Abstract
Background:
Stereotactic body radiotherapy has been suggested to provide high rates of local control for locally advanced pancreatic cancer. However, the close proximity of highly radiosensitive normal tissues usually causes the labor-intensive planning process and may impede further escalation of the prescription dose.
Purpose:
The present study aims to evaluate the consistency and efficiency of Pinnacle Auto-Planning for pancreas stereotactic body radiotherapy with original prescription and escalated prescription.
Methods:
Twenty-four patients with pancreatic cancer treated with stereotactic body radiotherapy were studied retrospectively. The prescription is 40 Gy over 5 consecutive fractions. Most of patients (n = 21) also had 3 other different dose-level targets (6 Gy/fraction, 5 Gy/fraction, and 4 Gy/fraction). Two types of plans were generated by Pinnacle Auto-Planning with the original prescription (8 Gy/fraction, 6 Gy/fraction, 5 Gy/fraction, and 4 Gy/fraction) and escalated prescription (9 Gy/fraction, 7 Gy/fraction, 6 Gy/fraction, and 5 Gy/fraction), respectively. The same Auto-Planning template, including beam geometry, intensity-modulated radiotherapy objectives and intensity-modulated radiotherapy optimization parameters, were utilized for all the auto-plans in each prescription group. The intensity-modulated radiotherapy objectives do not include any manually created structures. Dosimetric parameters including percentage volume of PTV receiving 100% of the prescription dose, percentage volume of PTV receiving 93% of the prescription dose, and consistency of the dose-volume histograms of the target volumes were assessed. Dmax and D1 cc of highly radiosensitive organs were also evaluated.
Results:
For all the pancreas stereotactic body radiotherapy plans with the original or escalated prescriptions, auto-plans met institutional dose constraints for critical organs, such as the duodenum, small intestine, and stomach. Furthermore, auto-plans resulted in acceptable planning target volume coverage for all targets with different prescription levels. All the plans were generated in a one-attempt manner, and very little human intervention is necessary to achieve such plan quality.
Conclusions:
Pinnacle3 Auto-Planning consistently and efficiently generate acceptable treatment plans for multitarget pancreas stereotactic body radiotherapy with or without dose escalation and may play a more important role in treatment planning in the future.
Keywords: stereotactic body radiotherapy, pancreatic cancer, automated treatment planning, dose escalation, personalized treatment
Introduction
It is difficult to overstate the exigent need to identify novel therapeutic strategies for pancreatic cancer, one of the most lethal malignancies carrying a dire prognosis.1-5 Stereotactic body radiotherapy (SBRT), or stereotactic ablative body radiotherapy (SABR), has been documented to improve tumor control by delivering ablative doses with tolerable side effects.5 As a result of its success in medically inoperable early-stage lung cancer,6-9 the spectrum of SBRT clinical implementation continues to broaden to other tumor sites including the pancreas. Stereotactic ablative body radiotherapy has been demonstrated to be well tolerated and effective for locally advanced pancreatic cancer.5,10-17 Technical advances, such as advanced radiation delivery, real-time image guidance, and adaptive radiotherapy, have enabled the realization of dose escalation in SBRT.18-20 Recent studies and ongoing clinical trials of dose escalation on pancreas SBRT21-32 further suggested dose escalation is likely to increase patients’ survival benefits, indicating that the role of SBRT in pancreatic cancer management is likely to be further expanded.
However, treatment planning is still a challenging step for pancreas SBRT, because SBRT delivers ablative fractional doses sufficient to cause irreparable damage to proximate organs at risk (OARs) such as the duodenum, stomach, and small intestine. Combinational treatment regimens could potentially cause synergistic toxicities,33 emphasizing the criticality of OAR sparing. Dealing with risky close OARs often requires the effort of repetitive and meticulous design of artificial planning structures and frequentative adjustments of optimization objectives. Furthermore, this time-consuming and labor-intensive task often has to be completed in a short period of time considering the direness of pancreatic cancer. Therefore, plan quality may be suboptimal and inconsistent due to patient’s anatomy, planner’s experience, and limited time for planning. This challenging task could become more strenuous if the regimen of pancreas SBRT is further tailored to specific individual, with dose escalation or de-escalation, in the future resulted from the intensive effort and achievements of personalized biomarker developments and artificial intelligence in health care.34-40
Pinnacle3 Auto-Planning (AP) is a volume-driven automatic planning process, which is designed to improve planning efficiency while maintaining or improving plan quality. It utilizes progressive optimization to automatically create planning structures based on the desired target coverage and OAR sparing as well as anatomical relationships among the planning target volume (PTVs) and OARs, and iteratively prioritizes and adjusts the planning objectives during optimization. Pinnacle3 AP has been documented to generate acceptable plans with consistent quality and expedited planning processes for various tumor sites.41-48 Therefore, AP could potentially represent a feasible solution to generate acceptable pancreas SBRT plans with consistency and efficiency.
Furthermore, we envision that more clinical trials, aming at personalized medicine, will commence with customized treatment regimen, and the automated planning may play a more important role in radiotherapy treatment planning. In this study, we aim to evaluate the consistency and efficiency of the Pinnacle3 AP, with or without dose escalation, on pancreas SBRT with multiple dose-level targets following our institutional clinical trial.
Materials and Methods
Patient Selection
Twenty-four patients were selected for this retrospective study, which was approved by the institutional review board and ethics committee. All patients underwent consecutive 5-fraction SBRT treatments with the total dose of 40 Gy. Most of the patients have 3 other different dose-level targets, that is, 6 Gy/fraction, 5 Gy/fraction, and 4 Gy/fraction, following our institutional trial of SBRT for pancreatic cancer.
