Abstract
WRKY transcription factor (TF) family regulates many functions in plant growth and development and also during biotic and abiotic stress. In this study, 101 WRKY TF gene models in indica and japonica rice were used to conduct evolutionary analysis, gene structure analysis, and motif composition. Co-expression analysis was carried out first by selecting the differentially expressing genes that showed a significant change in response to the pathogens from Rice Oligonucleotide Array Database (ROAD). About 82 genes showed responses to infection by Magnaporthe oryzae or Xanthomonas oryzae pv. oryzae. Co-expression gene network was constructed using direct neighborhood and context associated inbuilt mode in RiceNetv2 tool. Only 41 genes showed interaction with 2299 non-WRKY genes. Variations exist in the structure and evolution of WRKY genes among indica and japonica genotypes which have important implications in their differential roles including disease resistance. WRKY genes mediate a complex networking and co-express along with other WRKY and non-WRKY genes to mediate resistance against fungal and bacterial pathogens in rice.
Keywords: co-expression, gene structure, phylogeny, rice, WRKY transcription factor
Introduction
Transcription factors (TFs) play a key role in regulating the expression of several genes by binding to the promoter regions in turn leading to transcription. Transcription factors control various biological processes through gene regulatory network and signal transduction that results in changes in plant physiology, biochemistry, and morphology. Rice WRKY TF genes are involved in seed germination, plant growth and development, signal transduction, and metabolic processes. These participate under various biotic and abiotic stress responses such as salt, drought, freezing, blast, and leaf blight diseases. During these changes, WRKY genes are regulated by multiple factors that involve phosphorylation. A number of WRKY proteins have been reported to be phosphorylated by Mitogen-Activated Protein Kinases (MAPKs).1
WRKY TFs are defined based on the presence of the conserved DNA binding regions referred to as a WRKY domain. The WRKY domain is known to have 60 amino acids comprising a high conserved WRKYGQK sequence adjacently placed to zinc finger structures, generally C2H2 or C2HC. Depending on the number of WRKY domains and the type of zinc finger structure, the genes were classified into groups.2–4 Evolutionary analysis suggests that group I is the oldest due to the presence of 2 WRKY domains and groups II and III have originated from group I.4–7 This was further supported by a comparison between domain sequences of WRKY genes in Arabidopsis, rice group I and slime mold.4 Thereby, WRKY genes are usually classified into 3 groups.2–5,7,8 Emergence of a fourth group as a separate classified set of WRKY proteins containing a complete WRKY motif but lacking a complete zinc finger. These sequences usually represent either pseudogenes or sequencing and assembly errors.4
Variations in the WRKY domain is also reported not only in rice5 but also in other plants like the occurrence of WRKYGKK domain variant has been reported in WRKY proteins in a wide variety of plant species including physic nut,9 bread wheat,10 canola,11 tomato,12 Arabidopsis,2 soybean,13 turkey berry, and eggplant.14 This implies that the domain has undergone changes during the course of evolution. Comparison of WRKY TF gene models of both Oryza sativa subsp. indica and O sativa subsp. japonica was carried out by Ross et al4 and identified unique genes that are formed due to duplication (leading to tandem repeats) and deletion of WRKY genes which supports the conclusion drawn by Xie et al.5 Even though these genes were not evenly distributed across all the chromosomes but the densities on each of the 12 chromosomes were found similar in both the subspecies. Some of these genes were present on the chromosomes as clusters.
Analysis of rice WRKY genes has been reported.2–4 Jimmy and Babu15 enlisted 34 reports of WRKY family genes involved in biotic stress. Around 45 OsWRKYs were induced during bacterial blight and fungal blast caused by Xanthomonas oryzae pv. oryzae (Xoo) and Magnaporthe oryzae, respectively.16 Ramamoorthy et al17 have reported several OsWRKY co-regulated under hormone treatment and abiotic stress. WRKYs were reported to be differentially regulated and significantly co-express during biotic or abiotic stress conditions.3 Lee et al18 have predicted a few genes using in silico analysis and proved that a few genes co-express and therefore differentially regulate the expression of Xa21 gene. A large number of genes responsible for resistance and defense were found to be putatively co-expressed along with WRKY22.19 These examples reveal that WRKY TF family functions with a well tuned and complex network of co-expressing genes. Nevertheless, later annotations and update of genome databases indicate a variation in a number of WRKY genes in indica and japonica rice than when they were previously analyzed. According to the Plant transcription factor database v3.0, there are 111 and 129 WRKY TF gene models in indica and japonica rice, respectively. Out of these gene models, 107 and 126 sequences in indica and japonica rice, respectively, have coding regions but only 101 sequences in indica and japonica rice were classified into groups as other sequences were found to be putative gene sequences. With the objective of improving our understanding based on the updated sequence data on rice WRKY TFs, an evolutionary analysis was carried out to reveal any variations in the structure of these genes in indica and japonica rice. Establishing co-expression networks of WRKY genes were attempted to explore the possible mechanisms mediated by WRKY genes in rice disease resistance.
Materials and Methods
Data sets and sequence retrieval
Amino acid sequences of rice WRKY TF gene models (101 of both indica and japonica rice) were retrieved from the Plant Transcription Factor Database v3.0 (PlnTFDB, http://plntfdb.bio.uni-potsdam.de/).20
Classification of WRKY TF Sequences
Protein sequences of rice WRKY proteins were aligned using ClustalW method embedded in MEGA v6.0 (Molecular Evolutionary Genetics Analysis Version 6.0)21 and the classification into groups was based on the WRKY domains as in previous literature. The proteins sequences were divided into groups I, II, III, and IV. Group I was divided into 2 subgroups. Group Ia consisted of amino acid sequences that had 2 WRKY domains and C2H2 zinc fingers whereas amino acid sequences with 2 WRKY domains and C2HC zinc finger were grouped into subgroup Ib. Group II WRKY proteins contain 1 WRKY domain with C2H2 zinc fingers. Group III WRKY proteins contain 1 domain with a C2HC zinc finger. Group IV contains partial incomplete sequences that had WRKY domain but no zinc fingers.
Phylogenetic Analysis and Alignment Using MEGA v6.0
The amino acid sequences of rice WRKY genes were manually aligned in FASTA format and saved as FASTA file. The respective FASTA files of both indica and japonica rice varieties were analyzed and aligned using the ClustalW embedded in MEGA v6.0 program. The aligned sequences were saved as a MEGA file. These MEGA files were used to construct the unrooted phylogenetic tree for both indica and japonica rice using the Neighbor-joining method with a bootstrap test (1000 replicates) using MEGA.
