Abstract
Background:
Skin injuries remain common in neonates admitted to neonatal intensive care units. While predicting neonates at risk of skin injury may assist in reducing the incidence of injury, currently there is limited evidence on which tool may be superior.
Methods:
A prospective study was completed during November-December 2016 to evaluate the predictive value of the Skin Risk Assessment and Management Tool (SRAMT). Comparisons were made between SRAMT and Neonatal/Infant Braden-Q Scale (BQS) as well as staff’s capacity to predict a neonate’s risk of skin injury. Data collected included gestation, weight, day of assessment, injury types, causation, medical devices in situ and risk scores.
Results:
In total, 248 assessments were completed with 38% (93) recorded skin injuries. Median (interquartile range) gestation and weight at assessment were 36.7 (26.86-56.86) weeks and 2.44 (0.99-4.06) kg, respectively. Receiver operating characteristic curve analysis showed the SRAMT had AUC (SE) of 0.94 (0.02) compared with 0.82 (0.03) for BQS (0.011, P < .001). The SRAMT and BQS had sensitivity of [(90.0 (80.5-95.9), 72.86 (60.9-82.8)] and specificity [(88.46 (81.7-93.4), 79.23 (71.2-85.8)], respectively.
Conclusion:
In this study, the SRAMT’s capacity to predict neonates at risk of injury was higher than the Neonatal BQS and staff. Predicting injuries remains complex and often multifactorial.
Keywords: Infant, risk assessment, pressure injury, skin injury
Introduction
Neonatal skin is physiologically and developmentally different to paediatric and adult skin.1–6 Functional and structural skin development is dynamic process; by week 4, the foetus already has 2 distinct layers of skin: a bottom cell layer, known as the basal layer, and an outer layer called the periderm.7,8 The skin of a preterm newborn is characterised by less functionality and perceived to be at greater risk of injury. The adaptive flexibility of skin maturation in neonates results in the unique properties of infant skin.8 Infants have the ability to restore skin and maintain a barrier function. Regulatory mechanisms control the epidermal and dermal development, eccrine sweating, sebum secretion, skin surface acidity, transepidermal water loss (TEWL) capacitance and natural moisturising factors (NMF) which develop during the physiologic maturation process.9 Development of the skin barrier increases with gestational age, and the epidermal maturation is complete at 34 weeks of age, with preterm newborns’ skin being comparable with a full-term newborns’ by 2 to 3 weeks.7
Moreover; premature or critically ill infants are placed at higher risk due to intrinsic and extrinsic factors related to their condition, and/or iatrogenic factors they may be exposed to during their admission to a neonatal intensive care unit (NICU).2 Over the past 10 years, scientific improvements in the intensive care of lower gestation (22-26 weeks) neonates, as well as infants born with complex cardiac, respiratory and metabolic conditions, have reduced mortality and improved morbidity. This is only achieved through long periods of intensive care supported by lifesaving medical devices and highly trained staff.5
The risk of skin injury during this period of intensive care remains significant with rates of neonatal skin injury ranging from 9.25% to 43.1%.10 While there remains limited evidence that considers age of life at time of injury; previous research has shown the rate of iatrogenic events is about 57% at gestational ages of 24 to 27 weeks, compared with 3% at term.11 The infant’s gestation or condition at birth has a direct relation to the intrinsic factors that increase their risk of a skin injury.12 These factors may include gestation, birth weight, skin integrity, immobility, impaired tissue perfusion, surgery, sepsis and malnutrition.3,4 Research has reported a direct correlation between lower gestation when the skin is at its most fragile and higher risk of skin injury.7 When considering extrinsic factors, previous research has highlighted infants requiring medically supportive devices being at high risk of skin injury.2,6,10 Equipment such as continuous positive air pressure (CPAP), endotracheal intubation (ETT) equipment, monitoring probes and electrodes frequently causes neonatal skin injuries.2,10 Such injuries have the potential to cause long-term disfigurement.1,3,5,6,10
Over the past 10 years, Health Services has moved from focusing on treatment of injury and disease to focus on prevention.11 The first stage of managing skin injuries is prevention through routine skin assessment based on the skin physiology at the given gestational age and age of life. Routine skin assessment is an essential part of reducing the risk of acquired skin injuries during an admission to an NICU.13,14 To assist staff in predicting infants at risk of injury, skin risk assessment tools are being integrated into daily care plans of neonates.13–19 Most recently, a Delphi study highlighted the need for a neonatal skin risk assessment tool that focuses on the iatrogenic and traumatic skin issues significant to infants of varying gestational age and illness.1 Since reporting these results in 2017, no further evidence has been published on the effectiveness of using skin tools to predict neonates at risk of skin injuries.18,19
In 2017, our team developed and evaluated a skin risk assessment and management tool (SRAMT).20 The tool is composed of 3 sections; risk assessment, care protocol and management guidelines. The team conducted an evaluation of the tool 12 months post introduction to clinical practice. Results showed a reduction in neonates who acquired skin injuries from 37 (n = 60; 61.7%) in 2010, to 12 (n = 30; 40%) in 2012 post introduction (OR = 0.41; 95% confidence interval [CI] = 0.17-1.02; P value = .085).20 When publishing the first article, we acknowledged further research was essential to evaluate the tool’s effectiveness in predicting risk of skin injury.20
Methodology
Aims
To evaluate the SRAMT’s effectiveness compared with the Neonatal/Infant Braden-Q Scale (BQS) in predicting neonates’ risk of acquiring skin injuries during their admission to the neonatal unit.
