Abstract
Objective
To propose and test the validity of a new syndrome called retrograde cricopharyngeus dysfunction (R-CPD) that explains inability to belch and the associated symptoms of loud gurgling noises, chest and abdominal pain/distention, and excessive flatulence, as well as to report the results of botulinum toxin (BT) injection into the cricopharyngeus muscle (CPM) for both diagnosis and treatment of R-CPD.
Study Design
To develop a case series of consecutive patients matched to the syndromic features of R-CPD, inject the CPM with BT as a concurrent diagnostic and therapeutic maneuver, and assess results.
Setting
Bastian Voice Institute (Downers Grove, Illinois).
Subjects and Methods
Consecutive (unselected) patients presenting with inability to belch and associated symptoms were matched to the proposed syndrome of R-CPD, treated with BT, and followed for effect on symptoms over time.
Results
All 51 patients achieved ability to belch and relief of associated symptoms, and the majority seem to have “retrained” the ability to belch on a potentially “permanent” basis.
Conclusion
R-CPD can be diagnosed syndromically, using a symptom complex; clinical diagnosis is validated by relief of symptoms after BT injection; and BT into the CPM is an efficacious treatment, whose benefit appears to often last longer than the pharmacologic duration of action of BT.
Keywords: belch, burp, flatulence, upper esophageal sphincter, cricopharyngeus, botulinum toxin
Study Highlights
- What is current knowledge?
- There are patients with lifelong inability to belch and debilitating associated symptoms who fail to be diagnosed or treated despite repeated interactions with the medical community because the syndrome of retrograde cricopharyngeus dysfunction (R-CPD) is virtually unknown.
- What is new here?
- Codification of the syndrome of R-CPD to facilitate straightforward recognition/diagnosis.
- The first known report of results of botulinum toxin injection into the cricopharyngeus muscle to serve as a combination diagnostic test/treatment for R-CPD.
Introduction
In November 2015, we encountered a desperate patient who described the severe, daily constellation of symptoms listed in Table 1 . He had seen numerous doctors and undergone many tests, yet without a diagnosis or any relief. Via deduction, the author posited the explanation to be a new diagnosis he called “retrograde cricopharyngeus dysfunction” (R-CPD). All of the patient’s symptoms disappeared after cricopharyngeus muscle (CPM) injection of botulinum toxin (BT). Without the knowledge of the authors, that initial patient posted his experience to the Internet (Reddit). Additional patients self-presented when their Internet searches stumbled upon Reddit. After diagnosing and successfully treating these additional patients, the author searched the literature and found 3 case reports describing elements of this disorder, but using other terminology. Kahrilas et al,1 Waterman and Castell,2 and Tomizawa et al3 each described a single patient who was unable to belch. One described chest pain, gurgling, and an inability to vomit,1 and a second described chest pain with acid reflux.2 The third complained of “unendurable” abdominal bloating.3 The 3 authors each performed manometric and esophageal fluoroscopy studies to support their diagnosis of a disorder of the “belch reflex.” They advised lying in a head-down position for pain relief. They also suggested other treatment ideas, although with none of these subsequently reported in the literature, and none proposed BT injection of the upper esophageal sphincter (UES).
Table 1.
Syndromic Features of Retrograde Cricopharyngeus Dysfunction.
| 1. The inability to belch |
| 2. Abdominal bloating and discomfort/nausea, or chest pain, especially after eating |
| 3. Socially awkward gurgling noises from the chest and lower neck as though the esophagus is churning and straining to eject the air |
| 4. Excessive flatulence |
| 5. Social inhibition (a result of 2, 3, and 4) |
| 6. Difficulty vomiting (common but not universal) |
To date, the authors have treated 121 individuals with what we continue to call R-CPD. Such patients experience the distressing, debilitating, and often socially crippling symptoms described here on a daily basis. None had been previously diagnosed or successfully treated despite intense search for answers from the medical community. The details of this intense search are fascinating but beyond the scope of this article.
Our objective is to report here on the first 51 sequential patients we diagnosed with R-CPD, rather than all 121, because at the time of data analysis and manuscript preparation, we had not yet reached at least 6 months after BT injection. We have attempted to answer 4 questions: (1) Is the constellation of symptoms described by this cohort ( Table 1 ) sufficient to establish R-CPD as a robust new “syndrome?” (2) Does the codified syndrome for R-CPD have diagnostic “power” for new patients? (3) Does botulinum toxin validate the diagnosis by restoring the ability to belch and abolishing associated symptoms? (4) Do any individuals experience benefit beyond the duration of action of the botulinum toxin?