Simulation and Structure Delineation
All the patients were immobilized with BlueBAG immobilization system (Medical Intelligence, Schwabmünchen, Germany), followed by a free-breathing 3-D computed tomography (CT) scan (FB-CT) and a 4D-CT scan using a Sensation Open CT system (Siemens Medical Solutions, Malvem, Pennsylvania). An abdomen compression belt system (Anzai Medical Systems, Tokyo, Japan) and the Real-time Position Management system (Varian Medical Systems, Palo Alto, California) were used to account for respiratory motion. A slice thickness of 2 mm was used for both CT scans.
Each patient also underwent high-resolution dynamic contrast-enhanced T1-weighted magnetic resonance (MR) imaging, which were registered to the simulation CT scans in the Varian Eclipse treatment planning system (Varian Medical Systems). Target volumes and OARs were delineated on the registered FB-CT, 4D-CT, and MR images in the Eclipse TPS by attending radiation oncologist. Computed tomography images with the associated anatomical structure sets were transferred to the Pinnacle3 TPS (version 9.10, Philips Medical Systems, Fitchburg, Wisconsin) using the DICOM-RT protocol.
Treatment Planning
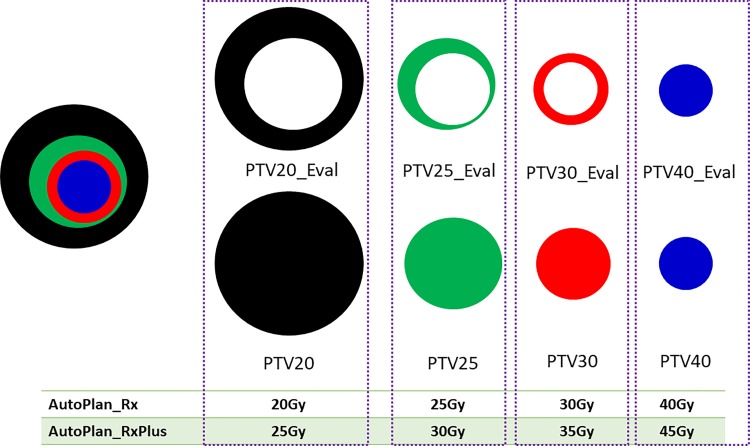
The AP module in the Pinnacle3 TPS was used to generate auto-plans for the selected cases. Briefly, the prescriptions, dose objectives of the OARs (maximum dose and dose–volume constraints), and their respective priorities were all predefined in the AP technique. Intensity-modulated radiotherapy (IMRT) technology was utilized for all the auto-plans, which consists of 18 coplanar static beams (20-degree apart) with collimator angles of 90 degree. All the plans were created on the FB-CT images using 10xFFF photon beams of a TrueBeam STx linear accelerator (Varian Medical Systems, Palo Alto, California). Two types of auto-plans were generated by Pinnacle3 AP, as illustrated in Figure 1: one with the original prescription as the patient was treated, which includes dose levels of 8 Gy/Fx, 6 Gy/Fx, 5 Gy/Fx, and 4 Gy/Fx (hereafter referred to as “AutoPlan_Rx”) and the other with the escalated prescription (9 Gy/Fx, 7 Gy/Fx, 6 Gy/Fx, 5 Gy/Fx; hereafter referred to as “AutoPlan_RxPlus”). During the optimization, the AP engine, as previously described,41,49 prioritizes optimization goals according to the anatomical relationship among the PTVs and OARs, and iteratively adjusts the optimization objectives to generate the plans.
Figure 1.
Schematic representation of the target volumes and prescriptions.
Plan Evaluation
The V100% (percentage volume of PTV receiving 100% of the prescription dose) and V93% (percentage volume of PTV receiving 93% of the prescription dose) of the PTV were used to assess plan quality. The V100% and V93% were obtained by dose–volume histogram (DVH) from the TPS. We utilized maximum dose (Dmax) and D1 cc (minimum dose to 1 cc of the most irradiated organ) to evaluate the OAR sparing of the auto-plans for the duodenum, stomach, and small intestine.
Software
The figures were generated in Prism 8 (GraphPad Software, San Diego, California) or Python 3.6 (Python Software Foundation).
Results
Dose Coverage and Conformity of Target
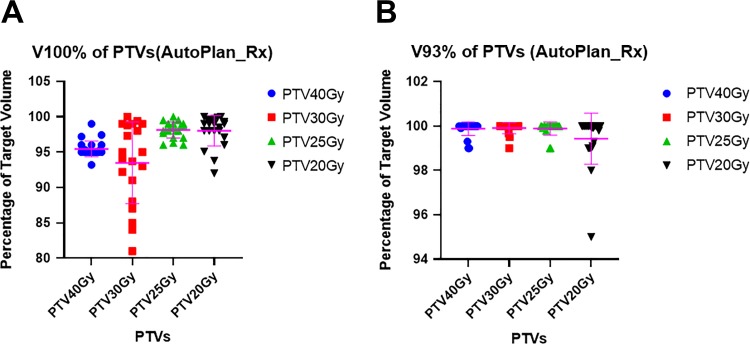
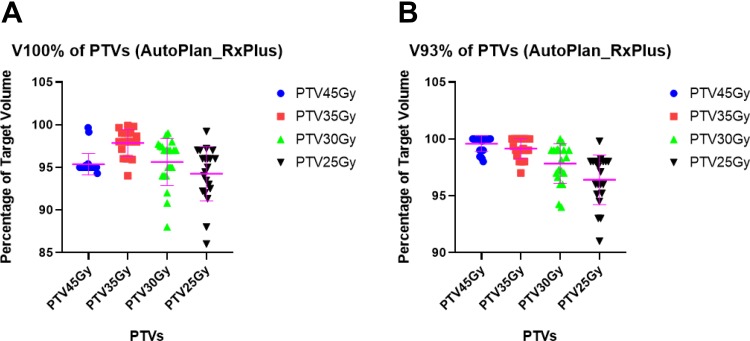
As shown in Figures 2 and 3, AP achieved the mean V40 Gy of 95.51% ± 1.25% for 40Gy-target; V30 Gy of 93.41% ± 5.79% for 30Gy-target; V25 Gy of 98.20% ± 1.20% for 25Gy-target, and V20 Gy of 98.33% ± 1.96% for 20Gy-target for the plans with the original prescription. For the plans with escalated prescriptions, AP achieved the mean V45 Gy of 95.38% ± 1.26% for 40Gy-target, V35 Gy of 97.85% ± 1.57% for 30Gy-target, V30 Gy of 95.63% ± 2.76% for 25Gy-target, and 94.26% ± 3.18% for 20Gy-target, respectively. According to our institutional protocol, we would like to have V93% more than 99%. Auto-Planning achieved V93% (93% of 40 Gy, 30 Gy, 25 Gy, and 20 Gy) of 99.88% ± 0.31%, 99.91% ± 0.25%, 99.89% ± 0.30%, and 99.43% ± 1.15% for 40Gy-target, 30Gy-target, 25Gy-target, and 20Gy-target, respectively, for the plans with original prescriptions. Similarly, for the plans with escalated prescriptions, AP achieved V93% (93% of 45 Gy, 35 Gy, 30 Gy, and 25 Gy) of 99.58% ± 0.69%, 99.15% ± 0.89%, 97.84% ± 1.74%, and 96.41% ± 2.17% for 40Gy-target, 30Gy-target, 25Gy-target, and 20Gy-target, respectively. In addition, the global maximum dose is within the 40Gy-target for all the auto-plans.