Study design
Over a 6-week period (3 days a week) during November-December 2016, a prospective observational study was undertaken. During the study period, no babies were admitted that had a genetic dermatologic condition, such as ichthyosis congenita or epidermolysis bullosa, that would result in exclusion. All neonatal skin injuries, whether pressure induced or from iatrogenic causes, were referred to as a ‘skin injury’ in the study.
Setting
This study was undertaken in a tertiary NICU in Australia that provides intensive and special care for 700 neonates per annum, born between 24 and 44 weeks gestation. The unit is staffed by approximately 100 part and full-time nurses.
Ethics approval
Ethics approval to complete the study was obtained (ACT-HEC ETH 15:147). As daily skin assessment is currently part of current practice, consent was not required. Posters were placed around the unit and information sheets were available if requested for by staff and parents.
Staff education
During a period of 4 weeks prior to commencing the study, the project coordinator (PC) held 8 in-service sessions for staff (morning and night shifts), aimed to standardise knowledge and clinical use of the SRAMT and the BQS. The PC was also available to provide small groups and one to one education throughout the study period. Staff members (n = 81) attended either in-service or small group education sessions. Attention was given to the BQS as staff had not previously used it in clinical practice.
Data collection
To assist with capturing new injuries, assessments were carried out 3 days a week for 6 weeks. On each assessment day, the PC updated the recruit list and handed out study packs to the staff member caring for each neonate. Assessments were carried out on 63 neonates over the 6 weeks. All new babies were included on each assessment day and weekly during their admission (1-6 assessments); 20-25 neonates were conveniently selected, based on clinical factors such as staff workload, plans for transfer, discharge or theatre on the day of assessment.
The staff member caring for the neonate completed a risk assessment score using the SRAMT and the BQS. While some neonates were admitted to the unit for the entire 6 weeks, skin risk assessments were completed by different nurses, at differing gestations and changing clinical requirements, thus generating 248 comparisons of the 2 tools to assess their predictive effectiveness. To assess staff capacity to predict a neonate’s risk of injury, 2 experienced nurses (more than 5 years’ experience in NICU with postgraduate certificate or master’s degree, who attended 3 expert group meetings aimed at standardising risk classification) categorised neonates at each assessment as extreme, high, medium or low risk of developing a skin injury. A study demographic form was also completed on each neonate. Data collected included gestation, weight, day of examination, injury types, causation, medical devices in situ and risk scores. Causation was determined by visual assessment during routine skin assessment and review of clinical records. All babies were visually assessed independently by the expert nurses who graded the baby as extreme, high, medium or low risk without knowledge of the scores completed by staff. To assess staff consistency with scoring, the PC completed a daily audit as part of her data collation process (2-3 per day).
Data analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) (version 24; SPSS Inc, Chicago, Illinois, USA) software. The clinical and demographic characteristics were compared by χ2 test with continuity correction and the t test where appropriate. To evaluate the effectiveness of the SRAMT in predicting the risk of a skin injury, we compared the SRAMT and the BQS with staff grading. The receiver operating characteristic (ROC) curve demonstrates the relation between the percentage of those correctly predicted to have the outcome (true positive) and the percentage of those incorrectly predicted to have the outcome (false positive) across a full range of cutoff points.21–23 The area beneath the ROC curve ranges from 0.5 for chance performance to 1.0 for perfect prediction.21–23 The best combined sensitivity and specificity with the optimal cutoff value and the area under the curve (AUC) with the standard error (SE) and 95% confidence interval (CI) were calculated for each score. We performed a stepwise multiple logistic regression elimination method to establish independent influence of the 8 subscales of the SRAMT, to assess whether any were of more value in predicting risk of a skin injury to assist in the next revision of the tool.24
Results
During the study period, 248 assessments were completed on 63 neonates. The median gestation and birth weight at time of assessment were 36.7 (26.86-56.86) weeks and 2.44 (0.99-4.06) kg with no statistically significant difference between the injury and no injury groups (Table 1).
