Abstract
Objective. To describe pediatric Medicaid patients with pediatric emergency department (PED) visits for anaphylaxis who received epinephrine auto-injector (EAI) prescriptions in the ED versus those who did not; and to compare patients who filled their prescriptions versus those who did not. Methods. We conducted a cross-sectional study of Medicaid patients aged 0 to 21 years presenting to 2 PEDs, with symptoms meeting the National Institute of Allergy and Infectious Diseases criteria for anaphylaxis, between July 2012 and July 2014. Results. We identified 86 patients across the 2 hospitals with a confirmed diagnosis of anaphylaxis in the PED. Of these, 55 (64%, 95% confidence interval [CI] = 53% to 74%) received a prescription for an EAI during their ED visit. Forty-two (68%; 95% CI = 56% to 80%) received a prescription for EAI in Hospital 1 versus 13 (54%; 95% CI = 33% to 74%) in Hospital 2. Medicaid prescription fill rates were available for Hospital 1. Of the 42 who received an EAI prescription, 36 (86%; 95% CI = 75% to 96%) filled these prescriptions with Medicaid. Of the 20 (32%) out of 62 patients with anaphylaxis who did not receive prescriptions for an EAI, only 5 had previously filled prescriptions for epinephrine. Conclusion. Previous Medicaid patient prescription adherence data suggested that these patients would have a low EAI prescription fill rate. We found Medicaid patients who received prescriptions for an EAI after the ED visit for anaphylaxis filled them; however, a considerable proportion of anaphylaxis visits had no EAI prescription provided at discharge.
Keywords: emergency department, pediatric emergency department, food allergy and anaphylaxis network, epinephrine auto injector, national institute of allergy and infectious disease, international classification of diseases-9, electronic medical record
Introduction
Anaphylaxis is an allergic reaction that can be rapid in onset and result in death.1 Every 3 minutes a food allergy reaction sends someone to the emergency department (ED), resulting in 200 000 ED visits per year.2 The current prevalence of anaphylaxis in the general population is at least 1.6% and probably higher.3 The incidence of anaphylaxis appears to be increasing, particularly in children, adolescents, and young adults.4
Epinephrine is the only treatment shown to be effective in the management of anaphylaxis.1,5-7 In patients with known or suspected allergies at risk for anaphylaxis, it is imperative to have rapid access to a previously prescribed epinephrine auto-injector (EAI). Access to and availability of the medication can be lifesaving. An analysis of a cohort of patients with fatal anaphylaxis showed that in approximately 40% of fatal cases, epinephrine was not available at the time of the reaction. Fatalities occur when epinephrine use is delayed or not given.8 Current anaphylaxis guidelines recommend that all patients who have had anaphylaxis to a particular allergen, or who are deemed to be at risk for future anaphylaxis, be prescribed an EAI.5,6
A major barrier to completion of a medication course is obtaining the medication. Studies from pediatric EDs (PEDs) demonstrate varying rates of unfilled prescriptions from 16% to 35% and from 16% to 24% depending on the source of data, survey versus claims.9 Prior medication studies have noted that younger children are more likely to have their prescriptions filled,10 that the medication fill rates for children with private insurance are higher than for children with Medicaid coverage,10 and specifically for anaphylaxis, that there are inadequacies in prescribing of EAIs after anaphylaxis ED visits in adults.10 In Maryland alone, over half a million children were enrolled in CHIP and Medicaid in 2013 and 2014.11 Few studies have assessed the current barriers related to anaphylaxis management and treatment in the PED12,13 and no studies have evaluated barriers to anaphylaxis treatment specifically in pediatric Medicaid patients.
The main objectives of this study are to describe the EAI prescription rate and prescription fill rate in a pediatric Medicaid population after a PED visit for anaphylaxis to better understand modifiable barriers to filling EAI prescriptions. Based on previous Medicaid patient prescription adherence data, it was hypothesized that our patients would have a low EAI prescription fill rate.