Figure 2.
Evaluation of V100% and V93% for auto-plans with original prescriptions. A: V100% of PTVs; B: V93% of PTVs.
Figure 3.
Evaluation of V100% and V93% for auto-plans with escalated prescriptions. A: V100% of PTVs; B: V93% of PTVs.
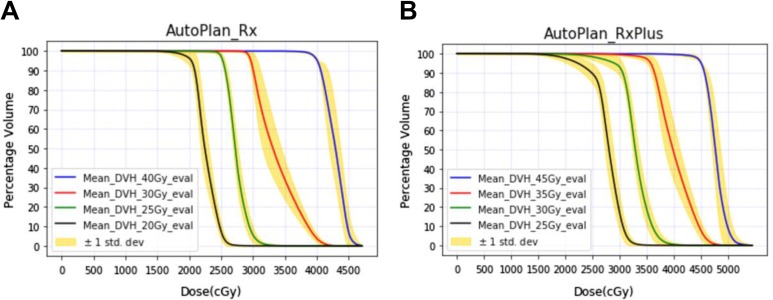
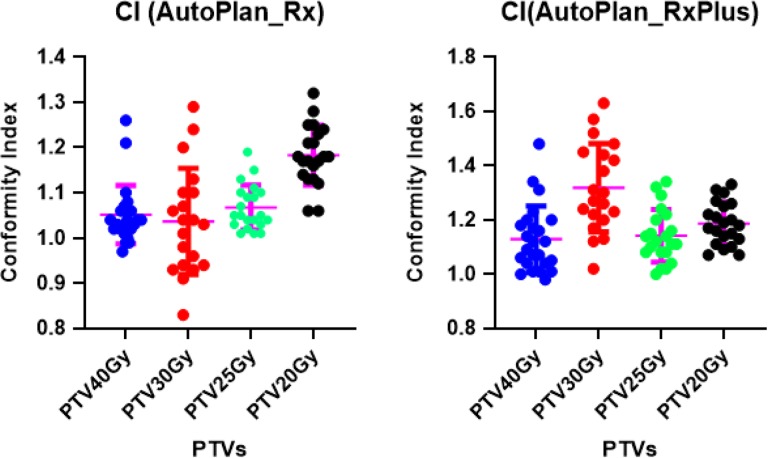
The mean DVH of the PTV evaluation structures (Figure 1) was plotted with standard deviation of the curves, as shown in Figure 4. As the lower dose target always encompasses the higher dose target, the true intended dose is pointing to those evaluation target volumes (Figure 1). As visualized in the DVH plots, all the auto-plans, with original or escalated prescriptions, have achieved every DVH in a relatively narrow range, indicating that all the plans were generated in a consistent way having similar plan quality. It’s worth mentioning, nevertheless, that the coverage of PTV20Gy_eval and PTV25Gy_eval decreased significantly in auto-plans with escalated prescription (Figure 4B). This is because we have an objective to keep less than 3% of the duodenum, stomach, and small intestine from receiving dose more than 25 Gy, and PTV25Gy and PTV20Gy may have significant overlap with these three OARs. Therefore, it is reasonable to lose coverage when we escalated the prescription dose by 5 Gy. The conformity indices of all the auto-plans are summarized in Figure 5. Our results showed that in most of the cases, our primary target, PTV40Gy, has achieved good conformity for both original and escalated prescriptions. The conformity indices of other targets (30 Gy, 25 Gy, and 20 Gy) were comprised in varying degrees to achieve other optimization goals, such as OAR sparing. Similar observations of dose conformity were also reported in previous study.42
Figure 4.
Mean DVH plots with standard deviation for all the auto-plans with or without dose escalation. DVH indicates dose–volume histogram. A: the mean DVH plots for original prescription; B: the mean DVH plot for escalated prescription.
Figure 5.
Conformity Index of AutoPlan_Rx and AutoPlan_RxPlus. Conformity Index of auto-plans with original prescription (left); Conformity index of auto-plans with escalated prescription (right).