Table 1.
Study Group Demographics.
| Demographics | NO INJURY (n=155) | INJURY (n=93) | TOTAL GROUP (n=248) |
|---|---|---|---|
| Gestational age (weeks) | 32.80 (24.0-41.5) | 33.10 (24.40-41.60) | 32.90 (24.0-41.60) |
| Birth weight (kg) | 1989 (659-4990) | 2021 (730-4990) | 2001 (650-4990) |
| Gestation on assessment (weeks) | 37.27 (27.0-44.2) | 35.79 (26.86-56.86) | 36.70 (26.86-56.86) |
| Weight on assessment (kg) | 2573 (960-4918) | 2222 (990-4918) | 2444 (990-4060) |
| Gender (male) | 96(61.9%) | 67(72.0%) | 163 (65.7%) |
Data are presented as median (interquartile range) or n (%).
Types and cause of injuries
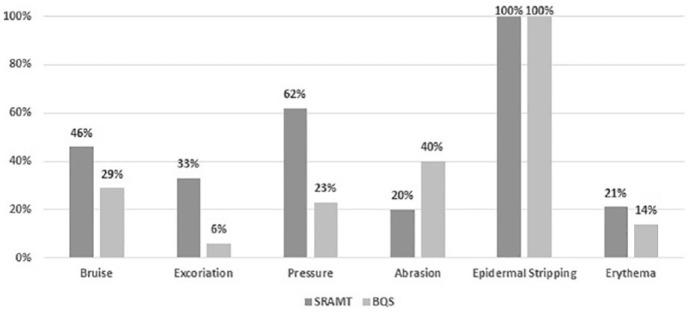
Skin injuries were recorded on 93 of the 248 (38%) assessments. Injury rates lowered progressively as gestational age increased with 15/28 (54%) in neonates <32 weeks, 44/102 (44%) in neonates 32 to 36 weeks and 33/118 (28%) in neonates >36 weeks. Results identified 4 main types (bruises, excoriation, erythema and pressure injuries) and 4 main causes (venepuncture, moisture, heel lance, pressure injuries from devices). The most common injury was bruises 44 (47%), with the main causes being equipment used for blood collecting such as cannulas and heel lance (see Table 2). Data reported neonates <36 weeks were at higher risk of injuries such as bruises, epidermal stripping and abrasions, whereas for neonates <36 weeks, the most common injury reports were excoriation and erythema related to incidences of diaper dermatitis. Pressure injuries correlate to the need for intensive care supportive equipment e.g: ventilator, continuous positive air pressure, transcutaneous monitoring equipment and total parental nutrition.
Table 2.
Types and Skin Injury Causation.
| Type of injury No (%) | Cause of injury No (%) | ||
|---|---|---|---|
| Bruise | 44 (47%) | Venepuncture | 33 (35%) |
| Excoriation | 15 (16%) | Moisture | 25 (27%) |
| Erythema | 14 (15%) | Heel lance | 12 (13%) |
| Pressure | 13 (14%) | Pressure from device | 8 (8.5%) |
| Abrasion | 5 (5%) | Position | 5 (5%) |
| Epidermal Stripping | 1 (1.5%) | Procedure | 1 (1.5%) |
| Other | 1 (1.5%) | Tapes | 1 (1.5%) |
| Total | 93 (100%) | Other/unknown | 8 (8.5%) |
| Total | 93 (100%) | ||
Data are presented as n (%).
Predicting neonates at risk of skin injuries
The SRAMT (0.936 [0.0218]) had significantly greater area under the curve than the BQS (0.826 [0.0032]) correctly predicting more neonates at risk of skin injuries (Figure 1 and Table 3). When reviewing the optimal cutoff value for each tool (SRAMT = 19, BSQ = 24), results highlighted a significant difference in the sensitivity [(90.0 (80.5-95.9), 72.86 (60.9-82.8)] and specificity [(88.46 (81.7-93.4), 79.23 (71.2-85.8)] of the 2 tools (Table 3).
Figure 1.

ROC curve for skin risk assessment and management tool (SRAMT) and Neonatal/Infant Braden-Q Scale (BQS) injury predictability.
Table 3.
