190 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.08, 0.44] |
| 3 Serious adverse events | 10 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Sodium benzoate versus placebo | 1 | 16 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Sodium benzoate versus lactulose/lactitol | 2 | 101 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.44, 2.68] |
| 3.3 Glycerol phenylbutyrate versus placebo | 1 | 178 | Risk Ratio (IV, Random, 95% CI) | 1.63 [0.85, 3.13] |
| 3.4 Ornithine phenylacetate versus placebo | 2 | 264 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.62, 1.36] |
| 3.5 AST‐120 versus lactulose | 1 | 41 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Polyethylene glycol versus lactulose | 3 | 190 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.18, 1.82] |
| 4 Non‐serious adverse events | 8 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Sodium benzoate versus placebo | 1 | 16 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Sodium benzoate versus lactulose/lactitol | 2 | 182 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.96, 1.32] |
| 4.3 Glycerol phenylbutyrate versus placebo | 1 | 178 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.88, 1.21] |
| 4.4 Ornithine phenylacetate versus placebo | 2 | 269 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.78, 1.51] |
| 4.5 Polyethylene glycol versus lactulose | 2 | 137 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.43, 1.18] |
| 5 Ammonia | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Sodium benzoate versus placebo | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐32.0 [‐46.85, ‐17.15] |
| 5.2 Sodium benzoate versus lactulose/lactitol | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 9.00 [‐1.10, 19.11] |
| 5.3 Glycerol phenylbutyrate versus placebo | 1 | 178 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐23.37, ‐0.63] |
| 5.4 Ornithine phenylacetate versus placebo | 1 | 231 | Mean Difference (IV, Random, 95% CI) | ‐27.10 [‐48.55, ‐5.65] |
| 5.5 AST‐120 versus placebo | 1 | 98 | Mean Difference (IV, Random, 95% CI) | ‐22.0 [‐26.75, ‐17.25] |
| 5.6 AST‐120 versus lactulose | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 5.2 [‐2.75, 13.15] |
| 5.7 Polyethylene glycol versus lactulose | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐29.28 [‐95.96, 37.39] |
What's new
| Date | Event | Description |
|---|---|---|
| 10 July 2019 | Amended | Adjustment to the number of trials at low risk of bias following removal of the 'For‐profit bias' category The units of ammonia measurements provided A table is added, specifying details of the ammonia measurements in the included trials |
History
Protocol first published: Issue 8, 2016 Review first published: Issue 6, 2019
| Date | Event | Description |
|---|---|---|
| 30 June 2019 | Amended | Sumary of Findings tables amalgamated |
Differences between protocol and review
We changed the title to more precisely reflect the modes of action of the interventions included in the review and to make it clear that we only included adult participants, and we changed the wording of the review objectives to reflect the changes we had made to the title. We also updated the methods according to the current recommendations of Cochrane Hepato‐Biliary. The updates include changes to the wording of the 'Risk of bias' assessment; obligatory inclusion of observational studies for the assessment of adverse events; and searching of the LILACS database. We did not include liver‐related mortality as a secondary outcome as most trials do report this separately. We upgraded blood ammonia from an exploratory to a secondary outcome as these pharmacotherapies specifically target ammonia. There were insufficient data to include Number Connection Test results as an exploratory outcome. We did not undertake subgroup analyses because the number of randomised clinical trials identified was too small. We did not undertake sensitivity analyses because the outcome data sets were complete in the intervention and control groups in all of the included trials. We did not undertake Trial Sequential Analyses because the number of events, participants, and trials was insufficient.
Characteristics of studies
Characteristics of included studies [author‐defined order]
| Methods | Sodium benzoate: single‐centre, double‐blind, placebo‐controlled, randomised clinical trial | |
| Participants |
Included participants: cirrhosis with acute Grade 3 or 4 hepatic encephalopathy (n = 18); 2 participants were excluded shortly after inclusion because of gastrointestinal bleeding. Age: not reported Proportion of men: not reported Aetiology of the liver disease: not reported MELD score: not reported |
|
| Interventions |
Intervention comparison: sodium benzoate vs placebo Sodium benzoate: 7.2 g/24 h administered orally or via nasogastric tube (n = 7) Placebo: (not detailed) administered orally or via nasogastric tube (n = 9) Duration of treatment: 7 days Co‐intervention: participants in both groups received lactose enemata (200 g/L) thrice‐daily |
|
| Outcomes |
Neurocognitive assessment: Modified PSE Index comprising:
Others:
|
|
| Inclusion period | Not reported | |
| Country of origin | Mexico | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status: abstract
Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel using a placebo |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | Low risk | Blinding of outcome assessment using a placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two participants were excluded due to gastrointestinal bleeding. The allocation group of these participants was not reported. |
| Selective reporting (reporting bias) | Low risk | The trial reported clinically relevant outcomes. We did not have access to the trial protocol. |
| Other bias | Low risk | None identified |
| Overall assessment Non‐mortality outcomes | High risk | |
| Overall assessment Mortality outcomes | High risk | |
| Methods | Sodium benzoate: single‐centre, double‐blind, randomised clinical trial | |
| Participants |
Included participants: overt hepatic encephalopathy, Grade 2‐4, associated with cirrhosis or creation of a surgical portal‐systemic anastomosis with evidence of fibrosis (n = 74) Age (mean ± SD):
Proportion of men:
Aetiology of liver disease (sodium benzoate: lactulose group):
MELD score: not reported |
|
| Interventions |
Intervention comparison: sodium benzoate vs lactulose Sodium benzoate: 5 g/30 mL tap water, given orally or via nasogastric tube twice daily (n = 38); standard dose 10 g/day for 7.9 ± 6.4 days Lactulose: 30 mL/8 h administered orally or by nasogastric tube; dose adjusted to achieve 3 semi‐soft stools daily (n = 36); mean dose 66.8 ± 24.8 mL/day for 8.8 ± 7.1 days Duration of treatment: until complete recovery ‐ maximum follow‐up 42 days Co‐intervention: standard treatment was continued in both groups and included twice‐daily bowel washes with tap water, maintenance of fluid and electrolyte levels, restriction of oral intake of proteins to 20 g/day in participants in whom oral intake was possible; antibiotics were prescribed to treat infections in 7 participants receiving sodium benzoate and in 1 participant receiving lactulose. |
|
| Outcomes |
Neuropsychiatric assessment PSE Sum and Index comprising:
Additional psychometric tests including:
Visual, auditory and somatosensory evoked potentials Others:
|
|
| Inclusion period | January 1990‐June 1991 | |
| Country of origin | India | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status: full paper
Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not provided |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | An independent healthcare worker administered the treatments. However, it may be obvious to a participant if they are receiving lactulose, due to dose titration and the laxative effect |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | Low risk | The assessment of response was conducted by a blinded trial author |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants who did not complete some of the tests were not included in the analyses. However, it is unclear whether all participants were included in the assessment of the primary outcomes. |