Dose to OARs
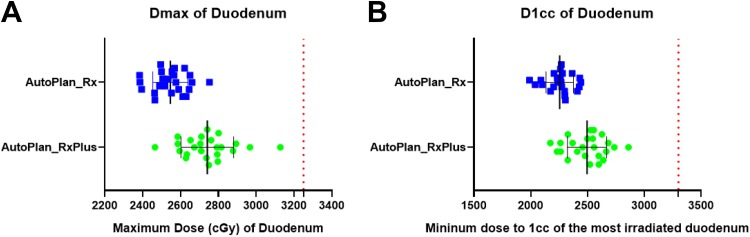
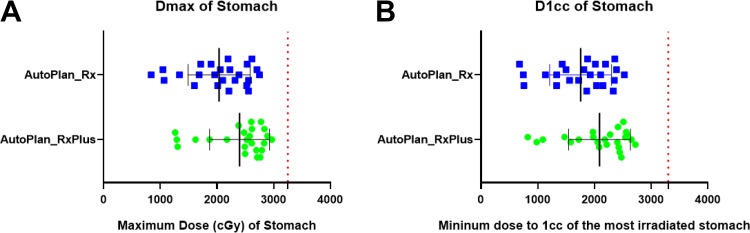
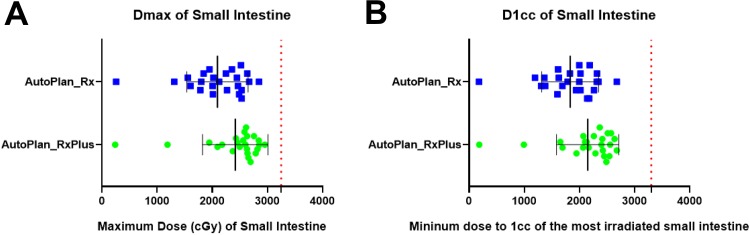
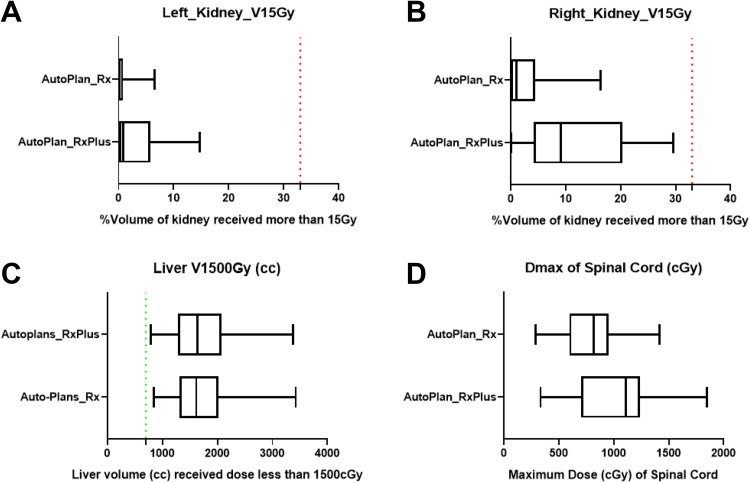
Maximum doses, as well as D1 cc to the OARs (duodenum, stomach, and small intestine), are plotted in Figures 6 to 8. Our institute recommends 32.5 Gy as the highest maximum dose allowed to these OARs. All of the auto-plans had a Dmax under 32.5 Gy, and achieved well-acceptable D1 cc based on previous clinical experience from a multi-institutional study,50 recommending the D1 cc below 33 Gy (Figures 6 -8). In addition, the kidneys, spinal cord, and liver are well spared based on American Association of Physicists in Medicine task group report 10151 and our institutional protocol (Figure 9). Briefly, our planning objectives specified the kidneys should not have over 33% of its volume receiving dose more than 15 Gy, and should have at least 700 cc of liver received dose less than 1500 cGy.
Figure 6.
Dmax and D1 cc of the duodenum for auto-plans with original and escalated prescriptions. A: Maximum dose of duodenum for auto-plans with original and escalated prescriptions; B: D1cc of stomach for auto-plans with original and escalated prescriptions.
Figure 7.
Dmax and D1 cc of the stomach for auto-plans with original and escalated prescriptions. A: Maximum dose of stomach for auto-plans with original and escalated prescriptions; B: D1cc of stomach for auto-plans with original and escalated prescriptions.
Figure 8.
Dmax and D1 cc of the small intestine for auto-plans with original and escalated prescriptions. A: Maximum dose of small intestine for auto-plans with original and escalated prescriptions; B: D1cc of small intestine for auto-plans with original and escalated prescriptions.
Figure 9.
Dosimetry of the kidneys, liver, and spinal cord for auto-plans with original and escalated prescriptions. A: Relative volume of left kidney receiving more than 15Gy for auto-plans with original and escalated prescriptions. B: Relative volume of right kidney receiving more than 15Gy for auto-plans with original and escalated prescriptions. C: Absolute volume of liver receiving less than 15Gy for auto-plans with original and escalated prescriptions. D: Maximum dose of spinal cord for auto-plans with original and escalated prescriptions.
Planning Efficiency
For all the auto-plans with the original or escalated prescriptions, Pinnacle3 AP generates dosimetrically acceptable for all 24 cases. All the plans with the same prescription utilized the same planning template (AP techniques). The same planning template means all the plans utilized the same beam geometry, IMRT objective, and IMRT optimization, and there was no manually-generated planning structures. Furthermore, after the automated planning process, there was no manual postoptimization adjustment of either IMRT objectives or planning structures. In other words, all the auto-plans were generated in a one-attempt manner. The consistent plan quality and little human intervention has demonstrated that AP could alleviate intensive effort from planners and expedited the planning process.
Discussion
Burgeoning technical advances have provided the impetus for the evolution of radiotherapy, which allows improved accuracy of target delineation, motion management, radiation delivery, and dose escalation. Stereotactic body radiotherapy (or SABR) came to light with implementations of all of these technical advances. Owing to its promising clinical results, SBRT becomes a more and more popular treatment option in contemporary clinical practice.6-8,52-55 Additionally, emerging data are positing that ablative radiation dose given by SBRT could potentially galvanize the immune response to further enhance tumoricidal effects.56,57 These encouraging results are likely to expand the role of SBRT in the multidisciplinary oncologic management.