While information about this disorder has been shared for more than 3 years with many physicians in the United States, England, Australia, Canada, Belgium, New Zealand, Germany, Turkey, and so on, this publication appears to represent the first formal definition and codification of the syndrome of R-CPD and results of its treatment with BT injection.
Statement of Ethics in Human Research
Aspire IRB (Pasadena, California) has officially approved this study with a waiver of consent.
Materials and Methods
Our general study design was to abstract existing data from Bastian Voice Institute charts of the first 51 of our caseload with R-CPD. This therefore represents a sequential case series. We tabulated the symptoms described below at the time of diagnosis and at various intervals after BT injection. Presence or absence of symptoms (yes/no) are the main data studied. Although not the focus of our study, we also summarized prior workup(s) performed elsewhere that had failed to yield a diagnosis.
The setting of this study was a tertiary care laryngology practice. The participants were individuals who sought our help based upon the Internet postings of the index and many subsequent patients with R-CPD symptoms. Our data source was the binary (yes/no) patient report of symptoms during in-person encounters and, after treatment, via telephone and encrypted email. All of these data existed as a part of the clinical process.
The statistics we employed were simple and as displayed in Table 2 , Figure 1 , and Figure 2 .
Table 2.
Patient Profile for Cohort of 51 Patients with Retrograde Cricopharyngeus Dysfunction.
| Patient Profile | Value |
|---|---|
| Total patient population, No. (%) | 51 (100) |
| Male, No. (%) | 30 (58.80) |
| Female, No. (%) | 21 (41.20) |
| Mean age, y | 30 |
| Age range, y | 16-63 |
Figure 1.

Prior tests and treatments that had not provided a diagnosis. CT, computed tomography; EGD, esophagogastroduodenoscopy; PPI, proton pump inhibitors.
Figure 2.
Prevalence of symptoms associated with retrograde cricopharyngeus dysfunction: at diagnosis, 1 week after botulinum toxin, and at 6 months posttreatment. Percent based on denominator (number evaluable at each time point).
Our diagnostic and therapeutic clinical protocol was as follows: (1) we obtained yes/no data about the ability to belch and associated symptoms of the emerging diagnosis of R-CPD as shown in Table 1 . (2) In an office setting, we performed an upper aerodigestive tract neurological examination. (3) We conducted a videoendoscopic swallowing study (VESS).4 This videodocuments (a) structure and neurological function of tongue, palate, pharynx, and vocal cords; (b) presence or absence of pooling of saliva; and (c) ability to swallow blue-stained applesauce, water, and orange-colored crackers. (4) We injected 50 units of BT into the cricopharyngeus muscle via upper esophagoscopy during brief, outpatient general anesthesia. (5) Again, as a part of our clinical routine, we obtained the same binary yes/no data about the Table 1 syndromic symptoms that we had already obtained at the first meeting.
As for source of patients and clinical decision making, every patient came to the author’s practice “from the Internet.” The author had no role in this Internet activity. No patient had received a diagnosis elsewhere. The author explained to each patient the concept of R-CPD and the logistics, technical details, risks, and potential benefits of a diagnostic/therapeutic BT injection. The experiences of patients already treated were summarized for them as well. Every patient was strongly motivated and indicated his or her perception of severity of the disorder as “6” or “7” on a 7-point maximum-severity Likert scale included in their intake questionnaires.
In more detail, the injection technique was as follows: after induction of general anesthesia, a laryngoscope was used as an upper esophagoscope. The CPM bulge was visualized and palpated, especially when overlying mucosa was redundant, and injected in several locations ( Figure 3 ) using a 25-gauge butterfly needle held with a laryngoscopy forceps. Deep and superficial components of the sphincter were targeted. A total dose of 50U in 2 mL was used.
Figure 3.

Intraoperative view of a botulinum toxin injection. Four locations in the cricopharyngeus bulge were injected. Here, we see (a) the right paramedian and (b) left paramedian. Deeper injections are not shown.
In 2 patients, the laryngoscope was barely long enough. The backup plan—use of the Weerda bivalve upper esophagoscope employed by the author to perform laser cricopharyngeus myotomy for patients with antegrade CPD ± Zenker’s diverticulum—was not needed in any of the patients.