| Selective reporting (reporting bias) | Low risk | The trial reported clinically relevant outcomes in full. The original trial protocol was not available. |
| Other bias | Low risk | None identified |
| Overall assessment Non‐mortality outcomes | High risk | |
| Overall assessment Mortality outcomes | High risk | |
| Methods | Sodium benzoate: multicentre, double‐blind, randomised clinical trial | |
| Participants |
Included participants: cirrhosis with chronic Grade I or 2 hepatic encephalopathy (n = 27) Age (mean ± SD):
Proportion of men:
Aetiology of liver disease:
MELD score: not reported |
|
| Interventions |
Intervention comparison: sodium benzoate vs lactitol both given orally as a syrup Sodium benzoate: mean 5.6 g/day (n = 12) Lactitol: mean 29.1 g/day (n = 15) Duration of treatment: 2 weeks Co‐intervention: all participants received lactitol, titrated to produce 2‐3 soft stools/day, for 1 week prior to randomisation |
|
| Outcomes |
Neurocognitive assessment: PSE Sum and Index comprising:
|
|
| Inclusion period | Not reported | |
| Country of origin | Mexico | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status:
We contacted the trial authors who provided additional data and information regarding randomisation and funding. Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, and personnel measuring participants' mental state, were blinded to their allocation group. |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | Low risk | Personnel measuring participants' mental state were blinded to their allocation group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data and all participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | No reporting bias identified |
| Other bias | Low risk | None identified |
| Overall assessment Non‐mortality outcomes | Low risk | |
| Overall assessment Mortality outcomes | Low risk | |
| Methods | Glycerol phenylbutyrate: multicentre, double‐blind, placebo‐controlled, randomised clinical trial | |
| Participants |
Included participants: cirrhosis with 2 episodes of hepatic encephalopathy at West Haven Grade 2 or higher in the previous 6 months, 1 of which was within 3 months of randomisation (n = 178); 91% in the glycerol phenylbutyrate group and 92% in the placebo group had no evidence of hepatic encephalopathy at trial entry but the majority were on treatment with lactulose or rifaximin or both. Age (mean ± SD):
Proportion of men:
Aetiology of liver disease: not reported MELD score (mean ± SD):
|
|
| Interventions |
Intervention comparison: oral glycerol phenylbutyrate vs placebo Glycerol phenylbutyrate: 6 mL twice daily (n = 90) Placebo: identically presented but not specified (n = 88) Duration of treatment: 16 weeks Co‐intervention: Lactulose use at baseline (mL/day)
Rifaximin use at baseline (mg/day); participants in the USA were eligible if they had experienced at least 1 of their 2 qualifying episodes of hepatic encephalopathy after ≥ 1 month rifaximin treatment
|
|
| Outcomes |
Neuropsychiatric assessment:
Others:
|
|
| Inclusion period | 1 June 2010‐31 October 2011 | |
| Country of origin | 51 centres, including 35 in the USA, 7 in the Ukraine, and 9 in Russia | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status: full paper
Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All trial personnel, participants and caregivers were blinded to treatment group assignment. |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | Low risk | The primary efficacy measure was adjudicated by the blinded investigators during the study and analysed after unblinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data. However, more participants randomised to glycerol phenylbutyrate exited the study before completion than participants receiving placebo. |
| Selective reporting (reporting bias) | Low risk | All clinically relevant outcomes were reported. |
| Other bias | Low risk | None |
| Overall assessment Non‐mortality outcomes | High risk | |
| Overall assessment Mortality outcomes | High risk | |
| Methods | Ornithine phenylacetate (OCR‐002): multicentre, double‐blind, placebo‐controlled, randomised clinical trial | |
| Participants |
Included participants: cirrhosis, with an acute episode of overt hepatic encephalopathy ≥ Grade 2 and hyperammonaemia, whose mental status did not improve after a minimum of 12 h of 'standard care' (n = 231) Age: not reported Proportion of men: USA 59%; elsewhere 72% Aetiology of liver disease: not reported MELD score (mean): USA 20; elsewhere 18 |
|
| Interventions |
Intervention comparison: ornithine phenylacetate vs placebo Ornithine phenylacetate: 10 g/day, 15 g/day or 20 g/day, by continuous IV infusion (n = 116); dose pre‐determined by the level of hepatic decompensation Placebo: identically presented 5% dextrose by continuous IV infusion (n = 115) Duration of treatment: 5 days Co‐intervention: all participants received 'standard care'" based on 'gut flora modification'" but this was not specified although 135 (58.4%) participants were taking rifaximin (mean dose: 1057 mg/day; range: 400‐1650/day); 12 (5.2%) were taking L‐ornithine L‐aspartate (dose range 9‐20g/day) and an unspecified proportion were taking lactulose. |
|
| Outcomes |
Neurocognitive assessment:
|
|
| Inclusion period | November 2013‐December 2016 | |
| Country of origin | Australia, Austria, Belgium, Bulgaria, Czech Republic, Denmark, Estonia, France, Germany, Hungary, Israel, Italy, Netherlands, New Zealand, Russian Federation, Spain, USA | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status: 3 separate abstracts at the same conference
Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, care providers, investigators and outcomes assessors were blinded. |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | Low risk | Participants, care providers, investigators and outcomes assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. All participants were included in the analyses |
| Selective reporting (reporting bias) | High risk | The primary end‐point in the registered trial protocol is 'change from baseline in hepatic encephalopathy stage'. However, in the published abstracts the primary endpoint reported is 'the time to meaningful clinical improvement in hepatic encephalopathy symptoms assessed using the Hepatic Encephalopathy Staging Tool' together with a number of other time‐related variables. |
| Other bias | High risk | Differences were reported for a number of variables in participants recruited in the USA and those recruited elsewhere in the world, which is an additional source of bias. |
| Overall assessment Non‐mortality outcomes | High risk | |
| Overall assessment Mortality outcomes | High risk | |
| Methods | Ornithine phenylacetate (OCR‐002): two‐centre, double‐blind, placebo‐controlled, randomised clinical trial | |
| Participants |
Included participants: cirrhosis, enrolled within 24 h of an upper gastrointestinal bleed (n = 38). Three people in each group had hepatic encephalopathy at the time of randomisation Age (mean ± SD):
Proportion of men:
Aetiology of liver disease (ornithine phenylacetate: placebo group):
MELD score (mean ± SD):
|
|
| Interventions |
Intervention comparison: ornithine phenylacetate vs placebo Ornithine phenylacetate: 10 g/day by continuous IV infusion at a rate of 0.416 g/h (n = 20) Placebo: identically presented glucose‐saline solution by continuous IV infusion (n = 18) Duration of treatment: 5 days Co‐intervention: all participants received standard care which included:
|
|
| Outcomes |
Neurocognitive assessment:
Others:
|
|
| Inclusion period | September 2012 ‐ August 2014 | |
| Country of origin | Spain | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status: full paper
Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Correspondence with the trial authors confirmed the use of sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded. The pharmacy department prepared the infusion solutions independently. |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | Low risk | Data were independently monitored by a separate institution. A data‐blind review was conducted before locking the trial database. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Correspondence with the trial authors confirmed use of ITT analyses. |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported. |
| Other bias | Low risk | None identified |
| Overall assessment Non‐mortality outcomes | Low risk | |
| Overall assessment Mortality outcomes | Low risk | |
| Methods | AST‐120 (spherical carbon absorbant): multicentre, double‐blind, placebo‐controlled, dose‐ranging, randomised clinical trial | |
| Participants |
Included participants: compensated cirrhosis (MELD score < 25) and covert hepatic encephalopathy (minimal and Grade 1) (n = 148) Age (mean): 55 years Proportion of men: not reported Aetiology of liver disease:
MELD score (mean): 10 |
|
| Interventions |
Intervention comparison: oral AST‐120 vs placebo AST‐120: 6 g/day (n = 50), 12 g/day (n = 50) Placebo: no details provided (n = 48) Duration of treatment: 8 weeks Co‐intervention: none reported |
|
| Outcomes |
Neurocognitive assessment:
|
|
| Inclusion period | March 2009‐June 2010 | |
| Country of origin | USA | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status: abstract; unpublished information about trial methods (randomisation) received from the trial authors
Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel using placebo |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | Low risk | Blinding of outcome assessment using placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for and included in the analyses |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes were reported. Outcomes in the published abstract correspond with the published protocol. |
| Other bias | Low risk | None identified |
| Overall assessment Non‐mortality outcomes | Low risk | |
| Overall assessment Mortality outcomes | Low risk | |
| Methods | AST‐120 (spherical carbon absorbant): multicentre, open‐label, clinical trial | |
| Participants |
Included participants: cirrhosis (MELD score ≤ 15) and hepatic encephalopathy Grades 1 or 2 using West Haven Criteria (n = 47) Age: not reported Proportion of men: not reported Aetiology of liver disease: not reported MELD score: not reported |
|
| Interventions |
Intervention comparison: oral AST‐120 vs lactulose AST‐120: 2 g 4 times/day (n = 24) Lactulose: no details reported (n = 23) Duration of treatment: 4 weeks Co‐intervention: some participants were taking lactulose on admission: this was stopped in the participants randomised to AST‐120 |
|
| Outcomes |
Neurocognitive assessment:
|
|
| Inclusion period | September 2007 ‐ June 2009 | |
| Country of origin | USA | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status: abstract
Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial without blinding |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | High risk | Open‐label trial without blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Evaluable data are available for 41 participants: 3 participants randomised to lactulose were excluded because of non‐compliance; 3 participants randomised to AST‐120 were excluded for 'other reasons' |
| Selective reporting (reporting bias) | Low risk | The outcomes reported in the published abstract correspond to the outcomes listed in the trial protocol. |
| Other bias | Low risk | None identified |
| Overall assessment Non‐mortality outcomes | High risk | |
| Overall assessment Mortality outcomes | High risk | |
| Methods | PEG: single‐centre, double‐blind, randomised clinical trial | |
| Participants |
Included participants: cirrhosis with an acute episode of overt hepatic encephalopathy (n = 40) Age (mean ± SD):
Proportion of men:
Aetiology of liver disease (PEG: lactulose group):
MELD score (median ± interquartile range):
|
|
| Interventions |
Intervention comparison: PEG plus lactulose vs lactulose PEG: 280 g PEG in 4 L water as a single dose over 30‐120 min. In addition lactulose was administered in the same amount enterally or rectally as in the control group (n = 21) Lactulose: 20‐30 g lactulose administered orally or via nasogastric tube (at least 3 doses in 24 h) or 200 g administered via rectal tube (n = 19) Duration of treatment: 24 h Co‐intervention: none reported |
|
| Outcomes |
Neurocognitive assessment:
Others
|
|
| Inclusion period | September 2015 ‐ January 2016 | |
| Country of origin | Iran | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status: full paper
Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, block randomisation (block size 6 participants) |
| Allocation concealment (selection bias) | Low risk | One team member was responsible for prescribing the trial drugs, while assessments of HESA scores at presentation and at 24 h were done by another team member blinded to the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. The statistical analysis was undertaken by a blinded analyst. |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | Low risk | Outcome assessors were independent from the people who prescribed the study drugs. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four of the original 48 eligible participants refused consent, while a further 4 (1 allocated to PEG; 3 allocated to lactulose) were excluded from the analyses because they had received sedative drugs. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes are reported. |
| Other bias | Low risk | None identified |
| Overall assessment Non‐mortality outcomes | High risk | |
| Overall assessment Mortality outcomes | High risk | |
| Methods | PEG: single‐centre, open‐label, randomised clinical trial. The HELP study | |
| Participants |
Included participants: cirrhosis and any grade of overt hepatic encephalopathy (n = 50) Age (mean ± SD):
Proportion of men:
Aetiology of liver disease (PEG, lactulose group):
MELD score (mean ± SD):
|
|
| Interventions |
Intervention comparison: PEG vs lactulose PEG: 3350‐electrolyte solution; 4 L given as a single dose over 4 h (n = 25) Lactulose: 20‐30 g lactulose administered orally or via nasogastric tube (at least 3 doses in 24 h) or 200 g administered via rectal tube (n = 25) Duration of treatment: 24 h Co‐intervention: participants were allowed to receive a single dose of lactulose prior to randomisation, and after 24 h of treatment regardless of their group allocation. |
|
| Outcomes |
Neuropsychiatric assessment:
Others:
|
|
| Inclusion period | January 2011‐June 2012 | |
| Country of origin | USA | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status: full paper
Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number and treatment assignment |
| Allocation concealment (selection bias) | Low risk | Participants' allocation was concealed in opaque sealed envelopes. In some participants one investigator obtained consent, randomised the participants and ensured that the appropriate study medication was administered while the follow‐up was undertaken by another investigator. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | Low risk | A separate, blinded investigator conducted the assessment of neuropsychological status after randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All analyses were conducted as ITT. Two participants did not achieve the full 24‐h follow‐up and so data are missing for the assessment of neuropsychological status. One participant in the PEG group did not receive the allocated treatment and so was treated with a lactulose enema. Two participants in the PEG group did not have follow‐up HESA scores; of these one became alert and oriented and refused assessment while the other improved to point of discharge home in less than 24 h. Consequently, these two participants were not included in either the initial or 24‐h analysis. |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported. However, non‐serious adverse events were not extractable. |