Gene Structure Analysis
The accession IDs of the OsWRKY coding sequences belonging to japonica rice variety were obtained from the Rice Genome Annotation Project (RGAP; http://rice.plantbiology.msu.edu/).22 The exon-intron structures of these sequences were determined by manual input of accession IDs into the Gene Structure Display Server tool (GSDS v2.0).23 The format for the input was selected as GenBank Accession numbers/GI. The color and width were manually selected in the GSDS tool.
Conserved Motif Analysis of WRKY Amino Acid Sequences
The WRKY amino acid sequences were manually edited to remove the gaps and were saved as FASTA file. Sequences of both the rice varieties were separately uploaded in MEME (Multiple Expectation Maximization for Motif Elicitation) online tool (http://meme-suite.org/).24 The optimized parameters were any number of repetitions: the optimum width of each motif (between 6 and 50 residues) and maximum number of motifs (15). The output data were saved as separate files. Motif Alignment and Search Tool (MAST) program was used to search the detected motifs in protein databases.
Co-expression Network of WRKY Genes
Normalization
WRKY gene locus IDs were retrieved from the Rice Annotation Project Database25 using the “Keyword search” option to download all the available WRKY genes using “WRKY” as “Search Text.” The obtained gene locus IDs were used to download the expression profiles of WRKY genes using Rice Oligonucleotide Array Database (ROAD; http://www.ricearray.org/).26 The genes were searched for their response against the 2 pathogens: Xoo and M oryzae. Gene Expression Data (GED) data sets that had an expression for both or either of the pathogens were selected. The GED data set accession IDs were GSE7256, GSE16793, GSE18361, OS4, and GSE19844 from the selected platform Affymetrix; GSE5906, GSE6244, and GSE62444 from the selected platform Agilent22K; and GSE9653 from the selected platform NSF20K. Normalization and expression data analysis were performed as described.26
Prioritization
The corresponding MSU/TIGR gene ID of the differentially expressed genes (DEGs) among the WRKY genes obtained from ROAD were used to draw the gene network on the basis of a prioritization server for O sativa—RiceNetv2 (http://www.inetbio.org/ricenet/Network_nga_form_conv.php).27 RiceNet uses embedded Cytoscape for visualization of the network and provides searching and analyzing information on the molecular interactions around a set of genes. Prioritization was obtained on the basis of both network direct neighborhood and context associated hubs. The locus IDs of the nodes (genes) from the gene prioritization on the basis of the direct neighborhood network and the context associated hubs obtained from RiceNet v2 were used to derive gene descriptions and functions from the RAP-DB through “batch retrieval” option.
Results
Indica and japonica rice genotypes vary in their WRKY gene groups
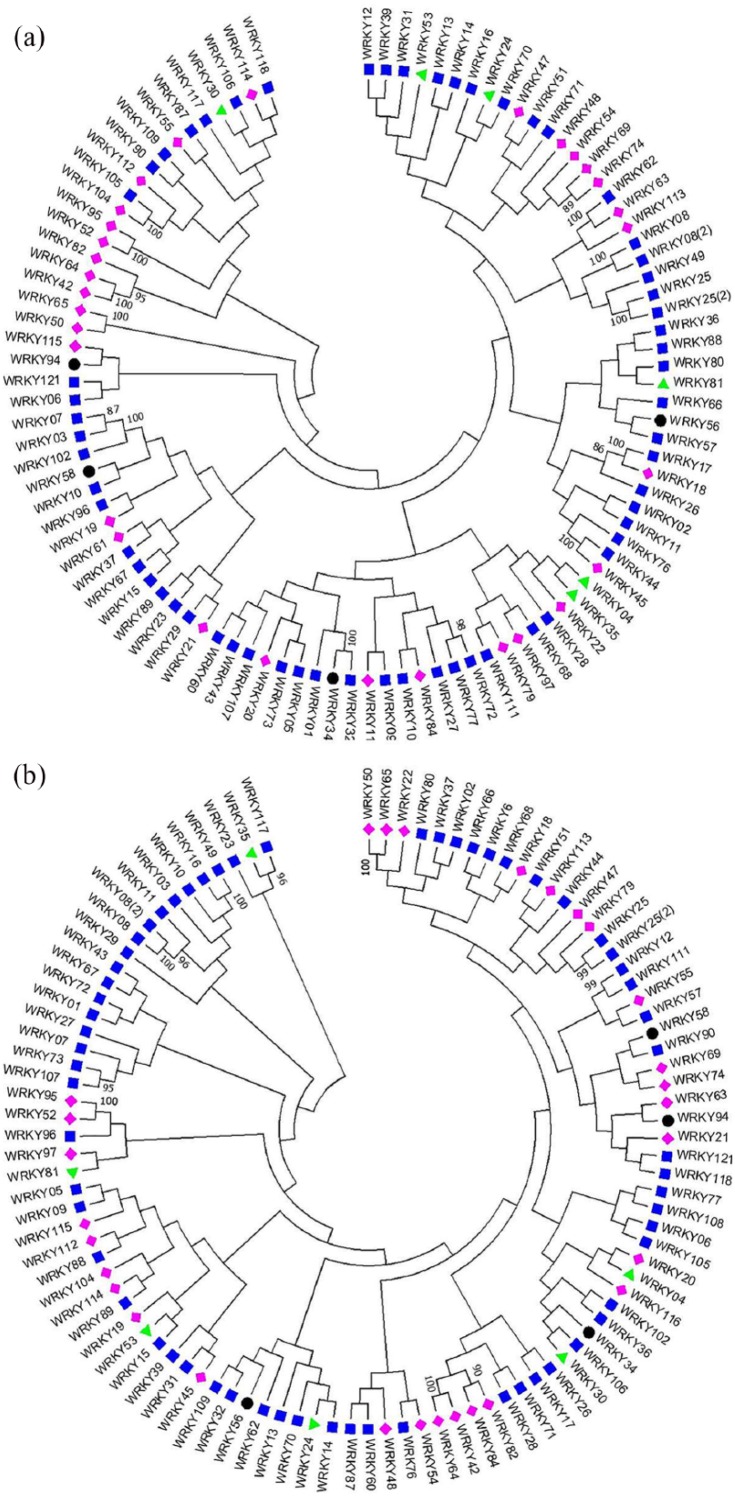
WRKY genes were found to be unevenly distributed in the chromosomes of indica and japonica (Supplementary Table S1). Annotations of known japonica rice WRKY sequences are according to the nomenclature as provided by the Rice WRKY Working Group.6 Phylogenetic trees of WRKY TF gene models and protein sequences of both indica and japonica rice are shown in Figure 1. Amino acid sequences were further classified into 4 main phylogenetic groups to facilitate the analysis of rice WRKY gene evolution. Group I was divided into Ia and Ib based on the fact that the zinc finger motif in both the N- and C-terminal WRKY domain in Ia genes are of C2H2 type; however, in group Ib it is of C2HC type. C-terminal of the group Ia genes contain a conserved intron in the region encoding WRKY domain but in group Ib, both the N- and C-terminal WRKY domains contain a conserved intron.