Sensitivity and Specificity of skin risk assessment and management tool (SRAMT) and Neonatal/Infant Braden-Q Scale (BQS).
| Outcome | Skin injury | |
|---|---|---|
| Score | SRAMT | BQS |
| n total | 248 | 248 |
| n affected (%) | 93 | 93 |
| Optimal cutoff value | 19 | 24 |
| Sensitivity (95% CI) | 90.0 (80.5-95.9) | 72.86 (60.9-82.8) |
| Specificity (95% CI) | 88.46 (81.7-93.4) | 79.23 (71.2-85.8) |
| AUC (SE) | 0.936 (.0218) | 0.826 (.0032) |
| AUC 95% CI | 0.893-.966 | 0.767-.876 |
| Diff (SE) | 0.110 (0.0262) | |
| Diff 95% CI | 0.0587-0.161 | |
| P value | <0.0001 | |
n total: number of infants entered into score; n affected: number of infants matching outcome definition; optimal cutoff value: score result allowing best discrimination between affected/unaffected; sensitivity: % affected above cutoff score (true positive) with (95% confidence interval); specificity: % unaffected below cutoff score (true negative) with (95% confidence interval); AUC (SE): area under the curve of receiver operating characteristic curve with (standard error); Diff (SE): difference between AUC’s with (standard error).
In comparing the optimal cutoff value for each tool, the SRAMT correctly predicted 42% of the infants at risk of skin injury compared with 24% predicted by the BSQ (Figure 2). Further analysis was undertaken to evaluate gaps in the current SRAMT. Data showed neonates identified with injuries such as bruises, excoriation and erythema were frequently categorised as low risk. Comparison between the SRAMT and staff assessment of neonate’s risk showed the SRAMT correctly predicted more neonates at risk of injury (42% vs 39%) (see Figure 3).
Figure 2.
Correctly predicted infants at risk of injury.
Figure 3.
Comparison of SRAMT and staff assessment of risk of injury.
To establish independent influence of the 8 subscales on predicting a skin injury and to assist in improving the tool, a multiple logistic regression model was undertaken. Results (OR, 95% CI) have identified respiratory support and blood collection as factors that significantly increase the risk of skin injury (Table 4).
Table 4.
Multiple logistic regression model of the factors associated with skin injury.
| Variable | OR (95% CI) | P value |
|---|---|---|
| Gestation | 1.760 (0.883-3.506) | .108 |
| Sensory perception | 1.874 (0.826-4.253) | .133 |
| Activity/mobility | 1.375 (0.705-2.684) | .350 |
| Moisture | 2.582 (1.445-4.614) | .001 |
| Respiratory support | 3.704 (2.158-6.358) | .000 |
| Skin integrity | 2.073 (1.333-3.224) | .001 |
| Blood collection | 3.062 (1.595-5.878) | .001 |
| Nutrition | 1.874 (1.147-3.062) | .012 |
Bold: highest risk. Data are presented as odd ratio (OR) (95% confidence interval [CI]).
Discussion
To meet the National Safety and Quality Health Service Standards, NICUs have been directed to introduce a skin risk assessment tool. While many other NICUs in Australia have chosen to introduce a modified version of the BQS, we reviewed current literature regarding current tools in use, to find no tool had been developed specifically for neonates or that had been classified as the most effective tool.20 The SRAMT team has taken the next step to develop a tool specifically for neonates that considers all types of skin injuries.20
This pragmatic study has been undertaken to evaluate the SRAMT’s effectiveness in predicting neonates at risk of acquiring skin injuries. The study has shown the SRAMT had significantly higher sensitivity and specificity than the BQS in predicting neonates at risk of injury in the study population. Results have reported the most common injuries being bruises, excoriation, erythema and pressure. Once again as shown in our previous study, the cause of injuries related to 2 main groups: (1) medical devices (eg, blood collection and CPAP devices) and (2) routine skin care.20 Results in this study provide evidence that the incidence of injury is higher in the most premature group of our NICU population (<32 weeks) and progressively reduces as gestation increases, but it should be acknowledged all neonates admitted to a NICU are at risk of acquiring a skin injury. It is also of interest that infants of higher gestational age were more likely to be scored as low risk than lower gestation infants, when scored using the current tool. This reinforces the importance of this evaluation in gathering evidence to assist us in revising the tool.
When comparing the 2 tools, it is important to remember that the SRAMT has been designed to predict all types of skin injuries, unlike the Neonatal BSQ which is based on the Braden Scale for predicting pressure injury risk in adults. The Scale was piloted with 32 neonates which showed reliability was high for the subscales of general physical condition, activity and nutrition, but low in the other 4 subscales. For predictive validity, sensitivity was 83% and specificity was 81%.15,16 More recently, García-Molina et al25 conducted an observational study to assess the validity of the Spanish version of the Scale in pressure ulcers (PU), showing a sensitivity of 91.18%, specificity of 76.50%, positive predictive value of 36.05% and negative predictive value of 98.35%.