| Other bias | High risk | The blood urea nitrogen level was significantly higher in the participants randomised to PEG |
| Overall assessment Non‐mortality outcomes | High risk | |
| Overall assessment Mortality outcomes | High risk | |
| Methods | PEG: single‐centre, open label, randomised clinical trial | |
| Participants |
Included participants: people admitted with cirrhosis and an acute episode of overt hepatic encephalopathy (n = 100). Age (mean ± SD):
Proportion of men:
Aetiology of liver disease: all participants had hepatitis C‐related cirrhosis |
|
| Interventions |
PEG: single dose (3 sachets if participant < 75 kg or 4 sachets if participant > 75 kg) administered orally over 3‐4 h or via a nasogastric tube at a rate of 20‐30 mL/min. Each sachet contained 64 g PEG dissolved in 1 L tap water. Lactulose: 20‐30 mL lactulose orally or via nasogastric tube, given as 3 doses over 24 h. 200 mL lactulose and plain water also given as a retention enema every 4 h. |
|
| Outcomes |
Primary: at least 1 scale improvement in the HESA score after 24 h Secondary: length of hospital stay, adverse events |
|
| Inclusion period | May‐December 2017 | |
| Country of origin | Egypt | |
| Outcomes included in meta‐analyses |
|
|
| Notes |
Publication status: full paper
Funding:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation (block size 10 participants) |
| Allocation concealment (selection bias) | Low risk | Participant allocation was in opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators were blinded to the random code sequence. Participants and personnel administering the treatments were not blinded |
| Blinding of outcome assessment (detection bias) Non‐mortality outcomes | Low risk | Participants were identified only by code numbers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analyses. Those lost to follow‐up were evaluated using ITT analyses except for adverse events |
| Selective reporting (reporting bias) | Low risk | All clinically relevant predefined outcomes were reported. Changes in blood ammonia levels were not reported |
| Other bias | Low risk | None identified |
| Overall assessment Non‐mortality outcomes | High risk | |
| Overall assessment Mortality outcomes | High risk | |
CHESS: Clinical Hepatic Encephalopathy Staging Scale; HESA: Hepatic Encephalopathy Scoring Algorithm; ITT: intention‐to‐treat; IV: intravenous; MELD: Model for End‐stage Liver Disease; PEG: polyethylene glycol; PHES: Psychometric Hepatic Encephalopathy Score; PSE: Portal‐Systemic Encephalopathy; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; SD: standard deviation
Characteristics of excluded studies [author‐defined order]
| Study | Reason for exclusion |
|---|---|
| Campollo 1992 |
Sodium benzoate: 18 participants with cirrhosis and chronic persistent hepatic encephalopathy were treated for 6 months with oral sodium benzoate in a mean dose of 6.4 g/day given as an 8% solution. We excluded this study as it was observational. |
| Mendenhall 1986 |
Sodium benzoate: double‐blind, cross‐over, randomised clinical trial, undertaken in 8 participants with overt hepatic encephalopathy allocated to oral sodium benzoate or sodium phenylacetate We excluded this trial because it compared sodium benzoate with another ammonia‐lowering agent. |
| Panella 1993 |
Sodium benzoate: double‐blind, cross‐over, randomised clinical trial undertaken in 7 participants with cirrhosis and stable Grade 1‐2 hepatic encephalopathy randomised to sodium benzoate or placebo as an adjuvant to treatment with branched chain amino acids; participants were crossed over to the alternative arm after 7 days. Lactulose and the antibiotic Colimicin were added to standard treatment but it is unclear whether this was provided equally in both groups. Participants receiving adjuvant sodium benzoate showed a significant reduction in blood ammonia levels compared to the group receiving placebo and a trend to a greater reduction in Number Connection Test times and the PSE Index. We excluded this trial as it was not a direct comparison of the drug of interest against either placebo or another active agent |
| Ghabril 2013 |
Glycerol phenylbutyrate: open‐label, phase IIa study designed to evaluate the safety, tolerability and effect on blood ammonia concentrations of 2 doses of glycerol phenylbutyrate in 15 participants with cirrhosis and a history of at at least 2 previous episodes of overt hepatic encephalopathy We excluded this trial as it was observational. |
| Weiss 2018 |
Sodium phenylbutyrate: 18 participants with cirrhosis, overt hepatic encephalopathy and hyperammonaemia admitted to an ICU were given sodium phenylbutyrate 200 mg/kg/day orally or via a nasogastric tube until blood ammonia levels returned to normal on 2 consecutive occasions or until neurological status improved. Other treatments such as lactulose or rifaximin, were maintained if prescribed before inclusion. The results in this group were compared with those in an historical control group (matched for age, sex, MELD score, and severity of hepatic encephalopathy using West Haven Criteria), managed in the same unit, using the same guidelines. We excluded this trial as it was observational. |
ICU: intensive care unit; MELD: Model for End‐stage Liver Disease; PSE: Portal‐Systemic Encephalopathy (see Table 4)
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title |
AST‐120 Randomized trial comparing the efficacy of AST‐120 versus lactulose for treatment of mild hepatic encephalopathy |
| Methods | Open‐label, randomised clinical trial |
| Participants | End‐stage liver disease with a MELD score < 16 and Grade 1‐2 hepatic encephalopathy (n = 40) |
| Interventions |
AST‐120: 2 g sachets 4 times/day Lactulose: as previously prescribed or started at 30 mL twice and titrated to produce 2‐3 soft stools/day |
| Outcomes |
Primary: change in West Haven grade over 4 weeks Secondary: change in the HESA, ammonia levels, serum bile acid and amino acid profiles, itching (visual analogue scale), asterixis, clinical laboratory tests, clinical examination |
| Starting date | September 2007 |
| Contact information | Paul Pockros, Scripps Clinic |
| Country of origin | USA |
| Notes | Phase 2 study; completed on 30 May 2014 |
| Trial name or title |
PEG: To compare efficacy and safety of lactulose versus polyethylene glycol for treatment of overt hepatic encephalopathy in cirrhotics; a randomised controlled trial |
| Methods | Open‐label, randomised clinical trial |
| Participants | Cirrhosis and hepatic encephalopathy of Grade 2 and above (n = 110) |
| Interventions |
PEG: 17 g administered orally or via nasogastric tube 3‐4 times/day Lactulose: 20‐30 g lactulose administered orally or via nasogastric tube (at least 3 doses in 24 h) or 200 g administered via rectal tube |
| Outcomes |
Primary: complete reversal of hepatic encephalopathy (Grade 0) Secondary: improvement in hepatic encephalopathy by 2 grades; length of ICU stay; presence of EED changes; adverse events |
| Starting date | 1 August 2017 |
| Contact information | Dr Abhinav Verma, abhinav.3183@gmail.com |
| Country of origin | India |
| Notes | The estimated final data collection date for the primary outcome measure and for study completion was 31 July 2018. |
EEG: electroencephalogram;HESA: Hepatic Encephalopathy Scoring Algorithm; ICU: intensive care unit; MELD: Model for End‐stage Liver Disease; PEG: Polyethylene glycol;
Contributions of authors
HDZ drafted the review, and identified and selected trials, conducted the data extraction, analyses and interpretation of the results. APZ identified and selected trials and assisted with the data extraction. LLG and MYM identified and selected trials, contributed to the analyses and interpretation of the results, and revised the review.
All authors participated in the final revision of the review and have approved of the submitted version.
Sources of support
Internal sources
No funding, Other.
External sources
No funding, Other.