Four patients chose to have the injection procedure in-office with local anesthesia under electromyogram (EMG) guidance. The skin overlying the cricothyroid membrane was infiltrated with 2% xylocaine with epinephrine 1:100,000. Then, 1 mL was also introduced into the subglottis for topical anesthesia. A Teflon-coated EMG needle was used to pierce the cricothyroid membrane. From there, the needle traversed the lumen, posterior cricoid plate, and posterior cricoarytenoid muscles to reach the cricopharyngeus muscle. The posterior cricoarytenoid (PCA) muscles were protected from injection by having the patients “sniff” and avoiding injecting where a burst of EMG signal resulted.
Results
Patient demographics are found in Table 2 . The first 51 patients accumulated across more than 2 years came from 20 states and 3 foreign countries. Prior tests and treatment trials are summarized in Figure 1 .
The syndromic criteria for R-CPD to which all 51 patients were evaluated are found in Table 1 and are further described below as follows: 50 of 51 said they had recognized the inability to belch “as long as I can remember”; the 1 outlier said he could belch up until 2 years earlier. A few could release air by inducing hard gagging or vomiting several times per day. At least 15 (29%) had been told they could not be burped in infancy and had colic, projectile vomiting, or “incredible gassiness.” Information about ability to burp in infancy was not available for the remaining 36 patients.
Another symptom comprising the syndrome and found in 49 of 51 were discomforts that might be speculated as due to distention of the esophagus, stomach, and intestines: central sharp chest pain, abdominal bloating and distention, and occasionally nausea, especially after eating. Pain or terrible pressure was reported variably in the lower throat, chest, abdomen, and back. Numerous patients said, “I begin the day with a flat stomach, and by the end of the day I appear pregnant.” Another patient’s wife said, “At the end of the day, my husband’s stomach protrudes and is as hard as a rock.” See also Figure 4 for a visual depiction of these symptoms. Many patients also had gastroesophageal reflux symptoms causing significant heartburn; in none had PPIs (proton pump inhibitors) provided relief of the symptoms of R-CPD, however.
Figure 4.
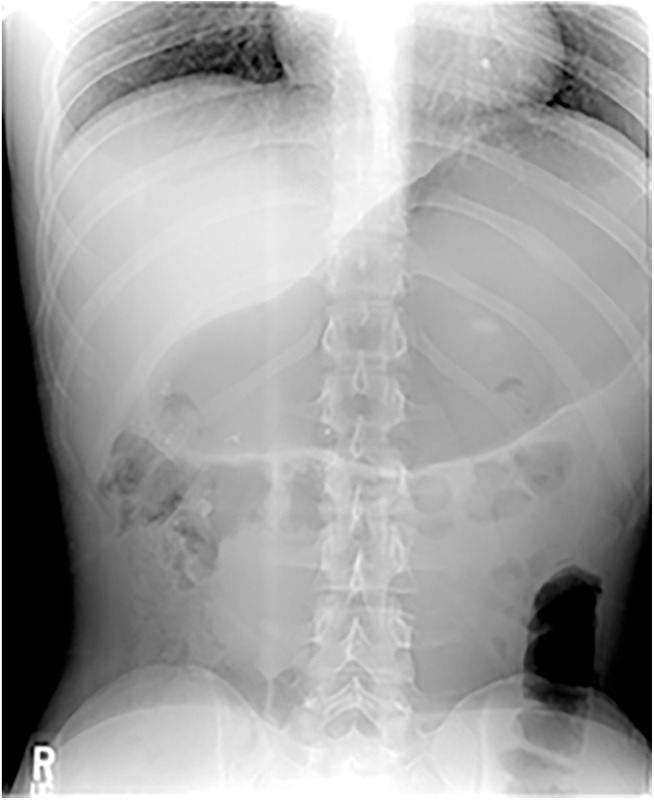
Abdominal film of a patient with retrograde cricopharyngeus dysfunction (R-CPD). While such a film by itself does not “make” the diagnosis of R-CPD as compared with “match” to the syndromic features, this film makes the nearly universal symptoms of feeling bloated, visible abdominal distention, and flatulence “visible.” Photo credit: Urgent Care 24/7–Midtown (Savannah, Georgia).