Methods
We conducted a cross-sectional study of a Medicaid population of patients aged 0 to 21 years presenting to 2 PEDs (Hospital 1—an urban academic tertiary care Children’s Hospital center located in Baltimore, Maryland; and Hospital 2—an urban tertiary care Children’s Hospital, located in St. Petersburg, Florida) with symptoms meeting National Institute of Allergy and Infectious Diseases (NIAID) and Food Allergy and Anaphylaxis Network (FAAN) criteria for anaphylaxis, between July 2012 and July 2014.14 The primary outcome was the proportion of patients with anaphylaxis who received an EAI prescription. A secondary outcome was the proportion of patients who filled their EAI prescription. To explore barriers to EAI use for anaphylaxis in pediatric patients with Medicaid, comparisons were made between groups based on patient demographics, past medical history, symptoms and care prior to the ED visit, and factors related to the ED visit.
Hospital 1 ED in this study saw 31 000 patients annually over this time span, and 68% of patients were on Medicaid. Hospital 2 ED, during this period, saw 51 850 patients annually, and of these, 68.7% patients used Medicaid during their ED visit. All electronic medical records (EMRs) were reviewed by 3 study reviewers, and the initial 10% of records reviewed were compared with assess internal validity of the data abstraction forms. The EMR was initially filtered by all Medicaid patients seen over the 2-year time period from July 2012 to July 2014 (see Figure 1). Records for all Medicaid patients with the International Classification of Diseases, Ninth Revision (ICD-9), Clinical Modification, codes for anaphylaxis, allergy, and allergic reaction (995.0, 995.3, 708*, V15.06) were evaluated for data elements supporting a diagnosis of anaphylaxis per the expert panel NIAID and FAAN definitions, using a data abstraction form (Figure 1). Pediatric patients who were found to have local allergic reactions, hives, chronic urticaria, or contact dermatitis per NIAID and FAAN definitions were excluded. For patients who met the criteria for anaphylaxis and who received a prescription for an EAI from the ED, confirmation of prescription filling was obtained from state Medicaid records for Hospital 1. For patients not prescribed an EAI at the PED visit, history of filling a prior script in the last year was queried from the state Medicaid record. We were unable to obtain the Medicaid records for Hospital 2 because of the inability to obtain data from a multitiered pediatric Medicaid program within a reasonable time period.
Figure 1.
Data abstraction flow chart.
Data elements abstracted from the EMR of patients meeting the definition of anaphylaxis included the following: diagnosis; age; gender; past history of allergic reaction, past history of food and environmental allergies, anaphylaxis, asthma, or eczema; signs/symptoms leading to and at PED presentation, epinephrine administration prior to and in the ED; time to PED epinephrine administration, duration of observation in the ED; evidence of prescription for EAI on discharge; PED disposition, anaphylaxis education, and allergist referrals, and prior history of EAI use. The form used to abstract information from the EMR is included in Figure 1.
Statistical Methods
Patient PED visits for anaphylaxis to Hospital 1 (Maryland) and Hospital 2 (Florida) were described using univariate statistics such as means and proportions, where appropriate. Data elements listed above were assessed within each site to determine the statistical significance of the bivariate association of these factors to a prescription for EAI, and then to the filling of a script for an EAI. Student’s t tests were used to compare continuous valued characteristics such as child’s age, and Fisher’s exact tests to compare categorical factors of interest.
Ethical Approval and Informed Consent
This study was approved by the Johns Hopkins School of Medicine Internal Review Board (IRB), which approved data abstraction from both Hospital 1 and Hospital 2. The IRB study number was IRB00027986 (Determining the Prescription Rate and Fill Rate of Epinephrine Auto-Injectors in Medicaid Pediatric Patients Diagnosed With Anaphylaxis in the Emergency Department; PI: Jennifer Anders).
Results
At the Hospital 1, there were 250 charts reviewed for potential anaphylaxis, with 74 patients meeting the definition of anaphylaxis per criteria noted above. Of these 74, 12 were not identifiable by Maryland Medicaid, primarily due to missing identification numbers. These 12 patients did not differ demographically from the other patients (data not shown). Of the remaining 62, there were 42 (68%; 95% confidence interval [CI] = 56% to 80%) who received prescriptions for EAIs during the documented visit (Figure 2). Of these 42, 36 (86%; 95% CI = 75% to 96%) are indicated to have filled these prescriptions with Medicaid. At Hospital 2, of 599 charts reviewed, 24 PED visits with symptoms of anaphylaxis were identified. Of these 24 visits, 13 (54%; 95% CI = 33% to 74%) were discharged from the ED with a prescription for an EAI (Figure 3).