Declarations of interest
Harry Zacharias: none Antony Zacharias: none Lise L Gluud: none Marsha Y Morgan: none
Edited (no change to conclusions)
References
References to studies included in this review
- Bajaj JS, Sheikh MY, Chojkier M, Balart L, Sherker AH, Vemuru R, et al. AST‐120 (spherical carbon adsorbent) in covert hepatic encephalopathy: results of the Astute trial. Journal of Hepatology 2013;58(Suppl 1):S84. [DOI: 10.1016/S0168-8278(13)60192-0] [DOI] [Google Scholar]; Bajaj JS, Sheikh MY, Chojkier M, Balart LA, Sherker AH, Vemuru RP, et al. Su1685 AST‐120 (spherical carbon adsorbent) in covert hepatic encephalopathy: results of the Astute trial. Gastroenterology 2013;144(5 (Suppl 1)):S997. [Google Scholar]
- Gonzalez A, Neri M, Campollo O, Amacio O. Treatment of acute portal systemic encephalopathy (PSE) grades III and IV with sodium benzoate and lactose. Hepatology (Baltimore, Md.) 1994;19(4 Suppl):67I. [Google Scholar]
- Naderian M, Heshmatollah A, Saeedi M, Sohrabpour A. Polyethylene glycol and lactulose alone in the treatment of hepatic encephalopathy in patients with cirrhosis: a non‐inferiority randomised control trial. Middle East Journal of DIgestive Diseases 2017;9(1):12‐9. [PUBMED: 28316761] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros P, Hassanein T, Vierling J, Heuman D, Hillebrand D, Chojkier M. Phase 2, multicenter, randomised study of AST‐120 (spherical carbon adsorbent) vs. lactulose in the treatment of low‐grade hepatic encephalopathy (HE). Journal of Hepatology2009; Vol. 50, issue Suppl 1:S43. [CN‐00715816]
- Rahimi R, Singal A, Cuthbert J, Rockey D. Lactulose vs polyethylene glycol 3350‐electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomised clinical trial. Journal of the American Medical Association Internal Medicine 2014;174(11):1727‐33. [PUBMED: 25243839] [DOI] [PMC free article] [PubMed] [Google Scholar]; Rahimi RS, Singal AG, Cuthbert JA, Rockey DC. A randomised trial of polyethylene glycol 3350‐electrolyte solution (PEG) and lactulose for patients hospitalized with acute hepatic encephalopathy. Hepatology (Baltimore, Md.) 2012;56(Suppl):915A‐6A. [Google Scholar]
- Jurek M, Mokhtarani M, Vierling J, Coakley D, Ganju J, Rowell R, et al. Variation in liver biochemistries in patients with decompensated cirrhosis: implications for assessing drug‐induced liver injury in clinical trials. Pharmaceutical Medicine 2016;30(2):95‐101. [Google Scholar]; Rockey DC, Vierling JM, Mantry P, Ghabril M, Brown RS Jr, Alexeeva O, et al. Randomized, double‐blind, controlled study of glycerol phenylbutyrate in hepatic encephalopathy. Hepatology (Baltimore, Md.) 2014;59(3):1073‐83. [PUBMED: 23847109] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata HH, Elfert AA, Abdin AA, Soliman SM, Elkhouly RA, Hawah NI, et al. Randomized controlled trial of polyethylene glycol versus lactulose for the treatment of overt hepatic encephalopathy. European Journal of Gastroenterology & Hepatology 2018;30(12):1476‐81. [DOI: 10.1097/MEG.0000000000001267] [DOI] [PubMed] [Google Scholar]
- Rahimi RS, Safadi R, Thabut D, Bajaj JS, Bhamidimarri KR, Pyrsopoulos NT, et al. STOP‐HE: a randomised, double‐blind, placebo‐controlled study of OCR‐002 in patients with hepatic encephalopathy. Hepatology (Baltimore, Md.) 2017;66(Suppl 1):276A. [Google Scholar]; Safadi R, Rahimi RS, DiLiberti CE, Wang L, Pyrsopoulos NT, Pothoff AP, et al. OCR‐002 (ornithine phenylacetate) is a potent ammonia scavenger as demonstrated in phase 2b STOP‐HE study. Hepatology (Baltimore, Md.) 2017;66(Suppl 1):126A. [Google Scholar]; Thabut D, Stadlbauer V, Bhamidimarri KR, Pothoff A, Bukofzer S, on behalf of the STOP‐HE study group. Geographic differences for patients enrolled in STOP‐HE: a randomised, phase 2 study of OCR‐002 for hepatic encephalopathy. Hepatology (Baltimore, Md.) 2017;66(Suppl 1):274A. [Google Scholar]
- Sushma S, Dasarathy S, Tandon RK, Jain S, Gupta S, Bhist MS. Sodium benzoate in the treatment of acute hepatic encephalopathy: a double‐blind randomised trial. Hepatology (Baltimore, Md.) 1992;16(1):138‐44. [PUBMED: 1618465] [DOI] [PubMed] [Google Scholar]
- Uribe M, Bosques F, Marín E, Cervera E, Gil S, Poo JL, et al. Sodium benzoate in portalsystemic encephalopathy induced blood ammonia normalization and clinical improvement. Interim report of a double blind multicenter trial [Derivacion metabolica del amonio plasmatico con benzoato de sodio, como metodo de tratamiento en pacientes con cirrosis y encefalopatia portosistemica. Informe interino de un estudio multicentrico, doble ciego]. Revista de Investigación Clínica 1990;42(Suppl):149‐54. [PUBMED: 19256155] [PubMed] [Google Scholar]
- Ventura‐Cots M, Arranz JA, Simón‐Talero M, Torrens M, Blanco A, Riudor E, et al. Safety of ornithine phenylacetate in cirrhotic decompensated patients: an open‐label, dose‐escalating, single‐cohort study. Journal of Clinical Gastroenterology 2013;47(10):881‐7. [PUBMED: 23751856] [DOI] [PubMed] [Google Scholar]; Ventura‐Cots M, Concepción M, Arranz JA, Simón‐Talero M, Torrens M, Blanco‐Grau A, et al. Impact of ornithine phenylacetate (OCR‐002) in lowering plasma ammonia after upper gastrointestinal bleeding in cirrhotic patients. Therapeutic Advances in Gastroenterology 2016;9(6):823‐35. [PUBMED: 27803737] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Campollo O, Gil S, Olvera G, Poo JL, Uribe M. Efficacy and safety of sodium benzoate for the long‐term treatment of chronic hyperammonemic hepatic encephalopathy. Hepatology (Baltimore, Md.) 1992;16(Suppl 1):248A. [Google Scholar]
- Ghabril M, Zupanets I, Vierling J, Mantry P, Rockey D, Wolf D, et al. Glycerol phenylbutyrate in patients with cirrhosis and episodic hepatic encephalopathy: a pilot study of safety and effect on venous ammonia concentration. Clinical Pharmacology in Drug Development 2013;2(3):278‐84. [PUBMED: 27121790] [DOI] [PubMed] [Google Scholar]
- Mendenhall CL, Rouster S, Marshall L, Weesner R. A new therapy for portal systemic encephalopathy. American Journal of Gastroenterology 1986;81(7):540‐3. [PUBMED: 3717115] [PubMed] [Google Scholar]
- Panella C, Guglielmi FW, Mastronuzzi T, Contento F, Siciliano N, Francavilla A. Oral Na‐benzoate in the treatment of chronic hepatic encephalopathy. Italian Journal of Gastroenterology 1993;25:228. [Google Scholar]; Panella C, Guglielmi FW, Mastronuzzi T, Contento F, Siciliano N, Francavilla A. Oral sodium benzoate in the treatment of chronic hepatic encephalopathy. Gut 1993;34(Suppl 3):S43. [Google Scholar]
- Tripon S, Mallet M, Lodey M, Guiller E, Rudler M, Mouri S, et al. Sodium phenylbutyrate administration to avoid neurological worsening in cirrhotic patients with hepatic encephalopathy admitted in ICU. Journal of Hepatology 2016;64(2 Suppl):S288. [Google Scholar]; Weiss N, Tripon S, Lodey M, Guiller E, Junot H, Monneret D, et al. Treating hepatic encephalopathy in cirrhotic patients admitted to ICU with sodium phenylbutyrate: a preliminary study. Fundamental & Clinical Pharmacology 2018;32(2):206‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- NCT00558038. Safety and efficacy of AST‐120 compared to lactulose in patients with hepatic encephalopathy (AST015). clinicaltrials.gov/ct2/show/NCT00558038 (first posted 14 November 2007).