However, challenges associated with SBRT have also arisen in the form of treatment planning: the need for accurate image registration among different image modalities, more stringent OAR dose constraints, and rapid dose fall-off surrounding the target. These notions are especially true for pancreas SBRT, largely due to the anatomic relationship between the target and highly radiosensitive OARs that are in close proximity to the target. Additionally, limited time and inconsistent experiences from different planners could cause suboptimal and inconsistent plan quality.58 Since the primary goal and benefits of automated planning is to automate the trial-and-error planning process—thereby potentially improving planning efficiency and consistency—evaluating automated planning on onerous pancreas SBRT cases is, therefore, a logical progression.
The most salient observation herein was the consistency and efficiency achieved by Pinnacle AP. All auto-plans, regardless of prescription, afforded similar and sufficient PTV coverage in a one-attempt manner. Moreover, data distribution of V100% and V93% (Figures 2 and 3) as well as DVH plot of the PTV evaluation structures (Figure 4) indicated that this automatic planning platform generates plans with consistent plan quality for the selected cases. Meanwhile, the auto-plans managed to control the doses to highly radiosensitive OARs satisfactorily. In a dose-escalation setting, this platform is also able to generate plans with sufficient PTV coverage as well as adequate OAR sparing. Furthermore, all the auto-plans, planned either with the original or escalated prescriptions, were generated in a less manually cumbersome manner, since all the plans were generated without any reoptimization by the planner. Our results suggest that Pinnacle3 AP could represent a solution to reduce possible disproportionate plan quality partially resulting from uneven experience from the planners, patient anatomy, or limited time for planning. However, the conformity indices of some of the targets may not be as perfect as we want. In our case, due to multiple targets with different prescriptions, the automatically generated planning objectives may be contradicting to themselves (e.g., the objectives of ring structure of PTV40 may be contradicting to those of PTV25). Our results, along with others’ experience with head and neck cases,42 may suggest the capabilities to better optimize plans with multiple targets are needed for the next-generation automated planning platform.
It should be noted that our study does not intend to demonstrate automated planning is superior to manual planning, but rather suggests that AP is able to generate acceptable treatment plans with consistency and efficiency. First, in our opinion, it is difficult to quantitatively evaluate “the level of education and experience of the planners” and whether the manual plan was pushed to its limit. Therefore, it is not easy to make a convincing statement about the comparison results, no matter which turns out to be better. Second, manual planning could achieve very good plan with meticulous adjustments of the planning structures and objectives on specific anatomy. It is very likely, though, at the expense of cumbersome planning structure tuning and manual trial-and-error process. This is especially true for the cases such as pancreas SBRT where the OARs are potentially in risky close proximity to the targets. Contrariwise, our purpose of the study is to contribute to fueling the broadened implementation of automated planning in clinical practice, the landscape of which may have foreseeable paradigm shifting because of the impact of machine artificial intelligence and automation.
In this study, we utilized a multiple static beam setup instead of volumetric-modulated arc therapy (VMAT), primarily due to the long computation time of auto-VMAT plans. It generally would take more than 7 hours to finish one auto-VMAT plan, with frequent “out-of-memory” interruptions on our nonclinical Pinnacle workstation because of the limited memory and computation resources. It took approximately 30 to 40 minutes to complete each auto-plan with our computational resources. Another limitation of this study is the lack of larger sample size, which is necessary to further corroborate the effectiveness and efficiency of Pinnacle3 AP on pancreas SBRT planning. Furthermore, we did not perform gamma analysis of intensity-modulated radiation therapy quality assurance (IMRT QA) for the auto-plans due to decommission of our linear accelerator, albeit our group has previously demonstrated the high passing rate of IMRT QA for other Pinnacle auto-plans.44 We also did not analyze the gradient indices due to our plans have multiple targets with different prescriptions (Figure 1). The demand of dose coverage for lower prescription target could be contradictory to the conventional rapid dose fall-off requirement for SBRT planning for one target case. The study is also limited by not directly demonstrating the clinical benefits utilizing this automated planning platform in accordance with dosimetry. Conversely, as a retrospective study, only dosimetric end points were evaluated herein; actual clinical implementation has yet to be conducted. In addition, all patients were treated and replanned following only 1 fractionation schedule, so the Pinnacle AP was not assessed for other clinically implemented fractionation schedules,59-62 which may have different OAR constraints. At last, we did not systematically investigate the design, current limitations as well as the potential improvements for this automated planning platform owing to inadequate access and resources to experiment on this commercially available product as a prototype.
We envision that automated treatment planning may play a more important role in radiotherapy. First, adaptive radiotherapy (adaptive RT) has been gradually implemented in the clinical practice.36,63-65 As the expeditious and consistent planning (and re-planning) is the key for adaptive RT, popularization of adaptive RT would demand further implementation of automated planning in a wider ranging disease sites. In this regard, our study would serve as a stepping stone to future practice of automated planning. Moreover, without the limitations of manual planning, such as inadequate time and unwillingness to approach the limit of the potential dose escalation, this automatic planning platform could represent a possible strategy to expeditiously generate quality plans with more aggressive prescription doses than originally intended. Second, recent breakthrough in artificial intelligence and other treatment modality (eg, immunotherapy) may invigorate the effort for clinicians to further explore potentially additive or synergistic clinical benefits of various dosing (escalation or de-escalation) and sequencing of SBRT when it is given with other treatment modalities. It is anticipated that automated planning is necessary to address the increased complexities in the future, such as various prescription levels and frequent re-planning.