Figure 1.
Neighbor-joining bootstrap consensus tree of amino acid sequences of rice WRKY TF sequences: (A) WRKY proteins in indica rice; (B) WRKY proteins in japonica rice. The phylogenetic trees were constructed using neighbor-joining method with bootstrap test (1000 replicates) in MEGA6.
The colors show the groups: green triangle (group Ia), green triangle-unfilled (group Ib), blue rectangles (group II), pink diamonds (group III), black circles (group IV). Sequences with numbers in the parentheses represents duplicated genes. Bootstrap values above 70% are given on the tree. Numbers in parentheses are assigned by MEGA software for the duplicated genes appearing in the both rice genome.
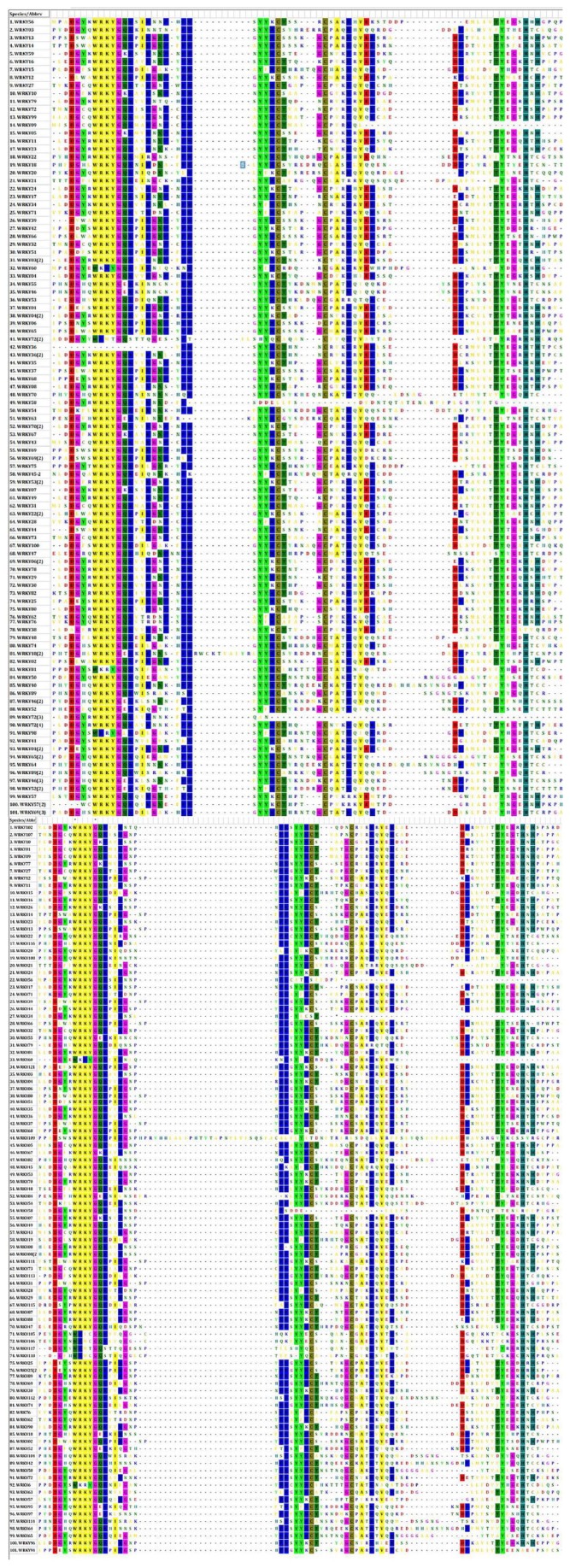
Alignment of 101 amino acid sequences of both indica and japonica WRKY proteins exhibit a prominent feature of the WRKY domain, which was a heptapeptide (WRKYGQK) motif at the N-terminus followed by a zinc finger motif (Figure 2). Some of the motifs exhibit variations such as WRKYGKK, WRKYGEK, WKKYGQK, and WRMCGQK as observed in both indica and japonica. In addition, WRMTGQS was unique to indica; however, WRICGQK, WRMTGQS, and WRMCGQS were observed only in japonica (Table 1). Such variants were not reported earlier. Our observations are more up to date as the sequences were retrieved a decade later, compared with the existing reference analysis of Xie et al.5 Alignment of indica and japonica rice amino acid sequences revealed that group Ib genes had evolved into group II completely in both the genotypes. Group I in both the genotypes were identified by the presence of 2 domains and C2H2 zinc finger. Group II contains 1 single WRKY domain and C2H2 zinc finger. Group III had a single domain and a C2HC zinc finger. Group IV contains partial sequence with and without WRKY domain or zinc finger. List of genes divided into each group is given in Supplementary Tables S2 and S3 for indica and japonica rice, respectively. Number of WRKY genes classified into each groups and subgroups in indica and japonica rice is given in Table 2.
Figure 2.
Amino acid sequence alignment of WRKY proteins of indica and japonica rice: (A) indica rice and (B) japonica rice.
The highlighted lines represent the highly conserved sequences of WRKY family. The sequences were aligned by ClustalW in MEGA6 and the conserved amino acid residues were determined by inbuilt toggle conserved sites option. Residues that are highly conserved within each of the groups are shown in colors. WRKY domain was highlighted in yellow.
Table 1.
Variants of the WRKYGQK amino acid residues in indica and japonica rice.
| Variants | Indica rice | Japonica rice |
|---|---|---|
| WRKYGKK | WRKY59, WRKY79 | WRKY10, WRKY77, WRKY26, WRKY67, WRKY67.2 |
| WRKYGEK | WRKY55, WRKY46, WRKY52, WRKY57 | WRKY55, WRKY55.2, WRKY84, WRKY07, WRKY18, WRKY52, WRKY46, WRKY46.2, WRKY97.2 |
| WKKYGQK | WRKY60 | WRKY61, WRKY106, WRKY117, WRKY118 |
| WRICGQK | – | WRKY105 |
| WRMCGQK | WRKY80 | WRKY106 |
| WRMTGQS | – | WRKY117 |
| WRMCGQS | – | WRKY118 |
| WRMTGQS | WRKY72 | – |
Table 2.