- NCT03448770. To compare efficacy and safety of lactulose versus polyethylene glycol for treatment of overt hepatic encephalopathy in cirrhotics: a randomised controlled trial. clinicaltrials.gov/ct2/show/nct03448770 (first posted 28 February 2018).
Additional references
- American Association for the Study of Liver Diseases, European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. Journal of Hepatology 2014;61(3):642‐59. [DOI] [PubMed] [Google Scholar]
- Acharya C, Bajaj J. Current management of hepatic encephalopathy. American Journal of Gastroenterology 2018;113(11):1600‐12. [DOI] [PubMed] [Google Scholar]
- Ahuja N, Ally W, Caldwell S. Direct acting inhibitors of ammoniagenesis: a role in post‐TIPS encephalopathy?. Annals of Hepatology 2014;13(2):179‐86. [PubMed] [Google Scholar]
- Allampati S, Duarte‐Rojo A, Thacker L, Patidar K, White M, Klair J, et al. Diagnosis of minimal hepatic encephalopathy using strop EncephalApp: a multicentre US‐based, norm‐based study. American Journal of Gastroenterology 2016;111(1):78‐86. [PUBMED: 26644276] [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology 2008;135(5):1591‐600. [PUBMED: 18723018] [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology (Baltimore, Md.) 2009;50(6):2014‐21. [DOI: 10.1002/hep.23216; PUBMED: 19787808] [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 2010;138(7):2332‐40. [PUBMED: 20178797] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, et al. Review article: the design of clinical trials in hepatic encephalopathy ‐ an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Alimentary Pharmacology & Therapeutics 2011;33(7):739‐47. [PUBMED: 21306407] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, et al. The multi‐dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. American Journal of Gastroenterology 2011;106(9):1646‐53. [PUBMED: 21556040] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass N. Review article: the current pharmacological therapies for hepatic encephalopathy. Alimentary Pharmacology & Therapeutics 2007;25(S1):23‐31. [DOI] [PubMed] [Google Scholar]
- Berry SA, Lichter‐Konecki U, Diaz GA, McCandless SE, Rhead W, Smith W, et al. Glycerol phenylbutyrate treatment in children with urea cycle disorders: pooled analysis of short and long‐term ammonia control and outcomes. Molecular Genetics and Metabolism 2014;112(1):17‐24. [PUBMED: 24630270] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco Vela CI, Bosques Padilla FJ. Determination of ammonia concentrations in cirrhosis patients ‐ still confusing after all these years?. Annals of Hepatology 2011;10(Suppl 2):S60‐5. [PUBMED: 22228884] [PubMed] [Google Scholar]
- Bosoi CR, Parent‐Robitaille C, Anderson K, Tremblay M, Rose CF. AST‐120 (spherical carbon adsorbent) lowers ammonia levels and attenuates brain edema in bile duct‐ligated rats. Hepatology (Baltimore, Md.) 2011;53(6):1995‐2002. [PUBMED: 21384402] [DOI] [PubMed] [Google Scholar]
- Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, et al. How to formulate research recommendations. BMJ (Clinical Research Ed.) 2006;333(7572):804‐6. [PUBMED: 17038740] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J, Rimola A, Ventura PJ, Navasa M, CireraI I, Reggiardo V, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. Journal of Hepatology 1999;30(5):890‐5. [PUBMED: 10365817] [DOI] [PubMed] [Google Scholar]
- Butterworth R. Complications of cirrhosis III. Hepatic encephalopathy. Journal of Hepatology 2000;32(Suppl 1):171‐80. [DOI] [PubMed] [Google Scholar]
- Butterworth RF. The liver‐brain axis in liver failure: neuroinflammation and encephalopathy. Nature Reviews. Gastroenterology & Hepatology 2013;10(9):522‐8. [PUBMED: 23817325] [DOI] [PubMed] [Google Scholar]
- Cadranel JF, Lebiez E, Martino V, Bernard B, Koury S, Tourbah A, et al. Focal neurological signs in hepatic encephalopathy in cirrhotic patients: an underestimated entity?. American Journal of Gastroenterology 2001;96(2):515‐8. [PUBMED: 11232699] [DOI] [PubMed] [Google Scholar]
- Chu NS, Yang SS, Liaw YF. Evoked potentials in liver diseases. Journal of Gastroenterology and Hepatology 1997;12(Suppl):S288‐93. [PUBMED: 9407349] [DOI] [PubMed] [Google Scholar]
- Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, et al. Comparison of lactulose and neomycin in the treatment of chronic portal‐systemic encephalopathy. A double blind controlled trial. Gastroenterology 1977;72(4 Pt 1):573‐83. [PUBMED: 14049] [PubMed] [Google Scholar]
- Jongh FE, Janssen HL, Man RA, Hop WC, Schalm SW, Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen‐positive cirrhosis of the liver. Gastroenterology 1992;103(5):1630‐5. [PUBMED: 1426884] [DOI] [PubMed] [Google Scholar]
- Las Heras J, Aldámiz‐Echevarría L, Martínez‐Chantar ML, Delgado T. An update on the use of benzoate, phenylacetate and phenylbutyrate ammonia scavengers for interrogating and modifying liver nitrogen metabolism and its implications in urea cycle disorders and liver disease. Expert Opinion on Drug Metabolism & Toxicology 2017;13(4):439‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
- Dhiman RK, Saraswat VA, Verma M, Naik SR. Figure connection test: a universal test for assessment of mental state. Journal of Gastroenterology and Hepatology 1995;10(1):14‐23. [PUBMED: 7620102] [DOI] [PubMed] [Google Scholar]
- D’Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Digestive Diseases and Sciences 1986;31(5):468‐75. [PUBMED: 3009109] [DOI] [PubMed] [Google Scholar]
- D’Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44:217‐31. [DOI] [PubMed] [Google Scholar]
- Efrati C, Masini A, Merli M, Valeriano V, Riggio O. Effect of sodium benzoate on blood ammonia response to oral glutamine challenge in cirrhotic patients: a note of caution. American Journal of Gastroenterology 2000;95(12):3574‐8. [PUBMED: 11151894] [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy‐‐definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology (Baltimore, Md.) 2002;35(3):716‐21. [PUBMED: 11870389] [DOI] [PubMed] [Google Scholar]
- Gluud LL, Vilstrup H, Morgan MY. Non‐absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Systematic Review 2016;5:CD003044. [DOI: 10.1002/14651858.CD003044.pub4; PUBMED: 27153247] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh ET, Stokes CS, Sidhu SS, Vilstrup H, Gluud LL, Morgan MY. L‐ornithine L‐aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD012410.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster University (developed by Evidence Prime). GRADEpro. Version accessed 16 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
- Groeneweg M, Quero JC, Bruijn I, Hartmann IJ, Essink‐bot ML, Hop WC, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology (Baltimore, Md.) 1998;28(1):45‐9. [PUBMED: 9657095] [DOI] [PubMed] [Google Scholar]
- Guérit JM, Amantini A, Fischer C, Kaplan PW, Mecarelli O, Schnitzler A, et al. Neurophysiological investigations of hepatic encephalopathy: ISHEN practice guidelines. Liver International 2009;29(6):789‐96. [PUBMED: 19638107] [DOI] [PubMed] [Google Scholar]
- Hadjihambi A, Arias N, Sheikh M, Jalan R. Hepatic encephalopathy: a critical current review. Hepatology International 2018;12(Suppl 1):135‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [PUBMED: 16345038] [DOI] [PubMed] [Google Scholar]
- Hassanein TI, Hilsabeck RC, Perry W. Introduction to the Hepatic Encephalopathy Scoring Algorithm (HESA). Digestive Diseases and Sciences 2008;53(2):529‐38. [DOI: 10.1007/s10620-007-9895-0; PUBMED: 17710551] [DOI] [PubMed] [Google Scholar]
- Haussinger D, Kircheis G, Fischer R, Schliess F, vom Dahl S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low‐grade cerebral edema?. Journal of Hepatology 2000;32(6):1035‐8. [PUBMED: 10898326] [DOI] [PubMed] [Google Scholar]
- Higgins JP, White IR, Wood AM. Imputation methods for missing outcome data in meta‐analysis of clinical trials. Clinical Trials 2008;5(3):225‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017 Available from: www.training.cochrane.org/handbook.