Our future work includes the comparison the plan quality, planning time between Pinnacle AP with other automated planning platform such as Eclipse RapidPlan (RP). RapidPlan is another frontrunner in the field of automated planning, which utilizes statistical or machine learning methods to build predictive models for DVH estimation based on extracted “experience” from historical planning data. In contrast to an iterative approach of progressive optimization based predominantly on planning structures implemented in AP, RP requires a library of acceptable plans as the existing knowledge to configure a statistical model. We will conduct another study to compare the knowledge-based planning with Pinnacle AP when our clinical trial enrolls more patients for the RP model to be more statistical meaningful.
Conclusions
In summary, our pilot study showed that Pinnacle3 AP could efficiently generate treatment plans with consistent plan quality for pancreas SBRT planning, with or without dose escalation. It may contribute to more personalized, multidisciplinary oncologic management in the foreseeable future.
Acknowledgment
This work is partially supported by Philips Medical System under research agreement.
Abbreviations
- adaptive RT
adaptive radiotherapy
- AP
Auto-Planning
- DVH
dose–volume histogram
- IMRT
intensity-modulated radiotherapy
- OARs
organs at risk
- PTV
planning target volume
- RP
RapidPlan
- SABR
stereotactic ablative body radiotherapy
- SBRT
stereotactic body radiotherapy
- VMAT
volumetric-modulated arc therapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shuo Wang, PhD  https://orcid.org/0000-0002-3814-606X
https://orcid.org/0000-0002-3814-606X
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442 PubMed PMID: 29313949. [DOI] [PubMed] [Google Scholar]
- 2. Trakul N, Koong AC, Chang DT. Stereotactic body radiotherapy in the treatment of pancreatic cancer. Semin Radiat Oncol. 2014;24(2):140–147. doi:10.1016/j.semradonc.2013.11.008 PubMed PMID: 24635871. [DOI] [PubMed] [Google Scholar]
- 3. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi:10.1016/S0140-6736(10)62307-0 PubMed PMID: 21620466; PubMed Central PMCID: PMCPMC3062508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saif MW. Pancreatic neoplasm in 2011: an update. JOP. 2011;12(4):316–321. PubMed PMID: 21737886. [PubMed] [Google Scholar]
- 5. Abrams RA, Lowy AM, O’Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1751–1756. doi:10.1245/s10434-009-0413-9 PubMed PMID: 19390900. [DOI] [PubMed] [Google Scholar]
- 6. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. doi:10.1016/S1470-2045(15)70168-3 PubMed PMID: 25981812; PubMed Central PMCID: PMCPMC4489408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140(2):377–386. doi:10.1016/j.jtcvs.2009.12.054 PubMed PMID: 20400121. [DOI] [PubMed] [Google Scholar]
- 8. Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28(6):928–935. doi:10.1200/JCO.2009.25.0928 PubMed PMID: 20065181. [DOI] [PubMed] [Google Scholar]
- 9. Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiotherapy for large (non-small-cell lung cancerpooled analysis. Cancer. 2016. doi:10.1002/cncr.30375 PubMed PMID: 27741355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63(2):320–323. doi:10.1016/j.ijrobp.2005.07.002 PubMed PMID: 16168826. [DOI] [PubMed] [Google Scholar]
- 11. Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1017–1021. doi:10.1016/j.ijrobp.2003.11.004 PubMed PMID: 15001240. [DOI] [PubMed] [Google Scholar]
- 12. Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):678–686. doi:10.1016/j.ijrobp.2008.01.051 PubMed PMID: 18395362. [DOI] [PubMed] [Google Scholar]
- 13. Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e615-e622. doi:10.1016/j.ijrobp.2011.04.045 PubMed PMID: 21658854. [DOI] [PubMed] [Google Scholar]
- 14. Rwigema JC, Parikh SD, Heron DE, et al. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34(1):63–69. doi:10.1097/COC.0b013e3181d270b4 PubMed PMID: 20308870. [DOI] [PubMed] [Google Scholar]
- 15. Didolkar MS, Coleman CW, Brenner MJ, et al. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg. 2010;14(10):1547–1559. doi:10.1007/s11605-010-1323-7 PubMed PMID: 20839073. [DOI] [PubMed] [Google Scholar]
- 16. Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86(3):516–522. doi:10.1016/j.ijrobp.2013.02.022 PubMed PMID: 23562768. [DOI] [PubMed] [Google Scholar]
- 17. Verma V, Li J, Lin C. Neoadjuvant therapy for pancreatic cancer: systematic review of postoperative morbidity, mortality, and complications. Am J Clin Oncol. 2016;39(3):302–313. doi:10.1097/COC.0000000000000278 PubMed PMID: 26950464. [DOI] [PubMed] [Google Scholar]
- 18. Dawson LA, Jaffray DA. Advances in image-guided radiation therapy. J Clin Oncol. 2007;25(8):938–946. doi:10.1200/JCO.2006.09.9515 PubMed PMID: 17350942. [DOI] [PubMed] [Google Scholar]
- 19. Guckenberger M, Wilbert J, Richter A, Baier K, Flentje M. Potential of adaptive radiotherapy to escalate the radiation dose in combined radiochemotherapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79(3):901–908. doi:10.1016/j.ijrobp.2010.04.050 PubMed PMID: 20708850. [DOI] [PubMed] [Google Scholar]
- 20. Song W, Schaly B, Bauman G, Battista J, Van Dyk J. Image-guided adaptive radiation therapy (IGART): Radiobiological and dose escalation considerations for localized carcinoma of the prostate. Med Phys. 2005;32(7 pt 1):2193–2203. doi:10.1118/1.1935775 PubMed PMID: 28493587. [DOI] [PubMed] [Google Scholar]
- 21. Qing SW, Ju XP, Cao YS, Zhang HJ. Dose escalation of stereotactic body radiotherapy (SBRT) for locally advanced unresectable pancreatic cancer patients with CyberKnife: protocol of a phase I study. Radiat Oncol. 2017;12(1):6 doi: 10.1186/s13014-016-0760 -1 PubMed PMID: 28069017; PubMed Central PMCID: PMCPMC5223403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma SJ, Prezzano KM, Hermann GM, Singh AK. Dose escalation of radiation therapy with or without induction chemotherapy for unresectable locally advanced pancreatic cancer. Radiat Oncol. 2018;13(1):214 doi:10.1186/s13014-018-1158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaorsky NG, Lehrer EJ, Handorf E, Meyer JE. Dose escalation in stereotactic body radiation therapy for pancreatic cancer: a meta-analysis. Am J Clin Oncol. 2019;42(1):46–55. doi:10.1097/COC.0000000000000472 PubMed PMID: 29965809. [DOI] [PubMed] [Google Scholar]
- 24. Colbert LE, Rebueno N, Moningi S, et al. Dose escalation for locally advanced pancreatic cancer: how high can we go? bioRxiv. 2018:324129 doi:10.1101/324129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dose Escalation Trial of Stereotactic Body Radiation Therapy (SBRT) in Combination With GC4419 in Pancreatic Cancer. https://ClinicalTrials.gov/show/NCT03340974.