Figure 2.
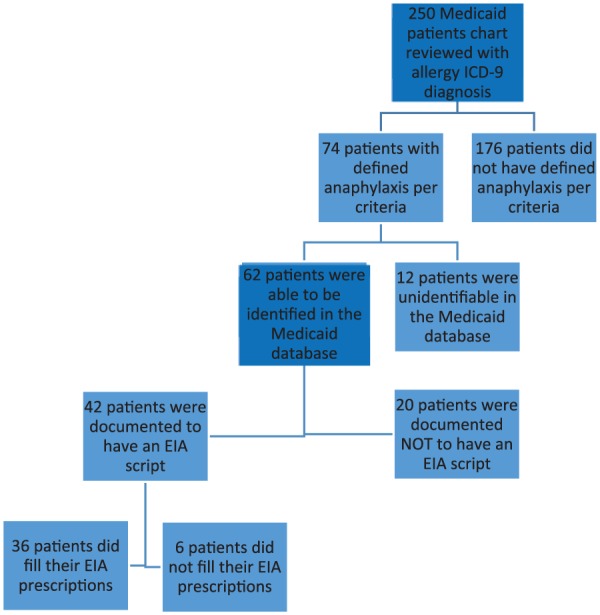
Diagram of study flow for hospital 1.
Figure 3.
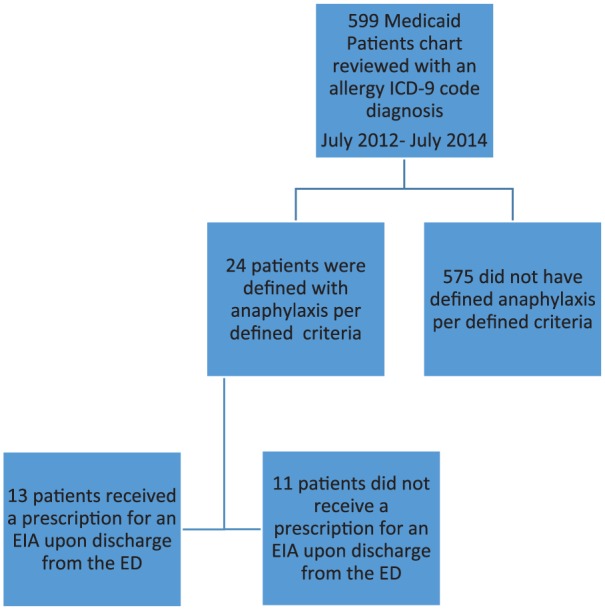
Diagram of study flow for hospital 2.
Among the 62 visits for anaphylaxis to Hospital 1, those complaining primarily of rash, hives, or itching were less likely to receive an EAI prescription (odds ratio [OR] = 0.11, P < .001). Also, those who did not have a history of food allergies were less likely to receive an EAI prescription (OR = 0.22, P = .013). Neither demographic factors nor past medical histories were related to prescription receipt in the 24 PED visits to Hospital 2 (Table 1).
Table 1.
Demographics and Medical History of Those Who Received a Prescription for an Epinephrine Auto-Injector Versus Those Who Did Not, Among Medicaid Patients Presenting With Anaphylaxis at the Pediatric Emergency Departments of Hospital 1 and Hospital 2a.