A third primary symptom, found in 50 of 51, was gurgling noises from the chest and lower neck or abdomen. One patient said, “I can’t eat for 6 hours before going to class because the sounds would be so distracting to other students sitting near me.” The sounds were described as “loud gurgling,”“frogs croaking,”“dinosaur noises,”“strangled whale,” or “monster sounds.”
The fourth primary symptom comprising the syndrome of R-CPD was less universal and found in 43 of 51: excessive flatulence. This was a significant issue for all but 8 patients. One young woman said, “Everywhere I go, I scan my surroundings for a private place to go briefly to release gas.” See also the air in the colon in Figure 4 .
Other common symptoms or characteristics of patients with R-CPD for which we do not have formal data deserve mentioning. Because the symptoms of R-CPD are dramatically amplified during and after eating and drinking, patients often described social anxiety caused by their gurgling noises, bloating, and flatulence. Many would not eat outside of their own home and avoided carbonated or alcoholic beverages. Two patients had difficulty traveling by air, and they reported that their unrequited need to burp and associated symptoms (again summarized in Table 1 ) were intensified upon ascent. Many also described difficulty vomiting. Some were never able to vomit, even after food poisoning, while still others reported being able to vomit only after violent and painful heaving. Several noted when vomiting, they initially released “incredible” amounts of air.
The result of upper aerodigestive tract examination and videoendoscopic swallowing study was that no patient had focal neurological findings in tongue, palate, pharynx, or larynx. Furthermore, videoendoscopic swallowing studies were in the aggregate normal. (Two patients had mild, subclinical postswallow hypopharyngeal residue.)
At the time of CPM injection, the ease of visualization of the CPM varied between patients due to dental/jaw/neck anatomy. Two patients were extraordinarily challenging to inject due to retrognathism and a narrow mandibular arch. Due to the effort required in one, a scrape was created on the posterior pharyngeal wall. She developed fever and mild swelling in the area of the abrasion. This resolved with 2 doses of intravenous antibiotics followed by completion of the antibiotic course by oral route. Mucosal redundancy was extraordinary in a few patients, somewhat like finding the muscle behind a redundant and billowing shower curtain. Here, the muscle was identified mostly by palpation. Esophageal dilation beyond the sphincter, a common author observation during upper esophagoscopy but without objective measurement, is demonstrated to some degree in Figure 5 .
Figure 5.
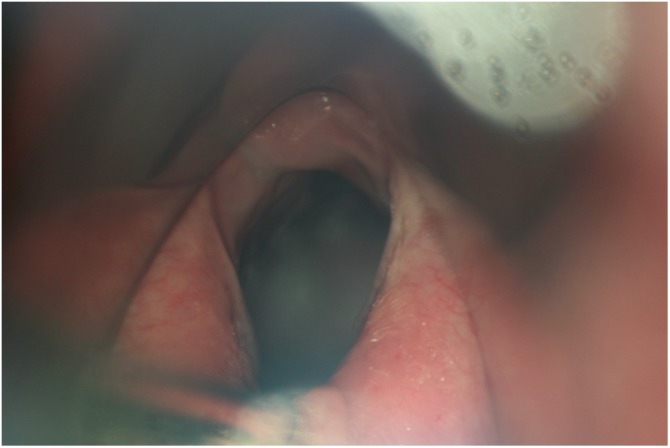
Patulous esophagus. The cricopharyngeus muscle (CPM) is being retracted posteriorly with a suction cannula (under arrow). As an unvalidated observation, the esophagus seen beyond the CPM typically appears to be abnormally open and even dilated.
The effect of BT injection on symptoms is displayed in Figure 2 .
Every patient experienced either complete or at least a major reduction of all symptoms. All 51 patients were able to belch after injection. At this writing, all 51 patients had their injection 6 months ago or longer.
A description of the composite (and 2 outlier) postinjection experiences follows here and is summarized as well in Figure 2 . Aside from the outliers already described, postoperative discomfort was very tolerable. Virtually every patient said that he or she began to experience “micro-burps” 24 to 48 hours after injection. All had to interact with their newfound ability to belch to control initiation or timing. In this way, across the first few weeks, they made belching routine and more predictable. Several “slow starters” mentioned that turning the head seemed to help. A few mentioned that a belch would happen involuntarily when they would cough. Several experienced acid brash upon belching. Fifty of 51 patients were relieved of their abdominal bloating, and all but 4 were relieved of their gurgling noises and excessive flatulence. Each patient described transient low throat “lodgment” of solids beginning the second or third day after the injection. We speculated that this was the result of food pausing within a flaccid UES. This problem was easily managed by ingesting soft, wet foods in small amounts followed by a liquid “chaser” just until the patient became unconcerned by this initially unfamiliar swallowing sensation.