Number of WRKY genes from indica and japonica in each group and subgroup.
| Group/sub group | Gene number |
||
|---|---|---|---|
| Japonica rice (Wu et al2) | Indica rice (present study) | Japonica rice (present study) | |
| Ia | 14 | 11 | 6 |
| Ib | 20 | 01 | – |
| II | 30 | 54 | 61 |
| III | 36 | 31 | 30 |
| IV | – | 04 | 4 |
| Total | 100 | 101 | 101 |
Structure of WRKY Genes: Similarities and Differences Among Indica and Japonica Genotypes
Multiple Expectation Maximization for Motif Elicitation online tool24 helped in identifying 15 motifs in the amino acid sequences of WRKY proteins in indica and japonica rice. Motif boxes identified in WRKY protein sequences are given in Supplementary Tables S4 and S5. Motifs 1, 2, 3, 4, and 5 (indica) and motifs 1, 2, 3, 4, and 6 (japonica) were characterized as WRKY domains (Supplementary Figures S1 and S2). WRKY members within the same subgroups were found to share a similar motif composition. In the amino acid sequences of indica rice WRKYs, motif 9 and 15 are unique to the group Ia. Motifs 12, 13, and 14 are found in the entire group I members. Motif 8 was observed in group Ib and in all the members of group II; however, motif 7 was observed in group Ia and only a few members of group II. In japonica rice, motifs 5, 7, 8, 9, and 14 are unique to group I. Motif 12 was found in all members of the group I and 2. Motif 11 was found in all members of group II. Motif 10 and 15 show exceptions in their occurrence. Motif 10 was found only in 3 members of group I and only in 1 member of group IV.
Exon-intron structure analysis was carried out using the GSDS tool to gain an insight into the evolution of WRKY genes of japonica rice (Supplementary Figure S3). This was not done in indica rice genes as accession numbers were not available. Out of the 101 WRKY genes in japonica rice as per the PTFD database, accession numbers of only 76 genes were available in RGAP database. Most WRKY genes had 2 introns. Out of 76 genes, 39 had 2 introns, 13 had 1 intron, 7 had 3 introns, 6 had 4 introns, 7 had 5 introns, and 1 had 8 introns. Three of the WRKY genes had no intron. Most genes showed introns at the 3′ end of the genes. Large uninterrupted introns were observed in 15 genes at the 3′ end, whereas uninterrupted introns at the 5′ end were observed in WRKY18, WRKY28, and WRKY49.
WRKY Genes Co-express With Other WRKY and Non-WRKY Genes to Mediate Disease Resistance in Rice
To analyze the response of WRKY genes to pathogen infection (M oryzae and Xoo), microarray data were collected from ROAD database.26 The genes which showed a significant change (fold change ⩽0.5 or ⩾2) in response to the pathogens were selected. Analysis of the microarray data revealed 82 out of 110 WRKY genes that showed responses to infection by M oryzae or Xoo. The heat maps of the differentially expressing WRKY genes were from 3 different platforms: Affymetrix, Agilent 22K, and NSF 20K. Three platforms were selected to select DEGs that should be pathogen-responsive as well as disease resistance genes that already exist in ROAD. Supplementary Figure S4 represents heat maps for 3 different experiments under Affymetrix platform: (1) GSE19844—transcriptomics-based identification of Xoo strain BAI3 Talc targets in rice; (2) GSE18361 Rice root infected with M oryzae strain Guy11; and (3) GSE7256—Virulent infection by M oryzae. Supplementary Figures S5 and S6 represents heat map GSE16793—Rice undergoing infection by Xoo or by Xanthomonas oryzae pv. oryzicola (Xoc) using 60 samples and OS4—Rice cultivars IRBB7, IRBB5, and IR24 undergoing infection by Xoo. Supplementary Figure S7 represents 3 heat maps for Agilent22K: (1) GSE6244—indica rice cultivar IET8585 (Ajaya) infected with Xoo; (2) GSE5906—bacterial lipopolysaccharides induce defense responses; (3) GSE8518—biotrophic invasion by M oryzae. Supplementary Figure S8 represents 1 NSF20K heatmap for array data for rice to identify gene to dissect rice defense response pathways. The total number of arrays considered from all 3 platforms was 270 (105 from japonica, 51 from indica, and 114 from mixed samples).
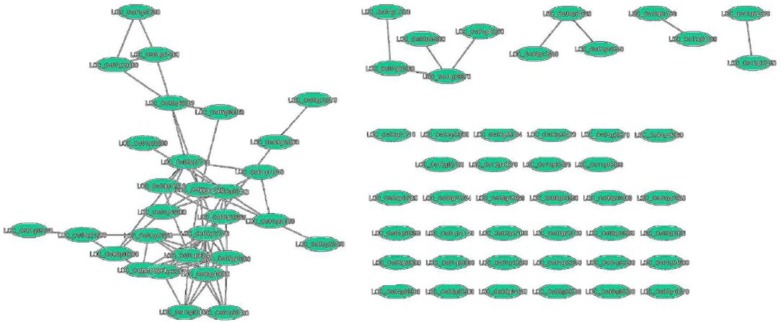
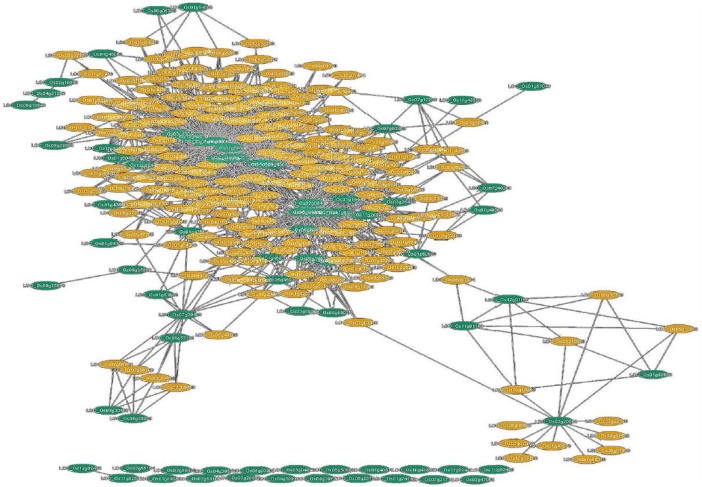
Co-expression of WRKY genes with other genes in rice genome that have significant changes in their expression in response to pathogens were used to construct the expression network using RiceNet tool.18,27 The network provided the likelihood (designed log network score, LLS) of the genes that are participating in the conditioned process in each data set. Bootstrapping value for all LLS evaluations was 0.632.18,27 The backbone of the network was formed by the guide genes. These are DEGs that were obtained from ROAD database that were also observed to be linked to one another. WRKY TF networking genes (top 100 genes) in mediating disease resistance is given in Supplementary Table S6. In the co-expression network, the input WRKY genes are the guide genes and henceforth referred to as guide WRKY genes. Interaction among the guide WRKY gene was observed for 41 genes out of 82 guide WRKY genes (Figure 3 and Supplementary Table S7). These 41 genes co-expressed along with 2299 non-WRKY genes. As depicting a large number of genes in the Cytoscape was difficult, about 100 genes co-expressing along with the 41 guide WRKY genes are shown in Figure 4. Out of the 41 guide WRKY genes, 14 were already reported to be involved in resistance to both fungal and bacterial pathogens.15 WRKY64, WRKY62, and WRKY77 were reported for resistance against bacterial pathogens, whereas WRKY22, WRKY24, WRKY69, WRKY74, WRKY108, and WRKY113 were reported for resistance against fungal