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
- Jackson CD, Gram M, Halliday E, Olesen SS, Sandberg TH, Drewes AM, et al. New spectral thresholds improve the utility of the electroencephalogram for the diagnosis of hepatic encephalopathy. Clinical Neurophysiology 2016;127(8):2933‐41. [DOI: 10.1016/j.clinph.2016.03.027; PUBMED: 27236607] [DOI] [PubMed] [Google Scholar]
- Jalan R, Wright G, Davies NA, Hodges SJ. L‐Ornithine phenylacetate (OP): a novel treatment for hyperammonemia and hepatic encephalopathy. Medical Hypotheses 2007;69(5):1064‐9. [PUBMED: 17467190] [DOI] [PubMed] [Google Scholar]
- Jawaro T, Yang A, Dixit D, Bridgeman M. Management of hepatic encephalopathy. Annals of Pharmacology 2016;50(7):569‐77. [DOI] [PubMed] [Google Scholar]
- Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. The clinical course of alcoholic liver cirrhosis: a Danish population‐based cohort study. Hepatology (Baltimore, Md.) 2010;51(5):1675‐82. [DOI: 10.1002/hep.23500; PUBMED: 20186844] [DOI] [PubMed] [Google Scholar]
- Jover‐Cobos M, Noiret L, Sharifi Y, Jalan R. Ornithine phenylacetate revisited. Metabolic Brain Disease 2013;28(2):327‐31. [PUBMED: 23456516] [DOI] [PubMed] [Google Scholar]
- Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Haussinger D. Critical flicker frequency for quantification of low‐grade hepatic encephalopathy. Hepatology (Baltimore, Md.) 2002;35(2):357‐66. [PUBMED: 11826409] [DOI] [PubMed] [Google Scholar]
- Kircheis G, Knoche A, Hilger N, Manhart F, Schnitzler A, Schulze H, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009;137(5):1706‐15. [PUBMED: 19686744] [DOI] [PubMed] [Google Scholar]
- Kornerup L, Gluud L, Vilstrup H, Dam G. Update on the therapeutic management of hepatic encephalopathy. Current Gastroenterology Reports 2018;20(5):20‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauridsen MM, Jepsen P, Vilstrup H. Critical flicker frequency and continuous reaction times for the diagnosis of minimal hepatic encephalopathy: a comparative study of 154 patients with liver disease. Metabolic Brain Disease 2011;26(2):135‐9. [PUBMED: 21484318] [DOI] [PubMed] [Google Scholar]
- Lee B, Rhead W, Diaz GA, Scharschmidt BF, Mian A, Shchelochkov O, et al. Phase 2 comparison of a novel ammonia scavenging agent with sodium phenylbutyrate in patients with urea cycle disorders: safety, pharmacokinetics and ammonia control. Molecular Genetics and Metabolism 2010;100(3):221‐8. [PUBMED: 20382058] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood AH. Blood ammonia levels and hepatic encephalopathy. Metabolic Brain Disease 2004;19(3‐4):345‐9. [PUBMED: 15554426] [DOI] [PubMed] [Google Scholar]
- Mardini H, Saxby BK, Record CO. Computerized psychometric testing in minimal encephalopathy and modulation by nitrogen challenge and liver transplant. Gastroenterology 2008;135(5):1582‐90. [DOI: 10.1053/j.gastro.2008.06.043; PUBMED: 18647604] [DOI] [PubMed] [Google Scholar]
- Matoori S, Leroux JC. Recent advances in the treatment of hyperammonemia. Advanced Drug Delivery Reviews 2015;90(1):55‐68. [DOI] [PubMed] [Google Scholar]
- McGuire BM, Zupanets IA, Lowe ME, Xiao X, Syplyviy VA, Monteleone J, et al. Pharmacology and safety of glycerol phenylbutyrate in healthy adults and adults with cirrhosis. Hepatology (Baltimore, Md.) 2010;51(6):2077‐85. [PUBMED: 20512995] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological expectations of Cochrane intervention reviews ‐ Version 1.07. community.cochrane.org/mecir‐manual 2018 (accessed 5 March 2019).