While the duration of therapeutic benefit of BT injection for laryngeal dystonia is typically 4 months, the benefit of lower esophageal sphincter injection for achalasia is considered to be much longer. Therefore, we defined long-term results as those assessed at least 6 months after injection. Eleven of 38 patients for whom we have comprehensive long-term results became less able or unable to burp again, and their other symptoms also returned between 8 and 20 weeks after injection. This happened to a lesser degree with 1 additional patient who lost ability for a week and then regained ability when she was counseled to turn her head and to use less effort to push air out of the esophagus. Four of the patients who had lost the ability to belch have undergone a second Botox injection. One underwent this second injection in the operating room again under brief general anesthesia as before, and 3 had the injection procedure in-office. All 4 patients have since regained the ability to belch and associated symptoms are again resolved. One, who had the second injection in-office, lost the ability the second time after 3 months. He has since had a third injection in-office, which he noted worked better than the first 2, with easier ability to belch and relief of related symptoms.
Discussion
This report may be the first description of the complete syndrome of R-CPD. Furthermore, it appears to be the first to report the use of BT to simultaneously diagnose and to treat this condition in a large caseload. We were unable to find reports of the results of other treatments such as “fundoplication, cricopharyngeal myotomy, operant conditioning, and periodic nasogastric intubation.”1,2
The main result of our study is that it appears that R-CPD can be identified robustly using the combination of preliminary identification of key symptoms that comprise a “syndrome” of R-CPD, followed by confirmation of the diagnosis by achieving relief of these same symptoms after BT injection into the CPM. Preliminarily, it is appearing that many seem to “retrain” the sphincter permanently in retrograde function.
BT injection into the CPM has been previously reported for a different condition: a swallowing problem seen mostly in older persons caused by antegrade CPD (A-CPD).5-7 In the senior author’s extensive experience with that condition, A-CPD is much better treated with cricopharyngeus myotomy, and none of the patients with R-CPD reported here had any symptoms or findings of A-CPD.
For persons with the R-CPD symptoms on a longstanding/lifelong basis, it appears reasonable to diagnose the disorder syndromically. In those who match the syndrome, BT injection of the CPM can then serve as the definitive test to validate (or not) the syndromic diagnosis. R-CPD as a diagnosis was validated by BT injection in all 51 of the patients reported here.
Our reasons for this simple and direct diagnostic model are as follows: (1) the extensive testing many of our patients had undergone elsewhere (eg, esophagoscopy, barium studies, and esophageal manometry) had failed to make a diagnosis of R-CPD. While radiographic imaging using barium contrast easily points to a diagnosis of A-CPD, it does not diagnose R-CPD. Common (incorrect) diagnoses after extensive conventional workup done elsewhere (barium studies, esophagoscopy, manometry, etc) were acid reflux, irritable bowel syndrome, or “stress.” No patient had noted relief from treatment of these conditions. (2) As shown here, our simple diagnostic model appears to diagnose new patients robustly. (3) Given the duration of symptoms, it does not seem necessary to rule out ominous diagnoses. (4) Each of our 51 patients in a sense diagnosed himself or herself from Internet postings of other sufferers who had already been diagnosed. Hence, physicians can likely use the same syndromic criteria with even deeper understanding and skill. (5) The 15 (29%) of patients diagnosed by the author using only the syndromic criteria, office examination and VESS responded to BT equally, with cost savings and protection from the “medical jadedness” and even cynicism that so many patients experienced after their prolonged but fruitless search for an explanation for and relief from their R-CPD symptoms. One man said, “I read about all of the people who had so many tests and nothing came of it for them, so I decided to just come directly to you for the Botox injection that had helped them all so much.”
Of course, other clinicians may prefer to expand our diagnostic protocol and attempt to discover objective measures that have utility in further defining and more scientifically validating this disorder, particularly for research purposes. Furthermore, use of a placebo arm of treatment for a study group would also be of interest.