| Hospital 1 |
Hospital 2 |
|||||
|---|---|---|---|---|---|---|
| All Anaphylaxis Patients (n = 62) | Patients Receiving Rx (n = 42) | Patients Not Receiving Rx (n = 20) | All Anaphylaxis Patients (n = 24) | Patients Receiving Rx (n = 13) | Patients Not Receiving Rx (n = 11) | |
| Age in years, mean (SD) | 9.3 (6.2) | 9.3 (6.0) | 9.5 (6.6) | 8.4 (5.5) | 7.9 (4.6) | 9.0 (6.6) |
| Gender | ||||||
| Male | 31 (50%) | 24 (57%) | 7 (35%) | 13 (54%) | 7 (54%) | 6 (55%) |
| Female | 31 (50%) | 18 (43%) | 13 (65%) | 11 (46%) | 6 (46%) | 5 (45%) |
| History of food allergies* | ||||||
| No | 29 (49%) | 15 (38%) | 14 (74%) | 15 (62%) | 6 (46%) | 9 (82%) |
| Yes | 30 (51%) | 25 (63%) | 5 (26%) | 9 (38%) | 7 (54%) | 2 (18%) |
| History of allergies to medicine | ||||||
| No | 55 (92%) | 38 (93%) | 17 (89%) | 5 (21%) | 2 (15%) | 3 (27%) |
| Yes | 5 (8%) | 3 (7%) | 2 (11%) | 19 (79%) | 11 (85%) | 8 (73%) |
| History of anaphylaxis | ||||||
| No | 46 (81%) | 33 (83%) | 13 (76%) | 6 (25%) | 4 (31%) | 2 (18%) |
| Yes | 11 (19%) | 7 (17%) | 4 (24%) | 18 (75%) | 9 (69%) | 9 (82%) |
| History of asthma | ||||||
| No | 32 (54%) | 24 (59%) | 8 (44%) | 9 (38%) | 6 (46%) | 3 (27%) |
| Yes | 27 (47%) | 17 (41%) | 10 (56%) | 15 (62%) | 7 (54%) | 8 (73%) |
| History of seasonal allergies | ||||||
| No | 45 (78%) | 30 (73%) | 15 (88%) | 2 (8%) | 2 (15%) | 0 (0%) |
| Yes | 13 (22%) | 11 (27%) | 2 (12%) | 22 (92%) | 11 (85%) | 11 (100%) |
| History of eczema | ||||||
| No | 41 (71%) | 28 (68%) | 13 (76%) | 23 (96%) | 13 (100%) | 10 (91%) |
| Yes | 17 (29%) | 13 (32%) | 4 (24%) | 1 (4%) | 0 (0%) | 1 (9%) |
Significance determined by Student’s t or Fisher’s exact test.
P < .05 at Hospital 1.
Patients at Hospital 1 who received an epinephrine prescription were more likely to have received an EAI at home (OR = 9.24, P = .024) or in the ED (OR = 4.16, P = .044; Table 2). In contrast, those visiting Hospital 2 were less likely to receive a prescription if they had received an EAI in the ED (OR = 0.15, P = .047).
Table 2.
Emergency Department (ED) Course for Those Who Received a Prescription for an Epinephrine Auto-Injector Versus Those Who Did Not, Among Medicaid Patients Presenting With Anaphylaxis at the Pediatric Emergency Departments of Hospital 1 and Hospital 2.
| Hospital 1 |
Hospital 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| All Anaphylaxis Patients (n = 62) | Patients Receiving Rx (n = 42) | Patients Not Receiving Rx (n = 20) | P a | All Anaphylaxis Patients (n = 24) | Patients Receiving Rx (n = 13) | Patients Not Receiving Rx (n = 11) | P b | |
| Epinephrine at home | .024 | .813 | ||||||
| No | 47 (76%) | 28 (67%) | 19 (95%) | 18 (75%) | 9 (69%) | 9 (82%) | ||
| Yes | 15 (24%) | 14 (33%) | 1 (5%) | 6 (25%) | 4 (31%) | 2 (18%) | ||
| Epinephrine in ED | .044 | .047 | ||||||
| No | 41 (66%) | 24 (57%) | 17 (85%) | 10 (42%) | 8 (62%) | 2 (18%) | ||
| Yes | 20 (32%) | 17 (40%) | 3 (15%) | 14 (58%) | 5 (38%) | 9 (82%) | ||
| Time to Epinephrine (minutes), mean (SD) | 40.3 (22.6) | 40.7 (24.4) | 38.3 (10.2) | .789 | 28.6 (19.8) | 29.5 (21.0) | 21.0 (NA)b | 1.000 |
| Observed in ED more than 2 hours | .006* | 1.000 | ||||||
| No | 9 (15%) | 2 (5%) | 7 (35%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Yes | 53 (85%) | 40 (95%) | 13 (65%) | 24 (100%) | 13 (100%) | 11 (100%) | ||
| Time observed (hours), mean (SD) | 3.5 (2.1) | 4.1 (1.8) | 2.3 (2.1) | .002* | 8.5 (6.0) | 9.8 (5.8) | 6.9 (6.0) | .171 |
Significance determined by Student’s t or Fisher’s exact test.