pathogens. WRKY28, WRKY45, WRKY53, WRKY71, and WRKY76 were reported for their role in resistance to both bacterial and fungal pathogens.15 In total, 31 guide WRKY genes were found to be disconnected in the network. This indicates that these genes do not take part in the main network but may have individual networks that contribute to disease resistance in rice. Out of the 31 disconnected genes (Supplementary Table S8), 10 were known for their involvement in rice disease resistance. Out of the 82 input genes, 7 were not found in RiceNet but were found to be valid genes (Supplementary Table S9). The co-expression network revealed that each non-WRKY gene was connected to at least 3 to 5 guide WRKY genes. The co-expressed genes are known to be involved in physiology, pathogen resistance, negative regulation, and defense responses similar to that of the WRKY genes. The result obtained was represented using Figure 4 and Supplementary Table S10 that only represents 100 out of 2299 genes in the Cytoscape plugin. Network analysis indicated WRKY07, WRKY24, WRKY28, WRKY53, WRKY71, and WRKY108 were linked with OsMSRMK2 (multiple stress-responsive MAP kinase genes 2). Perforin protein was found linked with WRKY28, WRKY51, WRKY53, and WRKY71; bZIP50 linked with WRKY15, WRKY53, WRKY71, and WRKY113; OsMKK6 linked with WRKY71; Patatin-related phospholipase A II eta (OspPLAIIeta) linked with WRKY53; Phytoalexin-deficient 4 (OsPAD4) linked with WRKY53 and NBS-LRR (Nucleotide Binding Sites–Leucine Rich Repeat) linked with WRKY74.
Figure 3.
Guide genes that are connected to one another. Guide genes are also called as input genes. These genes help in determining the co-expressing genes.
Genes appearing continuous are disconnected from the guide genes.
Figure 4.
Co-expression network map of WRKY genes. Constructed using direct neighborhood method.
Green oval symbol represents guide genes and orange oval symbol represents co-expressing genes.
Descriptions of all the 2299 genes that interact along with guide WRKY genes were found using RAP-DB database and were classified depending on their interaction with the guide WRKY genes. The co-expressing 2299 genes were sorted based on their functions provided by the RiceNet tool. These genes were sorted into those involved in defense, negative regulation, pathogen resistance, hormone-responsive genes, MAPK responsive genes, physiological response, and unknown function (Supplementary Figure S9 and S10). This suggests that WRKY TF mediate a complex networking among them as well as the co-expressing non-WRKY gene toward disease resistance.
Context associated hubs involve the use of DEGs as input. This involves the study of central hubs and their neighbors that form subnetwork which was associated with the stress context. The context associated hubs are based on the guide genes that may or may not be connected with each other. The genes of the central hubs may or may not be DEGs. The same 82 guide WRKY genes (DEGs) were used to determine the central hubs. This analysis resulted in 100 co-expressing genes which were recognized to form a subnetwork with a positive P value (Supplementary Table S11). Out of these 100 co-expressing genes, 10 genes were the input DEG. The genes that formed a subnetwork to these DEG were found to be either transcription factors or defense genes. The context associated hubs are DEG dependent. Each DEG/set of DEGs will reveal a subnetwork of genes that form a hub. A single subnetwork gene in the hub was represented in the figure (Supplementary Figure S11).
Discussion
Oryza sativa L. is a model organism containing the smallest genome. Oryza sativa subsp. japonica rice contains a haploid chromosome number of 12, containing 370 Mb with 30 000 protein-coding genes,28 whereas subsp. indica rice contains a haploid chromosome number of 12, containing 466 Mb with 46 022 to 55 615 coding genes.29 The average number of exons and introns per gene are same in indica and japonica sequences.30 Chromosome 4 is larger in the case of japonica rice than that of indica because of expansion of japonica sequence by insertions. Khush31 stated that Oryza nivara populations are of polyphyletic origin from both indica and japonica rice which helps to conclude that japonica might have evolved earlier than indica as more insertions have been added to the chromosomes of japonica. Comparatively, the number of stress-responsive genes are more in japonica rice (1716) than indica rice (1118)32 but indica rice is better resistant to diseases than japonica rice.33
The WRKY genes were divided into 4 groups unlike the previous grouping carried out.2–5 This was due to the fact that some of the sequences were partial which are grouped in group IV. The number of genes in group I reduced due to gene loss, and the number of genes in group II and III have increased due to gene duplication. Gene loss was evident from the number of variants that exist in a group. Comparison of the amino acid sequences of indica and japonica rice has helped to report mutational changes giving rise to a greater number of variants in the domain structures in the family (Table 2) than already reported.2–5 Such variants were observed more in indica than in japonica rice and maximum in group I members. Gene loss in group I members caused no reduction in the number of WRKY family members as gene duplication observed in group II and group III has compensated. One WRKY gene in indica was grouped into subgroup Ib, whereas in japonica rice, no WRKY gene containing C2HC zinc finger was found. Gene structure analysis revealed that genes of the same group exhibit similar intron-exon patterns at least for the known genes obtained in the RAP-DB database.