Speculatively, after BT injection, transient dysphagia might be explained as follows: the food material is delivered to a post-BT adynamic upper esophageal sphincter that did not (by comparison with normal swallowing) clamp down immediately after bolus passage and “send” the bolus onward to the next segment of the esophagus. Esophageal dilation/damage caused by years of R-CPD might have also impaired esophageal transit.
We cannot explain why many appear to maintain the ability to belch long after the botulinum toxin has worn off. Conversely, we cannot explain why, at an average of 16.7 months postinjection, 11 of the 51 did lose the ability to belch. Possibly, for these 11, placement may have been suboptimal or the dose insufficient. Still, relief of their symptoms, even for a few months, validates the diagnosis of R-CPD.
Generalizability
Other clinicians can master the syndromic features of R-CPD without difficulty. No special training beside the description and photo provided here would be necessary for many clinicians who work routinely around the CPM for other disorders such as A-CPD. Once R-CPD is more widely known, patients will not need to travel from “20 states and 3 foreign countries” for diagnosis and treatment. In fact, accumulation of this large of a series may not happen again as future patients find a source of both diagnosis and treatment close to home.
Future Studies
What is the pathophysiology of R-CPD?
Why do some achieve seemingly “permanent” benefit from a single injection of BT, while others do not?
For those who lose benefit, does myotomy work equally well and permanently?
What is the role of aerophagia in persons with R-CPD? Are there persons who cannot belch but have little or no distress because they swallow less air?
What is the familial incidence of R-CPD? Several patients mentioned a family member with the same problem.
What is the typical neuromuscular junction distribution within the CPM, for purposes of optimizing BT placement?
What is the best method of injection in an office setting? [In progress.]
Should pediatricians put R-CPD into the differential diagnosis of infants whose parents cannot burp them, when those babies also experience projectile vomiting after feedings, extreme colic, unusual flatulence, and even failure to thrive? This question has already been communicated to a nearby major pediatric hospital.
Conclusion
R-CPD appears to be effectively diagnosed by matching the patient’s symptoms with the codified syndromic features described here. The diagnosis can then be validated by BT injection into the CPM. In the majority, 1 injection appears to “retrain” the UES/CPM for its retrograde function.
Author Contributions
Robert W. Bastian, original idea/conception and design of the work, and clinical activity that provided the information; writing the paper and revising it, final approval of the version to be published, and the person accountable for all aspects of the work and answering questions regarding its accuracy and integrity; Melissa L. Smithson, collection of data, tabulation and analysis of data, editorial suggestion, manuscript preparation, and final sign-off of the manuscript.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
Footnotes
No sponsorships or competing interests have been disclosed for this article.
References
- 1. Kahrilas PJ, Dodds WJ, Hogan WJ. Dysfunction of the belch reflex: a cause of incapacitating chest pain. Gastroenterology. 1987;93:818-822. [DOI] [PubMed] [Google Scholar]
- 2. Waterman DC, Castell DO. Chest pain and inability to belch. Gastroenterology. 1989;96:274-275. [DOI] [PubMed] [Google Scholar]
- 3. Tomizawa M, Motoyasu K, Takashi A, et al. A case of inability to belch. J Gastroenterol Hepatol. 2001;16:349-351. [DOI] [PubMed] [Google Scholar]
- 4. Bastian R. Videoendoscopic evaluation of patients with dysphagia: an adjunct to the modified barium swallow. Otolaryngol Head Neck Surg. 1991;104:339-350. [DOI] [PubMed] [Google Scholar]
- 5. Schneider I, Thumfart WF, Pototschnig C, Eckel HE. Treatment of dysfunction of the cricopharyngeal muscle with botulinum A toxin: introduction of a new, noninvasive method. Ann Otol Rhinol Laryngol. 1994;103:31-35. [DOI] [PubMed] [Google Scholar]
- 6. Murry T, Wasserman MS, Carrau RL, Castillo B. Injection of botulinum toxin A for the treatment of dysfunction of the upper esophageal sphincter. Am J Otolaryngol. 2005;26:157-162. [DOI] [PubMed] [Google Scholar]
- 7. Kelly EA, Koszewski IJ, Jaradeh SS, Merati AL, Blumin JH, Bock JM. Botulinum toxin injection for the treatment of upper esophageal sphincter dysfunction. Ann Otol Rhinol Laryngol. 2013;122:100-108. [DOI] [PMC free article] [PubMed] [Google Scholar]