Only one patient reported time to epinephrine receipt.
P < .05 at Hospital 1.
In addition, those receiving prescriptions at Hospital 1 were observed in the PED for a longer period (average 4.1 vs 2.3 hours, P = .002), while observation time was not a factor at Hospital 2.
Patients from both hospitals were unlikely to get an allergy referral even on receiving an EAI prescription (Table 3). However, most patients at both hospitals were provided with a referral to their primary care physician and educational material about anaphylaxis, with no differences in those who received a prescription compared with those who did not, at both Hospitals 1 and 2. Finally, there was no significant differences in any of these factors with regard to prescription fill rates for Hospital 1 (data not shown).
Table 3.
Emergency Department Disposition for Those Who Received a Prescription for an Epinephrine Auto-Injector Versus Those Who Did Not, Among Medicaid Patients Presenting With Anaphylaxis at the Pediatric Emergency Departments of Hospital 1 and Hospital 2a.
| Hospital 1 |
Hospital 2 |
|||||
|---|---|---|---|---|---|---|
| All Anaphylaxis Patients (n = 62) | Patients Receiving Rx (n = 42) | Patients Not Receiving Rx (n = 20) | All Anaphylaxis Patients (n = 24) | Patients Receiving Rx (n = 13) | Patients Not Receiving Rx (n = 11) | |
| Allergist referral | ||||||
| No | 52 (84%) | 34 (81%) | 18 (90%) | 19 (79%) | 12 (92%) | 7 (64%) |
| Yes | 10 (16%) | 8 (19%) | 2 (10%) | 5 (21%) | 1 (8%) | 4 (36%) |
| Primary care follow-up recommended | ||||||
| No | 10 (16%) | 4 (10%) | 6 (30%) | 5 (21%) | 2 (15%) | 3 (27%) |
| Yes | 52 (84%) | 38 (90%) | 14 (70%) | 19 (79%) | 11 (85%) | 8 (73%) |
| Education on anaphylaxis documented | ||||||
| No | 3 (5%) | 1 (2%) | 2 (10%) | 9 (38%) | 3 (23%) | 6 (55%) |
| Yes | 59 (95%) | 41 (98%) | 18 (90%) | 15 (62%) | 10 (77%) | 5 (45%) |
Significance determined by Student’s t or Fisher’s exact test.
Discussion
Despite published guidelines and research supporting the importance of epinephrine in the treatment of anaphylaxis, care remains suboptimal.1 Both patients and clinicians have poor knowledge of how and when to use an EAI, and of clinical guidelines for epinephrine use in anaphylaxis.1,12 Previous studies have shown that the majority of patients discharged after treatment for anaphylaxis in the ED did not receive an EAI prescription or allergist referral.12,13,15-18 In this study, we found that 64% of all patients studied with a diagnosis of anaphylaxis received a prescription for an EAI during their ED visit, with similar rates in Hospital 1 (68%) and Hospital 2 (54%).
Hospital 2 had more patients with the initial coding for anaphylaxis, but less patients with a confirmed diagnosis of anaphylaxis after extensive chart review. For Hospital 2 only 4% (24 out of 599 charts) of patients coded for anaphylaxis met the definition of anaphylaxis compared with 30% (74 out of 250 charts) of patients at Hospital 1. The initial ICD-9 coding for anaphylaxis for Hospital 2 was more loosely defined than Hospital 1. For Hospital 2, a large number of charts coded general rashes, contact dermatitis, and marine animal stings as an allergic reaction, and after scrutiny on chart review, these patients did not meet the definition of anaphylaxis. There was less discrepancy for Hospital 1 in coding for allergic reactions and anaphylaxis and the confirmed diagnosis of anaphylaxis. It is possible the hospital type may have affected the variation in coding. Hospital 1 is a tertiary pediatric academic center with predominantly subspecialized board-certified pediatric emergency medicine physicians, whereas Hospital 2 is a community-based pediatric tertiary care center with a combination of providers for pediatric emergency care. Provider differences at both hospitals, as highlighted above, could also account for a potential source of differences in patient care and diagnosis.