Phylogenetic trees lead to grouping based on zinc finger-type and domain-type exhibited by rice WRKY TFs. Group I members were found to be placed within group II members in both indica and japonica. Xie et al5 found group Ib genes embedded within the group III, thereby inferring that group Ib gave rise to group III. Based on the amino acid sequences of WRKY proteins in japonica rice, members belonging to group I was found closely associated with a group III member. In indica rice, group I genes are closely placed with group II genes. This indicated that group II would have originated from group I in indica rice due to the loss of N-terminus. Similarly, in japonica rice, group III would have originated from group II. Group IV are rarely reported and they contain partial sequences.1,5 These results follow the same lines to that reported by Ross et al.4 The domain duplication scenario in WRKY sequences was reported to explain the evolution of group I genes from either C2H2-C2H2 or C2HC-C2HC type of zinc finger. This was reported on the basis that no C2H2-C2HC or C2HC-C2H2 type of zinc finger was found in WRKY amino acid sequences. This also indicated group Ib evolved from group III WRKY domain that had the C2HC type of zinc finger due to intramolecular duplication. Similarly, group II gene evolution was based on the domain feature loss and gain of the WRKY domain. Group II genes are known to have evolved from group Ia due to the loss of N-terminus but independent of the loss of C-terminus.5
Gene duplications, recombination, and sequence divergence have long been considered as the main sources of evolutionary momentum. On the basis of the phylogenetic analysis, WRKY genes such as WRKY93 and WRKY94 genes are considered as duplicated genes. Each of these genes contains 2 WRKY domains, which are generated probably due to the domain duplication clustered in the same clade in subgroup IIIa. Wu et al2 reported tandem duplication events occurred in the WRKY gene arrangements in O nivara which were also observed in other plants. Similarly, Ross et al4 and Li et al34 proposed gene duplication and loss that caused an increase in the number and density of WRKY TFs. The motifs that were found using the MEME software were predicted motifs that may be responsible for acting as binding site of many regulatory proteins. Most motifs may be related to the positive or negative regulation of the WRKY TFs. Therefore, these motifs are directly related to the regulation of the expression of the WRKY genes during various physiological changes. Two short sequences such as PTDDS and EDLEEK in the N-terminus of WRKY62 was required for its repressor activity in plants. They represent new types of repression motifs.35 This may be related to the reported negative feedback regulation of the IIa subfamily of WRKYs but also provide evidence for alternative splicing of WRKY TFs during the plant defense response.35 Therefore, the group IIb members out of all the WRKY networking genes may be predicted to be involved in a network as a repressor of other proteins or in negative feedback mechanism in defense response.
WRKY TF gene network regulates via complex networking of genes in response to biotic stress. This involves a series of signal transduction of genes through TFs or other proteins. In this study, 82 WRKY TFs were found interacting with other genes to modulate biotic stress resistance. Network analysis indicated that certain WRKYs co-express along with other genes involved in MAPK cascade pathway. It was already known that a number of WRKY proteins have been reported to be phosphorylated by MAPKs. The MAPK cascade plays an important role in regulating various responses and also regulates the intra- and extra-cellular signaling in plants.36,37 Therefore, it was predicted that WRKY TFs co-expressed with genes involved in MAPK cascade that were the core genes that get activated first during a pathogen attack. Tracing the genes that were connected to these core WRKY genes, the network by which WRKY TFs activates other proteins were predicted. According to the Gramene database,38 these genes also have physiological response functions. WRKY TFs on activation further activates a deep transcriptional reprogramming of the cell that involves defense genes such as genes for secondary metabolites (phytoalexins like momilactones, phenylamides), pathogenesis-related (PR) proteins (PR3—chitinase 11, chitinase 6, chitinase 10; PR4c, PR10a, PR10b; and root-specific PR10), cell wall strengthening proteins (callose, pectin, lignin, and cutin), and programmed cell death and hypersensitive reactions. These are the genes that are usually present downstream to the WRKY genes.
It was observed that WRKY53 co-expressed with β-1,3-glucan and glucan endo-1,3-β-glucosidase GII precursor. β-1,3-glucan was responsible for callose deposition on the plant cell wall39 and glucan endo-1,3-β-glucosidase GII precursor was a cell wall-associated gene.40 Other TFs such as NAC, ERF, bZIP, and MYB are observed to co-express along with WRKY TFs. Plant resistance (R) genes such as those protein products containing nucleotide-binding sites and NBS-LRR motifs were observed to co-express along with certain WRKY TFs. Pathogenesis-related protein genes were also found as the hub genes when the WRKY genes were used as input for the context associated hub analysis. Hubs constitute genes that consist of neighbors that have significant overlap to the DEG used as input to search the subnetwork (hub) forming genes.18,27 It was predicted that if a central hub gene of a subnetwork modulates stress response in this case disease resistance, many of its neighboring subnetwork genes could be DEGs. Our analyses indicated jumonji 709, fatty acid elongase, increased leaf inclination 7, VQ motif-containing protein 13, ZOS1-14-C2H2 zinc finger protein, Lil3 protein, TMV (tobacco mosaic virus) response related protein overlap with the direct neighborhood network. Therefore, these genes are predicted to be involved in disease resistance.
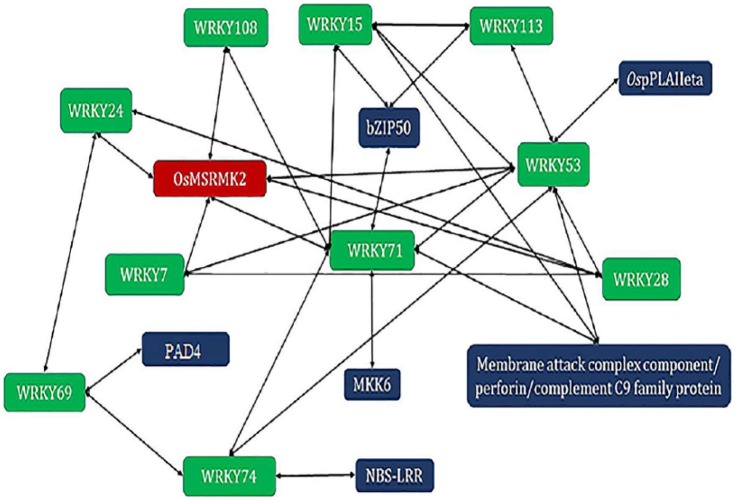
Cell wall strengthening provides mechanical defense against a pathogen that ramifies in the plant tissues. Callose deposition mainly defends against the invading pathogens41 followed by pectin,42 lignin,43 and cutin44 biosynthesis. NAC6 was reported to be up-regulated along with Pi54 gene over-expressed transgenic rice plants that confer resistance to M oryzae.42 A large number of R genes have a role in disease resistance in rice. ERF genes are also found to be expressed due to pathogenic infection in rice45 along with other TFs such as bZIP genes.46 PR1a was already reported to be induced by WRKY12 gene during SA and bacterial elicitor in the tobacco plant.47 PR1b was found to be expressed during the over-expression of WRKY03 in salicylic acid (SA)-dependent or jasmonic acid (JA)-dependent defense signaling cascades.48 Allelic genes WRKY45-1 and WRKY45-2 were reported to act antagonistically during the accumulation of signaling molecules (SA and JA) during pathogen attack.49 WRKY13 was found as a disconnected gene but it was found to interact with the core genes WRKY71 and WRKY28 via TIFY9. WRKY13 gene was reported to act as an activator of the SA-dependent pathway and a suppressor of the JA-dependent pathway50 and works against both bacterial and fungal pathogen.15 TIFY9 in rice performs role in JA biosynthetic process, responds to ethylene, auxin, abscisic acid, JA, and wounding.38 WRKY13 also binds to another TF gene, bZIP37 that also interacts in MAPK cascade as illustrated (Figure 5). bZIP37 is involved in ethylene biosynthetic process. It also responds to abscisic acid, SA and JA signaling and expressed during hypersensitive response.38 These could be candidate genes for developing multiple disease resistant rice varieties through molecular breeding/transgenic approaches. Conclusively, we observed variations exist in the structure and evolution of WRKY genes among indica and japonica genotypes, which have important implications in their differential roles including disease resistance. WRKY genes mediate complex networking and co-express along with other WRKY and non-WRKY genes to mediate resistance against fungal and bacterial pathogens in rice.