During the development of the SRAMT our team recognised some risks related to both pressure and other skin injuries that occur in neonates. The 3 subscales general physical condition, activity and nutrition, previously noted for high reliability, have been included but may have been renamed or combined in the SRAMT. Moisture has also been included but the descriptors have been modified to meet current neonatal practice. We have also added 4 subscales developed from the types and causes of injuries identified in our previous study: these are Current Gestational Age, Respiratory Support, Visual Examination of Skin Integrity and Blood Collection. Descriptors in these subscales are directly related to equipment, care and behaviour, commonly seen in clinical practice when caring for neonates. The SRAMT has been developed and evaluated in a neonatal population considering the nuances and all types of skin injuries common in a neonatal population. Our study has shown the SRAMT had higher predictive value in both sensitivity and specificity compared with the Neonatal BQS.16
Visscher and Taylor26 conducted a prospective study to determine the aetiology, severity and influence of gestational age on PU among hospitalised infants. Their study showed when comparing premature neonates with or without PU, those with PU were younger and weighed less at birth (P = .05).26 In contrast, the term infants with PU and without PU did not differ for any characteristics.27 Our study has shown no statistically significant different between the injury and no injury group at birth or time of assessment. When considering the reasons for this difference in the two studies results, we suggest it may be linked to the fact that devices accounted for 90.5% of the PU in premature neonates and 71.4% in term neonates,26 whereas when assessing all types of skin injuries, the causation is varied and often multifactorial relating to skin integrity, procedures undertaken and treatment requirements.
Recent literature has described how this implementation of skin risk assessment tool in conjunction with clinical guidelines can improve practice and clinical outcomes. Researchers have outlined that the implementation of a skin risk assessment tool may reduce the incidence of injury.19 It has also been discussed by previous researchers that it is not enough to just score an infant; a skin risk assessment tool needs to provide a skin care and documentation plan to standardised current practice in a NICU.14,18,19 Many risk assessment tools just provide staff caring for the neonate with a score which informs them that the neonate may or may not be at risk of a skin injury with no instructions on the timing and type of care required to reduce the risk of skin injuries. The SRAMT acknowledges all neonates are at risk and identifies levels of risk (low, mod, high extreme). The tool allows the user to move from risk assessment to a standardised care and documentation plan. Evidence from our previous study showed a significant improvement since the introduction of the SRAMT in standardising care and documentation of skin injuries.20 The final section of the tool outlines guidelines regarding skin care practices and products currently used in the NICU, providing a complete skin assessment and care guide for staff at the bedside (Appendix 1).
There have been suggestions that clinical assessment may be adequate in predicting infants at risk of skin injury. We acknowledge the previous research that has recommended such a tool should be used as part of routine clinical assessment.1,18,19 In this project, we were keen to see whether the SRAMT would predict as accurately as members of nursing team who had experience in managing neonates across gestation and acuity levels. This study had shown the SRAMT was more accurate than the experienced staff. Currently approximately 50% of our staff are classed as transition (<2 years NICU experience). All staff receive regular education updates on all aspects of skin care management; the SRAMT has an educative function aimed at standardising skin care across NICU.
Preventing and caring for neonates who acquire a skin injury has become a continuous quality improvement (QI) project in our NICU. While the SRAMT assists in predicting neonates at risk of injury, we continue to update skin care clinical guidelines. By implementing new practices and products and updating staff education, we continue to foster evidence-based research aiming to minimise skin injuries to neonates cared for in our service.
Limitations
Due to limitations in current literature regarding aetiology, severity and influence of gestational age regarding all types of skin injuries, it remains a dilemma for our team in the usefulness of comparing data from our study with studies that have only reviewed pressure injuries, which may reduce the transferability of our results. We also acknowledge this study has been only been held in 1 centre with a small sample size. A structured education programme was conducted on both tools that included 5 examples of different injuries (gestation, causation, risks) to evaluate staff proficiency over 4 weeks. Subsequently, when they compared the use of the 2 tools during conduct of the study, staff may have perceived the SRAMT as easier to use. We acknowledge the SRAMT needs further review and testing across a larger neonatal population.
Post evaluation
Utilising the results from this project, we undertook a QI project to revise the tool to improve its predictive capacity. A project team that included the study primary investigator, clinical educator, tissue viability and senior clinical nurse was formed. The team reviewed the current tool, and based on results and clinical feedback, subscale descriptors have been updated to clarify the language and assist staff in grading neonates more accurately. The causes of injuries outlined in the clinical guidelines and preventive measures have been aligned with injury causation previously identified. The clinical guidelines and preventive measures have also been revised to meet current practices and include new products that have been introduced over the past 3 years. An example of this is the blood collection: aiming to reduce the incidence of bruises. QI projects have been conducted to assess causation and clinical care practices and reduce the incidence of blood collection. QI projects resulted in updated guidelines, additions to the SRAMT as well as the introduction of a venepuncture education package for staff. We also recognise the importance of working with industry through feedback to product consultants related to improvements they might make to equipment, as well as requests for equipment that is more suitable to the neonatal population.