- 26. Stereotactic Body Radiotherapy Dose Escalation in Pancreatic Cancer. https://ClinicalTrials.gov/show/NCT02454140.
- 27. A Dose Escalation Trial of Stereotactic Body Radiotherapy (SBRT) After Induction Chemotherapy for Locally Advanced Pancreatic Cancer. https://ClinicalTrials.gov/show/NCT02643498.
- 28. MR Guided Dose Escalated RT + Concurrent Chemotherapy in Unresectable Pancreatic Cancer. https://ClinicalTrials.gov/show/NCT01972919.
- 29. A Dose Escalation Trial of SBRT After Induction Chemotherapy for Locally Advanced Pancreatic Cancer. https://ClinicalTrials.gov/show/NCT02873598.
- 30. Prior P, Chen X, Botros M, et al. MRI-based IMRT planning for MR-linac: comparison between CT- and MRI-based plans for pancreatic and prostate cancers. Phys Med Biol. 2016;61(10):3819–3842. doi:10.1088/0031-9155/61/10/3819 PubMed PMID: 27089554. [DOI] [PubMed] [Google Scholar]
- 31. Henke L, Kashani R, Robinson C, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126(3):519–526. doi:10.1016/j.radonc.2017.11.032 PubMed PMID: 29277446. [DOI] [PubMed] [Google Scholar]
- 32. Bohoudi O, Bruynzeel AME, Senan S, et al. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol. 2017;125(3):439–444. doi: 10.1016/j.radonc.2017.07.028 PubMed PMID: 28811038. [DOI] [PubMed] [Google Scholar]
- 33. Barney BM, Markovic SN, Laack NN, et al. Increased bowel toxicity in patients treated with a vascular endothelial growth factor inhibitor (VEGFI) after stereotactic body radiation therapy (SBRT). Int J Radiat Oncol Biol Phys. 2013;87(1):73–80. doi:10.1016/j.ijrobp.2013.05.012 PubMed PMID: 23920388. [DOI] [PubMed] [Google Scholar]
- 34. Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: Where do we stand? Clin Transl Radiat Oncol. 2018;8:4–11. doi:10.1016/j.ctro.2017.10.005 PubMed PMID: 29594236; PubMed Central PMCID: PMCPMC5862680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andreassen CN, Eriksen JG, Jensen K, et al. IMRT - Biomarkers for dose escalation, dose de-escalation and personalized medicine in radiotherapy for head and neck cancer. Oral Oncol. 2018;86:91–99. doi:10.1016/j.oraloncology.2018.09.001 PubMed PMID: 30409326. [DOI] [PubMed] [Google Scholar]
- 36. Martinez AA, Yan D, Lockman D, et al. Improvement in dose escalation using the process of adaptive radiotherapy combined with three-dimensional conformal or intensity-modulated beams for prostate cancer. Int J Radiat Oncol Biol Phys. 2001;50(5):1226–1234. PubMed PMID: 11483333. [DOI] [PubMed] [Google Scholar]
- 37. Feng M, Suresh K, Schipper MJ, et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol. 2018;4(1):40–47. doi:10.1001/jamaoncol.2017.2303 PubMed PMID: 28796864; PubMed Central PMCID: PMCPMC5766368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Multi-parametric Magnetic Resonance Imaging for Prostate Cancer Patients. https://ClinicalTrials.gov/show/NCT03180398. [Google Scholar]
- 39. Pembrolizumab With or Without Stereotactic Body Radiation Therapy in Treating Patients With Advanced or Metastatic Merkel Cell Cancer. https://ClinicalTrials.gov/show/NCT03304639. [Google Scholar]
- 40. A Study of Individualized Radiotherapy Based on a Prediction Model of Lymph Node Metastasis in Hepatocellular Carcinoma. https://ClinicalTrials.gov/show/NCT03416803. [Google Scholar]
- 41. Hazell I, Bzdusek K, Kumar P, et al. Automatic planning of head and neck treatment plans. J Appl Clin Med Phys. 2016;17(1):5901 PubMed PMID: 26894364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krayenbuehl J, Norton I, Studer G, Guckenberger M. Evaluation of an automated knowledge based treatment planning system for head and neck. Radiat Oncol. 2015;10:226 doi:10.1186/s13014-015-0533-2 PubMed PMID: 26555303; PubMed Central PMCID: PMCPMC4641383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quan EM, Chang JY, Liao Z, Xia T, Yuan Z, Liu H, et al. Automated volumetric modulated arc therapy treatment planning for stage III lung cancer: how does it compare with intensity-modulated radio therapy? Int J Radiat Oncol Biol Phys. 2012;84(1):e69-e76. doi:10.1016/j.ijrobp.2012.02.017 PubMed PMID: 22901421; PubMed Central PMCID: PMCPMC3428745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang S, Zheng D, Zhang C, et al. Automatic planning on hippocampal avoidance whole-brain radiotherapy. Med Dosim. 2017;42(1):63–68. doi:10.1016/j.meddos.2016.12.002 PubMed PMID: 28237294. [DOI] [PubMed] [Google Scholar]