Figure 5.
Core WRKY genes co-expressing with other genes involved in MAPK cascade.
Green rounded rectangles: WRKY TF genes; blue rounded rectangles: genes involved in MAPK cascade; red rounded rectangle: gene involved in activation. Green rounded rectangles: WRKY TF genes. WRKY genes are phosphorylated by MAPKs thereby leads to the activation of other genes, thus forming a network. The members of the WRKY family already reported to play a role in disease resistance in rice are used to built this network.
Supplemental Material
Supplemental material, Combined_pdf_-_Supplementary_Figures_xyz17870f46ce8b9 for Variations in the Structure and Evolution of Rice WRKY Genes in Indica and Japonica Genotypes and their Co-expression Network in Mediating Disease Resistance by John Lilly Jimmy and Subramanian Babu in Evolutionary Bioinformatics
Supplemental Material
Supplemental material, Jimmy_Manuscript_-_EB_-_Supplementary_Tables_xyz178700578937f for Variations in the Structure and Evolution of Rice WRKY Genes in Indica and Japonica Genotypes and their Co-expression Network in Mediating Disease Resistance by John Lilly Jimmy and Subramanian Babu in Evolutionary Bioinformatics
Acknowledgments
The authors sincerely acknowledge the support rendered by the Vellore Institute of Technology, Vellore, in carrying out our research.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: JJ obtained the data from the databases, performed all the data analysis, prepared the results, and contributed in writing the first draft of the manuscript. SB conceived the concept, planned the work, contributed to result interpretation and manuscript writing.
Availability of Data and Materials: All data used in the study are retrieved from publicly available databases. Plant Transcription Factor Database v3.0 (PTFD; http://planttfdb.cbi.pku.edu.cn/), Rice Genome Annotation Project (RGAP; http://rice.plantbiology.msu.edu/), Rice Annotation Project Database (RAP-DB; http://rapdb.dna.affrc.go.jp/gbl/), and Rice Oligonucleotide Array Database (ROAD; http://www.ricearray.org/) are the databases used.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Subramanian Babu  https://orcid.org/0000-0003-2781-7136
https://orcid.org/0000-0003-2781-7136
References
- 1. Xu H, Watanabe KA, Zhang L, Shen QJ. WRKY transcription factor genes in wild rice Oryza nivara. DNA Res. 2016;23:311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu KL, Guo ZJ, Wang HH, Li J. The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 2005;12:9–26. [DOI] [PubMed] [Google Scholar]
- 3. Berri S, Abbruscato P, Faivre-Rampant O, et al. Characterization of WRKY co-regulatory networks in rice and Arabidopsis. BMC Plant Biol. 2009;9:120. doi: 10.1186/1471-2229-9-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ross CA, Liu Y, Shen QJ. The WRKY gene family in rice (Oryza sativa). J Integr Plant Biol. 2007;49:827–842. doi: 10.1111/j.1744-7909.2007.00504.x. [DOI] [Google Scholar]
- 5. Xie Z, Zhang ZL, Zou Z, et al. Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 2005;137:176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rice WRKY Working Group. Nomenclature report on rice WRKY’s—conflict regarding gene names and its solution. Rice. 2012;5:3. doi: 10.1186/1939-8433-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ulker B, Somssich IE. WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol. 2004;7:491–498. [DOI] [PubMed] [Google Scholar]
- 8. Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. [DOI] [PubMed] [Google Scholar]
- 9. Xiong W, Xu X, Zhang L, et al. Genome-wide analysis of the WRKY gene family in physic nut (Jatropha curcas L.). Gene. 2013;524:124–132. [DOI] [PubMed] [Google Scholar]
- 10. Okay S, Derelli E, Unver T. Transcriptome-wide identification of bread wheat WRKY transcription factors in response to drought stress. Mol Genet Genomics. 2014;289:765–781. [DOI] [PubMed] [Google Scholar]
- 11. Yang B, Jiang Y, Rahman MH, Deyholos MK, Kav NN. Identification and expression analysis of WRKY transcription factor genes in canola (Brassica napus L.) in response to fungal pathogens and hormone treatments. BMC Plant Biol. 2009;9:68. doi: 10.1186/1471-2229-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang S, Gao Y, Liu J, et al. Genome-wise analysis of WRKY transcription factors in Solanum lycopersicum. Mol Genet Genomics. 2012;287:495–513. [DOI] [PubMed] [Google Scholar]
- 13. Yin G, Xu H, Xiao S, et al. The large soybean (Glycine max) WRKY TF family expanded by segmental duplication events and subsequent divergent selection among subgroups. BMC Plant Biol. 2013;13:148. doi: 10.1186/1471-2229-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang X, Deng C, Zhang Y, Cheng Y, Huo Q, Xue L. The WRKY transcription factor genes in eggplant (Solanum melongena L.) and Turkey berry (Solanum torvum Sw.). Int J Mol Sci. 2015;16:7608–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jimmy JL, Babu S. Role of OsWRKY transcription factors in rice disease resistance. Trop Plant Pathol. 2015;1:355–361. [Google Scholar]
- 16. Ryu H, Han M, Lee S, et al. A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 2006;25:836–847. [DOI] [PubMed] [Google Scholar]
- 17. Ramamoorthy R, Jiang S-Y, Kumar N, Venkatesh PN, Ramachandran S. A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol. 2008;49:865–879. [DOI] [PubMed] [Google Scholar]