In addition to obtaining a prescription from the clinician in the ED, a second issue in ensuring availability of an EAI for future episodes of anaphylaxis relies on filling that prescription. We only had complete prescription fill data for Hospital 1, where we found that the vast majority (86%) of prescriptions were indeed filled. This is encouraging compared with prior studies of overall prescription fill rates, with one demonstrating that children with private insurance (68%) were more likely to get a prescription filled compared with patients covered by Medicaid (57%; P = .03).11 That study also noted that the overall prescription fill rate was only 65% for high-urgency prescriptions (antibiotics, respiratory medications), with fill rates in the 0- to 3-year age group (75%) significantly higher than in the rest of the cohort (55%). In addition, the study also noted, despite the absence of copayments, pickup rates were lower for Medicaid patients. We did not find any age differences but also had a very small sample of these young children.
Additional findings of note include the fact that patients at Hospital 1 who received an EAI prescription were more likely to have received epinephrine at home (OR = 9.24, P = .024) or in the ED (OR = 4.16, P = .044). In addition, those receiving prescriptions at Hospital 1 were observed for a longer period (average 4.1 vs 2.3 hours, P = .002), although observation time was similar in these patient groups at Hospital 2.
A significant limitation of this study is the retrospective design and a small number of patients. We were also unfortunately unable to examine prescription fill rate data for the Hospital 2 cohort. There was difficulty in obtaining the state Medicaid data for Hospital 2, which required access to and use of multiple state databases based on patient age and Medicaid type. We were unable to obtain these data in the time period allotted. An additional limitation noted was the inability to obtain certain demographic data from the EMR regarding race/ethnicity, socioeconomic status, and maternal education in order to acknowledge their role or possible confounding effects of the observed study associations related to prescribing practices and medication access. The strengths of the study include the strict definition of anaphylaxis that was required, as well as the detailed clinical information extracted from the record of each individual patient.
Epinephrine is the mainstay treatment of anaphylaxis. It is imperative that patients receive an EAI at the time of an anaphylactic reaction. Previous literature has shown there are numerous barriers to receiving an EAI both within and outside of the hospital. In addition, more recently, there has been a marked surge in EAI costs adding to the obstacles of anaphylaxis treatment and management. The key is to be able to identify these modifiable barriers and provide solutions to overcome these barriers. This study has reemphasized lack of provider recognition of anaphylaxis and understanding its definition as one of the important barriers to EAI use in the ED. Interventions need to focus on health provider anaphylaxis education and health provider and practice changes within the ED. Anaphylaxis recognition should begin with medical trainees with the use of education modules and simulation during as part of the educational curriculum the pre- and post-education testing to assess for anaphylaxis recognition and appropriate administration of an EAI. Use of EMR is becoming mainstay of hospital-based care, and health providers’ technology-based solutions can be used to improve provider anaphylaxis recognition. EMR anaphylaxis pathways, based on signs and symptoms consistent with expert panel definition of anaphylaxis, can be developed and used to alert providers when to use EAIs in the ED. The pediatric Medicaid population is a particularly vulnerable population in relation to access to medications and was the focus of this study.
Conclusion
Few studies have assessed the Medicaid pediatric population in regard to access and potential barriers to EAI, a critical medication. The objective of this study was to describe Medicaid pediatric patients with PED visits for anaphylaxis who received EAI prescriptions in the ED versus those who did not, to assess the rate these prescriptions were filled, and to compare patients who filled their prescriptions and those who did not. We found instead that a large majority of Medicaid patients who received prescriptions for an EAI after the ED visit for anaphylaxis filled them; however, a considerable proportion of anaphylaxis visits had no EAI prescription provided at discharge. Future studies assessing the barriers to EAI use for anaphylaxis should focus on factors related to proper prescribing practices in the PED.