Conclusion
During an admission to a NICU, neonates are at high risk of skin injuries. In this study, the SRAMT’s capacity to predict neonates at risk of injury was better than the Neonatal BSQ and experienced staff. This study has also shown predicting all types skin injuries remains complex and is often multifactorial. Skin injury prediction and care is a topic that requires further research and QI projects to identify aetiology, severity and influence of gestational age on all types of skin injuries aimed to improve current clinical practices. Staff should acknowledge predicting neonates at risk of skin injury will only reduce injury rates if supported by skin care policy, guidelines and vigilance in daily care.
Acknowledgments
We acknowledge the staff and NICU management for their support of this project. Special thanks to the key members of our team: Wendy Burton and Leanne Ehrich, for their expert advice in every stage of this project; Jasmine Song and Joelle Auguta for their assistance with data collection for this study. We also acknowledge the ACT Nursing and Midwifery Research Office for the Practice Development Grant that assisted with study funding. Our group also acknowledge all the other teams across the world who have developed paediatric skin tools that we have learnt from; you have greatly assisted us in developing a tool to meet the needs of our NICU.
Appendix
Appendix 1.
Skin risk assessment and management tool
| Risk score | Category | Suggested action | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ⩽8 | Low risk | Continue daily assessment and documentation of skin integrity on a daily basis | ||||||||||
| 9-16 | Moderate risk | Reposition neonate every 6-8 hours. Assess and document skin integrity 6-8 hourly | ||||||||||
| 17-24 | High risk | Reposition neonate and equipment devices at least every 4-6 hours. Reassess and document every 4-6 hourly | ||||||||||
| 25-32 | Extreme risk | Inspect skin at least 2-4 hourly, ensuring equipment/objects are not pressing on the skin. Reassess and document every 2-4 hourly | ||||||||||
| Category | Please complete the assessment on admission and then assess and document below and in notes according to scoring guidelines | |||||||||||
| Score | Descriptor | Date/time | ||||||||||
| Day of life | ||||||||||||
| Current gestational age | 4 | Neonate ⩽28 weeks | ||||||||||
| 3 | Neonate >28 weeks and ⩽33 weeks | |||||||||||
| 2 | Neonate >33 weeks and ⩽38 week | |||||||||||
| 1 | Neonate >38 weeks | |||||||||||
| Sensory perception | 4 | Diminished level of consciousness/muscle relaxed/heavily sedated/cooling for HIE | ||||||||||
| 3 | Oversensitive to noise, lights and touch/easily agitated/difficult to calm | |||||||||||
| 2 | Easily agitated but calms with comfort measures/few self-calming behaviours | |||||||||||
| 1 | Responds appropriately to stimuli, alert, good self-calming behaviours | |||||||||||
| Activity/mobility | 4 | Makes no change in position—full assistance required | ||||||||||
| 3 | Makes occasional slight changes in body or extremity position | |||||||||||
| 2 | Makes frequent changes in body or extremity position, eg, turns head | |||||||||||
| 1 | Makes major and frequent changes in position, moving all extremities, turns head | |||||||||||
| Moisture | 4 | Constantly moist due to humidity/urine/wound/stoma/respiratory support/NAS | ||||||||||
| 3 | Skin often moist—linen needs to be changed <12 hours | |||||||||||
| 2 | Skin occasionally moist—needs linen change >12 hours | |||||||||||
| 1 | Skin usually dry, routine nappy changes and linen once / day | |||||||||||
| Respiratory support | 4 | Intubated and ventilated or CPAP ⩾6 cm H2O | ||||||||||
| 3 | CPAP ⩾5 cm H2O | |||||||||||
| 2 | High flow/low flow/micro low flow/Cot O2 | |||||||||||
| 1 | No respiratory support | |||||||||||
| Skin integrity (visual examination) | 4 | Extensive loss of skin integrity/wound/pressure area | ||||||||||
| 3 | Localised loss of skin integrity/broken area/oedema/nappy rash/excoriation | |||||||||||
| 4 | Minor skin irritation/redness | |||||||||||
| 1 | Skin integrity intact | |||||||||||