- 45. Wu B, Kusters M, Kunze-Busch M, et al. Cross-institutional knowledge-based planning (KBP) implementation and its performance comparison to Auto-Planning Engine (APE). Radiother Oncol. 2017;123(1):57–62. doi:10.1016/j.radonc.2017.01.012. PubMed PMID: 28202228. [DOI] [PubMed] [Google Scholar]
- 46. Kusters JMAM, Bzdusek K, Kumar P, et al. Automated IMRT planning in Pinnacle: a study in head-and-neck cancer. Strahlenther Onkol. 2017;193(12):1031–1038. doi:10.1007/s00066-017-1187-9 PubMed PMID: 28770294. [DOI] [PubMed] [Google Scholar]
- 47. Xia P, Kotecha R, Sharma N, et al. A treatment planning study of stereotactic body radiotherapy for atrial fibrillation. Cureus. 2016;8(7):e678 doi:10.7759/cureus.678 PubMed PMID: 27563504; PubMed Central PMCID: PMCPMC4985047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gintz D, Latifi K, Caudell J, et al. Initial evaluation of automated treatment planning software. J Appl Clin Med Phys. 2016;17(3):331–346. doi:10.1120/jacmp.v17i3.6167. PubMed PMID: 27167292; PubMed Central PMCID: PMCPMC5690942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xhaferllari I, Wong E, Bzdusek K, Lock M, Chen J. Automated IMRT planning with regional optimization using planning scripts. J Appl Clin Med Phys. 2013;14(1):4052 PubMed PMID: 23318393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121(7):1128–1137. doi:10.1002/cncr.29161 PubMed PMID: 25538019; PubMed Central PMCID: PMCPMC4368473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–4101. doi:10.1118/1.3438081 PubMed PMID: 20879569. [DOI] [PubMed] [Google Scholar]
- 52. Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18(4):1081–1087. doi:10.1245/s10434-010-1405-5 PubMed PMID: 21046264. [DOI] [PubMed] [Google Scholar]
- 53. Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572–1578. doi:10.1200/JCO.2008.19.6329 PubMed PMID: 19255321. [DOI] [PubMed] [Google Scholar]
- 54. Boike TP, Lotan Y, Cho LC, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol. 2011;29(15):2020–2026. doi:10.1200/JCO.2010.31.4377 PubMed PMID: 21464418; PubMed Central PMCID: PMCPMC3138546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109(2):217–221. doi:10.1016/j.radonc.2013.08.030 PubMed PMID: 24060175. [DOI] [PubMed] [Google Scholar]
- 56. Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13(8):516–524. doi:10.1038/nrclinonc.2016.30 PubMed PMID: 26951040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Verma V, Lin SH. Implications of the bystander and abscopal effects of radiation therapy. Clin Cancer Res. 2016;22(19):4763–4765. doi: 10.1158/1078-0432.CCR-16-1512. PubMed PMID: 27458248. [DOI] [PubMed] [Google Scholar]
- 58. Nelms BE, Robinson G, Markham J, et al. Variation in external beam treatment plan quality: an inter-institutional study of planners and planning systems. Pract Radiat Oncol. 2012;2(4):296–305. doi:10.1016/j.prro.2011.11.012 PubMed PMID: 24674168. [DOI] [PubMed] [Google Scholar]
- 59. Tozzi A, Comito T, Alongi F, et al. SBRT in unresectable advanced pancreatic cancer: preliminary results of a mono-institutional experience. Radiat Oncol. 2013;8:148 doi:10.1186/1748-717X-8-148 PubMed PMID: 23799996; PubMed Central PMCID: PMCPMC3707803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):181–188. doi:10.1016/j.ijrobp.2010.05.006 PubMed PMID: 21549517. [DOI] [PubMed] [Google Scholar]
- 61. Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78(3):735–742. doi:10.1016/j.ijrobp.2009.08.046 PubMed PMID: 20171803. [DOI] [PubMed] [Google Scholar]
- 62. Polistina F, Costantin G, Casamassima F, et al. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17(8):2092–2101. doi:10.1245/s10434-010-1019-y PubMed PMID: 20224860. [DOI] [PubMed] [Google Scholar]
- 63. Colvill E, Booth J, Nill S, et al. A dosimetric comparison of real-time adaptive and non-adaptive radiotherapy: a multi-institutional study encompassing robotic, gimbaled, multileaf collimator and couch tracking. Radiother Oncol. 2016;119(1):159–165. doi:10.1016/j.radonc.2016.03.006 PubMed PMID: 27016171; PubMed Central PMCID: PMCPMC4854175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nijkamp J, Pos FJ, Nuver TT, et al. Adaptive radiotherapy for prostate cancer using kilovoltage cone-beam computed tomography: first clinical results. Int J Radiat Oncol Biol Phys. 2008;70(1):75–82. doi:10.1016/j.ijrobp.2007.05.046 PubMed PMID: 17869445. [DOI] [PubMed] [Google Scholar]
- 65. Li Y, Hoisak JD, Li N, et al. Dosimetric benefit of adaptive re-planning in pancreatic cancer stereotactic body radiotherapy. Med Dosim. 2015;40(4):318-324. doi:10.1016/j.meddos.2015.04.002 PubMed PMID: 26002122. [DOI] [PubMed] [Google Scholar]