Footnotes
Author Contributions: SOA: conceptualized and designed the study, designed the data collection instruments, coordinated and supervised data collection at both sites, drafted the initial manuscript, and approved the final manuscript as submitted;
OB: carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted;
JP: carried out the initial and final analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted;
MS and JA: critically reviewed and revised the manuscript, and approved the final manuscript as submitted;
RW: aided in the conceptualization and design of the study, critically reviewed and revised the manuscript, and approved the final manuscript as submitted;
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Food Allergy Research and Education (FARE), # 90059409.
ORCID iD: Sylvia Owusu-Ansah  https://orcid.org/0000-0002-8201-4267
https://orcid.org/0000-0002-8201-4267
References
- 1. Campbell R, Li J, Nicklas R, et al. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113:599-608. [DOI] [PubMed] [Google Scholar]
- 2. Clark S, Espinola J, Rudders SA, Banerji A, Camargo CA., Jr Frequency of US emergency department visits for food-related acute allergic reactions. J Allergy Clin Immunol. 2011;127:682-683. [DOI] [PubMed] [Google Scholar]
- 3. Wood RA, Camargo CA, Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:461-467. [DOI] [PubMed] [Google Scholar]
- 4. Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9-e17. [DOI] [PubMed] [Google Scholar]
- 5. Sicherer S, Simons FER; Section on Allergy and Immunology. Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017;139:e20164006. [DOI] [PubMed] [Google Scholar]
- 6. Lieberman P, Nicklas R, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.e1-e42. [DOI] [PubMed] [Google Scholar]
- 7. Kemp SF, Lockey RF, Simons FE, et al. ; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis—a statement of the World Allergy Organization. World Allergy Organ J. 2008;1(7 suppl):S18-S26. doi: 10.1186/1939-4551-1-S2-S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30:1144-1150. [DOI] [PubMed] [Google Scholar]
- 9. Zweigoron R, Binns J, Tanz R. Unfilled prescriptions in pediatric primary care. Pediatrics. 2012;130:620-626. [DOI] [PubMed] [Google Scholar]
- 10. Kajioka EH, Itoman EM, Li ML, Taira DA, Li GG, Yamamoto LG. Pediatric prescription pick-up rates after ED visits. Am J Emerg Med. 2005;23:454-458. [DOI] [PubMed] [Google Scholar]
- 11. Statistical Enrollment Data System. FY 2014 unduplicated number of children ever enrolled in Medicaid and chip. https://www.medicaid.gov/chip/downloads/fy-2014-childrens-enrollment-report.pdf. Accessed May 29, 2019. [Google Scholar]
- 12. Song TT, Worm M, Lieberman P. Anaphylaxis treatment: current barriers to adrenaline auto-injector use. Allergy. 2014;69:983-991. [DOI] [PubMed] [Google Scholar]
- 13. Russell WS, Farrar JR, Nowak R, et al. Evaluating the management of anaphylaxis in US emergency departments: guidelines vs practice. World J Emerg Med. 2013;4:98-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391-397. [DOI] [PubMed] [Google Scholar]
- 15. Johnson TL, Parker AL. Rates of retrieval of self-injectable epinephrine prescriptions: a descriptive report. Ann Allergy Asthma Immunol. 2006;97:694-697. [DOI] [PubMed] [Google Scholar]
- 16. Campbell R, Luke A, Weaver AL, et al. Prescriptions for self-injectable epinephrine and follow-up referral in emergency department patients presenting with anaphylaxis. Ann Allergy Asthma Immunol. 2008;101:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clark S, Bock SA, Gaeta TJ, Brenner BE, Cydulka RK, Camargo CA; Multicenter Airway Research Collaboration-8 Investigators. Multicenter study of emergency department visits for food allergies. J Allergy Clin Immunol. 2004;113:347-352. [DOI] [PubMed] [Google Scholar]
- 18. Clark S, Long AA, Gaeta TJ, Camargo CA. Multicenter study of emergency department visits for insect sting allergies. J Allergy Clin Immunol. 2005;116:643-649. [DOI] [PubMed] [Google Scholar]