| Blood collection | 4 | Neonate requires cannulation/PICCS/daily blood collection | ||||||||||
| 3 | Neonate requires heel prick for blood collection | |||||||||||
| 2 | Blood collection weekly | |||||||||||
| 1 | No blood collection required | |||||||||||
| Nutrition | 4 | TPN + Lipids/IV Fluids/NBM/does not tolerate feeds | ||||||||||
| 3 | TPN + Lipids/IV Fluids/trophic feeds | |||||||||||
| 2 | TPN + Lipids/IV Fluids/gastric feeds increasing and tolerated | |||||||||||
| 1 | Full gastric feeds | |||||||||||
| Total | ||||||||||||
| Initials | ||||||||||||
| Type of injury | Cause of injury | Guidelines and preventive measures | ||||||||||
| Bruises | Venepuncture and heel lance | Try not to hold limb too tightly causing a tourniquet effect Warm site to improve perfusion Do not squeeze heel/wrist during blood collection (increases risk of bruising) Use heel lance appropriate for gestation (wrong size increases risk of bruising) After 2 attempts call senior staff member Hold site till bleeding stops—do not use cotton wool and adhesive tapes |
||||||||||
| Type of injury | Cause of injury | Guidelines and preventive measures | ||||||||||
| Epidermal stripping | Removal of adhesive tapes used to secure devices (tubes/lines) | Avoid products that bond to skin Double back tapes or fluff with cotton wool Use saturation wraps for holding lines in place instead of tapes(especially neonates <28 weeks) To assist in the removal of tapes use an alcohol free product, adhesive remover Remember remove in a ‘low and slow’ manner |
||||||||||
| Excoriation | Neonatal Abstinence Syndrome (NAS), skin infections (fungal), medications | Take preventive action by following Management of Nappy Rash Guideline Frequent nappy changes 3 to 4 hourly, apply skin barrier cream Assess cause of excoriation (swab/thrush) Treat cause of excoriation |
||||||||||
| Extravasation | Peripheral arterial line causing vasoconstriction Intravenous Infusions (especially hypertonic/ionic/acidic or alkaline) may infiltrate the vein causing swelling at the cannula site |
Secure all lines to maintain good visibility of the cannula site and surrounding tissue Position arterial line/site to allow constant monitoring Ensure brisk cap refill to all digits Remove line if fingertips are dusky/white in colour Try to infuse hypertonic/ionic/acidic or alkaline drugs via a central line Flush cannula to check patency before attaching intravenous infusion Check line/site/pressures hourly |
||||||||||
| Chemical burns | Alcohol based skin preparation solutions, eg, chlorhexidine, iodine and alcohol swabs | Skin preparation for procedures—only use chlorhexidine 0.2% (antiseptic) Ensure skin is cleansed straight away with normal saline or water to prevent burns. DO NOT use alcohol swabs to clean neonates skin, before venepunctures or cannulation |
||||||||||
| Thermal burns | Heat from monitoring Equipment Cold lights Saturation/Temperature probes |
Reset the time and temperature of transcutaneous oxygen monitor according to the age and gestation of the neonate Check light is on the correct setting Minimise the length of time light is used Resite probes 2-4 hourly Mefix underneath probe of neonates ⩽28 weeks |
||||||||||
| Pressure | All medical devices CPAP nasal prongs or mask Anatomical position/lying prone/NAS babies |
Avoid the infant lying on tubes or rolls of linen, such as lines/devices Increased risk of Injury if neonate is exposed to unrelieved pressure, humid microclimate Ensure prongs are nursed off the septum and not causing damage to the inner nares Ensure ears are not rolled up under hat and the neonate is not lying on one side constantly, ie, rotated from side to side Cover knees with duoderm to prevent rubbing injuries from being nursed prone |
||||||||||
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: ACT Nursing and Midwifery Research Office Practice Development Grant.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MB and ALM designed the study, MB coordinated data collection and finalised data review. ALM completed data analysis. MB, AMD and ALM reviewed study results. MB wrote the manuscript, with input from and review by AMD and ALM.