- Misel ML, Gish RG, Patton H, Mendler M. Sodium benzoate for treatment of hepatic encephalopathy. Gastroenterology & Hepatology 2013;9(4):219‐27. [PUBMED: 24711766] [PMC free article] [PubMed] [Google Scholar]
- Montagnese S, Amodio P, Morgan MY. Methods for diagnosing hepatic encephalopathy in patients with cirrhosis: a multidimensional approach. Metabolic Brain Disease 2004;19(3‐4):281‐312. [PUBMED: 15554423] [DOI] [PubMed] [Google Scholar]
- Montagnese S, Balistreri E, Schiff S, Rui M, Angeli P, Zanus G, et al. Covert hepatic encephalopathy: agreement and predictive validity of different indices. World Journal of Gastroenterology 2014;20(42):15756‐62. [DOI: 10.3748/wjg.v20.i42.15756; PMCID: PMC4229541; PUBMED: 25400460] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MY. Hepatic encephalopathy. In: Dooley JS, Lok ASF, Garcia‐Tsao G, Pinzani M editor(s). Sherlock's Diseases of the Liver and Biliary System. 13th Edition. London: Wiley‐Blackwell, 2018:Chapter 10: pp 151‐80. [ASIN: 1119237548; ISBN‐13: 978‐1119237549] [Google Scholar]
- O'Carroll RE, Hayes PC, Ebmeier KP, Dougall N, Murray C, Best JJ, et al. Regional cerebral blood flow and cognitive function in patients with chronic liver disease. Lancet 1991;337(8752):1250‐3. [PUBMED: 1674063] [DOI] [PubMed] [Google Scholar]
- Olesen SS, Gram M, Jackson CD, Halliday E, Sandberg TH, Drewes AM, et al. Electroencephalogram variability in patients with cirrhosis associates with the presence and severity of hepatic encephalopathy. Journal of Hepatology 2016;65(3):517‐23. [DOI: 10.1016/j.jhep.2016.05.004; PUBMED: 27184531] [DOI] [PubMed] [Google Scholar]
- Ortiz M, Córdoba J, Doval E, Jacas C, Pujadas F, Esteban R, et al. Development of a clinical hepatic encephalopathy staging scale. Alimentary Pharmacology & Therapeutics 2007;26(6):859‐67. [DOI: 10.1111/j.1365-2036.2007.03394.x; PUBMED: 17767470] [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Kirkham J, Dwan K, Kramer S, Green S, et al. Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.MR000035.pub2; MROOOO35] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantham G, Post A, Venkat D, Einstadter D, Mullen KD. A new look at precipitants of overt hepatic encephalopathy in cirrhosis. Digestive Diseases and Sciences 2017;62(8):2166‐73. [DOI: 10.1007/s10620-017-4630-y; PUBMED: 28560484] [DOI] [PubMed] [Google Scholar]
- Parsons‐Smith BG, Summerskill WH, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet 1957;270(7001):867‐71. [PUBMED: 13482229] [DOI] [PubMed] [Google Scholar]
- Poodad FF. Review article: the burden of hepatic encephalopathy. Alimentary Pharmacology & Therapeutics 2007;25(Suppl 1):3‐9. [DOI] [PubMed] [Google Scholar]
- Rahimi RS, Rockey DC. Hepatic encephalopathy: pharmacological therapies targeting ammonia. Seminars in Liver Disease 2016;36(1):48‐55. [PUBMED: 26870932] [DOI] [PubMed] [Google Scholar]
- Randolph C. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). San Antonio: The Psychological Corporation, 1998. [Google Scholar]
- Randolph C, Hilsabeck R, Kato A, Kharbanda P, Li YY, Mapelli D, et al. Neuropsychological assessment of hepatic encephalopathy: ISHEN practice guidelines. Liver International 2009;29(5):629‐35. [PUBMED: 19302444] [DOI] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Roman E, Córdoba J, Torrens M, Torras X, Villanueva C, Vargas V, et al. Minimal hepatic encephalopathy is associated with falls. American Journal of Gastroenterology 2011;106(3):476‐82. [DOI] [PubMed] [Google Scholar]
- Rose CF. Ammonia‐lowering strategies for the treatment of hepatic encephalopathy. Clinical Pharmacology and Therapeutics 2012;92(3):321‐31. [PUBMED: 22871998] [DOI] [PubMed] [Google Scholar]
- Royle P, Milne R. Literature searching for randomised controlled trials used in Cochrane Reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(1):591‐603. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Walters JR, Davies AP, Paton A. A 20‐year prospective study of cirrhosis. BMJ (Clinical Research Ed.) 1981;282(6260):263‐6. [PUBMED: 6779978] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savović J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [PUBMED: 22989478] [DOI] [PubMed] [Google Scholar]
- Savović J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [PUBMED: 22945832] [DOI] [PubMed] [Google Scholar]
- Schomerus H, Hamster W, Blunck H, Reinhard U, Mayer K, Dölle W. Latent portasystemic encephalopathy. I. Nature of cerebral functional defects and their effect on fitness to drive. Digestive Diseases and Sciences 1981;26(7):522‐30. [PUBMED: 7249898] [DOI] [PubMed] [Google Scholar]
- Schomerus H, Hamster W. Neuropsychological aspects of portal‐systemic encephalopathy. Metabolic Brain Disease 1998;13(4):361‐77. [PUBMED: 10206827] [DOI] [PubMed] [Google Scholar]
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. gdt.gradepro.org/app/handbook/handbook.html 2013 (accessed 24 January 2019).
- Sharma P, Sharma BC, Sarin SK. Predictors of non response to lactulose in patients with cirrhosis and hepatic encephalopathy. European Journal of Gastroenterology & Hepatology 2010;22(5):526‐31. [PUBMED: 20009938] [DOI] [PubMed] [Google Scholar]
- Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transplantation 2009;15(2):184‐92. [DOI] [PubMed] [Google Scholar]
- Stata Corp. Stata: Release 14. Texas, USA: Stata Corp, 2015.
- Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In‐hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clinical Gastroenterology and Hepatology 2012;10(9):1034‐41. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Malinchoc M, Kim WR, Kamath PS. Hepatic encephalopathy as a predictor of survival in patients with end‐stage liver disease. Liver Transplantation 2007;13(10):1366‐71. [DOI] [PubMed] [Google Scholar]
- Sturgeon J, Shawcross D. Recent insights into the pathogenesis of hepatic encephalopathy and treatments. Expert Review of Gastroenterology & Hepatology 2014;8(1):83‐100. [DOI] [PubMed] [Google Scholar]
- Taylor‐Robinson SD, Sargentoni J, Marcus CD, Morgan MY, Bryant DJ. Regional variations in cerebral proton spectroscopy in patients with chronic hepatic encephalopathy. Metabolic Brain Disease 1994;9(4):347‐59. [PUBMED: 7898401] [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;304(7872):81‐4. [PUBMED: 4136544] [DOI] [PubMed] [Google Scholar]
- Toris G, Bikis C, Tsourouflis G, Theocharis S. Hepatic encephalopathy: an updated approach from pathogenesis to treatment. Medical Science Monitor 2011;17(2):RA53‐63. [PUBMED: 21278704] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M, Adams RD, Cole M. The acquired (non‐Wilsonian) type of chronic hepatocerebral degeneration. Medicine 1965;44(5):345‐96. [PUBMED: 5318075] [DOI] [PubMed] [Google Scholar]
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology (Baltimore, Md.) 2014;60(2):715‐35. [PUBMED: 25042402] [DOI] [PubMed] [Google Scholar]
- Weissenborn K. Diagnosis of encephalopathy. Digestion 1998;59(Suppl 2):22‐4. [DOI] [PubMed] [Google Scholar]
- Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. Journal of Hepatology 2001;34(5):768‐73. [PUBMED: 11434627] [DOI] [PubMed] [Google Scholar]
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
- Wright G, Noiret L, Olde Damink SW, Jalan R. Interorgan ammonia metabolism in liver failure: the basis of current and future therapies. Liver International 2011;31(2):163‐75. [DOI: 10.1111/j.1478-3231.2010.02302.x; PUBMED: 20673233] [DOI] [PubMed] [Google Scholar]
- Zacharias HD, Jackson CD, Morgan MY, Olesen SS . Alimentary Pharmacology, Therapeutics. Stepwise diagnosis in covert hepatic encephalopathy – critical flicker frequency and MELD‐score as a first‐step approach. Replication and pitfalls. Alimentary Pharmacology & Therapeutics 2017;45(1):187‐9. [DOI: 10.1111/apt.13830; PUBMED: 27910151] [DOI] [PubMed] [Google Scholar]
- Zipprich A, Garcia‐Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver International 2012;32(9):1407‐14. [PUBMED: 22679906] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Zacharias HD, Zacharias AP, Oliveira Ferreira A, Morgan MY, Gluud LL. Ammonia scavenging agents for people with cirrhosis and hepatic encephalopathy. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012334] [DOI] [Google Scholar]