- 18. Lee I, Seo YS, Coltrane D, et al. Genetic dissection of the biotic stress response using a genome-scale gene network for rice. Proc Natl Acad Sci U S A. 2011;108:18548–18553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abbruscato P, Nepusz T, Mizzi L, et al. OsWRKY22, a monocot WRKY gene, plays a role in the resistance response to blast. Mol Plant Pathol. 2012;13:828–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin JP, Zhang H, Kong L, Gao G, Luo JC. PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2014;42:D1182–D1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawahara Y, de la Bastide M, Hamilton JP, et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice (N Y). 2013;6:4. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31:1296–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bailey TL, Boden M, Buske FA, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202-W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakai H, Lee SS, Tanaka T, et al. Rice Annotation Project Database (RAP-DB): an integrative and interactive database for rice genomics. Plant Cell Physiol. 2013;54:e6. doi: 10.1093/pcp/pcs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao P, Jung KH, Choi D, Hwang D, Zhu J, Ronald PC. The rice oligonucleotide array database: an atlas of rice gene expression. Rice (N Y). 2012;5:17. doi: 10.1186/1939-8433-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee T, Oh T, Yang S, et al. RiceNet v2: an improved network prioritization server for rice genes. Nucleic Acids Res. 2015;43:W122–W127. doi: 10.1093/nar/gkv253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. UniProt Consortium. UniProt: a hub for protein information. Nucleic Acids Res. 2015;43:D204–D212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu J, Hu S, Wang J, et al. A draft sequence of the rice genome (Oryza sativa L. Ssp. Indica). Science. 2002;296:79–92. [DOI] [PubMed] [Google Scholar]
- 30. Han B, Xue Y. Genome-wide intraspecific DNA-sequence variations in rice. Curr Opin Plant Biol. 2003;6:134–138. [DOI] [PubMed] [Google Scholar]
- 31. Khush GS. Origin, dispersal, cultivation and variation of rice. Plant Mol Biol. 1997;35:25–34. [PubMed] [Google Scholar]
- 32. Shameer K, Ambika S, Varghese SM, Karaba Udayakumar M, Sowdhamini R. STIFDB-arabidopsis stress responsive transcription factor data base. Int J Plant Genomics. 2009;2009:583429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Visser M, Isaacs N. Much Depends on Dinner: The Extraordinary History and Mythology, Allures and Obsession, Perils and Taboos of an Ordinary Meal. New York, NY: Grove Atlantic; 1986. [Google Scholar]
- 34. Li M-Y, Xu Z-S, Tian C, Huang Y, Wang F, Xiong A-S. Genomic identification of WRKY transcription factors in carrot (Daucus carota) and analysis of evolution and homologous groups for plants. Sci Rep. 2016;6:23101. doi: 10.1038/srep23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu J, Chen X, Liang X, et al. Alternative splicing of Rice WRKY62 and WRKY76 transcription factor genes in pathogen defense. Plant Physiol. 2016;171:1427–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar K, Sinha AK. Overexpression of constitutively active mitogen activated protein kinase kinase 6 enhances tolerance to salt stress in rice. Rice (N Y). 2013;6:25. doi: 10.1186/1939-8433-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adachi H, Nakano T, Miyagawa N, et al. WRKY transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. Plant Cell. 2015;27:2645–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tello-Ruiz MK, Stein J, Wei S, et al. Gramene 2016: comparative plant genomics and pathway resources. Nucleic Acids Res. 2016;44:D1133–D1140. doi: 10.1093/nar/gkv1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gupta SK, Rai AK, Kanwar SS, Chand D, Singh NK, Sharma TR. The single functional blast resistance gene Pi54 activates a complex defence mechanism in rice. J Exp Bot. 2012;63:757–772. [DOI] [PubMed] [Google Scholar]
- 40. Ricardi MM, Gonzalez RM, Zhong S, et al. Genome-wide data (ChIP-seq) enabled identification of cell wall-related and aquaporin genes as targets of tomato ASR1, a drought stress-responsive transcription factor. BMC Plant Biol. 2014;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Voigt CA. Callose-mediated resistance to pathogenic intruders in plant defense-related papillae. Front Plant Sci. 2014;5:168. doi: 10.3389/fpls.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shamimuzzaman M, Vodkin L. Genome-wide identification of binding sites for NAC and YABBY transcription factors and co-regulated genes during soybean seedling development by ChIP-Seq and RNA-Seq. BMC Genomics. 2013;14:477. doi: 10.1186/1471-2164-14-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li W, Tian Z, Yu D. WRKY13 acts in stem development in Arabidopsis thaliana. Plant Sci. 2015;236:205–213. [DOI] [PubMed] [Google Scholar]
- 44. Serrano M, Coluccia F, Torres M, Haridon FL, Metraux J-P. The cuticle and plant defense to pathogens. Front Plant Sci. 2014;5:274. doi: 10.3389/fpls.2014.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Du D, Hao R, Cheng T, et al. Genome-wide analysis of the AP2/ERF gene family in Prunus mume. Plant Mol Biol Rep. 2013;31:741–750. [Google Scholar]
- 46. E ZG, Zhang YP, Zhou JH, Wang L. Mini review roles of the bZIP gene family in rice. Genet Mol Res. 2014;13:3025–3036. [DOI] [PubMed] [Google Scholar]
- 47. van Verk MC, Pappaioannou D, Neeleman L, Bol JF, Linthorst HJ. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008;146:1983–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu XQ, Bai XQ, Qian Q, Wang XJ, Chen MS, Chu CC. OsWRKY03, a rice transcriptional activator that functions in defense signaling pathway upstream of OsNPR1. Cell Res. 2005;15:593–603. [DOI] [PubMed] [Google Scholar]
- 49. Tao Z, Liu H, Qiu D, et al. A pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol. 2009;151:936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qiu D, Xiao J, Ding X, et al. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact. 2007;20:492–499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Combined_pdf_-_Supplementary_Figures_xyz17870f46ce8b9 for Variations in the Structure and Evolution of Rice WRKY Genes in Indica and Japonica Genotypes and their Co-expression Network in Mediating Disease Resistance by John Lilly Jimmy and Subramanian Babu in Evolutionary Bioinformatics
Supplemental material, Jimmy_Manuscript_-_EB_-_Supplementary_Tables_xyz178700578937f for Variations in the Structure and Evolution of Rice WRKY Genes in Indica and Japonica Genotypes and their Co-expression Network in Mediating Disease Resistance by John Lilly Jimmy and Subramanian Babu in Evolutionary Bioinformatics