ORCID iD: Abdel-Latif E Mohamed  https://orcid.org/0000-0003-4306-2933
https://orcid.org/0000-0003-4306-2933
References
- 1. Vance DA, Demel S, Kirksey K, Moynihan M, Hollis K. A Delphi study for the development of an infant skin breakdown risk assessment tool. Adv Neonatal Care. 2015;15:150–157. [DOI] [PubMed] [Google Scholar]
- 2. August DL, Edmonds L, Brown DK, Murphy M, Kandasamy Y. Pressure injuries to the skin in a neonatal unit: fact or fiction. J Neonatal Nurs. 2014;20:129–137. [Google Scholar]
- 3. Dunk AM, Carville K. The international clinical practice guideline for prevention and treatment of pressure ulcers/injuries. J Adv Nurs. 2016;72:243–244. [DOI] [PubMed] [Google Scholar]
- 4. Baharestani MM, Ratliff CR. Pressure ulcers in neonates and children: an NPUAP white paper. Adv Skin Wound Care. 2007;20:208, 210, 212, 214, 216, 218–220. [DOI] [PubMed] [Google Scholar]
- 5. García-Molina P, Balaguer-López E, García-Fernández FP, Ferrera-Fernández MLÁ, Blasco JM, Verdú J. Pressure ulcers’ incidence, preventive measures, and risk factors in neonatal intensive care and intermediate care units. Int Wound J. 2018;15:571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ottinger D, Hicks J, Wilson S, Sperber K, Power K, Schierholz E. The pressure is on! Adv Neonatal Care. 2016;16:420–423. [DOI] [PubMed] [Google Scholar]
- 7. Fluhr JW, Darlenski R, Raieb A, et al. Functional skin adaptation in infancy – almost complete but not fully competent. Exp Dermatol. 2010;19:483–492. [DOI] [PubMed] [Google Scholar]
- 8. Nikolovski J, Stamatas GN, Kollias N, Wiegand BC. Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. J Invest Dermatol. 2008;128:1728–1736. [DOI] [PubMed] [Google Scholar]
- 9. Oranges T, Dini V, Romanelli M. Skin physiology of the neonate and infant: clinical implications. Adv Wound Care (New Rochelle). 2015;4:587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. August DL, New K, Ray RA, Kandasamy Y. Frequency, location and risk factors of neonatal skin injuries from mechanical forces of pressure, friction, shear and stripping: a systematic literature review. J Neonatal Nurs. 2018;24:173–180. [Google Scholar]
- 11. Sardesai SR, Kornacka MK, Walas W, Ramanathan R. Iatrogenic skin injury in the neonatal intensive care unit. J Matern Fetal Neonatal Med. 2011;24:197–203. [DOI] [PubMed] [Google Scholar]
- 12. Johnson D. Extremely preterm infant skin care: a transformation of practice aimed to prevent harm. Adv Neonatal Care. 2016;16:S26–S32. [DOI] [PubMed] [Google Scholar]
- 13. Ashworth C, Briggs L. Design and implementation of a neonatal tissue viability assessment tool on the newborn intensive care unit. Infant. 2011;7:191–194. [Google Scholar]
- 14. Grosvenor J, Hara M, Dowling M. Skin injury prevention in an Irish neonatal unit: an action research study. J Neonatal Nurs. 2016;22:185–195. [Google Scholar]
- 15. Noonan C, Quigley S, Curley MA. Skin integrity in hospitalized infants and children: a prevalence survey. J Pediatr Nurs. 2006;21:445–453. [DOI] [PubMed] [Google Scholar]
- 16. Dolack M, Huffines B, Stikes R, Hayes P, Logsdon MC. Updated neonatal skin risk assessment scale (NSRAS). Ky Nurse. 2013;61:6. [PubMed] [Google Scholar]
- 17. Lund CH, Osborne JW. Validity and reliability of the neonatal skin condition score. J Obstet Gynecol Neonatal Nurs. 2004;33:320–327. [DOI] [PubMed] [Google Scholar]
- 18. Kottner J, Hauss A, Schlüer AB, Dassen T. Validation and clinical impact of paediatric pressure ulcer risk assessment scales: a systematic review. Int J Nurs Stud. 2013;50:807–818. [DOI] [PubMed] [Google Scholar]
- 19. Grosvenor J, Dowling M. Prevention of neonatal pressure injuries. J Neonatal Nurs. 2018;24:122–125. [Google Scholar]
- 20. Broom M, Burton W, Ehrlich L, Abdel-Latif ME. Developing an Australian skin risk assessment and management tool for neonates. Wound Pract Res. 2017;25:1939–1948. [Google Scholar]
- 21. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 22. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 23. Beck A, Shultz EK. The use of relative operating characteristic (ROC) curves in test performance evaluation. Arch Pathol Lab Med. 1986;110:13–25. [PubMed] [Google Scholar]
- 24. Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: Wiley; 1989. [Google Scholar]
- 25. García-Molina P, Balaguer Lopez E, Verdu J, Nolasco A, Garcia Fernandez FP. Cross-cultural adaptation, reliability and validity of the Spanish version of the neonatal skin risk assessment scale. J Nurs Manage. 2018;26:744–756. [DOI] [PubMed] [Google Scholar]
- 26. Visscher M, Taylor T. Pressure ulcers in the hospitalized neonate: rates and risk factors. Sci Rep. 2014;4:7429. [DOI] [PMC free article] [PubMed] [Google Scholar]